Abstract
The efficacy of dopamine-agonists (DA) in patients with prolactinomas and that of somatostatin analogues (SSA) in those with GH- and TSH-secreting adenomas is well established. More recently, data are accumulating suggesting a potential therapeutic role of DA also in patients with ACTH-secreting and clinically non-functioning (NFA) pituitary adenomas. This review aims at summarizing published results of DA and SSA on tumor shrinkage in patients with different histotypes of pituitary adenomas. Results of tumor shrinkage are of clinical relevance as tumor size is the one of the most important determinant of surgical outcome. While reduction of tumor size more than 50% of baseline size in macroprolactinomas treated with DA is a frequent finding in patients with GH-secreting adenomas treated with SSA tumor shrinkage only recently is becoming frequent thanks to the availability of depot formulations. Data on tumor shrinkage in patients with TSH-secreting adenomas treated with SSA are limited because of the rarity of these tumors. Very recently, DA have been reported of some efficacy also in patients with ACTH-secreting adenomas but data are still very limited. NFA respond very scantly to both DA and SSA even if receptors targeting these drugs are present. Whether this is due to limited receptor number or alterations of post-receptor pathway is still unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The medical approach to pituitary adenomas has been greatly improved since the availability of compounds with dopamine-agonists (DA), such as bromocriptine (BRC), cabergoline (CAB), and quinagolide (CV), and of somatostatin analogs (SSA) provided in slow release formulations, such as lanreotide slow release (SR) and autogel and octreotide long-acting repeatable (LAR).
The efficacy of DA in patients with prolactinomas and that of SSA in those with GH- and TSH-secreting adenomas is well established [1–3]. More recently, data are accumulating suggesting a potential therapeutic role of DA also in patients with ACTH-secreting and clinically non-functioning (NFA) pituitary adenomas.
This review is focused on the results of DA and SSA on tumor shrinkage in patients with different histotype of pituitary adenomas. Results of tumor shrinkage are of clinical relevance as tumor size is the one of the most important determinant of surgical outcome.
2 Dopamine & somatostatin receptors
Classically, dopamine receptors have been divided into D1 receptors, that stimulate adenylyl cyclase activity (including D1 and D5) and D2 receptors that inhibit this enzyme (including D2, D3,D4). Receptors subtypes D3, D4 and D5 have less activity on PRL secretion [4]. Dopamine inhibition of PRL secretion is mediated by the D2 dopamine receptors expressed by normal and tumoral cells [4]. D2 receptors belong to the family of G protein-coupled receptors, characterized by a single polypeptide chain containing seven hydrophobic transmembrane domains: besides their effect on adenylyl cyclase, they are able to inhibit inositol phosphate production, with an effect that involves G proteins sensitive to pertussis toxin [5]. Two isoforms generated by alternative splicing of the D2 dopamine receptor have been described [6]. These two isoforms differ by a 29 amino acid additive sequence located within the third intra-cytoplasmic loop that interacts with G proteins: dopamine inhibition of adenylyl cyclase activity is observed with both isoforms. Stimulation of D2 receptors by dopamine reduces adenylyl cyclase activity that consequently reduces intracellular cyclic AMP levels in normal as well as in tumoral lactotrophs [7]. The inhibition of cAMP levels is a key step in the inhibition of PRL release by dopamine [4]. Dopamine and its agonists reduce the size of prolactinomas by inducing a reduction in cell volume (via an early inhibition of secretory mechanism, and a late inhibition of gene transcription and PRL synthesis), as well as causing peri-vascular fibrosis and partial cell necrosis [8]. There may also be a true anti-mitotic effect of these drugs. Histologically, there is a reduction in secretory activity and cell size, an increase in immunoreactive PRL cellular content and inhibition of exocytosis [9].
Similarly the inhibitory effects of somatostatin are mediated by somatostatin receptors (ssts), of which there are five different subtypes [10]. The sst2 and sst5 are the subtypes primarily involved in the control of GH secretion, although sst1 may also have a role [11]. The expression of ssts is similar in the normal pituitary and in GH-secreting pituitary tumors: sst2 and sst5 are the predominantly expressed subtypes, whereas sst4 is infrequently expressed [10]. The sst expression is variable both within GH-secreting tumors and between different GH-secreting tumors [10]. Native somatostatin inhibits the proliferation of both normal and tumor cells [11]. The antiproliferative action is mediated through the ssts by a variety of mechanisms and depend on the receptor subtype and the target tissue [12]. Specific mechanisms include cell cycle arrest and induction of apoptosis. Activation of sst1, -2, -4, and -5 all appear able to induce cell cycle arrest, whereas sst3, and possibly sst2, induce apoptosis [13]. In direct effects of somatostatin on growth factor production, inhibition of angiogenesis and suppressed IGF-I, are also involved although the underlying mechanisms are unclear [14]. The effects of SSA on tumor shrinkage are mediated decrease in cell proliferation, as demonstrated by a reduced expression of Ki-67 in pre-treated tumors as compared to untreated [15, 16] and by a slight downward trend of cell and cytoplasmic size in pre-treated tumors [17].
3 Available dopamine-agonists and somatostatin analogues
This class comprises Ergot derivatives (Bromocriptine, Pergolide, Metergoline, Lisuride, Terguride, Cabergoline) and Non ergot derivatives (Quinagolide) compounds. The ergot alkaloids can all be considered derivatives of the tetracyclic ergoline skeleton [18] and possess a wide spectrum of pharmacological action that includes central, neurohumoral and peripheral effects. These effects are mainly responses mediated by noradrenaline, serotonin and dopamine receptors. Their wide range of biological activities may be explained by assuming that ergot compounds interact with more than one receptor site, that the population of receptors sites they bind varies from organ to organ, and that affinity for receptor sites and intrinsic activity vary from compound to compound [18]. The octahydrobenzyl(g)-quinolines, a group of non ergot oral dopamine agonists with specific D2 receptor activity, were tested for their in vivo activity in suppressing basal serum PRL levels [18]. Within the class of non ergot derivatives, quinagolide (CV) is the most active octahydrobenzyl(g)-quinolines and compared to bromocriptine (BRC), was about 35 times more potent in the implantation inhibition test given subcutaneously and an even higher potency in the lactation inhibition test.
It is likely that all dopaminergic ergot derivatives share similar mechanisms of action [18].
