Abstract
Group 2 innate lymphoid cells (ILC2s) are recently identified members of the innate lymphoid cell (ILC) family. These cells play critical roles in the regulation of type 2 cytokine responses during helminth infection, allergic inflammation, tissue remodeling, and metabolic homeostasis. ILC2s differentiate from a bone marrow-derived common lymphoid progenitor and are found in humans and mice in lymphoid tissues and at multiple epithelial barrier surfaces such as the lung, intestine, and skin. These cells are activated by the epithelial cell-derived cytokines interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP) to produce the type 2 cytokines IL-4, IL-5, IL-9, and IL-13, thus contributing to type 2 immune responses. Emerging data suggest that these cells have additional functions, including interacting directly with other innate and adaptive cell types, promoting tissue remodeling, and influencing metabolic pathways, that help to regulate type 2 inflammatory responses while maintaining tissue homeostasis. This chapter focuses on the basic biology of ILC2 development, activation, and effector function and highlights recent advances in our understanding of how ILC2s contribute to type 2 cytokine-dependent immunity, inflammation, and tissue homeostasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Type 2 immune responses play a key role in the initiation, maintenance, resolution, or prevention of numerous human disease states, including infection with parasitic helminths, allergic diseases, fibrosis, and metabolic disorders [1–5]. Type 2 cytokine responses drive protective immunity to parasitic helminths as well as pathologic allergic inflammation associated with diseases such as asthma, atopic dermatitis (AD), and food allergy [1–5]. In addition, these responses are also associated with tissue remodeling and repair and metabolic homeostasis [1–5]. Thus, type 2 immune responses are linked to a wide array of diseases that together are responsible for a significant public health and economic burden worldwide, and a better understanding of the regulation of type 2 inflammation has the potential to inform treatment and management of many of these diseases [1–5].
Type 2 immune responses begin with the production of epithelial cell-derived cytokines including interleukin (IL)-25, IL-33, and thymic stromal lymphopoietin (TSLP) and antigen presentation by antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages [1–3, 6, 7]. These early responses in turn promote production of the cytokines IL-4, IL-5, IL-9, and IL-13, development and activation of CD4+ T helper type 2 (TH2) cells, antigen-specific immunoglobulin (Ig)E production, and recruitment of innate effector cell populations such as eosinophils, mast cells, and basophils to the epithelial barrier [1–3, 6]. Together, these responses act back upon the epithelial barrier to regulate effector mechanisms such as mucus production, smooth muscle contractility, and barrier permeability that mediate helminth expulsion, result in signs and symptoms of type 2 inflammation, or contribute to reparative or homeostatic processes [1–5].
While a significant body of literature describes how responses by adaptive T and B cells promote type 2 cytokine responses, less is known regarding the regulation of innate immune responses that drive the initiation, maintenance, and resolution of these responses [1, 7]. Importantly, studies in the last 5 years have identified a novel subset of innate immune cells, the group 2 innate lymphoid cells (ILC2s), that is found in humans and mice in multiple tissues and is critical in the development of type 2 cytokine responses [3, 7–11]. This chapter describes recent advances in our understanding of the development and activation of ILC2s and how these cells contribute to type 2 inflammation in the context of helminth infection and allergy. Additionally, emerging work is discussed that describes alternative roles of ILC2s in promoting tissue remodeling and metabolic homeostasis. In particular, recent studies are highlighted that reveal how ILC2 responses could be targeted therapeutically to treat diseases in which ILC2-associated type 2 inflammation plays a role.
Identification and Definition of ILC2s
ILC2s are a newly described subset of innate cells within the ILC family, which includes classical natural killer (NK) cells and lymphoid tissue-inducer (LTi) cells [3, 7–17]. All ILCs lack expression of cell lineage markers associated with T cells, B cells, DCs, macrophages, and granulocytes, but do express CD90 (Thy1), the stem cell factor (SCF) receptor c-Kit, CD25 (IL-2 receptor (R)α), and CD127 (IL-7Rα) [3, 7–17]. As NK cells appear to have distinct developmental and functional characteristics when compared to other ILC subsets described below [3, 7–17], here the term “ILC” will be used to refer to only non-NK cell members of the ILC family.
The ILC family is currently categorized into three major subsets. These subsets are comparable to the three major CD4+ T helper lineages and are distinguished by their differential requirements for transcription factors during development and expression of distinct transcription factors and effector cytokines by mature cells [8]. The group 1 ILCs (ILC1s) include newly described innate cells that are T-box expressed in T cells (T-bet)-dependent, produce interferon-γ (IFN-γ), and promote immunity to intracellular pathogens and intestinal inflammation (classical NK cells are also categorized into this group). The group 3 ILCs (ILC3s) include retinoic acid receptor-related orphan receptor γ (RORγt)-dependent LTis and other RORγt-dependent cells that respond to IL-23, produce IL-17A and/or IL-22, and support lymphogenesis in the fetus and in adults, immunity to extracellular bacteria, and inflammation at multiple mucosal and barrier surfaces [8, 10, 12, 13, 15–19].
