Abstract
TSH-producing pituitary adenoma is a rare cause of thyrotoxicosis, and the rarest of all types of secretory pituitary adenomas. We present a case of a 55-year-old man who, after 6 years of therapy for presumed hypothyroidism, was diagnosed with a TSH-producing pituitary adenoma. He was lost to follow-up after that, to present again with thyrotoxicosis and atrial flutter 10 years later. We review the presentation, evaluation, treatment, and differential diagnosis of TSH-producing adenomas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Objectives
TSH-producing pituitary adenoma is a rare cause of thyrotoxicosis, and the rarest of all types of secretory pituitary adenomas. We present a case of a 55-year-old man who, after 6 years of therapy for presumed hypothyroidism, was diagnosed with a TSH-producing pituitary adenoma. He was lost to follow-up after that, to present again with thyrotoxicosis and atrial flutter 10 years later. We review the presentation, evaluation, treatment, and differential diagnosis of TSH-producing adenomas.
Case Presentation
A 55-year-old man presented to his local emergency department a month after the progressive worsening of palpitations, anxiety, insomnia, and heat intolerance.
Sixteen years prior to his current presentation, he started complaining of episodic paresthesias in his extremities. Thyroid function testing revealed an elevated TSH of 7.3 mIU/L (0.3–6) and a total T4 of 7.3 μg/dL (4.5–12.5). He was started on levothyroxine therapy, 75 mcg daily. Four weeks later, the dose was increased to 88 mcg daily because of a persistently elevated TSH (7.6 mIU/L) with normal total T4 (8.3 μg/dL). In the interim, while being investigated for multiple sclerosis, an MRI of the brain showed white matter changes in keeping with the diagnosis. Incidentally, the pituitary gland showed “mild diffuse enlargement.” The patient responded well to pulse glucocorticoid therapy, and 4 months later, he was referred by his neurologist to endocrinology for evaluation of hypothyroidism. He denied symptoms suggestive of hypothyroidism, and his clinical examination revealed a normal sized thyroid gland, but he was noted to be tachycardic (heart rate 100–120 beats per minute). Still on 88 mcg of levothyroxine, his laboratory tests showed a TSH 3.6 mIU/L, T4 9.9 μg/dL, and a free thyroxine index (FTI) of 3.9 (1–4.3), all within normal limits. Both antimicrosomal and antithyroglobulin antibody titers were negative so he was referred back to his primary care physician to continue the care of the presumed hypothyroidism. In addition, he continued to receive occasional glucocorticoid pulse therapy for multiple sclerosis flair-ups.
Five years later, his levothyroxine dose was increased to 100 mcg daily by his primary care physician because of an elevated TSH, 12.4 mIU/L, although his FT4 remained just below the upper limit of the normal range, 1.57 ng/dL (0.62–1.61). A month later, his dose was further increased to 112 mcg daily due to persistently elevated TSH. Two months later, his TSH was 13.2 mIU/L, and for the first time, his FT4 1.69 ng/dL, and FT3 4.40 pg/mL (2.3–4.2) were both elevated. This result prompted a pituitary MRI and another referral to endocrinology. The MRI showed a left-sided 12 mm pituitary macroadenoma, almost abutting the optic chiasm. He was clinically asymptomatic, but his thyroid showed asymmetric enlargement and he continued to be tachycardic. His endocrinologist discontinued the levothyroxine therapy and discussed the need for surgery to remove the presumed TSH-producing adenoma. The patient, however, was reluctant and decided to defer surgery, but then was lost to follow-up for 10 years.
At the current emergency department visit, he was found to be in atrial flutter with a heart rate of 140 beats per minute. Thyroid function tests showed a TSH of 15.7 mIU/L (0.4–4.5), free T4 of 2.8 ng/dL (0.8–1.8), and total T3 of 248 ng/dL (80–200).