The first commercially available SSA was octreotide formulated for sc administration (OCT)(Novartis, Cambridge, MA) [19]; it possesses a high affinity for sst2 and sst5, but the affinity to sst2 being the former about 10 times greater than the latter [19]. OCT is administered with multiple daily injections (two to four) and its GH suppressive effect lasts at most 5 h after injection [19]. Lanreotide SR (LAN) is a medium-acting SSA with a sst binding profile similar to the OCT (affinity to sst2 approximately 10 times greater than sst5) [20]. The drug is encapsulated in microspheres that provide prolonged release over 10–14 days after im administration [21]. Lanreotide SR is provided in a 30 and 60 mg dosage (the latter only in Italy), and the pharmacological effect is manipulated by changing the dosing interval among every 7, 10, or 14 days [20]. Octreotide is also available in a long-acting formulation, LAR [22]. As with LAN, the active compound is encapsulated in microspheres of a biodegradable polymer. After an im injection, drug levels begin to rise over 7–14 days and plateau for 20–30 days [22]. Dosing every 4 weeks is typical, but dosing intervals beyond 4 weeks may be possible in some patients, particularly those with lower GH and IGF-I levels [23]. The pharmacological effect of the drug can be manipulated by varying the dose from 10 to 30 mg with a fixed dosing interval of 28 days. We have alao used up to 40 mg every 28 days [24]. More recently another long-acting formulation, lanreotide Autogel (ATG) (Ipsen Limited, Slough, UK), has become available in many European countries and in U.S. and Canada [25]. ATG naturally congeals into a slow-release aqueous gel that can be administered s.c. and its pharmacological effect can be manipulated by varying the dose from 60, 90, or 120 mg with a fixed 28 days administration chedule or by varying the interval between injection between 28–56 days maintaining the dose of 120 mg [26]. Another SSA in currently under experimental investigation: pasireotide (SOM 230) has high affinity for sst 1,2, 3 and 5 [27]. In patients with acromegaly, available data are only related to its effect on GH and IGF-I levels [28] and it will not be mentioned further in this review. However, in a study conducted in HMGA2 transgenic mice bearing GH/PRL-secreting adenomas, SOM 230 induced a greater tumor shrinkage than OCT [29].
4 Shrinkage of pituitary tumors after DA or SSA treatment
4.1 PRL-secreting adenomas
More than 25 years ago BRC was introduced into clinical practice as the first medical treatment for prolactinomas [1]. It has a relatively short elimination half-life, so that it is usually taken 2 or 3 times daily, although once daily may be effective in some patients. Generally, the therapeutic doses are in the range of 2.5–15 mg/day and most patients are successfully treated with 7.5 mg or less. However, doses as high as 20–30 mg/day may be necessary for patients who demonstrate resistance. For microprolactinomas BRC is successful in 80% to 90% of patients in normalizing serum PRL levels, restoring gonadal function and shrinking tumor mass [18]. For macroprolactinomas normalization of serum PRL levels and tumor mass shrinkage occur in about 70% of patients treated with bromocriptine even when given at low doses; visual field defects improve in the majority of patients [10]. In most patients, headache and visual field defects improve dramatically within days after the first administration of BRC with gonadal and sexual function improving even before complete normalization of serum PRL levels.
Cabergoline (CAB) is a D2 selective agonist widely used to treat prolactinomas. The beneficial effects of CAB in resolving hyperprolactinemia are widely known [30]. CAB treatment normalized PRL levels in 86% of 425 patients with available follow-up, (92% of 244 patients with idiopathic hyperprolactinemia or microprolactinoma, and 77% of 181 patients with macroprolactinoma); 13% had side effects but only 4% discontinued cabergoline therapy because of side effects [31]. Generally, the median dose of cabergoline at the start of therapy was 1 mg/week in patients with macroprolactinomas and 0.5 mg/week in those with idiopathic hyperprolactinaemia or microprolactinomas (0.5 mg/week) [30]. A remarkable tumor-shrinking effect of cabergoline has been observed in patients with macroprolactinomas [32]: 12–24 months treatment with cabergoline induced a greater than 20% decrease of baseline tumor size in more than 80% of cases with complete disappearance of tumor mass in 26–36% of cases (Fig. 1). Moreover, we also showed [33] that cabergoline treatment induced further tumor shrinkage in 60% of patients previously treated with other dopamine-agonists compared to 82.3% of previously untreated patients. Importantly, the tumor shrinking effect is very rapid and improvement of visual field defects can be detected even after the administration of the first tablet (Fig. 2).The superiority of CAB over BRC was supported by a comparative retrospective study by Di Sarno et al. [34] and by the evidence of further tumor shrinkage in patients previously intolerant or resistant to BRC and in those already responsive to it (Fig. 3).
Tumor shrinkage in individual patients with prolactinomas treated first-line with cabergoline at low doses. The superscript graph indicates the percent shrinkage in individual patients. Data derived from ref. [32]
Changes in tumor size and consistency (left) and visual field (right) before (top) and after 1 week (middle; administration of 1 tablet of cabergoline 0,5 mg) and 1 month (bottom; at a dose of 0.5 mg twice weekly starting from the second week) in a male patient aged 18 years and coming to our observation because of sudden reduction in visual loss. Colao A, personal observation
Tumor shrinkage in patients with prolactinomas treated with cabergoline. Patients were divided in 4 groups according with previous treatments. Data are shown as Mean ± SD and are derived from ref. [33]
Of the other dopamine-agonists used to treat prolactinomas, it is important to mention that several studies demonstrated that once-daily CV treatment in women with hyperprolactinaemia reduced PRL levels and tumor size and relieved gonadal dysfunction thereby restoring fertility [1]. Tumor mass was reduced by at least 30% in 8 of 13 men with macroprolactinomas [35] and in 21 of 26 patients with macroprolactinomas [36]. Though rare, a dissociation between PRL control and tumor shrinkage can be encountered [37].
A recommendation in patients with large macroadenomas, is to begin CAB treatment (as well as with any other DA) with very low doses as in some very responsive tumors treatment can be associated with remarkable early tumor changes and shrinkage leading to rhinorrhea that need surgical intervention [38]. In patients chronically treated with CAB and achieving significant tumor shrinkage withdrawal of treatment for as long as 5 years was not associated with a rebound in tumor re-growth [39]. In this study, however, patients with macroadenomas extending into critical areas such as cavernous sinuses or cerebral areas were excluded.
Though it is expected that SOM 230, by binding sst5, could have some role in treating patients with prolactinomas resistant to DA, there are no data at present. Experimental data recently published by Fusco et al. [40] showed that a sst5 agonist produced a dose-dependent inhibition of PRL release similar to that of cabergoline in DA-sensitive prolactinomas and a chimeric compound binding sst2 and D2 receptors produced a maximal PRL inhibition similar to that obtained with CAB. These data do not apparently support a role of SSt5 and its analogues in resistant prolactinomas, even if only a clinical experimentation will finally respond to this query.