In contrast, ILC2s are dependent upon and express the transcription factors retinoic acid receptor-related orphan receptor α (RORα) [20, 21] and GATA binding protein 3 (GATA3) [22–27] (Fig. 1). These cells respond specifically to the epithelial cell-derived cytokines IL-25, IL-33, and TSLP and the tumor necrosis factor (TNF) family member TNF-like ligand 1A (TL1A) to produce IL-4, IL-5, IL-9, IL-13, and/or the epidermal growth factor receptor (EGFR) ligand amphiregulin (Areg) [3, 7–11]. These effector functions then support the development of type 2 inflammation in the context of immunity and allergic disease, and also contribute to the ability of ILC2s to maintain tissue homeostasis by promoting wound healing, tissue remodeling, and metabolic homeostasis [3, 7–11, 28–48].
A network of transcription factors and cytokines directs ILC2 development. ILC2s arise from a CLP that expresses the integrin α4β7, the common γc receptor, and the transcription factors ID2 and PLZF. Signals from the cytokines IL-2, IL-7, IL-25, IL-33, and TSLP and the transcription factors GATA3, TOX, NFIL3, and TCF1, along with Notch signaling, guide the differentiation, maturation, and activation of ILC2s. These mature ILC2s are negative for lineage markers of T and B cells, monocytes, macrophages, and innate granulocytes, but they do express CD90 (Thy1), CD25, CD127, IL-25R, IL-33R, TSLPR, and CRTH2
While the categorization of ILCs into the distinct subsets described above has been useful in providing a rubric for understanding the developmental requirements and effector functions of these cells, it remains possible that additional innate cell subsets exist, or that there may be functional plasticity among different subsets of ILCs [8, 10]. For example, cells termed multipotent progenitor type 2 (MPPtype2) cells were originally thought to be an ILC-like population that promoted type 2 immunity to helminth infection [49]. However, subsequent studies showed that these cells were distinct from ILC2s, as MPPtype2 cells responded preferentially to IL-25 and exhibited a progenitor-like phenotype and function, while ILC2s responded preferentially to IL-33 and were terminally differentiated [50]. Regarding plasticity of ILC subsets, recent studies have shown that murine and human ILC3s could respond to IL-12 and IL-18, which mediates dynamic expression of RORγt and T-bet [51], loss of IL-17 and IL-22 expression, and acquisition of the ability to produce IFN-γ [51–54]. However, it remains unclear whether ILC2s demonstrate similar functional plasticity, and further studies will be required to fully dissect how ILC2s respond to changing environmental cues to modulate their characteristic effector functions.
Requirements for the Development of ILC2s
Following the identification of ILC2s and other ILC subsets, there has been tremendous interest in understanding the pathways that lead to the development and differentiation of ILC2s. All ILCs are derived from a bone marrow-resident common lymphoid progenitor that expresses the integrin α4β7 [55–58]. Downstream of this precursor, the development of the three ILC subsets has been shown to be dependent on the transcription factors inhibitor of DNA binding 2 (ID2) [55, 58–60] and promyelocytic leukemia zinc finger protein (PLZF) [59]. In addition, ILC development was dependent upon the common γ-chain (γc or CD132), IL-7, the Notch pathway, and the transcription factors thymocyte selection-associated high mobility group box protein (TOX), nuclear factor, interleukin 3 regulated (NFIL3), GATA3, and transcription factor 1 (TCF1) [8, 10, 19, 27, 28, 31, 35, 55, 59–68] (Fig. 1).
The transcription factor GATA3 is particularly key for the development and maintenance of ILC2s [22–27]. While deletion of this factor at the earliest stages of ILC development in hematopoietic stem cells prevented the development of all ILCs [27, 68], deletion of GATA3 in downstream precursors only prevented the development of ILC2s, suggesting that sustained GATA3 expression is uniquely required for ILC2 differentiation [22–27, 68]. Other transcription factors coordinate the effects of GATA3 on ILC2 development, including growth factor-independent 1 transcription repressor (GFI1), a transcription factor that targets the Gata3 gene and helps to maintain GATA3 expression [69]. In addition to GATA3, other transcriptional regulators contribute to the development of ILC2s [20, 21]. Together, these findings suggest a model in which multiple transcription factors converge in a regulatory network that controls the development of ILC2s (Fig. 1).