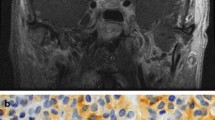
The patient was admitted to the hospital, and started on propranolol and methimazole in addition to anticoagulation. Further tests showed prolactin 10.9 ng/mL (2–18), cortisol 23.8 μg/dL (4–22), ACTH 23 pg/mL (10–60), FSH 2.7 IU/L (1.0–18.0), LH 1.4 IU/L (1.8–8.6), IGF-1 126 ng/mL (50–317). Alpha subunit was 2.5 ng/mL (<0.5). An MRI of the pituitary showed a hypoenhancing sellar mass measuring 2.5 cm in the greatest dimension with mild left optic chiasmal displacement (Fig. 6.1).
Other evaluations during his hospitalization revealed evidence of cardiomyopathy that was attributed to chronic tachycardia, and he underwent a successful ablation procedure restoring sinus rhythm. He was discharged home on carvedilol 12.5 mg twice daily, methimazole 20 mg three times daily, ramipril 2.5 mg daily, zolpidem and alprazolam as needed.
One month later, he presented to the endocrinology clinic with a TSH of 7.5 mIU/L, FT4 1.7 ng/dL, and total T3 206 ng/dL. Total testosterone was 581 ng/dL (250–1,100) and free testosterone was 52.3 pg/mL (35–155). The methimazole dose was increased to 30 mg three times daily and he was referred to us for further management and surgical intervention.
On his first presentation to our center, the patient described improvement in his thyrotoxic symptoms after 2 months of treatment with methimazole and beta blockers. He denied headaches, peripheral vision disturbances, weight loss, tremors, excessive sweating, or hyperdefecation. He also denied polyuria and polydipsia, change in shoe or ring size, or coarsening of his facial features. His libido remained intact and he denied galactorrhea or gynecomastia.
His past history was significant for multiple sclerosis and psoriasis, in addition to the pituitary adenoma. He had no family history of pituitary disease, but there was suspicion of thyroid disease in his father.
On examination his blood pressure was 126/72, heart rate 80 beats per minute and in sinus rhythm, temperature 97.2 °C, and his weight was 200 pounds. There was no lid lag or exophthalmos. His thyroid gland was normal in size and there were no nodules. Chest was clear to auscultation. Cardiovascular exam showed normal first and second heart sounds with no murmurs. The abdominal exam was unremarkable. There was no peripheral edema or tremor. His cranial nerve exam was intact, along with normal power and reflexes in his extremities. Psoriatic lesions on the extensor surfaces of his lower extremities were noted but the skin examination was intact otherwise.
Laboratory studies performed at the time (2 months after his diagnosis and initiation of therapy) showed a TSH of 68.59 mIU/L with a free T4 of 0.5 ng/dL. A formal visual field exam was normal. His methimazole dose was cut from 90 mg/day to 40 mg/day, but TSH continued to rise, reaching >150 mIU/L with FT4 0.2 ng/dL 2 weeks later, so methimazole was stopped. A month off methimazole, TSH was 18 mIU/L, FT4 1.7 ng/dL, Total T3 214 ng/dL, and thus he was restarted on 10 mg daily. Two months later, TSH was 57.7 mIU/L and FT4 1.1 ng/dL. The patient underwent transsphenoidal adenomectomy and pathology confirmed a pituitary adenoma. The immunohistochemistry was negative for ACTH, but focally positive for GH and prolactin. TSH staining was not performed.
On the first day after surgery, his TSH fell to 6.47 mIU/L. One month after surgery, his TSH was 2.95 mIU/L, FT4 0.6 ng/dL, and total T3 58 ng/dL. Prolactin was 4.2 ng/dL and morning cortisol was 17.7 μg/dL. Two months after surgery, TSH was 2.0 mIU/L, FT4 1.1 ng/dL and total T3 83 ng/dL. Other pituitary axes were within normal limits, but his total testosterone was 204 ng/dL, and is currently being watched for possible spontaneous recovery.
