Abstract
Timing is a very abstract representation that shares with other magnitudes, such as numerosity, the peculiarity of being independent from any particular sensory modality. Not only we can time stimuli in different modalities but we can also compare the durations of different visual, auditory and somatosensory stimuli. Furthermore, even though time is not directly associated with space, and we are inclined to consider space and time as two different perceptual dimensions of our existence, an increasing number of studies challenge this idea by showing that timing and spatial processing have some relationship that involves sharing computation resources and that time may have a spatial representation. A more general theory, called theory of magnitude (ATOM), considers both timing and spatial computations, together with other magnitudes, as originating from a general magnitude system [Walsh VA, Trends Cogn Sci 7(11):483–8, 2003]. The neural underpinnings of time and its relationship to the processing of spatial information have started to be investigated only recently, but the field is rapidly growing. It is addressing the representation of time in several cortical and subcortical brain areas. Information processing of time and space are not strictly specialized in neural and cognitive mechanisms and we believe that studying them only separately may restrict our understanding of these processes. In this chapter, we will firstly introduce the role of the prefrontal cortex (PF) in coding relative durations. We will point out that the comparison of durations makes use of intermediate computations based on the order of the events. Secondly, we will describe the comparison mechanisms that are implemented by PF to make perceptual decisions about durations in relation to those involved in making decisions about spatial locations and distances. We will distinguish the decision processes from the goal choices, and we will examine which computational resources are shared between different magnitudes and which are domain-specific. We will summarize our results within the context of a more general PF function in promoting the generation of goals from the current context, consisting of domain- and modality-specific coding of stimuli of different modalities or magnitudes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Timing Function in PF
Timing functions have been associated with many brain regions, including the cerebellum, basal ganglia, and posterior parietal cortex [1–5], as well as PF. Among such areas, the role of PF in temporal perception has been shown by several neuropsychological [6, 7] and neuroimaging [8, 9] studies. For example, patients with right PF lesions show deficits in timing tasks [6, 10–12]. Likewise, transcranial magnetic stimulation (TMS) to the right PF cortex has been shown to impair explicit timing tasks in the suprasecond range of durations [13, 14]. In monkeys, inactivation of the dorsolateral prefrontal cortex (PFdl) through injections of bicuculline, a GABAA antagonist, produces deficits in the duration discrimination task, and the same task activates the PFdl in the context of a parietal-frontal network, in a positron emission tomography study, with covariation of activations between parietal and PF areas [8].
PF involvement in explicit timing has been also shown by adopting the variable foreperiod paradigm in which a target is presented after a foreperiod of different duration. In this paradigm, a progressive increase of the likelihood that a target will appear with the passage of time is associated with a reduction of the reaction time of the response to the target appearance. The reaction time advantage for longer foreperiods has been found to be compromised in patients with right PF damage [15–17]. Similar conclusions come from a TMS study of the right PF cortex [18].
In addition to neuropsychological and neuroimaging studies, an increasing number of studies have focused on the single cell level in primates. Since the early work of Niki and Watanabe [19] that proposed a role of cortical neurons in encoding durations, neurophysiological experiments in primates have investigated temporal processing in parietal cortex [20–23], PF cortex [24–31], motor and premotor cortex [4, 26, 32, 33, 35–38], and basal ganglia neurons both in monkeys [39] and in rats [40, 41]. Some of these neurophysiological experiments, including ours [42, 43], have investigated perceptual timing using paradigms that required subjects to compare the durations of two stimuli, whereas others have focused on the motor aspects of timing. Another approach to the study of time is to consider a particular type of temporal expectation: the time to reward. Several neurophysiological studies have shown that PF activity is modulated by the time until reward [30, 44]. Notwithstanding the importance of these studies, the interpretation of their results is challenged by the correlation, intrinsic to these paradigms, between value and time, because an earlier expected reward brings in itself also a greater value to the animal.
We started our investigation of the role of PF on timing encoding several years ago [24] by studying implicit timing in PFdl and PF area 9, by using a saccade strategy task with three different durations (1, 1.5, or 2 s) of stimulus presentation. Although originally designed for studying the neural correlate of learning strategies, variable durations of the stimulus presentations allowed us to investigate the representation of the elapsed time as well. We showed that the activity of ~9 % of the neurons was modulated after the stimulus offset by the duration of the preceding stimulus presentation. This “elapsed time” modulation could not be explained by differences in saccadic reaction times. Most neurons showed a greater activity for either short (Fig. 1a) or long delays (Fig. 1b). A much smaller proportion of neurons (25 %) preferred intermediate delays. The modulation of the activity by stimulus duration was often preceded by a ramping up of the activity in the neurons with a long duration preference (Fig. 1b). It is worth noting that the elapsed-time effect on the neural activity emerged even though there was no requirement for the monkeys to time the stimulus duration.