Regulation of ILC2 Effector Functions
Studies in multiple models and in humans suggest that ILC2s promote type 2 inflammation that contributes to protective immunity to helminth parasites and allergic disease, while supporting the maintenance of tissue remodeling mechanisms and metabolic homeostasis through production of IL-4, IL-5, IL-9, IL-13, and Areg and interactions with other innate and adaptive immune cells [3, 7–11]. The factors that drive the acquisition of ILC2 effector functions include a diverse array of cytokines and lipids derived from epithelial cells and other immune cells that are produced in response to helminth parasites, viruses, and fungi as well as allergens [3, 7–11]. In particular, the epithelial cell-derived cytokines IL-25, IL-33, and TSLP are critically important for ILC2 development, activation, and acquisition of effector function [25, 28–30, 32, 34, 39, 42–44, 48, 50, 70–77]. Other cytokines, including the γc cytokines IL-2, IL-4, and IL-7, also support the development of ILC2s and promote their activation [3, 7–11, 77–80]. Two recent studies have shown that the TNF family member TL1A acted on ILC2s that express death receptor 3 (DR3) to drive allergic inflammation in the lung and protective immunity to helminth parasites [40, 41]. Finally, autocrine signaling by IL-9 produced by ILC2s was demonstrated to be important in promoting ILC2 survival [45] (Fig. 2).
Cytokines and bioactive lipids regulate ILC2 acquisition of effector function. The epithelial cell-derived cytokines IL-25, IL-33, and TSLP act on ILC2s, driving cell proliferation and production of the type 2 cytokines IL-4, IL-5, IL-9, and IL-13. IL-9 produced by ILC2s, in addition to other γc cytokines such as IL-2 and IL-7, promote ILC2 proliferation and survival. In addition, the TNF family member TL1A signals to its receptor, DR3, on the surface of ILC2s to elicit expression of effector cytokines. Finally, ILC2s also respond to the eicosanoids PGD2, LTD4, and LXA4 through the receptors CRTH2, cysteinyl leukotriene receptor 2 (CysLT2), and formyl peptide receptor 2/lipoxin A4 receptor (FPR2/ALX), respectively. PGD2 and LTD4 drive the activation of ILC2s, while LXA4 limits ILC2 responses
In addition to responding to cytokine stimuli, ILC2s also respond directly to bioactive lipids of the eicosanoid family that are produced in the context of allergic inflammation. Specifically, ILC2s express the prostaglandin D2 (PGD2) receptor chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2), and ligation of CRTH2 in vitro elicited chemotaxis of ILC2s and production of IL-5 and IL-13 [81–84]. ILC2s also responded to leukotriene D4 (LTD4) [85] and were inhibited by lipoxin A4 (LXA4) [81]. Together, these studies suggest that numerous proteins and lipids regulate ILC2 activation and effector function. However, additional studies will be required to more completely define the factors that regulate ILC2 activities in various tissues during the steady state or in the context of inflammation (Fig. 2).
ILC2s Contribute to Protective Immunity to Helminth Parasites
While the role of type 2 cytokines in mediating helminth expulsion has been appreciated for many years, the critical source of these cytokines in vivo was previously poorly defined [1, 2, 4, 6, 7, 86]. Nippostrongylus brasiliensis is a mouse-adapted intestinal nematode parasite used as a model of hookworm infection [87], and protective immunity in mice is dependent upon IL-13-mediated changes in the intestinal epithelium, including an increase in mucus production and changes in smooth muscle contractility, that lead to parasite expulsion [86]. Seminal work in 2006 showed that an innate cell population that expressed c-Kit and produced type 2 cytokines was required for resistance to N. brasiliensis, providing the first indication that innate cells, rather than adaptive CD4+ T cells, were required as a source of type 2 cytokines during helminth infection [88].
Subsequent studies formally defined this innate cell population as innate helper cells, nuocytes, or innate helper type 2 cells, again in the context of infection with N. brasiliensis [28–30], and these cells are now universally referred to as ILC2s [8]. During N. brasiliensis infection, ILC2s are the dominant producers of IL-13, and mice lacking ILC2s or IL-13 failed to efficiently expel parasites [28–30]. Adoptive transfer of IL-13-expressing ILC2s to mice deficient in ILC2s or IL-13 was able to promote parasite expulsion, thus identifying ILC2s as key players in mediating interactions between the immune system and the epithelial barrier that are required for protection against helminth parasites [28–30] (Fig. 3). While it remains unclear exactly how ILC2s contribute to protective immune responses during infection with helminths aside from N. brasiliensis, there are studies that suggest that ILC2s do contribute to immunity to diverse helminth species, such as Strongyloides venezuelensis [89, 90]. Similarly, Areg has been shown to be required for expulsion of the nematode parasites Trichuris muris [91], and ILC2s are a predominant source of Areg in some contexts [35], suggesting that ILC2s may contribute to immunity to T. muris as well.