How the Diagnosis Was Made
The lack of suppressible TSH despite escalating levothyroxine therapy is certainly unusual in primary hypothyroidism. Nevertheless, the patient did not manifest frankly elevated thyroid hormone levels until a few years after his “hypothyroidism” diagnosis. This peculiar situation may be explained by the well described variation in the biological activity of the secreted TSH molecules, where despite being measurably elevated by TSH immunoassays, they may not have enough biological activity to produce high levels of thyroid hormones in vivo (see below). It is likely that as the tumor grew over time, it was able to produce more bioactive TSH, leading to frank elevation in thyroid hormones, which eventually lead to the correct diagnosis 6 years later. Retrospectively, his negative thyroid antibodies at the time of the “hypothyroidism” diagnosis are supportive of the hypothesis that he did not have primary hypothyroidism, but rather the elevated serum TSH levels due to the TSH-producing adenoma. Unfortunately, he was lost to follow-up for many years to later present with thyrotoxic symptoms and atrial flutter.
At the time of his latest presentation, both serum TSH and thyroid hormone levels were elevated suggesting secondary hyperthyroidism. With the elevated alpha subunit and the MRI evidence of an enlarging macroadenoma, it was clear that this was a TSH-producing adenoma.
An important and characteristic feature of adenoma thyrotrophs is their relative insensitivity to the negative feedback of thyroid hormones, leading to the hypersecretion of TSH despite the hyperthyroidism that was seen in this case. However, there is still integrity of the negative feedback of thyroid hormone, evidenced by the sharp rise of serum TSH levels when the patient was rendered hypothyroid on high-dose methimazole therapy.
Lessons Learned
TSH-secreting pituitary adenomas are considered the least common of all types of pituitary adenomas, accounting for 0.6–1.5 % of them. Thyrotrophs, which represent 5 % of adenohypophyseal cells, originate from the same common progenitor cell that expresses Pit-1 transcription factor along with somatotrophs (growth hormone-secreting cells) and lactotrophs (prolactin-secreting cells). Therefore, TSH-producing adenomas cosecreting GH or PRL are seen in up to one fourth of patients [1].
The majority (75 %) of TSH-producing adenomas are macroadenomas (measuring >10 mm) at the time of diagnosis, with no gender difference. The mean age at presentation is 45 years, with a wide age range (8–84). Those tumors are almost always benign but can be locally invasive, especially in patients with non-intact thyroid glands [2].
Patients typically present with overt thyrotoxicosis and diffuse goiters mimicking Graves’ disease, while others present with manifestations of a mass effect related to the pituitary tumor, resulting in headaches, visual field compromise, and/or loss of other anterior pituitary functions. In most patients, thyrotoxic symptoms are mild to moderate in severity [1]. Severe cardiovascular symptoms like atrial fibrillation or decompensated heart failure are less commonly reported compared to primary thyrotoxicosis. Nevertheless, similar to our patient, atrial fibrillation had been reported as the presenting symptom [3].
In most patients, a latency of a few years separates the onset of thyrotoxic symptoms from the establishment of the correct diagnosis; 6 ± 2 years in patients with intact thyroid glands, and 12 ± 3 years in patients who underwent unnecessary thyroidectomy or radioactive iodine ablation due to the erroneous diagnosis of Graves’ disease [4]. This latency seems to be shorter (4 ± 6 years) in more recent large series [5]. About one-third of patients with TSH-producing adenoma undergo unnecessary thyroidectomy or thyroid ablation due to misdiagnosis [6].
On clinical examination, up to 93 % of patients have diffuse or multinodular goiters due to chronic thyrotropin stimulation [1]. Other features of Graves’ disease like exophthalmos, pretibial myxedema, and positive antithyroid autoantibodies are absent. Nevertheless, cases of TSH-producing adenomas in patients with autoimmune thyroid disease including Graves’ disease and Hashimoto’s thyroiditis have been reported, with the clue to diagnosis of the latter being the lack of suppressibility of serum TSH by escalating thyroxine hormone replacement doses [7].
Laboratory investigations in patients with TSH-producing adenomas typically show elevated thyroid hormone levels with elevated, inappropriately normal, or incompletely suppressed TSH level (range from 0.4 to 393 mIU/L) [2]. This wide range of serum TSH levels despite frankly elevated thyroid hormone levels has been attributed to variations of the biologic activity of the secreted TSH molecules. It is important to stress that about 30 % of patients with TSH-producing adenomas present with serum TSH level in the normal range, which highlights the importance of measuring serum FT4 level in all patients with suspected thyrotoxicosis (and all patients with known pituitary disease).
