Abstract
The cytoskeleton is a group of dynamic proteins that permits the cells to have the proper shape and to perform any cellular movement, as well as the movement of structures inside the cell. To migrate the cell must emit specialized protrusions, especially the lamellipodium. Composed of actin filaments, the lamellipodium is formed at the leading edge of cells and allows them to advance on the substrate, which is the first step in migration. Chemokines are attractant molecules that induce the migratory phenotype and consequently lamellipodium formation. Its signaling though G their protein-coupled receptors leads to activation of pathways that are transduced into cytoskeleton changes.
In the second part of the chapter, we discuss the importance of the cytoskeleton for the functioning of specific cell types, initially addressing the importance of actin filaments, microtubules and intermediate filaments for the stem cells. Then we review, as an example, the cytoskeleton proteins of three types of glial fibrillary acidic protein (GFAP)-expressing glial cells, in particular the intermediate filament GFAP, in health and disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glial Fibrillary Acidic Protein
- Schwann Cell
- Actin Filament
- Intermediate Filament
- Glial Fibrillary Acidic Protein Expression
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The cytoskeleton is a dynamic network of proteins organized in fibrillar or globular filaments in the cell cytoplasm [1]. Three types of filaments are common to many eukaryotic cells: (1) intermediate filaments provide mechanical strength and resistance to shear stress; (2) microtubules determine the positions of membrane-enclosed organelles and direct intracellular transport; and (3) actin filaments determine the shape of the cell’s surface and are necessary for cell migration. Actin filaments also interact with accessory proteins that link them to other cellular components, as well as to each other [1].
This chapter initially addresses the role of the cytoskeleton in one of its main functions, cell migration. The dynamics and the mechanical aspects of the actin filaments are essential to this process, as are the signaling pathways induced by the chemokines and their receptors. We also give special attention to the cytoskeletal proteins of the stem cells, in the origin of cell functions. Finally, the chapter discusses the participation of the cytoskeleton in functions performed by different types of glial cells, focusing on the role of a particular intermediate filament, the glial fibrillary acidic protein (GFAP), in the health and disease.
An Overview of Cell Migration
Cell migration has fascinated cell biologists, biochemists, and recently also physicists and mathematicians. This is not surprising, since it is an essential process that occurs during different stages and at different times, ranging from organism development to normal adult life and also during disease states [2–4].
Cells in multicellular organisms can move in different directions, through the extracellular matrix, over each other, or even between each other. Cells move in three basic steps: (1) extending the plasma membrane forward at the front, or leading edge, of the cell in a protrusion; (2) moving the cell body; and (3) retracting the rear part of the cell [5]. These steps involve two main cytoskeleton filaments, microtubules and actin filaments; the microtubules are required for polarization [6, 7] while the actin filaments are the main players during migration and protrusion formation [8].
Cells are able to extend four different types of protrusions at the leading edge, lamellipodia, filopodia, blebs, and invadopodia. All these structures have their own functions and contribute to cell migration in specific ways. Lamellipodia are able to extend long distances through the extracellular matrix, pulling cells through the tissues [2]. Filopodia explore the cell’s surroundings [9, 10]. Membrane blebs help in cell migration during development [11], and invadopodia are protrusions that allow degradation of the extracellular matrix, and help cells to pass through tissues [12].
In the following parts of this section we discuss the mechanical aspects of the formation of a protrusive migratory structure called the lamellipodium, and how the plasma membrane regulates the behavior of this structure, as well as its influence during cell migration. We also discuss some chemokines that induce migratory processes followed by cytoskeletal changes.
Lamellipodium
The thin protrusive region at the leading edge of migrating fibroblasts in culture was termed the “lamellipodium” by Abercrombie et al. [13]. Abercrombie et al. [14] showed that these structures contain actin filaments arranged in a branched structure, but not microtubules. First described in fibroblasts, lamellipodia have also been observed in many other cell types such as precursor cells, epithelial cells, and neural crest cells [2, 15].
For many years, a group of proteins called the Actin-Related Proteins 2/3 (Arp2/3) complex was thought to be the primary mediator of actin polymerization in lamellipodia. First described as a nucleator of actin polymerization [16], the Arp2/3 complex binds to actin filaments and induces the formation of branched actin networks [17]. Branched actin networks were also observed in electron-microscopy images of lamellipodia [18]. However, it is now known that the extent of actin filament branching can vary depending on the cell type and conditions, as a recent report found only a few branches in the leading edge of cells [19]. Not only branching but also the balance of other known actin-binding proteins can contribute to the extension of the lamellipodium. For example, more capping protein activity reduces actin length and increases nucleation by Arp2/3 [20]. On the other hand, an increase in the expression of vasodilator-stimulated phosphoprotein (VASP) (a protein known to promote filament elongation) was reported to generate longer filaments [21, 22]. More recently, other actin nucleators were found to contribute to lamellipodial protrusion, including several members of the Formin family of proteins. Formins were described as protecting actin filaments from capping and also as promoting filament elongation without branching. One of these proteins, diaphanous homolog 1 of Drosophila (mDia1), was first reported to localize at the lamellipodia of migrating cells [23].
The final essential factor in cell movement is the plasma membrane. The lamellipodial protrusion will encounter the physical barrier imposed by the membrane, and this barrier will also restrict cell migration [24].
Membrane Mechanical Properties Orchestrates Cell Migration
The mechanical characteristics of the plasma membrane, particularly its plasma membrane tension and bending modulus, play central roles in cell motility and cytoskeleton remodeling [25–28].
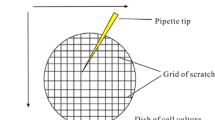
A direct way to assess these responses to forces is by measuring two elastic parameters of the cell membrane: its bending modulus [28] and its membrane tension [29], using a technique based on extracting the membrane tether from the cell by pulling on it with an attached microsphere trapped in an optical tweezers [30]. The experimental procedure is illustrated in Fig. 4.1. Analysis of the force-extension curve, together with measurement of the tether radius, yield these two elastic parameters and also information regarding the membrane–cytoskeleton interaction [31]. Tether pulling with optically trapped beads is the only known direct method for these measurements [26].
The mechanical load exerted by the membrane at the leading edge of cells can locally influence the dynamic growth and organization of the actin network [25, 32–34]. The high membrane tension in the lamellipodia of motile cells directly influences the protrusion [35–37]. Simultaneously at the rear of the cell, the same membrane load can exert a pulling force that induces retraction [38, 39]. However, this mechanical load imposed by the membrane was also reported to be influenced by forces generated from the actin cytoskeletal protrusion itself [27, 31, 32, 40, 41].
One possibility is that the membrane mechanical parameters are determined primarily through a balance of forces between the cytoskeleton and the hydrostatic pressure acting on the membrane [29, 42]. Another possibility is that these parameters are controlled mainly by the interaction between the membrane and the cytoskeleton [31, 43]. Regardless of the exact mechanism, membrane mechanical properties have emerged as important regulators that coordinate local dynamics over cellular scales.
Apart from these dynamic aspects, recent data also suggest that cell specialization and/or differentiation can account for the differences in the mechanical properties of the membrane, and that these differences are reflected in their specialized functions [44]. A question that remains unanswered is how the threshold value of these membrane parameters are set. The answer is still not clear, and may vary with different cell types. Pontes et al. [43] began to test this hypothesis by measuring the membrane tension and bending modulus for a variety of cells. These authors observed that the elastic parameters for neurons are close to those obtained for an isolated cell membrane (a membrane disconnected from the cytoskeleton), suggesting a weaker interaction between the membrane and the adjacent F-actin cortex in this cell type. They also observed that the parameters did not change within the different neuronal cell regions, i.e., the cell body, neurite and growth cone. They found very similar membrane mechanical parameters for astrocytes and glioblastoma cells, supporting the idea that these two cell types have the same origin and also share similar functions, for example giving support to neurons in the brain [45]. Macrophages and microglial cells have substantially higher values for the membrane mechanical parameters. When activated, these two phagocytic cells decrease their bending modulus by a factor of 3. This reduction can be interpreted as an easier way to bend the cell surface, which is advantageous during phagocytosis.
Taken together, these observations are striking examples which demonstrate that different cells performing different functions show different mechanical parameters. These new findings suggest the possibility of characterizing cells based not only on morphological and biochemical analyses, but now on their mechanical properties as well.
Chemokines Induce Migratory Processes
Chemokines are chemotactic cytokines, comprising a large superfamily of small peptides (approximately 8–17 kDa) that currently number 47 in humans [46, 47]. They can be classified according to their amino-acid structure into four groups, based on the variations of a conserved cysteine motif in the mature sequence of the proteins [48, 49]. Chemokines bind to the chemokine receptor subfamily of class A G-protein-coupled receptors (GPCRs), which comprises ten CCR family members, seven C-X-C chemokine receptors (CXCR) family members, the “C” sub-family of chemokine receptors 1 (XCR1) and CX3C chemokine receptor 1 (CX3CR1) [46, 50]. These GPCRs signal through heterotrimeric G-proteins, and regulate a diversity of signal transduction pathways involved in chemotaxis and cell survival.