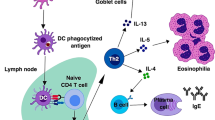
ILC2s respond to cues from the epithelium and interact with innate and adaptive cells to promote type 2 inflammation. Following exposure to helminth antigens or allergens, epithelial cells produce the cytokines IL-25, IL-33, and TSLP. ILC2s respond to these cytokines and express IL-5 and IL-13. IL-5 and IL-13 act on other innate cell types such as eosinophils and macrophages to promote tissue eosinophilia and alternative activation of macrophages. These innate responses, coupled with the action of ILC2-derived IL-13 directly on the epithelium, serve to mediate changes in epithelial barrier physiology that contribute to increased mucin production and smooth muscle contractility. In addition, activated ILC2s interact with CD4+ T cells via expression of MHC II, which drives T-cell production of IL-2 that acts back on ILC2s to support their continued proliferation, activation, and survival. Together, these ILC2-centric pathways contribute to type 2 inflammation that is protective during helminth infection and pathologic in the context of allergic disease
Numerous factors and pathways regulate ILC2 function during infection with helminth parasites. The cytokines IL-25 and IL-33 were required for the activation of these cells and subsequent parasite expulsion [28–30, 32], and expression of the signaling molecule NF-κB activator 1 (Act1) in epithelial cells was critical for the efficient production of IL-25 and IL-33 following infection [92]. Another cytokine, TL1A, also elicited ILC2 responses in the context of helminth infection [40, 41]. Recent work has shown that eicosanoids can also regulate ILC2 responses following helminth infection, as the PGD2 receptor CRTH2 mediated ILC2 accumulation and type 2 inflammation in the lung of mice that had been infected with N. brasiliensis [84]. A variety of transcription factors are required for optimal ILC2 responses. During N. brasiliensis infection, GATA3 was required for ILC2 development and function [22, 23], GFI1 regulated ILC2 responsiveness to IL-33 and supported maintained GATA3 expression [69], and TCF1 promoted ILC2 expansion [31]. Finally, very recent evidence suggests that interactions with T cells during infection are important in supporting ILC2 responses. Through expression of major histocompatibility class II (MHC II), ILC2s interacted with and activated naïve CD4+ T cells, resulting in the production of Tcell-derived IL-2 that supported ILC2 expansion, effector function, and parasite expulsion [33]. Collectively, these data show that ILC2s are regulated by various cytokines and transcription factors in order to allow ILC2s to interact with other immune cells and the epithelium to mediate protective immunity to helminth infection (Fig. 3).
ILC2s Promote Allergic Inflammation
ILC2s are potent sources of type 2 cytokines and are subject to complex regulation by a variety of pathways and factors [3, 7–11]. Thus, it is not surprising that ILC2 responses are not solely protective during helminth infection, but can also drive pathologic type 2 cytokine-associated responses associated with allergy [3, 7–11]. A significant body of work now supports a key role for ILC2s in the initiation and maintenance of allergic inflammation at mucosal and barrier surfaces in the context of multiple allergic diseases, including allergic asthma, allergic airway inflammation, chronic rhinosinusitis (CRS), AD, and food allergy [3, 7–11].
Shortly after the discovery of murine ILC2s in the intestine and fat-associated lymphoid clusters [28–30], these cells were also identified in the murine lung [35, 93]. Numerous studies have now established that IL-25, IL-33, and/or TSLP can elicit ILC2-derived IL-5 and IL-13 production that contributes to airway hyperresponsiveness and allergic airway inflammation in various murine models [20, 24, 26, 31, 37–41, 69, 74–76, 78, 85, 94–98] (Fig. 3). Notably, bioactive lipids such as eicosanoids can also promote ILC2 responses that regulate type 2 inflammation in the lung. For example, murine lung ILC2s responded to LTD4 by producing IL-4 and IL-5, which was associated with eosinophilia induced by exposure to Alternaria species [85]. Similarly, human ILC2s express the receptor for LXA4, which regulated IL-13 production by ILC2s [81].
Importantly, new studies suggest that ILC2s contribute to allergic inflammation in the lung through a variety of mechanisms in addition to their ability to produce IL-5 and IL-13. For instance, in response to IL-2 signals, murine lung ILC2s produced IL-9 that was necessary for their survival and effector function in response to challenge with papain [99]. This dependence on IL-2 signaling suggests that ILC2 activities are closely tied with those of T cells, which are the primary source of IL-2 [100]. In support of this concept, recent work has revealed that ILC2s and T cells interact to coordinately drive allergic lung inflammation. In vitro co-culture of ILC2s and CD4+ T cells led to Tcell proliferation and type 2 cytokine production, and co-transfer of these cells into mice that lacked both T cells and ILC2s drove allergic airway inflammation in response to ovalbumin or the cysteine protease bromelain [101] (Fig. 3). In addition, following exposure to papain, IL-13 from ILC2s promoted DC migration to the draining lymph node and priming of naïve T cells [102].