The picture can be less obvious in patients previously treated with thyroid radioablation or thyroidectomy, since they do not have elevated serum thyroid hormone levels. In such patients, TSH levels tend to be much higher than in patients with intact thyroid glands. This observation highlights a peculiar feature of the tumoral thyrotrophs: that they are relatively resistant to the suppressive effect of elevated thyroxine, but retain sensitivity to the lack of thyroid hormones.
Most TSH-secreting adenomas produce high levels of alpha-subunit and an alpha-subunit/TSH molar ratio greater than 1 is described in about 82 % of cases. This is not true, however, for microadenomas where normal serum levels of alpha-subunit is the rule [5]. Elevated alpha-subunit levels should be interpreted with care in post-menopausal women or men with primary hypogonadism, given that alpha-subunit levels are higher in these circumstances.
Several dynamic tests have been used to distinguish TSH-producing adenomas from other potential diagnoses, particularly in patients whose imaging studies are not revealing or those who present with microadenomas. Most TSH-producing adenomas (about 80 %) do not respond to intravenous TRH stimulation by doubling the basal serum level of TSH. In addition, the T3 suppression test fails to suppress serum TSH levels in patients with TSH-producing adenomas after 10 days of receiving high doses of T3, typically 80–100 mcg/day [6]. TRH is not available in the USA, and T3 suppression testing in patients with history of coronary artery disease or tachyarrhythmia is contraindicated.
MRI with gadolinium is the most commonly used imaging modality for diagnosis. Most TSH-producing tumors are macroadenomas, and in two-thirds of patients, the tumor invades the surrounding structures, including the cavernous sinuses, or extends superiorly compressing the optic chiasm [6].
Once the diagnosis is established, treatment is directed towards restoring euthyroid state and eliminating the local mass effects of the pituitary tumor. Surgical resection via transsphenoidal or transcranial approach is the mainstay of therapy. “Cooling off” the thyrotoxic state preoperatively is required in symptomatic patients, using beta blockers, antithyroid medications, or somatostatin analogs.
Because of the invasive nature of these tumors, and the fact that most are macroadenomas at the time of diagnosis, complete surgical resection may not always be possible. Medical therapy plays an important role in these situations. Surgical cure rates vary widely depending on criteria used to define cure, and the percentage of macro- and microadenomas in each series. Those criteria include reestablishment the euthyroid state, achievement of undetectable TSH 1 week after surgery, normalization of alpha-subunit or the alpha-subunit/TSH molar ratio, or suppression of serum TSH following T3 administration [4]. Post-operative evaluation for partial or complete hypopituitarism is essential.
Due to the expression of somatostatin receptors in TSH-producing adenomas, the preferred medical therapy is treatment with long-acting somatostatin analogs like octreotide or lanreotide. These agents have been shown to reduce serum TSH and alpha-subunit levels in almost all cases, in addition to shrinking tumor size. They are typically used in patients not cured by pituitary surgery, or those who are awaiting the effect of radiation therapy. Primary therapy with somatostatin analogs is restricted to specific situations only, as when surgery is contraindicated. Dopamine agonists have also been used, especially in mixed TSH/prolactin secreting tumors [8]. Radiotherapy, conventional fractionated or gamma knife, is indicated when surgery is contraindicated, as an adjuvant to surgery when remission is not achieved after surgery, or in rare cases of resistance to medical therapy.
Due to the rarity of this condition compared to primary thyrotoxicosis, it is not uncommon for some patients presenting with diffuse goiter and overt thyrotoxicosis to be misdiagnosed as having Graves’ disease, and many undergo radioactive iodine ablation or thyroidectomy before the correct diagnosis is eventually made. The importance of recognizing this pitfall is the deleterious effect shown in some studies, where thyroid ablation or resection may change the behavior of the adenoma into a more aggressive tumor with a tendency for local invasion [2]. This is similar to the development of Nelson’s syndrome following bilateral adrenalectomy to treat Cushing’s disease.