Chemokines were first described for their role in chemotaxis and migration of leukocytes to lymphoid tissues and sites of injury, and the signaling pathways activated by their receptors lead to changes and reorganization within the cytoskeleton proteins. They also proved to be important in the development and homeostasis of the immune system and various other organs, and in pathophysiological processes associated with osteoporosis [51], obesity and insulin resistance [52], viral infections [53, 54], immune responses [55, 56], mobilization of progenitors to the bone marrow [57] and autoimmune encephalomyelitis [58]. More recently, chemokines emerged as key mediators of cancer progression, by interfering with the homing of cancer cells to metastatic sites and the recruitment of a number of different cell types to the tumor microenvironment, such as tumor-associated macrophages, tumor-associated neutrophils, lymphocytes, cancer-associated fibroblasts, myeloid-derived suppressor cells and endothelial cells [48, 49, 59, 60, 61].
Processing the chemokine gradients into migratory or adhesive responses occurs in multiple dynamic steps that regulate changes in the cytoskeleton and cellular adhesion [46, 62–65]. Binding of the chemokines to their G-coupled receptors can lead to downstream activation of different signaling pathways (Fig. 4.2), such as protein kinase B (PKB/Akt) and mitogen-activated protein kinases (MAPKs) [66, 67]. Another activated pathway may be the Janus kinase family (JAK), activated in a Gαi-independent fashion [68, 69]. The Rho family of GTPases and their downstream effectors were also implicated in chemokine-elicited migration. One of the important groups of Rho effectors is the Rho-associated coiled-coil forming protein kinases (ROCK) I and II, which enhance myosin light chain (MLC) phosphorylation by both inhibiting MLC phosphatase and phosphorylating MLC, thereby regulating actin–myosin contraction [66, 70]. ROCK isoforms also regulate lymphocyte polarity and migration through members of the Ezrin/Radixin/Moesin (ERM) family of proteins [66, 71]. Rho GTPases also control cytoskeletal remodeling through effector proteins from the mDia family of formins, which, as mentioned above, are actin-nucleating proteins favoring the formation of long straight actin filaments. Lack of mDia1 expression significantly reduces T cell homing to secondary lymphoid organs [72].
Signaling pathways involved in cytoskeleton regulation. Chemokines bind to G-protein-coupled receptors, and consequently can activate different signaling pathways, such as protein kinase B (PKB), mitogen-activated protein kinases (MAPKs), the Janus kinases family (JAK) and the Rho family GTPases. All these signaling pathways are involved in modulating the cytoskeleton proteins, leading to their reorganization, more specifically of the F-actin filaments
Little is known about the correlation between chemokines and the cytoskeleton. At least C-C motif chemokine 19 (CCL19)/CCL21-CCR7 and CXCL12-CXCR4 constitute an exception.
The Influence of the Chemokines CCL21/CCL19 and Their Receptor CCR7 on the Cytoskeleton
From a physiological perspective, all these intracellular events that occur in the lymphocyte homing process depend on a combination of interactions between different chemokines and their receptors, according to the cell type involved: T-cell homing and traffic of lymphocytes into and within secondary lymphoid tissues rely largely on CCR7 and its ligands CCL21/CCL19 [73, 74], as well as a minor contribution from CXCL12-CXCR4 interactions [75, 76]. Bardi et al. [77] reported that both the CCR7-mediated polarization and chemotaxis are dependent on the Rho kinases, but not on MAPK extracellular signal-regulated protein kinase (ERK)-2, as previously described [78–80]. The C-C chemokine receptor type 7 (CCR7) and other chemokine receptors such as CXCR4 also activate leukocyte integrins, which are important for the endothelial adhesion and arrest of rolling lymphocytes [81–83], possibly through downstream activation of RhoA [84–86].
Although B cell integrin activation is also primarily induced by CCR7 and CXCR4, their homing also requires the activation of CXCR5, whose expression is restricted to B cells and a subset of CD4+ T cells [76].
The expression of CCR7 and CCL21 has been described in many cancers (especially melanoma, breast cancer, and head and neck cancers), and was correlated with actin polymerization and lamellipodium formation, which contribute to increased tumor-cell migration, invasion and metastatic potential [87–91].
The Influence of the Chemokine CXCL12 and Its Receptor CXCR4 on the Cytoskeleton
CXCL12, better known as stromal cell-derived factor-1 (SDF-1), was first described as pre-B cell growth-stimulating factor (PBSF) [92], and activates integrins in B-cells as mentioned above. The chemokine CXCL12 and its receptor CXCR4 are well known for their role in the metastasis of breast cancer [93, 94]. However, CXCL12 is constitutively expressed in a broad range of tissues, e.g. in bone marrow, spleen, liver, lung and brain, as well as in most types of tumors [92, 95]. This chemokine is the only known ligand for CXCR4, also known as Fusin/LESTR/CD184 [96–98]. CXCR4 is a G-protein-coupled seven transmembrane receptor and is widely expressed by many different cell types including hematopoietic cells, leukocytes, endothelial cells, central nervous system (CNS) cells, and cells of the gastrointestinal tract.
Physiologically, CXCL12 is important for the homing of CXCR4-expressing hematopoietic cells to the bone marrow [99] and for guiding CXCR4-positive cells from different tissues to their niche [100]. CXCL12/CXCR4 knockout is lethal and leads to several impairments in CNS development and hematopoiesis in mice [101]. Furthermore, the CXCL12-CXCR4 axis plays a role in angiogenesis and inflammation (e.g. recruitment of lymphocytes). The chemokine CXCL12 can also bind to another chemokine receptor, CXCR7/G Protein-Coupled Receptor (RDC-1) [102, 103]. However, the connection between this pathway and the cytoskeleton is poorly understood.
Li et al. [104] showed that after CXCR4 stimulation, a signaling pathway that leads to the reorganization of the actin-cytoskeleton becomes activated. After binding of CXCL12 to CXCR4, the heterodimeric G-protein dissociates into the Gαi- and Gβγ-subunits. The Gαi2-subunit interacts with the N-terminus of the engulfment and cell-motility protein 1 (ELMO1) which forms a complex with the DOCK180 (Dedicator of cytokinesis) protein. The ELMO1/DOCK180 complex serves as a guanine nucleotide exchange factor (GEF), activating the small GTP-binding protein (G protein) Rac1 [104].
It is well known that small GTPases such as RhoA, Rac and CDC42 control the dynamics of the cytoskeleton [105]. Rac, which is activated by ELMO1/DOCK180, can remove the capping proteins and activate the Arp2/3 complex, which induces the growth of actin filaments and the formation of new actin branches from existing ones [106, 107].
Another cytoskeleton modulation by CXCL12 is the activation of Focal Adhesion Kinase (FAK) and Paxillin. After CXCL12 binds to CXCR4, the Janus kinase 2 (JAK2) and the MAP-kinase ERK1/2 pathways become activated by phosphorylation. Activated JAK2 phosphorylates Signal transducer and activator of transcription 3 (STAT3), and pSTAT3 and pERK are able to phosphorylate FAK and Paxillin, activating these proteins, which leads to actin cytoskeleton reorganization [108]. In conclusion, CXCL12 in known to influence the cytoskeleton reorganization in two different ways, through the Gαi2-ELMO1/Dock180-Rac1 activation and the JAK2-pSTAT3/pERK-pFAK/paxillin activation.
Apart from the chemokines previously described in detail, the CXCL9/Mig chemokine is also known to activate the small GTPases Rac1 and RhoA via its receptor CXCR3 on human melanoma cells, also leading to cytoskeletal changes [109].
Role of Cytoskeleton in Maintenance of Stem-Cell Properties
Nowadays, the application of stem cells in regenerative medicine is one of the major fields in biomedical research. Because of their ability to self-renew and differentiate into specific lineages, stem cells play an important role in the development of cell-based therapies [110–112]. The implementation of these new therapies made it necessary to investigate the cellular and molecular mechanisms involved in the regulation of stem-cell differentiation, growth, and phenotypic expression. The most recent studies have indicated that the regulation of stem-cell growth and fate is also dependent on the crosstalk between the extracellular matrix (ECM) ligands and the stem-cell surface receptors [113, 114]. Therefore, during their differentiation into specific lineages, stem cells are subjected to extracellular stimuli that determine a number of morphological alterations associated with the expression of cytoskeletal proteins, actin filaments, microtubules, intermediate filaments, and their downstream effectors [115–117]. These alterations are also crucial in establishing the migration phenotype observed in different types of stem cells, as was previously documented in mesenchymal stem cells (MSCs) and in the embryonic neural stem cells (NSCs) [113, 115–118]. A brief summary of the most important conclusions that explain the role of the cytoskeleton in stemness maintenance and its contribution to stem-cell migration and differentiation is given below.
Increasing evidence shows that a diverse array of environmental factors contributes to the control of stem-cell activity, differentiation and migration. According to the tissue microenvironment factors, stem cells are believed to modulate their cytoskeleton, to migrate and move away from their niche, and then to differentiate [119]. Studies on myocardial development and on capillary endothelial cells have demonstrated that alterations in cell shape might regulate cellular differentiation [113, 115, 116, 118, 120]. These results show that the cytoskeleton is a key player in the differentiation and migration of stem cells [121] (Fig. 4.3).
Changes of the cytoskeleton in a neural stem cell during differentiation. During the differentiation process, the neural stem cell (represented by radial glia) undergoes cytoskeletal changes, in the microfilaments (MFs), microtubules (MTs) and intermediate filaments (IFs). Alterations in the stem-cell cytoskeleton may involve the disorganization of MFs, which become more dispersed; destabilization of the MTs; and also modulation of the expression of IF proteins such as Nestin and GFAP, leading to the differentiation of stem cells into astrocytes, for example
Recent studies showed that during osteogenic differentiation, the actin cytoskeleton of MSCs becomes more dispersed, similarly to that of osteoblasts, and the disruption of the actin cytoskeleton decreases osteogenesis in favor of adipogenesis [121]. These results have been crucial in the area of tissue engineering of bone and cartilage, which attempts to develop new therapeutic strategies for the treatment of musculoskeletal trauma and diseases [43, 122, 123]. A number of studies have shown that the stem-cell fate and the adhesive interactions between the stem cells and the substrate can be influenced through the control of their shape by artificial extracellular matrices.