4.2 GH-secreting adenomas
In normal subjects the administration of DA agonists stimulates GH secretion via a CNS-mediated mechanism but at least in half of the patients with GH-secreting adenomas, DA and its agonists inhibit GH secretion, presumably through the stimulation of D2 receptors, the same as that involved in the regulation of PRL secretion in normal and neoplastic lactotrophes [41]. DA-agonists are primarily effective in those GH-secreting tumors that co-secrete PRL or that exhibit immunostaining for PRL [2]. In a collection of 28 series including over 500 patients with acromegaly, BRC produced a symptomatic improvement in up to 70% of the patients but lowered GH levels below 5 μg/l and induced tumor shrinkage in only 10–15% of cases [41]. We also did not find tumor shrinkage in 31 of 34 patients treated with DA with the exception of two patients treated with CV and one with BRC-LAR [42]. In contrast, Abs et al. [43] reported tumor shrinkage in 13 of 21 patients with a tumor reduction of 50% in 5 GH/PRL-secreting adenomas. In another study, tumor shrinkage was documented in all the 3 patients whose pituitary imaging was repeated during CAB [44]. Actually, only in the few reports the adenoma size was described. In a study of six acromegalic patients who were treated with CAB, one exhibited a “marked shrinkage” in adenoma size by computerized tomography [45]. In a study of three patients treated with CAB, one, who had a previously excised somatomammotroph adenoma, experienced “neuroradiologic improvement,” as determined by high-resolution computerized tomography of the adenoma remnant [46]. Of note, a 69 year-old woman was reported to have 75% tumor shrinkage [47] and two other patients (48-year-old man and a 26-year-old woman) had a 94% and a 70% tumor shrinkage, respectively, [48] 6 months after starting CAB treatment. In all, only 39% of the acromegalic patients who were reported to receive CAB treatment for acromegaly had imaging during treatment, but volume measurements were reported only in a very few cases. Thus, prevalence and amount of tumor shrinkage after CAB treatment in acromegaly could have been underestimated. The combination treatment with DA and SSA was shown to better suppress GH/IGF-I levels [49] but results on tumor shrinkage are lacking. In the last decade, several studies have indicated that some tumor shrinkage is achieved using SSA as first-line treatment in contrast with the initial data (using OCT) that did not show significant shrinkage in most patients.
As reviewed by Bevan [50], many studies have analyzed tumor shrinkage as an outcome of SSA therapy. The definition of tumor shrinkage differed across studies but overall, 217 of 478 patients (45%) had a reduction in tumor size [50]. In patients treated first-line with SSA (defined as patients without prior surgery or radiation, although some patients may have been treated with BRC), 110 of 217 (51%) had tumor shrinkage while in patients treated after unsuccessful surgery and/or radiation, 22 of 82 (27%) had tumor shrinkage [50]. Similarly, Melmed et al. [51] reported that, for patients who experience significant shrinkage, an approximately 50% decrease in pituitary mass is achieved when a somatostatin analog is used exclusively or before surgery or radiotherapy. Fourteen studies including 424 patients provided a definition of significant tumor shrinkage, and the results showed that 36.6% (weighted mean percentage) of patients receiving primary SSA therapy for acromegaly experienced a significant reduction in tumor size. We analyzed results of treatment with SR and LAR for 12 months in 99 patients [52] and reported that tumor volume shrinkage was absent (<25% of baseline size) only in 22 patients (22.2%); mild (25–50% of baseline size) in 31 (31.1%), moderate (50–75% of baseline size) in 30 (30.3%) and notable (>75% of baseline size) in 14 patients (14.1%). We did not directly compare the effects of SR vs. LAR. However, in revising this data we did not find any difference in the amount of tumor shrinkage with the two analogues (Fig. 4). A very recent study has confirmed that results obtained with SR, LAR and ATG are similar [53]. In a study including patients treated only with SSA up to 18 years, the mean reduction in tumor volume was 43% (range 13–97%) and shrinkage >20% was obtained in 72% of the patients [54]. These data confirm previous data by Cozzi et al. [55] reporting tumor shrinkage by 62 ± 31% (range, 0–100%), in 82.1% of 67 patients treated up to 9 years with SSA only. In a study we conducted for 2 years of continuous treatment with LAR [56], we demonstrated that increased doses up to 40 mg every 28 days in the patients not responding to 30 mg for 1 year, increased their chance to control GH and IGF-I levels and increased the rate of tumor shrinkage. A detailed analysis of the results obtained in the 17 patients resistant to standard doses, we found a significant shrinkage (>25%) in 9 patients after 12 months (52.9%) when they received 30 mg every 28 days and in all 17 after 24 months when treated with 40 mg every 28 days (Fig. 5). Prevalence of shrinkage >25% compared to baseline was significantly lower in the resistant patients than those achieving disease control with a dose of LAR of 20 mg every 28 days [56]. These data confirm previous data showing that patients with controlled disease during SSA at standard doses have greater tumor shrinkage than those resistant [50, 52] but also demonstrate that significant shrinkage can be achieved also in the resistant patients if doses are adjusted to obtain the maximal hormone suppression. No data are currently available with ATG. A preliminary experience in 26 patients treated first-line with this SSA formulation had 30.6 ± 28.8% shrinkage after 6 months of treatment (95% CI 19.1–42.1%): similar to previous data shrinkage occurred both in patients with disease control and those who had not (Fig. 6) being slightly greater in the former [(n = 15) 38.8 ± 30.3% vs. (n = 11) 25.4 ± 19.6%, p = 0.072] (Colao et al. unpublished data). The evidence that tumor shrinkage occur also in patients not achieving disease control during SSA treatment has been also emphasized by two case reports in which notable tumor shrinkage was associated with very scant effects on GH and IGF-I levels possibly because of a differential expression of sst on tumor membranes being sst2 poorly expressed and sst3 and sst5 highly expressed [57, 58]. The effect of SSA on tumor shrinkage is reversible as demonstrated by tumor re-growth after stopping SSA treatment [59].
Individual percent changes in tumor volume of GH-secreting adenomas treated first-line with either octreotide-LAR or lanreotide-SR. The grey area corresponds to the −25% to + 25% change that was considered non significant according with our measurements. Data are collected after 12 months of continuous treatment and are derived from ref. [50]
Changes in tumor volume after 12 months of treatment with LAR at a dose of 30 mg/q28d and 24 months at a dose of 40 mg/q28d in patients with acromegaly proven to be resistant to the usual maximal dose of 30 mg/q28d. Superscript graph, comparison of tumor shrinkage of data collected in this resistant group with those collected in 24 patients considered to be sensitive to the drug as achieved control of acromegaly with a dose of 20 mg/q28d. Data are derived from ref. [54]
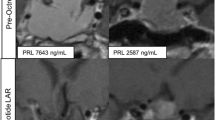
Two exemplary patients with GH-secreting adenomas showing tumor shrinkage after 12 months of treatment with lanreotide Autogel given as first-line for 12 months. Individual data of hormone control and tumor volume are given on the left. (Colao A, personal observation). On the left = clinical data of the two patients. On the right = MRI findings. A and B = study performed before treatment; C and D = study performed 12 months after treatment
As stated before a combined treatment SSA and DA could be beneficial also on tumor shrinkage as it has been demonstrated that sst2a and D2 receptors are frequently co-expressed in adenomas from acromegalic patients and thus immunohistochemistry may characterize receptor expression in pituitary adenomas to select patients responsive to different treatments [60].