Differential Diagnosis
-
1.
Graves’ disease: with the highly sensitive TSH immunoassays used nowadays, it is easier to distinguish Graves’ disease from a TSH-producing adenoma, since serum TSH levels as low as 0.4 mIU/L have been reported in cases of TSH-producing adenoma (TSH-oma), which would have been below the detection level of older TSH assays. Therefore, the lack of complete suppression of TSH when thyroid hormones are elevated should be an important clue to differentiating Graves’ disease from a TSH-producing adenoma.
-
2.
Resistance to thyroid hormone (RTH): Differentiating TSH-omas from resistance to thyroid hormone can be challenging, as both can present with elevated serum TSH and free thyroid hormone levels [9]. Key differences are that RTH is often a familial condition, is not associated with an elevated alpha-subunit, and peripheral markers of elevated thyroid hormone, such as sex hormone-binding globulin (SHBG) and carboxy-terminal collagen crosslinks (CTX), are not elevated. Dynamic testing with TRH stimulation or T3 suppression lead to elevation and suppression of serum TSH, respectively, in the case of RTH, but not TSH-omas. Finally, RTH is not associated with pituitary adenomas, but pituitary incidentalomas are not an uncommon finding in the general population, making such imaging findings nondefinitive.
-
3.
Euthyroid hyperthyroxinemia: Elevated thyroxine-binding globulin (TBG) levels (e.g., due to pregnancy, estrogen therapy or liver disease) can cause elevation of total thyroid hormone levels. Serum TSH is typically normal in these cases, and normal free thyroid hormone levels are the key to establishing the diagnosis. Other conditions that increase protein binding, such as that seen in familial dysalbuminemic hyperthyroxinemia, can lead to a similar picture although in that case, the free T4 can be artifactually elevated, leading to further diagnostic confusion.
Questions
-
1.
A 45-year-old woman with history of Graves’ disease with documented post ablative hypothyroidism is well controlled on l-thyroxine. She was noted to have elevated TSH despite escalating her l-thyroxine dose, which ultimately lead to symptomatic hyperthyroxinemia. Further testing revealed an elevated level of alpha-subunit but her pituitary MRI showed a normal gland. T3 suppression test was negative (TSH failed to suppress).
What is the most likely diagnosis?
-
A.
Resistance to thyroid hormone
-
B.
Ectopic TSH-producing adenoma
-
C.
Poor compliance to l-thyroxine
-
D.
Hashimoto’s thyroiditis
-
2.
A 50-year-old man developed palpitations, sweating, and weight loss. On examination, he appeared to be anxious and had a diffuse goiter. Laboratory tests showed TSH: 0.4 mIU/L (0.5–5), FT4: 2.8 ng/dL (0.8–1.7). Family history was positive for autoimmune thyroid disease. A diagnosis of Graves’ disease was made and he underwent radioiodine therapy. Two months later he developed hypothyroidism, and was started on l-thyroxine therapy, and was lost to follow up. Nine months later, he presented with bitemporal visual field defects, and an urgent MRI revealed a large 4 cm pituitary macroadenoma invading the cavernous sinuses and compressing the optic chiasm.
What is the likely etiology that explains the aggressive behavior of his tumor?
-
A.
Pituitary carcinoma
-
B.
Craniopharyngioma
-
C.
TSH-producing adenoma
-
D.
TSH/GH-producing adenoma
-
3.
A 60-year-old man with coronary artery disease presents with tachycardia. His TSH and FT4 were found to be elevated. A pituitary MRI showed a microadenoma. Alpha-subunit level was normal.
What is the best test to distinguish a TSH-producing adenoma from thyroid hormone resistance syndrome?
-
A.
Total T3
-
B.
T3 suppression test
-
C.
SHBG
Answers to Questions
-
1.