Notably, there is significant evidence to suggest that ILC2s play a role in asthma and upper and lower allergic airway inflammation in humans. Allergic rhinitis is characterized by type 2 cytokine responses in the upper airways and can be associated with the development of CRS [103]. Nasal polyps, a hallmark of CRS, had an enriched population of CRTH2-expressing ILC2s that responded to IL-25, IL-33, and TSLP [25, 34, 104, 105]. Additionally, ILC2s have been identified in the human adult and fetal lung that expressed IL-33R, CRTH2, and/or CD161 [34, 35, 81, 84], and levels of epithelial cell-derived cytokines and eicosanoids that activate ILC2s were elevated in the lung tissues of human asthmatics [81, 106–109]. Finally, ILC2s isolated from the peripheral blood of asthmatics were more numerous than ILC2s in the peripheral blood of healthy controls, and they also produced more IL-5 and IL-13 in response to stimulation [110]. Taken together, these studies indicate that eicosanoids and epithelial cell-derived cytokines activate ILC2s to produce cytokines and interact with innate and adaptive immune cells, leading to allergic airway inflammation in mice and humans (Fig. 3).
The importance of ILC2s in mediating type 2 inflammation in the upper and lower airways suggests that these cells contribute to other atopic diseases at different tissues sites. In support of this idea, levels of the epithelial cell-derived cytokines that activate ILC2s, including IL-25, IL-33, and TSLP, have been shown to be increased in the skin of patients with AD [111–114] and in the intestine of patients with food allergy [115–117]. Indeed, the ILC2 population was expanded in AD and AD-like lesions in humans and mice, respectively [43, 77], where these cells responded to IL-2, IL-25, IL-33, and TSLP, produced IL-5 and IL-13, and interacted with innate granulocyte populations to mediate allergic inflammation [44, 73, 77, 118]. While a role for ILC2s in food allergy has not yet been described, ILC2s in the intestine could drive inflammation in response to IL-25 in a murine model of oxazalone-induced colitis [119], suggesting that further research investigating the contribution of ILC2s to type 2 inflammation in the gastrointestinal tract is warranted. Collectively, studies in murine models of allergic disease and in human patients with allergic disease suggest that ILC2s play a key role in driving allergic inflammation at multiple mucosal and barrier surfaces, and that these cells and their effector functions could be targeted in the treatment of upper and lower allergic airway inflammation, AD, and potentially food allergy in humans.
ILC2s Support Tissue Remodeling and Wound Healing
While the role for ILC2s in promoting pathologic and protective type 2 inflammation is now well-established [3, 7–11], new data are emerging that highlight additional functions of ILC2s. In particular, ILC2s appear to contribute to tissue remodeling, wound healing, and tissue homeostasis in a number of contexts [3, 7–11]. A role for ILC2s in tissue remodeling was initially described during influenza A virus infection in mice, in which ILC2 depletion led to a defect in the ability of the lung epithelium to repair itself following virus-induced tissue damage [35]. In this context, lung ILC2s produced the EGFR ligand Areg, which was critical in mediating efficient repair of the lung tissue [35]. A recent study supports the idea that ILC2s have tissue-protective roles in the lung during infection with other pathogens, as IL-9 promoted survival of ILC2s in the lung that produced Areg and contributed to tissue repair following migration of the helminth N. brasiliensis through the lung [45]. The tissue-protective effects of ILC2s do not appear to be limited to the lung. Recent evidence suggests that cholangiocytes, the epithelial cells of the bile duct, also derive protective benefits from ILC2s. In response to IL-33, expansion of an IL-13-producing ILC2 population supported cholangiocyte proliferation in a murine model of biliary atresia [120]. Interestingly, this cholangiocyte hyperplasia was also associated with an increase in cholangiocarcinoma in mice, suggesting that precise regulation of ILC2-dependent tissue repair mechanisms is required to avoid pathologic outcomes [120].
In keeping with this idea, emerging evidence suggests that ILC2s can also contribute to dysregulated tissue remodeling associated with fibrosis and pathology [42, 72]. Following challenge with Schistosoma mansoni eggs, the development of pulmonary fibrosis was dependent upon IL-25-elicited ILC2s that produced IL-13, and the same study showed that ILC2 frequency and number were increased in the bronchoalveolar lavage (BAL) fluid of human patients suffering from idiopathic pulmonary fibrosis [48]. Also, in a model of hepatic fibrosis, ILC2s that responded to IL-33 and produced IL-13 were important in driving fibrotic processes [72]. Thus, similar to the capacity of ILC2s to contribute to both protective immune responses against helminth parasites and detrimental type 2 responses during allergic inflammation, the ability of ILC2s to drive tissue remodeling processes can be either beneficial or pathological. While the regulation of these activities is likely tissue- and disease-specific, further work is required to dissect the pathways that influence ILC2-mediated tissue remodeling and to determine whether these cells and their activities could be targeted to regulate tissue remodeling and homeostasis in various disease states.
ILC2s Promote Metabolic Homeostasis
Cutting edge studies in ILC2 biology have begun to focus on how ILC2s contribute to the maintenance of metabolic homeostasis [42, 46, 47, 121, 122]. Previous work has demonstrated that a type 2-polarized cytokine environment in adipose tissue elicits alternatively activated macrophage (AAM) activities and the accumulation of eosinophils, which in turn are associated with metabolic homeostasis [5, 123–125]. In contrast, classical activation of macrophages and TH1-polarized inflammation in the adipose is associated with obesity, insulin resistance, and metabolic disease [126–130]. These previous findings, coupled with the observations that ILC2s are potent sources of type 2 cytokines in other tissues [3, 7–11], prompted an investigation of the role of ILC2s in maintaining type 2 cytokine responses that support metabolic homeostasis.