Alterations in the cytoskeleton are also dependent on microtubules that contribute to migration and to stem-cell polarization. The regulation of microtubules is usually dependent on the Rho GTPases, in particular RhoA, Rac1 and Cdc42. Previous studies have reported that migrating hematopoietic stem and progenitor cells growing on MSCs display a polarized morphology, with the formation of an uropod at the rear pole and a leading edge at the front, which is involved in microtubule destabilization. The uropod formation seems to be dependent on the activity of RhoA and its downstream effector Rho-associated coiled-coil containing protein kinase (ROCK I). When RhoA is inhibited using the Rho kinase inhibitor (Y-27652) or RNA interference (RNAi), the polarization of the hematopoietic stem/progenitor cells (HSPCs) and their migration capability are considerably decreased, indicating the crucial role of microtubules in stem-cell migration [124, 125]. Vertelov et al. [126] showed that in hypoxic conditions, the human mesenchymal stem cells (hMSCs) showed increased RhoA activity, and consequently it may contribute not only to increasing migration, but also to preserving MSCs in an undifferentiated state, as compared to normoxic conditions. Moreover, the microtubules seem to be important to maintain the migration capacity as well as the polarity of NSCs [127].
Two of the most thoroughly studied IF proteins are nestin and GFAP [128] (Fig. 4.3). In the 1990s, nestin was first identified as a marker of neuroepithelial stem/progenitor cells in the CNS by Lendahl and collaborators [129]. Nowadays, it is considered to be a marker for distinguishing precursor from differentiated cells [130–132]. A study performed by Mellodew et al. [133] also showed that loss of nestin expression could be a predictive signal for differentiation of NSCs. GFAP is classically known as a marker of mature astrocytes. However, several studies have been conducted in order to evaluate its contribution to the maintenance of stem-cell features. Previous studies suggested that primary astrocyte cultures from the postnatal and adult mouse brain could contain GFAP-expressing cells that may act as multipotent NSCs when transferred to neurogenic conditions [134, 135]. GFAP functions are addressed in detail in section “GFAP Expression and Its Functions in Astrocytes”.
Cytoskeleton Alterations During Disease Progression: The Role of Stem Cells in Cancer
The cancer stem-cell theory predicts that not all cancer cells in a tumor exhibit the same tumor-growing ability, and that only a small population of cells with stem-cell properties drives tumor growth. The proliferation, survival and migration of tumor stem cells seem to be dependent on the local microenvironment. Although highly controlled during embryonic development, the ECM is commonly deregulated in cancer [136, 137] and seems to contribute to the development of chemo- and radioresistance of tumor cells. Under normal conditions, the ECM receptors allow stem cells to anchor to the local microenvironment where their properties can be maintained [136, 137]. This anchorage physically constrains stem cells to make direct contacts with the microenvironment cells, which produce paracrine-signaling molecules that are essential for maintaining stem-cell properties [136, 137].
Considering that tumor cells possess an increased proliferation and migration ability, we could hypothesize that due to the occurrence of genetic mutations and microenvironment alterations, the characteristics of the stem-cell cytoskeleton become distorted. The most recent studies have shown that cancer cells express the same cytoskeleton markers as benign stem cells [130]. Therefore, the problem seems to be associated with the degree of expression of the cytoskeleton markers and with the signaling pathways that become activated.
One of the first lines of evidence that the cytoskeleton is involved in the tumor phenotype was the experiment conducted by Vasioukhin et al. [138], who set up conditional gene targeting to knockout genes in the stem cells and basal epidermal layer of mouse skin. They started to knock out the α-catenin, and observed that mouse skin rapidly took on the appearance of squamous cell carcinoma in situ. More recently, Rampazzo et al. [139] demonstrated that the treatment of tumor stem cells isolated from glioblastoma samples, with Wnt ligands, or the induction of β-catenin overexpression mediates neuronal differentiation and halts proliferation in primary glioblastoma cells.
In prostate cancer, deregulation of the non-canonical Wnt/Ca2+ pathway leads to F-actin filament rearrangements and consequently to the reduction of cancer progression [140].
Understanding the complexities of the stem cell cytoskeleton in cell homeostasis and in tumor development is a challenging exercise, not only to understand the physiology of many diseases but also to implement new therapeutic strategies.
Role of Intermediate Filaments in Glial Cells: Example
Glial Fibrillary Acidic Protein (GFAP) in Health and Disease
The intermediate filaments are components of the cytoskeleton that are specific to each cell type. These filaments confer mechanical force and resistance on the cells, and are regulated developmentally and tissue-specifically. GFAP is the main intermediate filament (IF) protein in astrocytes, although other intermediate filament proteins such as nestin, vimentin and synemin can also be found in these cells [141]. A combination of vimentin and nestin is observed in immature astrocytes, while vimentin and GFAP are found in mature astrocytes [37, 142, 143] (Fig. 4.3). Only GFAP seems to be capable of forming homodimers [143].
GFAP was discovered in the brains of patients with multiple sclerosis. It was initially termed ‘plaque protein’ and was first isolated, purified and the amino-acid content determined over 40 years ago. Immunostaining for GFAP has been performed since 1975 [144, 145].
Although GFAP was originally thought to be an astrocyte-specific IF [145], curiously, several investigators have shown that it is present in different amounts in various types of tissues, such as enteric glia [146], Schwann cells [147], and even outside the CNS, in chondrocytes, fibroblasts and myoepithelial cells [147, 148], lymphocytes [149] and stellate cells from the liver, kidney, pancreas, lungs, and testes [150, 151].
Astrocytes are heterogeneous, and their biology varies according to the particular physiological state and time frame, and also to their location in the CNS [151, 152]. It is a challenging task to determine the specific role of GFAP in these multiple environments and in different physiological and pathological conditions.
The human GFAP polypeptide consists of 432 amino acids and has a molecular mass of 55 kDa. The gene contains 9 exons and spans over 10 Kb in chromosome 17p21 [153]. GFAP belongs to the family of type 3 intermediate filaments.
The constitutional transcription of the human GFAP gene is controlled by a TATA-like sequence CATAAA, located 29 base pairs downstream of the RNA start site [154]. Multiple sites seem to be involved in the regulation of GFAP expression, with important roles for phosphorylation and DNA methylation in GFAP transcription [155]. The demethylation of the GFAP promoter activates GFAP transcription [156]. GFAP expression during development is also controlled by acetylation in neural stem cells, and has been shown to be significantly reduced by acetylation in mature astrocytes [157, 158].
The expression of GFAP has multiple regulatory factors, including various hormones, cytokines and growth factors, including interleukins 1 and 2, tumor necrosis factor (TNF), ciliary neurotrophic factor (CNTF), basic fibroblast growth factor (bFGF), transforming growth factor beta 1 (TGFβ1) and glutamate. Interestingly, this regulation seems to be partially controlled by the interaction between astrocytes and cortical neurons, mainly through TGFβ1; and varies in different regions of the brain [159–163].
GFAP Alternative Splicing-Isoforms
At least nine isoforms of GFAP mRNA exist, and are generated by alternative mRNA splicing and polyadenylation signal selection [155, 164–169]. Please refer to Middeldorp and Hol for an excellent review of GFAP biology and GFAP isoforms [155]. Seven of these isoforms are present in humans.
GFAPα was the first to be identified, and is also the most abundant and the most studied.
GFAPβ has a transcription site located 169 nucleotides above the site for GFAPα, which corresponds to a 5′ region that is not transcribed in the main isoform [170]. GFAPβ is the main isoform in Schwann cells from the rat peripheral nervous system (PNS), but its mRNA comprises only 5–10 % of the total GFAP mRNA in the CNS. It has been found in normal hamster brain and in a case of human glioma [171].
GFAPγ was first described in spleen and bone marrow from mice. GFAPγ lacks exon 1 and includes the last 126 bp of intron 1–2, comprises around 5 % of all GFAP mRNA in the CNS in mice, and is also present in a small proportion in humans [167].
The splice variants GFAPΔEx6, GFAPΔ164, and GFAPΔ135 skip sequences in exon 6/7 and have been observed in tissue from patients with Alzheimer’s disease, focal lesions in chronic epilepsy, and a specific astrocyte subtype. These out-of-frame splice forms completely lack the tail domain [166].
GFAPκ is the result of an alternative splicing at the 3′ end of the GFAP pre-mRNA, and the consequent inclusion of an alternative exon termed 7B [164]. The C-terminal domain of GFAPκ is therefore different from those present in other isoforms.
GFAPζ was described in mice, originating from the initial report of a transcript including the last 284 bp of intron 8–9 [167, 172].
GFAPδ is the most often studied isoform, after the canonical form GFAPα. It was initially described in rats in 1999, from an alternative splicing, resulting in substitution of the last two exons by an alternative exon called 7+ [165]. Nielsen and coworkers [168] described the corresponding RNA in humans, terming it 7a, and naming the isoform GFAP epsilon; and more recently have come to a consensus on the name GFAPδ [173]. The exon 7a has its own polyadenylation signal inhibiting the expression of exons 8 and 9. It has been isolated only in mammals, and seems to be subject to a different evolutionary pressure than the other exons [174]. Among the higher primates, the exons 7+ are 100 % identical, with the exception of an alanine on codon 426, conserved in only 9 % of human alleles, and replaced by a valine in 21 % and by a threonine in 70 %. The potential phosphorylation of this threonine residue could explain a positive selection for this change [175].