Two examples of PF neurons encoding the duration of the previous stimulus. (a) Neuron with a preference for the shorter duration of the previous stimulus presentation. (b) Neuron with a preference for the longer durations. Activity (raster dots) is aligned on the end of the delay period (vertical lines), sorted by time to saccade onset (square marks). Light gray background shading indicates the delay period and the dark gray background shading indicates the post-delay period used for the analysis. Fix, onset of fixation; IS on, onset of IS; IS off, offset of IS; Sac on, onset of saccade. From Genovesio A., Tsujimoto S. Wise S.P J. Neurophysiol. 95, 3281-3285, 2006, with permission
Duration Task
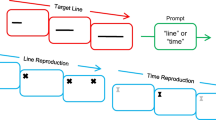
Figure 2a illustrates the duration discrimination task adopted to study PF in two macaques. In this task, the monkey viewed two stimuli, a red square or a blue circle, of different durations presented sequentially. The monkey’s task was to choose which of them lasted longer by touching a switch below it. The task was designed such that the monkey could not plan its motor response before the “go” signal (i.e., the targets appearance), because each choice stimulus could appear with the same probability on each side of the screen’s center.
(a) Duration discrimination task. When the monkey touched a central switch a white circle (pre-cue) appeared and the monkey was required to start fixation until a later “go” signal. After the pre-cue period, the monkey viewed the first stimulus (S1) followed by a first delay period (D1). After, the second stimulus appeared followed by a subsequent delay period (D2) of variable duration. After this second delay both stimuli reappeared, one to the left and the other to the right serving as a “go” signal and the monkey was required to choose the stimulus that lasted longer, indicating his decision by touching the switch below that stimulus. (b) MTS task. In this task the monkey viewed sequentially two identical stimuli called samples, either two red squares or two blue circles of different durations as those used in the “square set” of the duration task. The monkey’s task was to choose the target that had appeared twice. (c) Distance task. In this task one stimulus appeared above and the other below the reference point in an order determined randomly. After the appearance of the two targets the monkey was required to choose the farthest stimulus from the reference point. (d) Distributions of durations. The distribution of duration could belong to one of two sets either the ‘V’ or the “square” set. The distance task had a square distribution identical to the duration task only with distances instead of durations. (e) Penetration sites. Composite of the two monkeys. Abbreviation: AS arcuate sulcus, PS principal sulcus
The duration of the two stimuli was defined according to one of two duration sets termed “V” and “square” distributions (Fig. 2d). We adopted these two sets of stimulus durations for different purposes. The ‘V’ distribution prevented the subject from predicting the second stimulus duration based on the duration of the first stimulus, but it did not allow us to distinguish absolute from relative duration coding. The ‘square’ set had the advantage of varying systematically the duration differences.
Figure 2e shows the location of the recorded neurons. The recordings were made in two cortical areas: rostral to the arcuate sulcus in the caudal PF cortex (area 8) and in both banks of the principal sulcus and the adjacent convexity cortex in the PFdl (area 46). Because we did not find any dramatic differences between the two areas, we pooled together the results from the two areas in this chapter.
The duration task required the monkey to perform several computations on the duration information. The monkeys should (1) encode the S1 duration, (2) maintain that duration in memory in the D1 period until S2 occurred, and (3) compare their durations. In this chapter we will focus only on the comparison process, first in the delay period and then in the decision period.
Encoding of Relative Duration in the Delay Period
We examined the representation of relative duration in the second delay of the duration task that starts after the first stimulus is turned off, in terms of the order of presentation of the two sequentially presented stimuli and in terms of their features (blue or red). Using a two-way ANOVA, we found that the activity of ~30 % of the neurons depended on whether either S1 or S2 lasted longer. Moreover, approximately 25 % of neurons showed a second type of representation that depended on whether the blue or the red stimulus lasted longer. We will take up both types later in the chapter, when we will focus our attention on the decision period.
By using ANOVA, alone, we could not asses whether these two classes of neurons were encoding categorically which stimulus lasted longer or how much the stimuli differed in duration parametrically. Furthermore, we could not disentangle the encoding of absolute and relative duration coding. That could be addressed by a stepwise regression analysis. We applied this analysis only to the data collected with the “square” distribution, which varied the duration difference between stimuli in a highly graded manner. With a first analysis, we assessed the role of four factors related to the order of presentation: the absolute stimulus duration of both S1 and S2, which stimulus lasted categorically longer (S1 or S2), and their difference in duration. In a second analysis, we assessed the role of other four factors associated to the stimulus features: the absolute duration of both the red and the blue stimuli, which of them lasted longer (blue or red) and their difference in duration.