4.3 TSH-secreting pituitary adenomas
This adenoma histotype is rare and frequently at diagnosis tumors are macros presenting with mass effect symptoms such as headache, visual disturbance, together with variable symptoms and signs of hyperthyroidism [61]. The medical treatment of TSH-secreting adenomas mainly rests on the administration of SSA as DA failed to persistently block TSH secretion in almost all patients and caused tumor shrinkage only in those with combined excess of TSH and PRL. However, a few cases with tumor shrinkage with BRC or CAB have been reported [62]. In contrast, since somatostatin inhibits TSH secretion both in physiological conditions and in TSH-secreting pituitary adenomas and since TSH-secreting adenomas express SS receptors, SSA are a very efficient treatment in patients with TSH-secreting pituitary tumors to improve clinical signs, hormone levels and to produce tumor shrinkage [63–67]. OCT given s.c. induced an acute TSH suppression also reducing T4 deiodination in acromegalic patients [63]. In more than 90% of TSH-secreting adenomas, OCT s.c. suppressed TSH secretion and in about 50% of cases adenoma size was reduced [63–66]. OCT treatment was also considered useful preoperatively as it allowed an easier tumor removal [61] as it produces tumor shrinkage as reported in case reports [68]. The dose of OCT in patients with TSH-secreting adenomas to achieve TSH normalization was reported to be lower than that needed to suppress GH in GH-secreting adenomas [61, 63–68]. In four patients with thyrotropinoma a single im injection of 30 mg SR normalized TSH and thyroid hormone levels for 10–15 days [68]. SR treatment was similarly shown to suppress plasma TSH levels and to normalize thyroid hormone levels with no significant change in adenoma size [69, 70]. Another multicenter study reported treatment with LAR in TSH-secreting adenomas [71]. Eleven patients received OCT (at dosages of 200–900 μg/daily) first and then octreotide LAR (at 20 mg, every 28 days) after a 4-week washout period: both formulation significantly reduced TSH and thyroid hormone levels without causing significant side effects [71]. In our personal experience [68], in five cases with TSH-secreting pituitary adenomas, TSH levels were successfully normalized with either the analog or the formulation that we used, and thyroid hormones were normalized and remained normal throughout the treatment period. The dosage of sc OCT, SR, or LAR required to normalize TSH and thyroid hormone levels was indeed low (300 mg/day, 30 mg every 14 days, and 10 mg every 28 days, respectively) compared with dosages generally required in patients with GH-secreting adenomas; however, the few cases treated do not allow any firm conclusion, and dosage should be titrated on the basis on individual patients’ responsiveness and tolerance, as for patients bearing acromegaly.
4.4 ACTH-secreting adenomas (Cushing’s disease and Nelson’s syndrome)
The medical treatment of ACTH-secreting adenoma is reserved for patients with unsuccessful surgery [72] even after repeat pituitary surgery that is efficacious in approximately two-thirds (50–70%) of patients in a limited number of specialized centers [73]. The still most used drugs are adrenal-blocking drugs, which act directly at the adrenal level which include metyrapone, ketoconazole, aminoglutethimide, and mitotane [72]. These drugs are capable of lowering cortisol by directly inhibiting synthesis and secretion in the adrenal gland but are virtually devoid of any tumor shrinking affect [72]. Despite initial promise, subsequent studies do not support a routine clinical role for the use of peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists, such as rosiglitazone [73]. The dopamine receptors (mostly D2 but also D4) are expressed in more than 75% of ACTH-pituitary adenomas [74] but also in 83.3% of ectopic ACTH-secreting tumors [75] and in aldosterone- and cortisol-secreting adenomas, cortisol-secreting carcinomas, and clinically nonfunctioning adenomas [76]. However, in long-term studies with BRC, disease remission was confirmed in only a small minority of patients [77]. A small, short-term study suggests that CAB at dosages of 2–3.5 mg/week may be effective in treating a subset of patients with Cushing’s disease [74]. In an extension study of this latter enrolling 20 patients after unsuccessful surgery, we demonstrated that after 3 months of treatment, 15 (75%) of patients had normalization of free urinary cortisol that in 10 of them (50%) was maintained for 12 months (Pivonello and Colao, unpublished data).
However, tumor shrinkage after 6 months of CAB treatment was observed in 4 of the 10 patients responsive to the treatment; only 8 of 10, however had a visible tumor so that prevalence of tumor shrinkage was 50% (Fig. 7). In one patient we also reported that the treatment with CAB for 12 months at the dose of 2 mg a day was able to induce a complete clinical and biochemical remission of Nelson’s syndrome [78]. However, the use of CAB in Cushing’s and Nelson’s disease is still experimental and more data are required before proposing this treatment as an official therapy for ACTH-secreting tumors.
Individual changes in tumor volume after 3 and 12 months of treatment with CAB at a dose of 1–7 mg/week (median 3.5 mg/week) in patients with detectable ACTH-secreting adenomas on Magnetic Resonance Imaging. Superscript graph, Individual percent changes in tumor volume. Patients no. 4 and 15 did not complete 12 months of treatment. Patient no.9 did not have any change in tumor volume. (Pivonello R, personal observation)
The possible role of somatostatin analogs has also been reevaluated in the treatment of Cushing’s disease as a newer somatostatin analog, and SOM230 has been demonstrated to reduce ACTH secretion in cell culture of corticotroph tumor [79]. However, no definitive data are available on clinical trials; a preliminary experience on very short-term treatment, however, looks encouraging [80]. These data on the effectiveness of specific DA and SSA suggest that their combination may also be a possible therapeutic approach, considering that the combined use of CAB and SR was demonstrated to be of benefit in ectopic Cushing’s syndrome, especially when an escape from treatment with the only cabergoline occurs [81].
4.5 Clinically nonfunctioning adenomas (NFA)
NFA represent a very heterogeneous group of tumors since a consistent proportion of them (up to 90%) are shown to secrete low amounts of intact FSH and LH and/or their α- and β-subunits either in vitro or in vivo [82]. BRC was used in NFA with disappointing results [83], likely due to the lower amount and affinity of D2 receptors expressed on NFA than on prolactinoma cells. Pivonello et al. [84] demonstrated that D2 receptors were expressed in 67% of 18 NFA remnant tumors. In detail, the D2 (long) was found in 50%, D2 (short) in 17%, and both D2 isoforms in 33% of cases; the D4 receptor was also expressed in 17% of cases [84]. Only a few studies have reported on the efficacy of CV in the treatment of these tumors [85–87]. A significant tumor shrinkage was documented, however, only in 4 out of 12 reported patients [85–87]. CAB was administered to 10 patients with NFA who received a scintigraphy using 123I-methoxybenzamide (123I-IBZM) prior to the therapy, and we found a significant adenoma shrinkage only in the 2 out of 10 patients with NFA with intense pituitary uptake of 123I-IBZM [88]. In 18 patients with remnant NFA, 12 months of CAB treatment induced tumor shrinkage in 56% [84]. Tumor shrinkage was associated with D2 receptor expression mostly with D2 (short) expression [84].