Incorporation of exon 7a results in a substitution of the 42 C-terminal amino acids by a new c-terminal domain of 41 amino acids. Among the different isoforms, only GFAPδ and GFAPκ show a modification in the C-terminal portion. As a consequence, GFAPδ and GFAPκ are incapable of forming homodimers, but are able to form heterodimers with GFAPα and vimentin [164, 175]. GFAPκ mRNA comprises around 5–10 % of the total GFAP mRNA in humans [164, 175, 176].
GFAPδ is expressed in proliferating neurogenic astrocytes during development, in the adult human brain as well as in radial glia cells [169, 177–179]. It can be detected by immunohistochemistry, particularly in astrocytes of the glia limitans and in different forms of gliosis [172, 180], in contrast to earlier studies on post-mortem material [169]. Moreover, its expression seems to parallel that of vimentin in normal and reactive astrocytes, but not in glial tumors [180]. Interestingly, differential GFAP isoform expression in mice does not seem to be linked with aging or reactive gliosis [172]. The specific functions of the different GFAP isoforms have not been well established. These isoforms often differ from GFAPα in the C-tail domain, the region responsible for interaction with other cell components. The interaction of GFAPα was specifically shown with presenilins 1 and 2 [168] and αβcristallin [176]. Therefore an assembly-compromised role of GFAPδ as a modulator of the GFAP filament surface has been postulated [155].
GFAP Expression and Its Functions in Astrocytes
GFAP is phylogenetically ancient. The human GFAP polypeptide shows a 90 % homology with its murine and porcine counterparts, and about 85 % homology with goldfish GFAP [174].
During development, GFAP is expressed in radial glia, bipolar cells which express vimentin and nestin and which have been shown to be neural precursors [181, 182] (Fig. 4.3). Studies differ with respect to the exact moment when GFAP expression can first be detected in these cells, varying from gestational week 6–12; these differences are probably due to the location in the brain or to the detection techniques used [155, 183, 184]. Nervous-system neural precursor cells show a progressive shift in intermediate filament expression, from vimentin to GFAP. In the normal adult brain, only certain subpopulations of astrocytes seem to co-express vimentin and GFAP such as Bergmann Glia, subpopulations of corpus callosum, hippocampus, subpial, and rare white-matter astrocytes [185, 186].
The functions of GFAP are not yet completely elucidated, and include a role in the long-term maintenance of the brain parenchyma structure, the proper functioning of the blood–brain barrier [187], myelination [188], astrocyte proliferation [189, 190], and astrocytic modulation of some neuronal functions, such as the formation and protection of synapses [191, 192]. GFAP is involved in other important and fundamental cellular processes, and is probably implicated in astrocyte motility [193, 194] and exocytosis of astrocytic gliotransmitters [195, 196]. GFAP is also important in the regulation and maintenance of glutamate transporters in the astrocyte plasma membrane, a key mechanism for glutamate uptake and its metabolism and for the formation of GABA [197].
GFAP, Pathological States and Disease
GFAP Knockouts
Knockout mice for intermediate filaments (GFAP−/−, Vimentin−/−, GFAP−/−, and vimentin−/−) do not show major changes in their development, adult life, and reproduction [198–200]. These authors found no major differences in brain architecture and cellularity in comparison to wild-type animals. However, another group of researchers working on GFAP-null rodents reported contrasting results, showing myelination defects in the spinal cord, optic nerve and corpus callosum, and hydrocephalus in half GFAP-null mice after 18 months [201]. Astrocytes lacking intermediate filaments exhibit normal morphology, but lack the ability to form normal glial scars [202], have restricted motility in vitro [203], and are highly sensitive to ischemia and trauma [204]. GFAP−/− mice are also more sensitive to neurodegeneration induced by kainic acid or mechanical trauma, which is not observed in wild-type animals [205].
GFAP and Gliosis
GFAP expression rises as a consequence of inflammation and various CNS diseases such as trauma, ischemia, genetic diseases, toxic lesions, and degenerative diseases [206]. In all these situations, astrocytes react to injury, in a process usually called astrogliosis or simply gliosis. Despite having general morphological features in common, astrocytes may vary morphologically and chronologically in their responses according to the nature, intensity and localization of the lesion. The kinetics of this response is usually rapid, and can be detected 1 h after the insult, with a maximum intensity at between 3 and 7 days [207].
Astrocytic gliosis has classically been described morphologically by the hypertrophy of the cell soma and processes, which is roughly proportional to the severity of the insult and the proximity of the astrocyte to it. More recently it has been well established that there is an increase in the GFAP cellular content, and, depending on the severity of the reaction, also an increase in the number of astrocytes [208–210]. Constitutional GFAP expression is heterogeneous among different astrocyte populations, and in the normal state not all astrocytes express detectable levels of GFAP. With increasing intensity of gliosis, most astrocytes will express GFAP, and in severe gliosis one also observes astrocyte proliferation, with subsequent overlap and disruption of individual astrocyte domains [151]. Therefore, GFAP has been generally used as a marker of gliosis [206], even though astrocyte reactivity and GFAP upregulation due to different stimuli may be associated with different changes in transcriptome profiles and cell function [211]. In other words, GFAP levels can be generally increased in various CNS pathological states such as trauma, ischemia, infections, and neurodegenerative diseases.
Interestingly, low levels of GFAP can be detected in the cerebrospinal fluid (CSF) in healthy individuals. The reason has not been well established, but it has been postulated that the presence of GFAP might be related to some degree of astrocyte death and release of the protein into the extracellular space, as normal astrocytes do not secrete GFAP [212]. GFAP levels in the CSF can be elevated in association with several conditions, including traumatic, vascular, developmental, inflammatory, neoplastic, and degenerative diseases. We briefly describe certain diseases in which GFAP seems to have a particular role.
Alzheimer’s Disease
Alzheimer’s disease (AD) is characterized mainly by two neuropathological alterations, the formation of neurofibrillary tangles, and amyloid deposits in the brain [213]. Reactive astrogliosis has been well described in AD, although its role in this disease is not yet completely understood. Reactive astrogliosis in AD is usually focal, and reactive astrocytes are intimately associated with amyloid plaques or diffuse amyloid deposits, surrounding them and forming miniature scars all around [151]. The intensity of reactive astrogliosis, as determined by GFAP levels, has been reported to increase in parallel with increasing disease morphological burden [214, 215], and in some studies can be correlated with cognitive impairment [216].
Alexander’s Disease
A gradual increase in astrocyte GFAP content is usually observed during adult life in mice, primates, and humans [217, 218]. Mice induced to overexpress GFAP die for reasons that are currently unknown, and their astrocytes exhibit accumulation of the protein in numerous cytoplasmic Rosenthal fibers [219]. Likewise, Alexander’s disease, a rare human disease, is caused in 95 % of cases by mutations of GFAP, leading to its accumulation in the cytoplasm, associated with proteins such as small heat shock, ubiquitin and β cristallin in the form of Rosenthal fibers [220, 221]. There are two clinical forms, one with early onset, which progresses with childhood leukodystrophy with striking clinical signs of megalencephaly, seizures and psychomotor delay; and a later-onset form, often revealed by difficulty swallowing and speaking, autonomic dysfunction and ataxia. Several different mutations have been reported in the human GFAP gene in Alexander’s disease, mostly heterozygous missense changes predicting the production of full-length mutant and wild-type proteins, and subsequently alterations such as small in-frame insertions and deletions, and in-frame skipping of an entire exon or frameshifts at the extreme C-terminal end [222]. The pathophysiology is explained by the increase in the toxic function of abnormal tissue deposits [223]. For the list of GFAP mutations in this disease please refer to the Waisman Center of the University of Wisconsin-Madison (http://www.waisman.wisc.edu/alexander/mutations.html).
Interestingly, Rosenthal fibers are also present in certain pathological conditions, namely tumors such as pilocytic astrocytomas and gangliogliomas, and even some types of chronic gliosis, for example, those observed in the periphery of craniopharyngiomas or hemangioblastomas.
GFAP and Gliomas
The development of immunohistochemistry for GFAP was an important advance in surgical neuropathology, including the diagnosis of brain tumors. The expression of GFAP in primary glial tumors has been extensively studied since the beginning of the 1980s, and is now widely used in diagnostic neuropathology [224–226]. Independently of the histological grade, every tumor with astrocytic differentiation is expected to show at least some positivity for GFAP [227]. GFAP-positive cells can also be observed in some other glioma types, such as oligodendroglioma, where often small cells named “minigemistocytes” express GFAP [227]; and in ependymomas [226, 228]. A significant proportion of choroid-plexus tumors can also express GFAP, even focally [226].
Some studies have shown in vitro a negative correlation between GFAP expression levels and the malignant transformation of astrocytes [229–231]. However in experimental astrocytoma murine models, GFAP expression does not seem to affect tumor progression [232].
GFAP serum levels seem to be significantly elevated in patients with glioblastoma multiforme [233]. This implies damage to the blood–brain barrier, since this protein is not usually detectable in the serum or is present in very low levels. Some other conditions that can lead to its rise in the serum are stroke, hemorrhage, trauma and multiple sclerosis [212].