An initial study demonstrated that IL-13 played a role in limiting hyperglycemia, which is associated with insulin resistance. IL-13 limited glucose production by inhibiting genes involved in gluconeogenesis in the liver via signal transduction and activator of transcription 3 (STAT3) [121], suggesting that sources of IL-13, such as ILC2s, might contribute to glucose homeostasis. In addition, IL-5-responsive eosinophils in the adipose have been associated with the maintenance of healthy adipose tissue function in the lean state, and another study has shown that IL-33-elicited ILC2s that produced IL-5 maintained eosinophil and AAM populations in the fat [46]. Supporting a role for ILC2 effector functions in promoting metabolic homeostasis in the adipose, IL-25 treatment resulted in an increase in ILC2 populations in obese mice, associated with the expansion of eosinophil and AAM in the adipose, increased weight loss, and improved glucose tolerance. Similarly, ILC2 depletion in obese Rag1 −/− mice resulted in increased weight gain and glucose intolerance, and transfer of ILC2s to obese mice led to weight loss and improved insulin sensitivity [42].
In addition to their role in regulating immune cells that contribute to metabolic homeostasis in the fat, ILC2s that reside in the intestine produced IL-5 following caloric intake in response to vasoactive intestinal peptide (VIP), a neuropeptide produced following feeding that is tied to circadian rhythms. These data thus establish a link between ILC2s, caloric intake, and eosinophil responses that have been associated with metabolic homeostasis [47]. A very recent publication has also shown that IL-33-responsive ILC2s in the fat regulate adiposity and the recruitment of beige adipocytes that control caloric expenditure [122]. Together, these studies suggest that ILC2s may be key regulators of metabolic processes in the steady state. Of note, a very recent study has shown that micronutrient deficiencies appear to influence ILC2 responses that mediate protective immunity against pathogens [131], suggesting that ILC2s are also key players in coordinating nutrition and metabolism during infection and inflammation. Further studies will be needed to better understand how ILC2s sense nutritional status to regulate metabolic homeostasis during the steady state and following encounters with pathogens.
Conclusions and Future Directions
A rapidly advancing body of work highlights the key role that ILC2s play in protective and pathologic type 2 immune responses in the context of helminth infection, allergic disease, tissue remodeling and repair, and metabolic homeostasis [3, 7–11]. Together, these studies provide new insight into how the innate immune system contributes to type 2 cytokine responses that participate in a variety of key biologic processes [1]. However, numerous outstanding questions remain regarding the development, effector function, and regulation of ILC2s. The precise developmental paths that lead to ILC2 hematopoiesis remain unclear, and how ILC2 populations turn over in the steady state and change in the course of aging is unknown. Also, it seems likely that cytokines and factors in addition to the epithelial cell-derived cytokines and eicosanoids shape ILC2 responses. In particular, the pathways that negatively regulate ILC2 responses and the mechanisms that control ILC2 migration are poorly understood. Similarly, ILC2s likely produce cytokines or other factors in addition to type 2 cytokines and Areg. Further work will be required to more comprehensively profile the effector molecules produced by ILC2s and how these cells interact directly and indirectly with epithelial cells and innate and adaptive immune cells. Most importantly, our current understanding of ILC2 biology suggests that these cells and their effector functions could be targeted therapeutically in human diseases in which type 2 cytokine responses play a role, including helminth infection, allergic disease, fibrosis, and metabolic disease. Future studies that investigate ILC2 responses in humans and in murine models of human diseases could result in the development of innovative new therapies that target innate immune pathways involved in the pathogenesis of multiple inflammatory diseases.