NFA also express SS-receptors both in vitro [89–91] and in vivo [92–94]. Several clinical trials reported, however, that tumor reduction could be observed only in 11–13% of cases, indicating a weak correlation between SS-receptor expression and treatment efficacy with OCT in these patients [92–94]. In 9 patients with NFA we found a correlation between uptake of 111In-DTPA-D-phe1-octreotide and percent suppression of α-subunit levels after 6–12 month of OCT treatment at a dose of 0.3–0.6 mg/day [95]. Two patients with NFA (22.2%) had a significant tumor shrinkage (>30% of baseline size) during -term OCT therapy [95]. Although the results of chronic OCT treatment were controversial, it was reported to induce a rapid improvement of headache and visual disturbances, without any change in tumor volume [96, 97], together with variable inhibition of gonadotropin and α-subunit levels both in vitro and in vivo [98–100]. The analgesic effect of s.c. OCT was likely due to the in vitro evidence that OCT display an inhibition of the growth of new vessels and of endothelial cells, leading to hypothesize that the visual improvement was more likely to be due to a direct effect of OCT on the retina and the optic nerve than to an effect mediated by the SS-receptors expressed on the pituitary tumor [101]. Decrease of tumor volume by 30 ± 4% were reported in some patients with NFA treated with a combined OCT + CAB treatment [102]. There are no data on the effects of LAR, SR, or ATG in NFA.
5 Conclusions
Dopamine-agonists and somatostatin analogues are known to cause tumor shrinkage in a large proportion of patients with PRL-secreting (the former), GH-secreting and TSH-secreting adenomas (the latter). Data on tumor shrinkage in patients with GH-secreting adenomas have induces the investigators in changing the approach to these tumors suggesting a first-line with SSA in most patients with macroadenomas [103]. A few patients with GH-secreting and TSH-secreting adenomas also have some tumor shrinkage with DA. Only a few patients have been reported with significant tumor shrinkage of ACTH-secreting adenomas and NFA. With the future availability of new SSA able to target sst1–3 and sst5 and chimeric compounds able to target sst2 and D2, will potentially increase the results on tumor shrinkage also in these tumor histotypes.
References
Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27:485–534. doi:10.1210/er.2005-9998.
Melmed S, Casanueva FF, Cavagnini F, Chanson P, Frohman L, Grossman A, Acromegaly Treatment Consensus Workshop Participants, et al. Guidelines for acromegaly management. J Clin Endocrinol Metab 2002;87:4054–8. doi:10.1210/jc.2002-011841.
Caron P, Arlot S, Bauters C, Chanson P, Kuhn JM, Pugeat M, et al. Efficacy of the long-acting octreotide formulation (octreotide-LAR) in patients with thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 2001;86:2849–53. doi:10.1210/jc.86.6.2849.
Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225.
Caccavelli L, Cussac D, Pellegrini I, Audinot V, Jaquet P, Enjalbert A. D2 dopaminergic receptors: normal and abnormal transduction mechanisms. Horm Res. 1992;38:78–83.
Giros B, Sokoloff P, Martres MP, Riou JF, Emorine LJ, Schwartz JC. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342:923–6. doi:10.1038/342923a0.
Spada A, Nicosia S, Cortelazzi L, Pezzo G, Bassetti M, Sartorio A, et al. In vitro studies on prolactin release and adenylate cyclase activity in human prolactin-secreting pituitary adenomas. Different sensitivity of macro- and microadenomas to dopamine and vasoactive intestinal polypeptide. J Clin Endocrinol Metab. 1983;56:1–10.
Bevan JS, Webster J, Burke CW, Scanlon MF. Dopamine agonists and pituitary tumor shrinkage. Endocr Rev. 1992;13:220–40. doi:10.1210/er.13.2.220.
Trouillas J, Chevallier P, Claustrat B, Hooghe-Peters E, Dubray C, Rousset B, et al. Inhibitory effects of the dopamine agonists quinagolide (CV 205–502) and bromocriptine on prolactin secretion and growth of SMtTW pituitary tumors in the rat. Endocrinology. 1994;134:401–10. doi:10.1210/en.134.1.401.
Hofland LJ, Lamberts SWJ. Somatostatin receptor subtype expression in human tumors. Ann Oncol. 2001;12(Suppl 2):S31–6. doi:10.1023/A:1012420207244.
Culler MD, Taylor JE, Moreau JP. Somatostatin receptor subtypes: targeting functional and therapeutic specificity. Ann Endocrinol. 2002;63:2S5–2S12.
Ferjoux G, Bousquet C, Cordelier P, Benali N, Lopez F, Rochaix P, et al. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J Physiol. 2000;94:205–10.
Guillermet Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C. Physiology of somatostatin receptors. JEI 2005;28(11 Suppl International):5–9.
Garcıa de la Torre N, Wass JAH, Turner HE. Antiangiogenic effects of somatostatin analogues. Clin Endocrinol (Oxf). 2002;57:425–41. doi:10.1046/j.1365-2265.2002.01619.x.
Losa M, Ciccarelli E, Mortini P, Barzaghi R, Gaia D, Faccani G, et al. Effects of octreotide treatment on the proliferation and apoptotic index of GH-secreting pituitari adenomas. J Clin Endocrinol Metab. 2001;86:5194–200. doi:10.1210/jc.86.11.5194.
Thapar K, Kovacs KT, Stefaneanu L, Scheithauer BW, Horvath E, Lloyd RV, et al. Antiproliferative effect of the somatostatin analogue octreotide on growth hormone-producing pituitary tumors: results of a multicentre randomized trial. Mayo Clin Proc. 1997;72:893–900.
Ezzat S, Horvath E, Harris AG, Kovacs K. Morphological effects of octreotide on growth hormone-producing pituitary adenomas. J Clin Endocrinol Metab. 1994;79:113–8. doi:10.1210/jc.79.1.113.
Colao A, di Sarno A, Pivonello R, di Somma C, Lombardi G. Dopamine receptor agonists for treating prolactinomas. Expert Opin Investig Drugs. 2002;11:787–800. doi:10.1517/13543784.11.6.787.
Lamberts SWJ, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–54. doi:10.1056/NEJM199601253340408.
Freda PU. Somatostatin analogs in acromegaly. J Clin Endocrinol Metab. 2002;87:3013–8. doi:10.1210/jc.87.7.3013.
Colao A, Marzullo P, Ferone D, Marinò V, Pivonello R, Di Somma C, et al. Effectiveness and tolerability of slow release lanreotide treatment in active acromegaly. J Endocrinol Invest. 1999;22:40–7.
Lancranjan I, Bruns C, Grass P, Jaquet P, Jarvell J, Kendall-Taylor P, et al. Sandostatin LAR: a promising therapeutic tool in the management of acromegalic patients. Metabolism. 1996;45(Suppl 1):67–71. doi:10.1016/S0026-0495(96)90087-6.
McKeage K, Cheer A, Wagstaff AJ. Octreotide Long-Acting Release (LAR). A review of its use in the management of acromegaly. Drugs. 2003;63:2473–99. doi:10.2165/00003495-200363220-00014.
Colao A, Pivonello R, Auriemma RS, Galdiero M, Savastano S, Lombardi G. Beneficial effect of dose escalation of octreotide-LAR as first-line therapy in patients with acromegaly. Eur J Endocrinol. 2007;157(5):579–87. doi:10.1530/EJE-07-0383.