Enteric Glia Cytoskeleton
GFAP is also the main marker of enteric glia, a peripheral glial-cell type derived from the neural crest cells that has close morphological, molecular and functional similarities to astrocytes (reviewed by Coelho-Aguiar et al. [234]). These glial cells are crucial for the proper functioning of the gut. Their disruption in mice by the targeting of GFAP-positive cells leads to increased permeability of the mucosal epithelium, followed by an inflammation process and disruption of the ileum and jejunum structure [235–237].
Interestingly, as in the CNS, GFAP is also expressed in glia-like progenitors in the gut. Some of these GFAP-positive cells can generate multilineage colonies in vitro and also give rise to glial and neuronal cells in vivo in graft studies, in injury conditions or in experiments of myenteric plexus ablation with benzalkonium chloride detergent [238–245].
Similarly to reactive gliosis in the CNS, there is an increase of GFAP levels in enteric glia of inflamed gut regions, notably in inflammatory bowel diseases [246]. An increase in GFAP expression was also observed in colon biopsies of patients with Clostridium difficile infections [246]. Patients with Parkinson’s disease show α synuclein aggregates (the pathological trait of Parkinson’s disease) also in the ENS, and also an increased expression and reduced phosphorylation of GFAP, which is also observed in neurodegenerative processes in the CNS [247, 248]. These informations suggest an association between enteric inflammation and glial dysregulation.
Furthermore, specific roles are known for other components of the cytoskeleton in enteric glia, the F-actin filaments and microtubules. Little is known about this, but Ca2+ dependent responses have been identified in enteric glia, related to their functions in neurotransmission. Calcium enters enteric glial cells through a mechanism termed capacitative calcium entry, which is responsible for the maintenance of calcium storage in different cell types. It has been shown that in cultured myenteric glia, the disruption of the actin filaments or microtubules can decrease and even completely inhibit calcium entry [249]. These experiments confirmed the importance of the cytoskeleton for the physical interactions between the calcium storage organelle and the plasma membrane as a capacitative calcium-entry mechanism, which replenishes the cell after depletion of the intracellular calcium store. It is probable that the same events occur in astrocytes and other cell types to maintain their intracellular calcium store, but this has not yet been investigated.
Schwann Cell Cytoskeleton
Another neural crest-derived GFAP-expressing type of glia is the Schwann cells, which are responsible for the myelinization of the entire peripheral nervous system (PNS). Schwann cells can be found in myelinating or non-myelinating forms. This cell type is capable of great migratory and differentiation capacities during development and even in adults, to repair and replace the myelin of injured axons. The physical interaction of these cells with the surrounding microenvironment involves changes in the cytoskeleton.
These cells constitute the other example of peripheral glia that express GFAP. However, it is clear that they show a different GFAP isoform expression from that of astrocytes and enteric glia. Moreover, they express this IF at lower levels than astrocytes, and even more reduced levels in relation to enteric glia [250].
GFAP expression is a marker of non-myelinating Schwann cells. GFAP appears after differentiation of Schwann-cell precursors into immature Schwann cells [251]. Its expression depends on contact with non-myelinated axons, and is downregulated in myelinating Schwann cells. On the other hand, vimentin is expressed in myelin-forming Schwann cells. In experiments with sciatic-nerve transection, a reduction of GFAP mRNA levels was observed. Moreover, the immunodetection of GFAP also decreased, while vimentin expression increased [252].
Interactions among Schwann cells, the extracellular matrix and axons are mediated by surface receptors and are transduced by the cytoskeleton proteins. These interactions are essential to the recovery of neuronal transmission after axonal nerve injury. The axonal regeneration process follows well-established steps. After disruption of myelin sheets, Schwann cells dedifferentiate and proliferate. There is an increase in the expression of adhesion molecules and cytoskeleton proteins such as GFAP and vimentin. Then, these glial cells form bands of Büngner, which serve as a substrate for axonal regrowth. After that, they enwrap the axons and form myelin [253]. All these stages require continuous reorganization of the Schwann cell cytoskeleton.
In GFAP-null mice, the development of peripheral axons and their myelin is normal, as is their functioning. However, lack of GFAP leads to retardation in nerve regeneration after injury, probably because of a problem in Schwann cell proliferation. This study, developed by Triolo and coworkers [253], revealed that GFAP interacts with integrin αvβ8, which interacts with fibrin, thus acting in the early steps of Schwann-cell proliferation. These investigators also showed that vimentin interacts with integrin α5β1, which connects to fibronectin, acting in the subsequent steps of Schwann-cell proliferation and nerve regeneration [253].
Another cytoskeleton protein associated with integrins in Schwann cells is merlin, also known as schwannomin. Merlin is a perinuclear protein that translocates to cytoplasm during differentiation and becomes associated with integrin β1 in myelinating Schwann cells. This study suggests that merlin links the integrin to the microfilaments, supporting myelination [254]. Other proteins associated with intermediate filaments interact with integrins, such as hemidesmosomal 1 protein (HD1), plectin and dystonin [255]. Targeted disruption of dystonin, for example, results in problems in the interaction with the axon basement membrane and in PNS dysmyelination [256].
Conclusion
As set out in this chapter, the first step in cell locomotion is the formation of cytoskeleton protrusions such as lamellipodia at the periphery of a cell. This protrusion is composed of F-actin filaments. Study of the mechanical characteristics of cytoskeleton proteins and plasma membrane tension in lamellipodia was advanced by the use of optical tweezers, which enable measurement of the bending modulus and membrane tension. Important inductors of the migratory phenotype are the chemokines, small peptides that elicit cytoskeleton responses through signaling by their G protein-coupled receptors. CCL19/CCL21 and their receptor CCR7, and CXCL12 and its receptor CXCR4 have physiological roles in guiding different cell types to their niches, as well as acting in tumor-cell migration by eliciting reorganization of the actin cytoskeleton.
We next discussed the role of the cytoskeleton in the performance of stem cells. Actin microfilaments and microtubules are reorganized during differentiation, and are important for stem-cell fate and polarization. Intermediate filaments, specific to each cell type, may also be involved. Neural stem and precursor cells, for example, express nestin, while GFAP is typical for mature astrocytes but is also found in neural precursors. In the last section we explored the specific characteristics of the intermediate filament GFAP in its multiple isoforms. GFAP is essential for astrocyte functions, and its disruption is implicated in several disorders and diseases. Peripheral glial cells that also express GFAP are the enteric glial cells, which are the counterparts of astrocytes in the gut; and the Schwann cells, which are responsible for the myelination of the peripheral nerves.
Abbreviations
- AD:
-
Alzheimer’s disease
- Arp2/3:
-
Actin-related proteins 2/3
- bFGF:
-
Basic fibroblast growth factor
- CCL:
-
C-C motif chemokine
- CCR7:
-
C-C chemokine receptor type 7
- CDC42:
-
Cell division control protein 42 homolog
- CNS:
-
Central nervous system
- CNTF:
-
Ciliary neurotrophic factor
- CSF:
-
Cerebrospinal fluid
- CX3CR1:
-
CX3C chemokine receptor 1
- CXCR:
-
C-X-C chemokine receptor
- DOCK180:
-
Dedicator of cytokinesis protein
- ECM:
-
Extracellular matrix
- ELMO1:
-
Engulfment and cell-motility protein 1
- ERK:
-
Extracellular signal-regulated protein kinase
- ERM:
-
Ezrin/Radixin/Moesin
- FAK:
-
Focal adhesion kinase
- G proteins:
-
guanine nucleotide-binding proteins
- GABA:
-
Gamma amino butyric acid
- GEF:
-
Guanine nucleotide exchange factor
- GFAP:
-
Glial fibrillary acidic protein
- GPCRs:
-
G-protein-coupled receptors
- HD1:
-
Hemidesmosomal 1 protein
- hMSC:
-
Human mesenchymal stem cells
- HSPC:
-
Hematopoietic stem/progenitor cell
- IF:
-
Intermediate filament
- JAK:
-
Janus kinase
- MAPKs:
-
Mitogen-activated protein kinases
- mDia1:
-
Diaphanous homolog 1 of Drosophila
- MFs:
-
Microfilaments
- MLC:
-
Myosin light chain
- MSCs:
-
Mesenchymal stem cells
- MTs:
-
Microtubules
- NSCs:
-
Neural stem cells
- PBSF:
-
Pre-B cell growth-stimulating factor = SDF1, stromal cell-derived factor 1
- PKB:
-
Protein kinase B
- PNS:
-
Peripheral nervous system
- RAC1:
-
Ras-related C3 botulinum toxin substrate 1
- RDC-1:
-
G protein-coupled receptor = CXCL12
- RNAi:
-
RNA interference
- ROCK:
-
Rho-associated coiled-coil forming protein kinases
- SDF-1:
-
Stromal cell-derived factor-1= CXCL12
- STAT3:
-
Signal transducer and activator of transcription 3
- TGFβ1:
-
Transforming growth factor beta 1
- TNF:
-
Tumor necrosis factor
- VASP:
-
Vasodilator-stimulated phosphoprotein
- XCR1:
-
C sub-family of chemokine receptors 1
References
Alberts B (2002) Molecular biology of the cell, 4th edn, vol xxxiv. Garland Science, New York, 1464 p
Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10(7):445–457
Mogilner A (2009) Mathematics of cell motility: have we got its number? J Math Biol 58(1-2):105–134
Petrie RJ, Doyle AD, Yamada KM (2009) Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol 10(8):538–549
Ridley AJ et al (2003) Cell migration: integrating signals from front to back. Science 302(5651):1704–1709
Etienne-Manneville S, Hall A (2003) Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421(6924):753–756
Pletjushkina OJ et al (1994) Taxol-treated fibroblasts acquire an epithelioid shape and a circular pattern of actin bundles. Exp Cell Res 212(2):201–208