We found that the strongest signals were represented by the categorical representation of the relative duration based on the order of presentation of the two stimuli (12–19 %) and based on their stimulus features (12–19 %), in addition to the absolute duration of the second stimulus (13–16 %). The representation of the parametric difference between S1 and S2 durations reached ~10 %. In contrast, we found a very small proportion of neurons that encoded the difference between the blue and the red stimuli durations parametrically (5–7 %), which was very close to chance levels. Based on these results, we can propose two ways in which duration information could be compared by neurons in PF. For example, consider a trial in which the first stimulus is blue, the second is red, and the red stimulus is longer than the blue one. One way to determine that the blue stimulus is longer than the red stimulus would be by integrating two pieces of information: (1) the second stimulus lasted longer than the first stimulus, and (2) the second stimulus was red. Neurons showing an interactive effect by the two-way ANOVA for the relative duration based on the order of presentation and on the stimulus features reflected this computation [42]. Alternatively, the representation of blue-stimulus duration could be compared directly with a representation of the red-stimulus duration. We also found neurons showing a duration-color conjunction encoding, such as a preference for a long stimulus but only when it was blue. Such neurons that could represent the information required for this second type of computation. Both integrative processes are likely to coexist and they should not be considered as mutually exclusive.
General Considerations on the Representation of Time in PF
So far, we have described neurons that encoded relative durations. We found that the encoding of relative duration was represented in two “formats”, one associated to the order and the other to the stimulus features. The first can be thought as a conjunction of relative duration and order while the second as the conjunction of duration and stimulus color. The encoding of conjunctions of features represents a key difference with the parietal cortex. Such a role of PF in duration comparison agrees with an imaging study by Rao et al. [9] that associated PF activity to the later stage of the task corresponding to the duration comparison.
This result adds duration to the list of examples of conjunctive encoding identified by past studies using a variety of paradigms in PFdl [45–49]. For example, Tsujimoto and Sawaguchi [45] and Tsujimoto et al. [46, 50] have found neurons encoding conjunctions of goals and outcomes. In a cued strategy task Tsujimoto et al. [46] found neurons encoding the conjunction of stay and shift strategies with goals in PFdl, similar to the signal found by Genovesio et al. [47]. Another example can be found in a study by Hoshi and Tanji [49]. They have reported that PF neurons encoded the combination of arm (left/right) and spatial goal in a task in which two sequentially presented cues instructed which arm to use and the goal location.
We found that a substantial population of neurons encoded whether the red or the blue stimulus had lasted longer during the delay. Later in this chapter, by comparing the information derived from other tasks, we will be able to separate the decision process from the goal representation functions performed by different subpopulations of the neurons that encoded which stimulus blue or red lasted longer. Notwithstanding the fact that our experiment did not impose any requirement to the monkeys to report whether the first stimulus had lasted longer or shorter, we found neurons performing this intermediate computation. This result underlines the importance of ordering temporally the contents of our experience, such as the stimuli to compare in our task.
Furthermore, we found neurons that encoded higher order conjunctions, combining the information on which stimulus was the first based on the order of presentation with the information on which stimulus was the longest, blue or the red. Such a hypothetical neuron would show a specific preference: for example, for the red stimulus being both the first of the sequence and the longest stimulus. Neither a longer red second stimulus nor a blue first longer stimulus would activate such a neuron.
To date, only a few neurophysiological studies have investigated PF’s role in timing encoding [26–28, 38, 51, 52]. Three studies adopted a task similar to ours in PF [27, 28, 38] and we will compare their results to ours.
Oshio et al. [27] have described neurons encoding which stimulus had lasted longer but not neurons encoding duration differences and a relative duration encoding based on the order of stimulus presentation. We obtained different results, which are probably explained by some task differences. They used durations of the first stimulus that allowed the subject to predict the duration of the second upcoming stimulus, therefore no other duration comparison was necessarily required after the presentation of the first stimulus. Interestingly, when the second stimulus duration could not be predicted on the basis of the first stimulus duration, in a separate study on the basal ganglia, Chiba et al. [39] identified neurons with proprieties similar to those described in our study, such as the coding of the duration of the first stimulus presentation in the following delay period and the coding of whether the first or the second stimulus had lasted longer. It is likely that the differences between their PF and basal ganglia data depend on task differences, associated to the predictability of the duration of the comparison stimulus, rather than on different roles played by these two areas. In a more recent study, using the same task, Oshio et al. [28] recorded from PF using short and long stimuli that overlapped more than in their previous study [27], reducing the issue of predictability of the second stimulus duration. In this study the authors focused the analysis on the first stimulus period identifying neurons with both buildup and sustained activity in addition to others with phasic activities with unimodal peak times of response around 0.8 s. This duration corresponded roughly to the middle duration of the averages of the long and short stimuli. That suggests that the monkeys could have compared the duration of the first stimulus with this single filtering duration to separate durations into long and short categories.