Caron P, Cogne M, Raingeard I, Bex-Bachellerie V, Kuhn JM. Effectiveness and tolerability of 3-year lanreotide Autogel treatment in patients with acromegaly. Clin Endocrinol (Oxf). 2006;64:209–14. doi:10.1111/j.1365-2265.2006.02450.x.
Lombardi G, Minuto F, Tamburrano G, Ambrosio MR, Arnaldi G, Arosio M, et al. Efficacy of the new long-acting formulation of lanreotide (Lanreotide Autogel) in somatostatin analogues naive patients with acromegaly. J Endocrinol Invest. 2008; In press.
Bruns C, Lewis I, Briner U, et al. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–16. doi:10.1530/eje.0.1460707.
van der Hoek J, de Herder WW, Feelders RA, van der Lely AJ, Uitterlinden P, Boerlin V, et al. A single-dose comparison of the acute effects between the new somatostatin analog SOM 230 and octreotide in acromegalic patients. J Clin Endocrinol Metab. 2004;89:638–45. doi:10.1210/jc.2003-031052.
Fedele M, De Martino I, Pivonello R, Ciarmiello A, Del Basso De Caro ML, Visone R, et al. SOM230, A New Somatostatin Analogue, Is Highly Effective in the Therapy of Growth Hormone/Prolactin-Secreting Pituitary Adenomas. Clin Cancer Res. 2007;13:2738–44. doi:10.1158/1078-0432.CCR-06-2505.
Colao A, Lombardi G, Annunziato L. Cabergoline. Expert Opin Pharmacother. 2000;1:555–74. doi:10.1517/14656566.1.3.555.
Verhelst J, Abs R, Maiter D, van den Bruel A, Vandeweghe M, Velkeniers B, et al. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. 1999;84:2518–22. doi:10.1210/jc.84.7.2518.
Colao A, Di Sarno A, Landi ML, Cirillo S, Sarnacchiaro F, Facciolli G, et al. Long-term and low-dose treatment with cabergoline induces macroprolactinoma shrinkage. J Clin Endocrinol Metab. 1997;82:3574–9. doi:10.1210/jc.82.11.3574.
Colao A, Di Sarno A, Landi ML, Scavuzzo F, Cappabianca P, Pivonello R, et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patients. J Clin Endocrinol Metab. 2000;85:2247–52. doi:10.1210/jc.85.6.2247.
Di Sarno A, Landi ML, Cappabianca P, Di Salle F, Rossi FW, Pivonello R, et al. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86:5256–61. doi:10.1210/jc.86.11.5256.
Colao A, De Rosa M, Sarnacchiaro F, Di Sarno A, Landi ML, Iervolino E, et al. Chronic treatment with CV 205–502 restores the gonadal function in hyperprolactinemia males. Eur J Endocrinol. 1996;135:548–52.
Vance ML, Lipper M, Klibanski A, Biller BM, Samaan NA, Molitch ME. Treatment of prolactin-secreting pituitary macroadenomas with the long-acting non-ergot dopamine agonist CV 205–502. Ann Intern Med. 1990;112:668–73.
Cannavo S, Bartolone L, Blandino A, Spinella S, Galatioto S, Trimarchi F. Shrinkage of a PRL-secreting pituitary macroadenoma resistant to cabergoline. J Endocrinol Invest. 1999;22:306–9.
Cappabianca P, Lodrini S, Felisati G, Peca C, Cozzi R, Di Sarno A, et al. Cabergoline-induced CSF rhinorrhea in patients with macroprolactinoma. Report of three cases. J Endocrinol Invest. 2001;24:183–7.
Colao A, Di Sarno A, Cappabianca P, Di Somma C, Pivonello R, Lombardi G. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. 2003;349:2023–33. doi:10.1056/NEJMoa022657.
Fusco A, Gunz G, Jaquet P, Dufour H, Germanetti AL, Culler MD, et al. Somatostatinergic ligands in dopamine-sensitive and -resistant prolactinomas. Eur J Endocrinol. 2008;158:595–603. doi:10.1530/EJE-07-0806.
Jaffe CA, Barkan AI. Acromegaly: recognition and treatment. Drugs. 1994;47:425–45.
Colao A, Ferone D, Marzullo P, Di Sarno A, Cerbone G, Sarnacchiaro F, et al. Effect of different dopaminergic agents in the treatment of acromegaly. J Clin Endocrinol Metab. 1997;82:518–23. doi:10.1210/jc.82.2.518.
Abs R, Versholst J, Maiter D, Van Acker K, Nobels F, Coolens JL, et al. Cabergoline in the treatment of acromegaly: a study in 64 patients. J Clin Endocrinol Metab. 1998;83:374–8. doi:10.1210/jc.83.2.374.
Cozzi R, Attanasio R, Barausse M, Dallabonzana D, Orlandi P, Da Re N, et al. Cabergoline in acromegaly: a renewed role for dopamine agonist treatment? Eur J Endocrinol. 1998;139:516–21. doi:10.1530/eje.0.1390516.
Ferrari C, Paracchi A, Romano C, Gerevini G, Boghen M, Barreca A, et al. Long-lasting lowering of serum growth hormone and prolactin levels by single and repetitive cabergoline administration in dopamine-responsive acromegalic patients. Clin Endocrinol (Oxf). 1988;29:467–76. doi:10.1111/j.1365-2265.1988.tb03695.x.
Muratori M, Arosio M, Gambino G, Romano C, Biella O, Faglia G. Use of cabergoline in the long-term treatment of hyperprolactinemia and acromegalic patients. J Endocrinol Invest. 1997;20:537–46.
Rickels MR, Snyder PJ. Cabergoline decreases somatotroph adenoma size: a case report. Pituitary. 2004;7:107–10. doi:10.1007/s11102-005-5353-1.
Vilar L, Czepielewsk MA, Naves LA, Rollin GA, Casulari LA, Coelho CE. Substantial shrinkage of adenomas cosecreting growth hormone and prolactin with use of cabergoline therapy. Endocr Pract. 2007;13:396–402.
Colao A, Filippella M, Pivonello R, Di Somma C, Faggiano A, Lombardi G. Combined therapy of somatostatin analogues and dopamine agonists in the treatment of pituitary tumours. Eur J Endocrinol. 2007;156(Suppl 1):S57–63. doi:10.1530/eje.1.02348.
Bevan JS. The antitumoral effects of somatostatin analog therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:1856–63. doi:10.1210/jc.2004-1093.
Melmed S, Sternberg R, Cook D, Klibanski A, Chanson P, Bonert V, et al. A critical analysis of pituitary tumor shrinkage during primary medical therapy in acromegaly. J Clin Endocrinol Metab. 2005;90:4405–10. doi:10.1210/jc.2004-2466.
Colao A, Pivonello R, Auriemma RS, Briganti F, Galdiero M, Tortora F, et al. Predictors of tumor shrinkage after primary therapy with somatostatin analogues in acromegaly: a prospective study in 99 patients. J Clin Endocrinol Metab. 2006;91:2112–8. doi:10.1210/jc.2005-2110.
Murray RD, Melmed S. A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab. 2008;93:2957–68.