Euteneuer U, Schliwa M (1984) Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature 310(5972):58–61
Eilken HM, Adams RH (2010) Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol 22(5):617–625
Gupton SL, Gertler FB (2007) Filopodia: the fingers that do the walking. Sci STKE 2007(400):p. re5
Charras G, Paluch E (2008) Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol 9(9):730–736
Buccione R, Caldieri G, Ayala I (2009) Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev 28(1–2):137–149
Abercrombie M, Heaysman JE, Pegrum SM (1970) The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res 59(3):393–398
Abercrombie M, Heaysman JE, Pegrum SM (1971) The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res 67(2):359–367
Weijer CJ (2009) Collective cell migration in development. J Cell Sci 122(Pt 18):3215–3223
Mullins RD, Heuser JA, Pollard TD (1998) The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A 95(11):6181–6186
Campellone KG, Welch MD (2010) A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11(4):237–251
Svitkina TM, Borisy GG (1999) Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol 145(5):1009–1026
Urban E et al (2010) Electron tomography reveals unbranched networks of actin filaments in lamellipodia. Nat Cell Biol 12(5):429–435
Akin O, Mullins RD (2008) Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell 133(5):841–851
Bear JE, Gertler FB (2009) Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci 122(Pt 12):1947–1953
Breitsprecher D et al (2011) Molecular mechanism of Ena/VASP-mediated actin-filament elongation. EMBO J 30(3):456–467
Chesarone MA, DuPage AG, Goode BL (2010) Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol 11(1):62–74
Keren K (2011) Cell motility: the integrating role of the plasma membrane. Eur Biophys J 40(9):1013–1027
Houk AR et al (2012) Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148(1–2):175–188
Gauthier NC, Masters TA, Sheetz MP (2012) Mechanical feedback between membrane tension and dynamics. Trends Cell Biol 22(10):527–535
Masters TA et al (2013) Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc Natl Acad Sci U S A 110(29):11875–11880
Gittes F et al (1993) Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol 120(4):923–934
Diz-Munoz A, Fletcher DA, Weiner OD (2013) Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol 23(2):47–53
Bo L, Waugh RE (1989) Determination of bilayer membrane bending stiffness by tether formation from giant, thin-walled vesicles. Biophys J 55(3):509–517
Pontes B et al (2011) Cell cytoskeleton and tether extraction. Biophys J 101(1):43–52
Gauthier NC et al (2011) Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc Natl Acad Sci U S A 108(35):14467–14472
Keren K et al (2008) Mechanism of shape determination in motile cells. Nature 453(7194):475–480
Risca VI et al (2012) Actin filament curvature biases branching direction. Proc Natl Acad Sci U S A 109(8):2913–2918
Sheetz MP, Dai J (1996) Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol 6(3):85–89
Morris CE, Homann U (2001) Cell surface area regulation and membrane tension. J Membr Biol 179(2):79–102
Apodaca G (2002) Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol 282(2):F179–F190
Ofer N, Mogilner A, Keren K (2011) Actin disassembly clock determines shape and speed of lamellipodial fragments. Proc Natl Acad Sci U S A 108(51):20394–20399
Cai Y et al (2010) Cytoskeletal coherence requires myosin-IIA contractility. J Cell Sci 123(Pt 3):413–423
Hissa B et al (2013) Membrane cholesterol removal changes mechanical properties of cells and induces secretion of a specific pool of lysosomes. PLoS One 8(12):e82988
Lieber AD et al (2013) Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr Biol 23(15):1409–1417
Abu Shah E, Keren K (2013) Mechanical forces and feedbacks in cell motility. Curr Opin Cell Biol 25(5):550–557
Pontes B et al (2013) Membrane elastic properties and cell function. PLoS One 8(7):e67708
Janmey PA, McCulloch CA (2007) Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng 9:1–34
Faria J et al (2006) Interactive properties of human glioblastoma cells with brain neurons in culture and neuronal modulation of glial laminin organization. Differentiation 74(9–10):562–572
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12(2):121–127
Lazennec G, Richmond A (2010) Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med 16(3):133–144
Vindrieux D, Escobar P, Lazennec G (2009) Emerging roles of chemokines in prostate cancer. Endocr Relat Cancer 16(3):663–673
Ali S, Lazennec G (2007) Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev 26(3–4):401–420
Mantovani A, Bonecchi R, Locati M (2006) Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol 6(12):907–918
Binder NB et al (2009) Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med 15(4):417–424
Chavey C et al (2009) CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab 9(4):339–349
Kohlmeier JE et al (2008) The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 29(1):101–113
Yoder A et al (2008) HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell 134(5):782–792
Tiwari S et al (2009) Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat Immunol 10(8):907–917
Molon B et al (2005) T cell costimulation by chemokine receptors. Nat Immunol 6(5):465–471
Pitchford SC et al (2009) Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 4(1):62–72
Reboldi A et al (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10(5):514–523
Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7(3):211–217
Mantovani A (2009) Cancer: inflaming metastasis. Nature 457(7225):36–37
Mentlein R, Hattermann K, Held-Feindt J (2013) Migration, metastasis, and more: the role of chemokines in the proliferation, spreading, and metastasis of tumors. In: Resende RR, Ulrich H (eds) Trends in the cell proliferation and cancer research. Springer, Netherlands, pp 339–358
Moser B, Loetscher P (2001) Lymphocyte traffic control by chemokines. Nat Immunol 2(2):123–128
von Andrian UH, Mackay CR (2000) T-cell function and migration. Two sides of the same coin. N Engl J Med 343(14):1020–1034
Sallusto F, Mackay CR, Lanzavecchia A (2000) The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 18:593–620
Sanchez-Madrid F, del Pozo MA (1999) Leukocyte polarization in cell migration and immune interactions. EMBO J 18(3):501–511
Ridley AJ (2001) Rho GTPases and cell migration. J Cell Sci 114(Pt 15):2713–2722
Thelen M (2001) Dancing to the tune of chemokines. Nat Immunol 2(2):129–134
Mellado M et al (1998) The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol 161(2):805–813
Mellado M et al (2001) Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol 19:397–421
Amano M, Fukata Y, Kaibuchi K (2000) Regulation and functions of Rho-associated kinase. Exp Cell Res 261(1):44–51
Lee JH et al (2004) Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. J Cell Biol 167(2):327–337
Sakata D et al (2007) Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med 204(9):2031–2038
Gunn MD et al (1998) A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A 95(1):258–263
Stein JV et al (2000) The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J Exp Med 191(1):61–76
Campbell JJ et al (1998) Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279(5349):381–384
Okada T et al (2002) Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med 196(1):65–75
Bardi G, Niggli V, Loetscher P (2003) Rho kinase is required for CCR7-mediated polarization and chemotaxis of T lymphocytes. FEBS Lett 542(1–3):79–83
Boehme SA et al (1999) Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J Immunol 163(3):1611–1618
Bonacchi A et al (2001) Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem 276(13):9945–9954
Kampen GT et al (2000) Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood 95(6):1911–1917
Berlin-Rufenach C et al (1999) Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med 189(9):1467–1478
Laudanna C, Alon R (2006) Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb Haemost 95(1):5–11
Warnock RA et al (1998) Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med 187(2):205–216
Giagulli C et al (2004) RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity 20(1):25–35
Laudanna C, Campbell JJ, Butcher EC (1996) Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science 271(5251):981–983
Pasvolsky R et al (2008) RhoA is involved in LFA-1 extension triggered by CXCL12 but not in a novel outside-in LFA-1 activation facilitated by CXCL9. J Immunol 180(5):2815–2823
Cunningham HD et al (2010) Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl Oncol 3(6):354–361
Mburu YK et al (2012) Chemokine receptor 7 (CCR7) gene expression is regulated by NF-kappaB and activator protein 1 (AP1) in metastatic squamous cell carcinoma of head and neck (SCCHN). J Biol Chem 287(5):3581–3590
Jung JI et al (2015) High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: roles of adipocytes and M2-macrophages. Int J Cancer 136(2):258–270
Shields JD et al (2007) Chemokine-mediated migration of melanoma cells towards lymphatics–a mechanism contributing to metastasis. Oncogene 26(21):2997–3005
Shields JD et al (2007) Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11(6):526–538
Nagasawa T, Kikutani H, Kishimoto T (1994) Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A 91(6):2305–2309
Muller A et al (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410(6824):50–56
Liu Y et al (2010) Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res 29:16
Tashiro K et al (1993) Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science 261(5121):600–603
Loetscher M et al (1994) Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem 269(1):232–237
Feng Y et al (1996) HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272(5263):872–877
Bleul CC et al (1996) The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382(6594):829–833
Aiuti A et al (1997) The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 185(1):111–120
Reiss K et al (2002) Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience 115(1):295–305
Ma Q et al (1998) Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 95(16):9448–9453
Balabanian K et al (2005) The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 280(42):35760–35766
Odemis V et al (2012) The presumed atypical chemokine receptor CXCR7 signals through G(i/o) proteins in primary rodent astrocytes and human glioma cells. Glia 60(3):372–381