Abbreviations
- AAM:
-
Alternatively activated macrophage
- Act 1:
-
NF-κB activator 1
- AD:
-
Atopic dermatitis
- APC:
-
Antigen-presenting cell
- Areg:
-
Amphiregulin
- BAL:
-
Bronchoalveolar lavage fluid
- CRS:
-
Chronic rhinosinusitis
- CRTH2:
-
Chemoattractant receptor-homologous molecule expressed on T helper type 2 cells
- CysLT2:
-
Cysteinyl leukotriene receptor 2
- DC:
-
Dendritic cell
- DR3:
-
Death receptor 3
- EGFR:
-
Epidermal growth factor receptor
- FPR2/ALX:
-
Formyl peptide receptor 2/lipoxin A4 receptor
- γc:
-
γ-Chain
- GATA3:
-
GATA binding protein 3
- GFI1:
-
Growth factor-independent 1 transcription repressor
- ID2:
-
Inhibitor of DNA binding 2
- IFN-γ:
-
Interferon-γ
- Ig:
-
Immunoglobulin
- IL:
-
Interleukin
- ILC:
-
Innate lymphoid cell
- ILC1:
-
Group 1 innate lymphoid cell
- ILC2:
-
Group 2 innate lymphoid cell
- ILC3:
-
Group 3 innate lymphoid cell
- LTD4 :
-
Leukotriene D4
- LTi:
-
Lymphoid tissue inducer
- LXA4 :
-
Lipoxin A4
- MHC II:
-
Major histocompatibility class II
- MPPtype2 :
-
Multipotent progenitor type 2
- NFIL3:
-
Nuclear factor interleukin 3 regulated
- NK:
-
Natural killer
- PGD2 :
-
Prostaglandin D2
- PLZF:
-
Promyelocytic leukemia zinc finger protein
- R:
-
Receptor
- RORα:
-
Retinoic acid receptor-related orphan receptor α
- RORγt:
-
Retinoic acid receptor-related orphan receptor γ
- SCF:
-
Stem cell factor
- STAT3:
-
Signal transducer and activator of transcription 3
- T-bet:
-
T-box expressed in T cells
- TCF1:
-
Transcription factor 1
- TH2:
-
T helper type 2
- TL1A:
-
Tumor necrosis factor-like ligand 1A
- TNF:
-
Tumor necrosis factor
- TOX:
-
Thymocyte selection-associated high mobility group box
- TSLP:
-
Thymic stromal lymphopoietin
- VIP:
-
Vasoactive intestinal peptide
References
Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337(6093):431–5.
Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24(4):459–66.
Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):536–42.
Allen JE, Sutherland TE. Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin Immunol. 2014;26(4):329–40.
Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013;38(4):644–54.
Anthony RM, Rutitzky LI, Urban Jr JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7(12):975–87.
Oliphant CJ, Barlow JL, McKenzie AN. Insights into the initiation of type 2 immune responses. Immunology. 2011;134(4):378–85.
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–9.
Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol. 2013;25(6):738–44.
Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. 2012;12(4):445–57.
Koyasu S, Moro K. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Curr Opin Allergy Clin Immunol. 2011;11(2):109–14.
Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31(1):15–23.
Eberl G. Development and evolution of RORgammat+ cells in a microbe’s world. Immunol Rev. 2012;245(1):177–88.
Hoyler T, Connor CA, Kiss EA, Diefenbach A. T-bet and Gata3 in controlling type 1 and type 2 immunity mediated by innate lymphoid cells. Curr Opin Immunol. 2013;25(2):139–47.
Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3(4):292–303.
Vosshenrich CA, Di Santo JP. Developmental programming of natural killer and innate lymphoid cells. Curr Opin Immunol. 2013;25(2):130–8.
Pearson C, Uhlig HH, Powrie F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012;33(6):289–96.
Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+ CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7(4):493–504.
Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73.
Halim TYF, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37(3):463–74.
Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13(3):229–36.
Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13(1):58–66.
Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–48.
Klein Wolterink RG, Serafini N, van Nimwegen M, Vosshenrich CA, de Bruijn MJ, Fonseca Pereira D, et al. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2013;110(25):10240–5.
Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37(4):649–59.
KleinJan A, Klein Wolterink RG, Levani Y, de Bruijn MJ, Hoogsteden HC, van Nimwegen M, et al. Enforced expression of Gata3 in T cells and group 2 innate lymphoid cells increases susceptibility to allergic airway inflammation in mice. J Immunol. 2014;192(4):1385–94.
Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40(3):378–88.
Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–4.
Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–70.
Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107(25):11489–94.
Yang Q, Monticelli LA, Saenz SA, Chi AW, Sonnenberg GF, Tang J, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38(4):694–704.
Hung LY, Lewkowich IP, Dawson LA, Downey J, Yang Y, Smith DE, et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci U S A. 2013;110(1):282–7.
Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41(2):283–95.
Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–62.
Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–54.
Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129(1):191–8. e1-4.
Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188(3):1503–13.
Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42(5):1106–16.
Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129(1):216–27. e1-6.
Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7(4):958–68.
Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7(3):730–40.
Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191(11):5349–53.
Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra16.
Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–50.
Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210(13):2951–65.
Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–49.
Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–8.
Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111(1):367–72.
Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban Jr JF, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464(7293):1362–6.
Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, et al. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210(9):1823–37.
Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494(7436):261–5.
Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33(5):736–51.
Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107(24):10961–6.
Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14(3):221–9.
Cherrier M, Sawa S, Eberl G. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med. 2012;209(4):729–40.
Yoshida H, Kawamoto H, Santee SM, Hashi H, Honda K, Nishikawa S, et al. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167(5):2511–21.
Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, et al. Notch signaling is necessary for adult, but not fetal, development of RORgammat(+) innate lymphoid cells. Nat Immunol. 2011;12(10):949–58.
Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157(2):340–56.
Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508(7496):397–401.
Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207(2):273–80.
Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11(10):945–52.
Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397(6721):702–6.
Seillet C, Rankin LC, Groom JR, Mielke LA, Tellier J, Chopin M, et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. 2014;211(9):1733–40.