B: Resistance to thyroid hormone is not associated with an elevated alpha-subunit level. In addition, T3 suppression testing results in suppression of serum TSH level in patients with resistance to thyroid hormone, but not in TSH-omas, thus answer A is incorrect. Poor compliance to l-thyroxine and Hashimoto’s thyroiditis are also not associated with elevated level of alpha-subunit and should respond to a T3 suppression test, therefore answers C and D are incorrect. Finally, since the biochemical picture is suggestive of a TSH-producing adenoma but no tumor was identified on the pituitary MRI, one must think of an ectopic pituitary tumor and look for it in its most common location, the pharynx. The reason behind that is that the pituitary gland develops embryologically from Rathke’s pouch which originates from the primitive oral cavity. Ectopic TSH-producing adenomas have been reported with masses discovered in the nasopharynx.
-
2.
C: The clue to the correct diagnosis in this case is the lack of complete suppression of TSH when thyroid hormones are elevated. This is never seen in primary hyperthyroidism and should raise the suspicion of a secondary cause of the patient’s hyperthyroidism. Unfortunately, the patient was incorrectly diagnosed with Graves’ disease and underwent radioiodine therapy. The resultant primary hypothyroidism is known to alter the behavior of TSH-producing adenomas causing them to behave more aggressively with marked elevation of serum TSH levels and local tumor invasion, as a result of the lack of the negative feedback of thyroid hormones. Answer C is correct. There is no distant metastasis to suggest a pituitary carcinoma in this patient, thus answer A is incorrect. Craniopharyngioma can cause secondary hypothyroidism with low TSH, but that is associated with low FT4 as well, thus answer B is incorrect. Finally, there is no evidence to suggest that plurihormonal tumors cosecreting TSH and GH are more aggressive that tumors that secrete TSH alone, therefore answer D is incorrect.
-
3.
C: The T3 suppression test can help distinguish RTH from TSH-omas but administering high levels of T3 over 10 days can be risky in patients with coronary artery disease, thus answer B is incorrect. Total T3 does not help discrimiate the two diagnoses from each other, thus answer A is not correct. SHBG is a peripheral marker of elevated thyroid hormone actions, and thus is high in TSH-omas but not in RTH, therefore answer C is correct. Tachycardia has no discriminating value as it could be a manifestation of hyperthyroidism secondary to TSH-omas, or a finding in generalized RTH, as it is mediated by the thyroxine receptor alpha (TRα) isoform in the heart, which is not affected by the TRβ mutations that characterize RTH. Finally, it is important to note that alpha-subunit is usually normal in TSH-producing microadenomas, but elevated in macroadenomas.
References
Beck-Peccoz P, Persani L, Mannavola D, Campi I. Pituitary tumours: TSH-secreting adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23:597–606.
Rouach V, Greenman Y. Thyrotropin-secreting pituitary tumors. In: Melmed S, editor. The pituitary. Burlington: Academic Press; 2011. p. 619–36.
George JT, Thow JC, Matthews B, Pye MP, Jayagopal V. Atrial fibrillation associated with a thyroid stimulating hormone-secreting adenoma of the pituitary gland leading to a presentation of acute cardiac decompensation: a case report. J Med Case Rep. 2008;2:67.
Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the national institutes of health. J Clin Endocrinol Metab. 1999;84:476–86.
Socin HV, Chanson P, Delemer B, et al. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. Eur J Endocrinol. 2003;148:433–42.
Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev. 1996;17:610–38.
Losa M, Mortini P, Minelli R, Giovanelli M. Coexistence of TSH-secreting pituitary adenoma and autoimmune hypothyroidism. J Endocrinol Invest. 2006;29:555–9.
Colao A, Pivonello R, Di Somma C, Savastano S, Grasso LF, Lombardi G. Medical therapy of pituitary adenomas: effects on tumor shrinkage. Rev Endocr Metab Disord. 2009;10:111–23.
Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta. 2013;1830(7):3987–4003.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Alkabbani, A., Salvatori, R., Cooper, D.S. (2015). TSH-Secreting Pituitary Adenoma. In: Davies, T. (eds) A Case-Based Guide to Clinical Endocrinology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2059-4_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2059-4_6
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2058-7
Online ISBN: 978-1-4939-2059-4
eBook Packages: MedicineMedicine (R0)