Li H et al (2013) Association between Galphai2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat Commun 4:1706
Takabayashi T et al (2009) Lipopolysaccharides increase the amount of CXCR4, and modulate the morphology and invasive activity of oral cancer cells in a CXCL12-dependent manner. Oral Oncol 45(11):968–973
Dong X et al (2005) P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol 15(20):1874–1879
Sun CX, Magalhaes MA, Glogauer M (2007) Rac1 and Rac2 differentially regulate actin free barbed end formation downstream of the fMLP receptor. J Cell Biol 179(2):239–245
Gao H et al (2009) Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27(4):857–865
Robledo MM et al (2001) Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem 276(48):45098–45105
Lima FR et al (2012) Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta 1826(2):338–349
Singh A, Settleman J (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29(34):4741–4751
Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Guilak F et al (2009) Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5(1):17–26
Lutolf MP, Gilbert PM, Blau HM (2009) Designing materials to direct stem-cell fate. Nature 462(7272):433–441
Gage FH (2000) Mammalian neural stem cells. Science 287(5457):1433–1438
Mamsen LS et al (2012) The migration and loss of human primordial germ stem cells from the hind gut epithelium towards the gonadal ridge. Int J Dev Biol 56(10–12):771–778
Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414(6859):98–104
Treiser MD et al (2010) Cytoskeleton-based forecasting of stem cell lineage fates. Proc Natl Acad Sci U S A 107(2):610–615
Engler AJ et al (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Kinney MA, Saeed R, McDevitt TC (2014) Mesenchymal morphogenesis of embryonic stem cells dynamically modulates the biophysical microtissue niche. Sci Rep 4:4290
McBeath R et al (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6(4):483–495
Collart-Dutilleul PY et al (2014) Initial stem cell adhesion on porous silicon surface: molecular architecture of actin cytoskeleton and filopodial growth. Nanoscale Res Lett 9(1):564
Galkin VE, Orlova A, Egelman EH (2012) Actin filaments as tension sensors. Curr Biol 22(3):R96–R101
Fonseca AV, Corbeil D (2011) The hematopoietic stem cell polarization and migration: a dynamic link between RhoA signaling pathway, microtubule network and ganglioside-based membrane microdomains. Commun Integr Biol 4(2):201–204
Fonseca AV et al (2010) Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network. J Biol Chem 285(41):31661–31671
Vertelov G et al (2013) High targeted migration of human mesenchymal stem cells grown in hypoxia is associated with enhanced activation of RhoA. Stem Cell Res Ther 4(1):5
Titushkin I, Cho M (2007) Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J 93(10):3693–3702
Hyder CL et al (2011) Insights into intermediate filament regulation from development to ageing. J Cell Sci 124(Pt 9):1363–1372
Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60(4):585–595
Kleeberger W et al (2007) Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res 67(19):9199–9206
Suzuki S et al (2010) The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem 58(8):721–730
Wiese C et al (2004) Nestin expression–a property of multi-lineage progenitor cells? Cell Mol Life Sci 61(19–20):2510–2522
Mellodew K et al (2004) Nestin expression is lost in a neural stem cell line through a mechanism involving the proteasome and Notch signalling. Brain Res Dev Brain Res 151(1–2):13–23
Hitoshi S et al (2004) Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling. Genes Dev 18(15):1806–1811
Sachewsky N et al (2014) Primitive neural stem cells in the adult mammalian brain give rise to GFAP-expressing neural stem cells. Stem Cell Rep 2(6):810–824
Jinka R et al (2012) Alterations in cell-extracellular matrix interactions during progression of cancers. Int J Cell Biol 2012:219196
Moura-Neto V et al (2014) Glioblastomas and the special role of adhesion molecules in their invasion. In: Sedo A, Mentlein R (eds) Glioma cell biology. Springer, Vienna, pp 293–315
Vasioukhin V et al (2001) Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 104(4):605–617
Rampazzo E et al (2013) Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis 4:e500
Wang Q et al (2010) A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PLoS One 5(5):e10456
Jing R et al (2007) Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. J Cell Sci 120(Pt 7):1267–1277
Sancho-Tello M et al (1995) Developmental pattern of GFAP and vimentin gene expression in rat brain and in radial glial cultures. Glia 15(2):157–166
Eliasson C et al (1999) Intermediate filament protein partnership in astrocytes. J Biol Chem 274(34):23996–24006
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25(9–10):1439–1451
Eng LF et al (1971) An acidic protein isolated from fibrous astrocytes. Brain Res 28(2):351–354
Kato H et al (1990) Immunocytochemical characterization of supporting cells in the enteric nervous system in Hirschsprung’s disease. J Pediatr Surg 25(5):514–519
Hainfellner JA et al (2001) Fibroblasts can express glial fibrillary acidic protein (GFAP) in vivo. J Neuropathol Exp Neurol 60(5):449–461
Viale G et al (1991) Glial fibrillary acidic protein immunoreactivity in normal and diseased human breast. Virchows Arch A Pathol Anat Histopathol 418(4):339–348
Riol H et al (1997) Detection of the peripheral nervous system (PNS)-type glial fibrillary acidic protein (GFAP) and its mRNA in human lymphocytes. J Neurosci Res 48(1):53–62
Carotti S et al (2008) Glial fibrillary acidic protein as an early marker of hepatic stellate cell activation in chronic and posttransplant recurrent hepatitis C. Liver Transpl 14(6):806–814
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Brenner M (2014) Role of GFAP in CNS injuries. Neurosci Lett 565:7–13
Reeves SA et al (1989) Molecular cloning and primary structure of human glial fibrillary acidic protein. Proc Natl Acad Sci U S A 86(13):5178–5182
Nakatani Y, Brenner M, Freese E (1990) An RNA polymerase II promoter containing sequences upstream and downstream from the RNA startpoint that direct initiation of transcription from the same site. Proc Natl Acad Sci U S A 87(11):4289–4293
Middeldorp J, Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93(3):421–443
Namihira M et al (2009) Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell 16(2):245–255
Kanski R et al (2014) Histone acetylation in astrocytes suppresses GFAP and stimulates a reorganization of the intermediate filament network. J Cell Sci 127(Pt 20):4368–4380
Zhou Q et al (2011) Histone deacetylase inhibitors SAHA and sodium butyrate block G1-to-S cell cycle progression in neurosphere formation by adult subventricular cells. BMC Neurosci 12:50
de Sampaio e Spohr TC et al (2002) Neuro-glia interaction effects on GFAP gene: a novel role for transforming growth factor-beta1. Eur J Neurosci 16(11):2059–2069
Gomes FC et al (1999) Neurons induce GFAP gene promoter of cultured astrocytes from transgenic mice. Glia 26(2):97–108
Romao LF et al (2008) Glutamate activates GFAP gene promoter from cultured astrocytes through TGF-beta1 pathways. J Neurochem 106(2):746–756
Sousa Vde O et al (2004) Glial fibrillary acidic protein gene promoter is differently modulated by transforming growth factor-beta 1 in astrocytes from distinct brain regions. Eur J Neurosci 19(7):1721–1730
Rutka JT et al (1997) Role of glial filaments in cells and tumors of glial origin: a review. J Neurosurg 87(3):420–430
Blechingberg J et al (2007) Identification and characterization of GFAPkappa, a novel glial fibrillary acidic protein isoform. Glia 55(5):497–507
Condorelli DF et al (1999) Structural features of the rat GFAP gene and identification of a novel alternative transcript. J Neurosci Res 56(3):219–228
Hol EM et al (2003) Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol Psychiatry 8(9):786–796
Zelenika D et al (1995) A novel glial fibrillary acidic protein mRNA lacking exon 1. Brain Res Mol Brain Res 30(2):251–258
Nielsen AL et al (2002) A new splice variant of glial fibrillary acidic protein, GFAP epsilon, interacts with the presenilin proteins. J Biol Chem 277(33):29983–29991
Roelofs RF et al (2005) Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. Glia 52(4):289–300
Feinstein DL, Weinmaster GA, Milner RJ (1992) Isolation of cDNA clones encoding rat glial fibrillary acidic protein: expression in astrocytes and in Schwann cells. J Neurosci Res 32(1):1–14
Galea E, Dupouey P, Feinstein DL (1995) Glial fibrillary acidic protein mRNA isotypes: expression in vitro and in vivo. J Neurosci Res 41(4):452–461
Kamphuis W et al (2012) GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS One 7(8):e42823
Thomsen R et al (2013) Alternative mRNA splicing from the glial fibrillary acidic protein (GFAP) gene generates isoforms with distinct subcellular mRNA localization patterns in astrocytes. PLoS One 8(8):e72110
Singh R et al (2003) Genetic polymorphism and sequence evolution of an alternatively spliced exon of the glial fibrillary acidic protein gene, GFAP. Genomics 82(2):185–193
Nielsen AL, Jorgensen AL (2004) Self-assembly of the cytoskeletal glial fibrillary acidic protein is inhibited by an isoform-specific C terminus. J Biol Chem 279(40):41537–41545
Perng MD et al (2008) Glial fibrillary acidic protein filaments can tolerate the incorporation of assembly-compromised GFAP-delta, but with consequences for filament organization and alphaB-crystallin association. Mol Biol Cell 19(10):4521–4533
Leonard BW et al (2009) Subventricular zone neural progenitors from rapid brain autopsies of elderly subjects with and without neurodegenerative disease. J Comp Neurol 515(3):269–294
Middeldorp J et al (2010) GFAPdelta in radial glia and subventricular zone progenitors in the developing human cortex. Development 137(2):313–321
van den Berge SA et al (2010) Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP-delta. Aging Cell 9(3):313–326
Andreiuolo F et al (2009) GFAPdelta immunostaining improves visualization of normal and pathologic astrocytic heterogeneity. Neuropathology 29(1):31–39
Gotz M, Hartfuss E, Malatesta P (2002) Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res Bull 57(6):777–788
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184