Mielke LA, Groom JR, Rankin LC, Seillet C, Masson F, Putoczki T, et al. TCF-1 controls ILC2 and NKp46+ RORgammat+ innate lymphocyte differentiation and protection in intestinal inflammation. J Immunol. 2013;191(8):4383–91.
Gentek R, Munneke JM, Helbig C, Blom B, Hazenberg MD, Spits H, et al. Modulation of signal strength switches Notch from an inducer of T cells to an inducer of ILC2. Front Immunol. 2013;4:334.
Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38(4):769–81.
Geiger TL, Abt MC, Gasteiger G, Firth MA, O’Connor MH, Geary CD, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211(9):1723–31.
Serafini N, Klein Wolterink RG, Satoh-Takayama N, Xu W, Vosshenrich CA, Hendriks RW, et al. Gata3 drives development of RORgammat+ group 3 innate lymphoid cells. J Exp Med. 2014;211(2):199–208.
Spooner CJ, Lesch J, Yan D, Khan AA, Abbas A, Ramirez-Carrozzi V, et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nat Immunol. 2013;14(12):1229–36.
Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, et al. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40(3):414–24.
McSorley HJ, Blair NF, Smith KA, McKenzie AN, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014;7(5):1068–78.
McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39(2):357–71.
Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110(34):13921–6.
Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132(4):933–41.
Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43(2):488–98.
Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134(2):429–39.
Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):564–73.
Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163(2):92–105.
Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40(5):758–71.
Mirchandani AS, Besnard AG, Yip E, Scott C, Bain CC, Cerovic V, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192(5):2442–8.
Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5(174):174ra26.
Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133(4):1184–94.
Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133(3):899–901.e3.
Tait Wojno ED, Monticelli LA, Tran SV, Alenghat TA, Osborne LC, Thome JJ, et al. The prostaglandin D2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015; doi:10.1038/mi.2015.21.
Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132(1):205–13.
Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55.
Camberis M, Le Gros G, Urban J, Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol. 2003;Chapter 19:Unit 19.2.
Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203(4):1105–16.
Yasuda K, Muto T, Kawagoe T, Matsumoto M, Sasaki Y, Matsushita K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci U S A. 2012;109(9):3451–6.
Yasuda K, Matsumoto M, Nakanishi K. Importance of both innate immunity and acquired immunity for rapid expulsion of S. venezuelensis. Front Immunol. 2014;5:118.
Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314(5806):1746.
Kang Z, Swaidani S, Yin W, Wang C, Barlow JL, Gulen MF, et al. Epithelial cell-specific Act1 adaptor mediates interleukin-25-dependent helminth expulsion through expansion of Lin(-)c-Kit(+) innate cell population. Immunity. 2012;36(5):821–33.
Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–8.
Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303(7):L577–88.
Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY. IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130(5):1159–66. 5.
Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–63.
Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675.
Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133(4):1142–8.
Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12(11):1071–7.
Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–90.
Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4(+) T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69(10):1300–7.
Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40(3):425–35.
Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128(4):693–707. quiz 8-9.
Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188(4):432–9.
Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69(9):1154–61.
Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011;128(1):116–24.
Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):104–11. e1-9.
Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125(3):752–4.
Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131(6):1504–12.
Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134(3):671–8. 3.
Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–80.
Deleuran M, Hvid M, Kemp K, Christensen GB, Deleuran B, Vestergaard C. IL-25 induces both inflammation and skin barrier dysfunction in atopic dermatitis. Chem Immunol Allergy. 2012;96:45–9.
Hvid M, Vestergaard C, Kemp K, Christensen GB, Deleuran B, Deleuran M. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol. 2011;131(1):150–7.
Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimaki S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132(5):1392–400.
Blazquez AB, Mayer L, Berin MC. Thymic stromal lymphopoietin is required for gastrointestinal allergy but not oral tolerance. Gastroenterology. 2010;139(4):1301–9.
Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131(1):187–200. e1-8.
Herberth G, Daegelmann C, Roder S, Behrendt H, Kramer U, Borte M, et al. IL-17E but not IL-17A is associated with allergic sensitization: results from the LISA study. Pediatr Allergy Immunol. 2010;21(7):1086–90.
Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol. 2014;193(7):3717–25.
Camelo A, Barlow JL, Drynan LF, Neill DR, Ballantyne SJ, Wong SH, et al. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol. 2012;47(11):1198–211.
Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124(7):3241–51.
Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123(1):261–71.
Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–6.
Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7(6):485–95.
Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107(52):22617–22.
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–7.
Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261–86.
Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8(12):709–16.
Han JM, Levings MK. Immune regulation in obesity-associated adipose inflammation. J Immunol. 2013;191(2):527–32.
Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–74.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343(6169):432–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Tait Wojno, E.D. (2016). Innate Lymphoid Cells: An Emerging Population in Type 2 Inflammation. In: Gause, W., Artis, D. (eds) The Th2 Type Immune Response in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2911-5_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2911-5_2
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2910-8
Online ISBN: 978-1-4939-2911-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)