Maiza JC, Vezzosi D, Matta M, Donadille F, Loubes-Lacroix F, Cournot M, et al. Long-term (up to 18 years) effects on GH/IGF-1 hypersecretion and tumour size of primary somatostatin analogue (SSTa) therapy in patients with GH-secreting pituitary adenoma responsive to SSTa. Clin Endocrinol (Oxf). 2007;67:282–9. doi:10.1111/j.1365-2265.2007.02878.x.
Cozzi R, Montini M, Attanasio R, Albizzi M, Lasio G, Lodrini S, et al. Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metab. 2006;91:1397–403. doi:10.1210/jc.2005-2347.
Colao A, Pivonello R, Auriemma RS, Galdiero M, Savastano S, Lombardi G. Beneficial effect of dose escalation of octreotide-LAR as first-line therapy in patients with acromegaly. Eur J Endocrinol. 2007;157:579–87. doi:10.1530/EJE-07-0383.
Resmini E, Dadati P, Ravetti JL, Zona G, Spaziante R, Saveanu A, et al. Rapid pituitary tumor shrinkage with dissociation between antiproliferative and antisecretory effects of a long-acting octreotide in an acromegalic patient. J Clin Endocrinol Metab. 2007;92:1592–9. doi:10.1210/jc.2006-2084.
Casarini AP, Pinto EM, Jallad RS, Giorgi RR, Giannella-Neto D, Bronstein MD. Dissociation between tumor shrinkage and hormonal response during somatostatin analog treatment in an acromegalic patient: preferential expression of somatostatin receptor subtype 3. J Endocrinol Invest. 2006;29:826–30.
Besser GM, Burman P, Daly AF. Predictors and rates of treatment-resistant tumor growth in acromegaly. Eur J Endocrinol. 2005;153:187–93. doi:10.1530/eje.1.01968.
Ferone D, de Herder WW, Pivonello R, Kros JM, van Koetsveld PM, de Jong T, et al. Correlation of in vitro and in vivo somatotropic adenoma responsiveness to somatostatin analogs and dopamine agonists with immunohistochemical evaluation of somatostatin and dopamine receptors and electron microscopy. J Clin Endocrinol Metab. 2008;93:1412–7. doi:10.1210/jc.2007-1358.
Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumours. Endocr Rev. 1996;17:610–38. doi:10.1210/er.17.6.610.
Kienitz T, Quinkler M, Strasburger CJ, Ventz M. Long-term management in five cases of TSH-secreting pituitary adenomas: a single center study and review of the literature. Eur J Endocrinol. 2007;157:39–46. doi:10.1530/EJE-07-0098.
Warnet A, Lajeunie E, Gelbert F, Duet M, Chanson P, Cophignon J, et al. Shrinkage of a primary thyrotropin-secreting pituitary adenoma treated with the long-acting somatostatin analogue octreotide (SMS 201–995). Acta Endocrinol (Copenh). 1991;124:487–91.
Wemeau L, Dewailly D, Leroy R, D'Herbomez M, Mazzuca M, Decoulx M, et al. Long term treatment with a somatostatin analog SMS 201–995 in a patient with a thyrotropin and growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 1988;66:636–9.
Losa M, Giovanelli M, Persani L, Mortini P, Faglia G, Beck-Peccoz P. Criteria of cure and follow-up of central hyperthyroidism due to thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 1996;81:3084–90. doi:10.1210/jc.81.8.3084.
Chanson P, Weintraub BD, Harris AG. Treatment of TSH-secreting pituitary adenomas with octreotide: a follow-up of 52 patients. Ann Intern Med. 1993;119:236–40.
Fischler MP, Reinhart WH. TSH-secreting pituitary macroadenoma: rapid tumor shrinkage and recovery from hyperthyroidism with octreotide. J Endocrinol Invest. 1999;22:64–5.
Colao A, Filippella M, Di Somma C, Manzi S, Rota F, Pivonello R, et al. Somatostatin analogs in treatment of non-growth hormone-secreting pituitary adenomas. Endocrine. 2003;20:279–83. doi:10.1385/ENDO:20:3:279.
Gancel A, Vuillermet P, Legrand A, Catus F, Thomas F, Kuhn JM. Effects of a slow-release formulation of the new somatostatin analogue lanreotide in TSH-secreting pituitary adenomas. Clin Endocrinol (Oxf). 1994;40:421–8.
Kuhn JM, Arlot S, Lefebvre H, Caron P, Cortet-Rudelli C, Archambaud F, et al. Evaluation of the treatment of thyrotropin-secreting pituitary adenomas with a slow release formulation of the somatostatin analog lanreotide. J Clin Endocrinol Metab. 2000;85:1487–91. doi:10.1210/jc.85.4.1487.
Caron P, Arlot S, Bauters C, Chanson P, Kuhn JM, Pugeat M, et al. Efficacy of the long-acting octreotide formulation (octreotide-LAR) in patients with thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 2001;86:2849–53. doi:10.1210/jc.86.6.2849.
Pivonello R, De Martino MC, De Leo M, Lombardi G, Colao A. Cushing’s syndrome. Endocrinol Metab Clin North Am. 2008;37:135–49. doi:10.1016/j.ecl.2007.10.010.
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, et al. Treatment of ACTH-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93:2454–62.
Pivonello R, Ferone D, de Herder WW, Kros JM, De Caro ML, Arvigo M, et al. Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab. 2004;89:2452–62. doi:10.1210/jc.2003-030837.
Pivonello R, Ferone D, de Herder WW, Faggiano A, Bodei L, de Krijger RR, et al. Dopamine receptor expression and function in corticotroph ectopic tumors. J Clin Endocrinol Metab. 2007;92:65–9. doi:10.1210/jc.2006-0728.
Pivonello R, Ferone D, de Herder WW, de Krijger RR, Waaijers M, Mooij DM, et al. Dopamine receptor expression and function in human normal adrenal gland and adrenal tumors. J Clin Endocrinol Metab. 2004;89:4493–502. doi:10.1210/jc.2003-031746.
Miller JW, Crapo L. The medical treatment of Cushing’s syndrome. Endocr Rev. 1993;14:443–58. doi:10.1210/er.14.4.443.
Pivonello R, Faggiano A, Di Salle F, Filippella M, Lombardi G, Colao A. Complete remission of Nelson’s syndrome after 1-year treatment with cabergoline. J Endocrinol Invest. 1999;22:860–5.
Hofland LJ, van der Hoek J, Feelders R, et al. The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur J Endocrinol. 2005;152:645–54. doi:10.1530/eje.1.01876.
Boscaro M, Atkinson A, Bertherat J, et al. SOM230 Cushing’s disease study group. Early data on the efficacy and safety of the novel multi-ligand somatostatin analog, SOM230, in patients with Cushing’s disease. Presented at the 87th Annual Meeting of the Endocrine Society. San Diego (CA), June 4–7, 2005.
Pivonello R, Ferone D, Lamberts SW, Colao A. Cabergoline plus lanreotide for ectopic Cushing’s syndrome. N Engl J Med. 2005;352:2457–8. doi:10.1056/NEJM200506093522322.