deAzevedo LC et al (2003) Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol 55(3):288–298
Sarnat HB (1992) Regional differentiation of the human fetal ependyma: immunocytochemical markers. J Neuropathol Exp Neurol 51(1):58–75
Lazarides E (1982) Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem 51:219–250
Yamada T et al (1992) Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathol 84(2):157–162
Pekny M et al (1998) Impaired induction of blood-brain barrier properties in aortic endothelial cells by astrocytes from GFAP-deficient mice. Glia 22(4):390–400
Gimenez YRM et al (2000) Comparative anatomy of the cerebellar cortex in mice lacking vimentin, GFAP, and both vimentin and GFAP. Glia 31(1):69–83
Messing A et al (1998) Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol 152(2):391–398
Rutka JT et al (1994) Effects of antisense glial fibrillary acidic protein complementary DNA on the growth, invasion, and adhesion of human astrocytoma cells. Cancer Res 54(12):3267–3272
Emirandetti A et al (2006) Astrocyte reactivity influences the number of presynaptic terminals apposed to spinal motoneurons after axotomy. Brain Res 1095(1):35–42
Tyzack GE et al (2014) Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression. Nat Commun 5:4294
Elobeid A et al (2000) Effects of inducible glial fibrillary acidic protein on glioma cell motility and proliferation. J Neurosci Res 60(2):245–256
Yoshida T et al (2007) The functional alteration of mutant GFAP depends on the location of the domain: morphological and functional studies using astrocytoma-derived cells. J Hum Genet 52(4):362–369
Berg A et al (2013) Axonal regeneration after sciatic nerve lesion is delayed but complete in GFAP- and vimentin-deficient mice. PLoS One 8(11):e79395
Potokar M et al (2010) Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes. Glia 58(10):1208–1219
Sullivan SM et al (2007) Cytoskeletal anchoring of GLAST determines susceptibility to brain damage: an identified role for GFAP. J Biol Chem 282(40):29414–29423
Gomi H et al (1995) Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 14(1):29–41
McCall MA et al (1996) Targeted deletion in astrocyte intermediate filament (GFAP) alters neuronal physiology. Proc Natl Acad Sci U S A 93(13):6361–6366
Pekny M et al (1995) Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J 14(8):1590–1598
Liedtke W et al (1996) GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 17(4):607–615
Pekny M et al (1999) Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 145(3):503–514
Lepekhin EA et al (2001) Intermediate filaments regulate astrocyte motility. J Neurochem 79(3):617–625
Nawashiro H et al (1998) Mice lacking GFAP are hypersensitive to traumatic cerebrospinal injury. Neuroreport 9(8):1691–1696
Otani N et al (2006) Enhanced hippocampal neurodegeneration after traumatic or kainate excitotoxicity in GFAP-null mice. J Clin Neurosci 13(9):934–938
Eng LF, Ghirnikar RS (1994) GFAP and astrogliosis. Brain Pathol 4(3):229–237
Alonso G, Privat A (1993) Reactive astrocytes involved in the formation of lesional scars differ in the mediobasal hypothalamus and in other forebrain regions. J Neurosci Res 34(5):523–538
Faulkner JR et al (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9):2143–2155
Hatten ME et al (1991) Astroglia in CNS injury. Glia 4(2):233–243
Wanner IB et al (2013) Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 33(31):12870–12886
Anderson MA, Ao Y, Sofroniew MV (2014) Heterogeneity of reactive astrocytes. Neurosci Lett 565:23–29
Liem RK, Messing A (2009) Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest 119(7):1814–1824
Hardy J (2006) A hundred years of Alzheimer’s disease research. Neuron 52(1):3–13
Muramori F, Kobayashi K, Nakamura I (1998) A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer’s disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatry Clin Neurosci 52(6):593–599
Simpson JE et al (2010) Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging 31(4):578–590
Kashon ML et al (2004) Associations of cortical astrogliosis with cognitive performance and dementia status. J Alzheimers Dis 6(6):595–604, discussion 673–681
Porchet R et al (2003) Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics 3(8):1476–1485
Sloane JA et al (2000) Astrocytic hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain Res 862(1–2):1–10
Messing A, Brenner M (2003) GFAP: functional implications gleaned from studies of genetically engineered mice. Glia 43(1):87–90
Alexander WS (1949) Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain 72(3):373–381, 3 pl
Brenner M et al (2001) Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet 27(1):117–120
Flint D et al (2012) Splice site, frameshift, and chimeric GFAP mutations in Alexander disease. Hum Mutat 33(7):1141–1148
Brenner M, Messing A (2015) A new mutation in GFAP widens the spectrum of Alexander disease. Eur J Hum Genet 23(1):1–2
Tascos NA, Parr J, Gonatas NK (1982) Immunocytochemical study of the glial fibrillary acidic protein in human neoplasms of the central nervous system. Hum Pathol 13(5):454–458
de Armond SJ, Eng LF, Rubinstein LJ (1980) The application of glial fibrillary acidic (GFA) protein immunohistochemistry in neurooncology. A progress report. Pathol Res Pract 168(4):374–394
Louis DN et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Herpers MJ, Budka H (1984) Glial fibrillary acidic protein (GFAP) in oligodendroglial tumors: gliofibrillary oligodendroglioma and transitional oligoastrocytoma as subtypes of oligodendroglioma. Acta Neuropathol 64(4):265–272
Cruz-Sanchez FF et al (1988) An immunohistological study of 66 ependymomas. Histopathology 13(4):443–454
Rutka JT, Smith SL (1993) Transfection of human astrocytoma cells with glial fibrillary acidic protein complementary DNA: analysis of expression, proliferation, and tumorigenicity. Cancer Res 53(15):3624–3631
Toda M et al (1999) Suppression of glial tumor growth by expression of glial fibrillary acidic protein. Neurochem Res 24(2):339–343
Weinstein DE, Shelanski ML, Liem RK (1991) Suppression by antisense mRNA demonstrates a requirement for the glial fibrillary acidic protein in the formation of stable astrocytic processes in response to neurons. J Cell Biol 112(6):1205–1213
Wilhelmsson U et al (2003) Loss of GFAP expression in high-grade astrocytomas does not contribute to tumor development or progression. Oncogene 22(22):3407–3411
Gallego Perez-Larraya J et al (2014) Diagnostic and prognostic value of preoperative combined GFAP, IGFBP-2, and YKL-40 plasma levels in patients with glioblastoma. Cancer 120(24):3972–3980
Coelho-Aguiar JM et al (2015) The enteric glia: identity and functions. Glia 63(6):921–935
Aube AC et al (2006) Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 55(5):630–637
Bush TG et al (1998) Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93(2):189–201
Cornet A et al (2001) Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc Natl Acad Sci U S A 98(23):13306–13311
Almond S et al (2007) Characterisation and transplantation of enteric nervous system progenitor cells. Gut 56(4):489–496
Bondurand N et al (2003) Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 130(25):6387–6400
Hetz S et al (2014) In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One 9(4):e93605
Joseph NM et al (2011) Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest 121(9):3398–3411
Kruger GM et al (2002) Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35(4):657–669
Laranjeira C et al (2011) Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121(9):3412–3424
Liu MT et al (2009) 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 29(31):9683–9699
Rauch U et al (2006) Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int J Colorectal Dis 21(6):554–559
von Boyen GB et al (2011) Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol 11:3
Clairembault T et al (2014) Enteric GFAP expression and phosphorylation in Parkinson’s disease. J Neurochem 130(6):805–815
Devos D et al (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48
Lin T et al (2003) The role of the cytoskeleton in capacitative calcium entry in myenteric glia. Neurogastroenterol Motil 15(3):277–287
Jessen KR, Mirsky R (1985) Glial fibrillary acidic polypeptides in peripheral glia. Molecular weight, heterogeneity and distribution. J Neuroimmunol 8(4–6):377–393
Jessen KR, Mirsky R (2005) The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6(9):671–682
Bianchini D et al (1992) GFAP expression of human Schwann cells in tissue culture. Brain Res 570(1–2):209–217
Triolo D et al (2006) Loss of glial fibrillary acidic protein (GFAP) impairs Schwann cell proliferation and delays nerve regeneration after damage. J Cell Sci 119(Pt 19):3981–3993
Obremski VJ, Hall AM, Fernandez-Valle C (1998) Merlin, the neurofibromatosis type 2 gene product, and beta1 integrin associate in isolated and differentiating Schwann cells. J Neurobiol 37(4):487–501
Rezniczek GA et al (1998) Linking integrin alpha6beta4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the beta4 subunit and plectin at multiple molecular sites. J Cell Biol 141(1):209–225
Bernier G et al (1998) Dystonin is an essential component of the Schwann cell cytoskeleton at the time of myelination. Development 125(11):2135–2148
Acknowledgments
This work was supported by National Institute for Translational Neuroscience (INNT) from Ministry of Science and Technology; Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES) from the Ministry of Education; National Council for Technological and Scientific Development (CNPq); Rio de Janeiro State Research Foundation (FAPERJ); PhD program on Morphological Sciences (PCM) from the Federal University of Rio de Janeiro (UFRJ); Ary Frauzino Foundation to Cancer Research; and Pró-Saúde Hospital Administration - Beneficent Association for Social and Hospital Assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
de Mattos Coelho-Aguiar, J. et al. (2015). The Role of the Cytoskeleton in Cell Migration, Its Influence on Stem Cells and the Special Role of GFAP in Glial Functions. In: Schatten, H. (eds) The Cytoskeleton in Health and Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2904-7_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2904-7_4
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2903-0
Online ISBN: 978-1-4939-2904-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)