Colao A, Di Sarno A, Marzullo P, Di Somma C, Cerbone G, Landi ML, et al. New medical approaches in pituitary adenomas. Horm Res. 2000;53(Suppl 3):76–87. doi:10.1159/000023539.
Bevan JS, Burke CW. Non-functioning pituitary adenomas do not regress during bromocriptine therapy but possess membrane-bound dopamine receptors which bind bromocriptine. Clin Endocrinol (Oxf). 1986;25:561–72. doi:10.1111/j.1365-2265.1986.tb03610.x.
Pivonello R, Matrone C, Filippella M, Cavallo LM, Di Somma C, Cappabianca P, et al. Dopamine receptor expression and function in clinically nonfunctioning pituitary tumors: comparison with the effectiveness of cabergoline treatment. J Clin Endocrinol Metab. 2004;89:1674–83. doi:10.1210/jc.2003-030859.
Ferone D, Lastoria S, Colao A, Varrella P, Cerbone G, Acampa W, et al. Correlation of scintigraphic results using 123I-methoxybenzamide with hormone levels and tumor size response to quinagolide in patients with pituitary adenomas. J Clin Endocrinol Metab. 1998;83:248–52. doi:10.1210/jc.83.1.248.
Hedner P, Valdemarsson S. Reduced size of a hormonally silent pituitary adenoma during treatment with CV 205–502, a new dopamine agonist mainly stimulating D2 receptors. Neurosurgery. 1989;25:948–50. doi:10.1097/00006123-198912000-00015.
Kwekkboom DJ, Lamberts SWJ. Long-term treatment with dopamine agonist CV 205–502 of patients with clinically non-functioning, gonadotroph, or α-subunit secreting pituitary adenoma. Clin Endocrinol (Oxf). 1992;36:171–17. doi:10.1111/j.1365-2265.1992.tb00953.x.
Colao A, Ferone D, Lastoria S, Cerbone G, Di Sarno A, Di Somma C, et al. Hormone levels and tumor size response to quinagolide and cabergoline in patients with prolactin-secreting and clinically nonfunctioning pituitary adenomas: predictive value of pituitary scintigraphy with 123I-methoxybenzamide. Clin Endocrinol (Oxf). 2000;52:437–45. doi:10.1046/j.1365-2265.2000.00951.x.
Ikuyama S, Nawata H, Kato K, Karashima T, Ibayashi H, Nakagaki H. Specific somatostatin receptors on human pituitary adenoma cell membranes. J Clin Endocrinol Metab. 1985;61:98–103.
Reubi JC, Heitz PU, Landolt AM. Visualization of somatostatin receptors and correlation with immunoreactive growth hormone and prolactin in human pituitary adenomas: evidence for different tumor subclasses. J Clin Endocrinol Metab. 1987;65:65–73.
Greenman Y, Melmed S. Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab. 1994;78:398–403. doi:10.1210/jc.78.2.398.
Faglia G, Bazzoni N, Spada A, Arosio M, Ambrosi B, Spinelli F, et al. In vivo detection of somatostatin receptors in patients with functionless pituitary adenomas by means of a radio-iodinated analog of somatostatin. J Clin Endocrinol Metab. 1991;73:850–6. (123I)SDZ204–090.
Plockinger U, Reichel M, Fett U, Saeger W, Quabbe HJ. Preoperative octreotide treatment of growth hormone-secreting and clinically nonfunctioning pituitary macroadenomas: effect on tumor volume and lack of correlation with immunohistochemistry and somatostatin receptor scintigraphy. J Clin Endocrinol Metab. 1994;79:1416–23. doi:10.1210/jc.79.5.1416.
Lamberts SWJ, de Herder WW, van der Lely AJ, Hofland LJ. Imaging and medical management of clinically nonfunctioning pituitary tumors. Endocrinologist. 1995;5:448–51. doi:10.1097/00019616-199511000-00020.
Colao A, Lastoria S, Ferone D, Varrella P, Marzullo P, Pivonello R, et al. Pituitary uptake of In-111-DTPA-D-Phe1-octreotide in the normal pituitary and in pituitary adenomas. J Endocrinol Invest. 1999;22:176–83.
Borson-Chazot F, Houzard C, Ajzenberg C, Nocaudie M, Duet M, Mundler O, et al. Somatostatin receptor imaging in somatotroph and non-functioning pituitary adenomas: correlation with hormonal and visual responses to octreotide. Clin Endocrinol (Oxf). 1997;47:589–98. doi:10.1046/j.1365-2265.1997.3361119.x.
Warnet A, Harris AG, Renard E, Martin D, James-Deidier A, Chaumet-Riffaud P, and the French multicenter octreotide study group. A prospective multicenter trial of octreotide in 24 patients with visual defects caused by nonfunctioning and gonadotropin-secreting pituitary adenomas. Neurosurgery 1997;41:786–97. doi:10.1097/00006123-199710000-00005.
Gasperi M, Petrini L, Pilosu R, Nardi M, Marcello AA, Mastio F, et al. Octreotide treatment does not affect the size of most non-functioning pituitary adenomas. J Endocrinol Invest. 1993;16:541–3.
De Bruin TWA, Kwekkeboom DJ, van't Verlaat JW, Reubi JC, Krenning EP, Lamberts SW, Croughs RJ. Clinically nonfunctioning pituitary adenoma and octreotide response to long term high dose treatment, and studies in vitro. J Clin Endocrinol Metab. 1992;75:1310–7.
Klibanski A, Alexander JM, Bikkal HA, Hsu DW, Swearingen B, Zervas NT. Somatostatin regulation of glycoprotein hormone and free subunit secretion in clinically nonfunctioning and somatotroph adenomas in vitro. J Clin Endocrinol Metab. 1991;73:1248–55.
Katznelson L, Oppenheim DS, Coughlin JF, Kliman B, Schoenfeld DA, Klibanski A. Chronic somatostatin analog administration in patients with a-subunit-secreting pituitary tumors. J Clin Endocrinol Metab. 1992;75:1318–25. doi:10.1210/jc.75.5.1318.
Andersen M, Bjerre P, Schrøder HD, Edal A, Høilund-Carlsen PF, Pedersen PH, et al. In vivo secretory potential and the effect of combination therapy with octreotide and cabergoline in patients with clinically non-functioning pituitary adenomas. Clin Endocrinol (Oxf). 2001;54:23–30. doi:10.1046/j.1365-2265.2001.01172.x.
Colao A, Martino E, Cappabianca P, Cozzi R, Scanarini M, Ghigo E, A.L.I.C.E. Study Group. First-line therapy of acromegaly: a statement of the A.L.I.C.E. (Acromegaly primary medical treatment Learning and Improvement with Continuous Medical Education) Study Group. J Endocrinol Invest 2006;29:1017–20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colao, A., Pivonello, R., Di Somma, C. et al. Medical therapy of pituitary adenomas: Effects on tumor shrinkage. Rev Endocr Metab Disord 10, 111–123 (2009). https://doi.org/10.1007/s11154-008-9107-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-008-9107-z