Another neurophysiological study on timing used a matching-to-sample paradigm to examine duration coding in PFdl [29]. In contrast to our study, Sakurai et al. [29] did not report relative duration neurons. They identified only a small proportion of neurons designed as “comparison neurons” that might contribute to comparing the duration of the two stimuli. However, these neurons were defined only for having a phasic activity specifically associated with the presentation of the comparison stimulus and not with the sample presentation. As we have described before, contrary to their results, many neurons in our experiment encoded the relative duration. This discrepancy probably also results from task differences. As in the work by Oshio et al. [27], the monkeys studied by Sakurai et al. [29] could simply categorize stimuli as either short or long, rather than encoding the sample duration, and the use of only two durations (0.5 and 2.0 s) was the reason for this interpretational problem. Therefore, these “comparison neurons” might have represented a rank-order signal like the neuron in Fig. 3b, indicating that the stimulus presented was the second of the sequence, irrespective of any duration comparisons.
(a) Neuron encoding which stimulus was farther and longer based on the stimulus order. Background shading indicates the decision period (80–400 ms after the ideal decision point). This neuron showed an higher activity when the first stimulus was longer (S1 of greater magnitude) in the duration task. The same neuron showed an opposite preference for the second stimulus farther (S2 of greater magnitude) in the distance task. (b) Rank-order and color selective neuron in the duration task. This neuron showed a preference for the first red stimulus. (c) Neuron encoding the relative magnitude based on the stimulus features in the duration task but not in the distance task with a preference for longer blue stimuli in the duration task. (d) Neuron encoding the same goal in all three tasks. This neuron showed the same preference for the red goal in all three tasks. Modified from Genovesio, A., Tsujimoto, S., Wise, S.P. Neuron 74, 656-662, 2012, with permission
Order-encoding properties have been described previously by several studies for both colored patterns [53] and for spatial stimuli [54]. For example, in the experiment of Funahashi et al. [54] the monkeys performed a delayed sequential reaching task, in which they were required to remember the position of two of three cues and their temporal order of presentation. They found a consistent population of neurons that showed a rank-order activity either in combination with the cue position or irrespectively.
Sakurai et al. [29] also found neurons representing which stimulus was either short or long. Some neurons showed a phasic discharge when the stimulus could be categorized as either short or long. However, the experimental design could not rule out the possibility that these categories of neurons represented an abstract category instead than specific durations.
Other studies have focused on time reproduction [33, 51]. Yumoto et al. [51] have recorded from PF area 9 using a time reproduction task in which monkeys were trained to estimate and reproduce the duration of a visual stimulus with a button press. They found a first population of neurons which were modulated by the previous stimulus duration, similar to what we have previously described [24, 42]. They indentified also a second population of neurons modulated by the duration that the subjects needed to reproduce. Interestingly, only a minority of neurons belonged to both categories, pointing to a separation of functions between duration decoding and temporal organization of movement execution. Furthermore, inactivating the same area through injection of muscimol affected the reproduction of the duration interval. Specifically, this kind of temporary inactivation shortened the duration that the monkeys produced.
Another study [52] has examined the role of both PF and the caudate nucleus in a visuomotor task that required the monkeys to make sequential saccades to visual targets after short fixed intervals. This study did not impose any explicit training regarding the timing of the events. The authors identified a subpopulation of neurons with peaks of activity distributed in relationship to several task events that may represent time-stamps of different durations, as part of what the authors call the “infrastructure of neural representation of events and actions”. They found very similar phasic discharges in both areas that are known to be connected through cortico-basal ganglia loops, supporting their combined role in timing [55].
We found stronger effects in periarcuate cortex compared to PFdl. Although most of the periarcuate cortex recordings were located in the cortex rostral to the arcuate sulcus we have included in the analysis a small number of neurons within the dorsal premotor cortex (PMd). PMd neurons are known to be involved in other non-motor [56] and attentional function [57], and their role in timing is compatible with their other functions. Lebedev et al. [25] have shown that from the ensemble of neurons recorded in PMd neurons it could be decoded both the elapsed time information from the previous hand movement and the time until the onset of the next movement in a task in which the monkeys released a key after a temporal interval. Similarly Lucchetti and Bon [34] have shown a buildup activity for predictable delays before movements in PMd.
Interaction Between Duration and Other Magnitudes
Temporal and spatial perceptions can interfere with each other and produce misperceptions in both humans [58–60] and monkeys [61]. However, most of the studies initially have focused on the interaction between space and number rather than between space and time. Therefore, we describe first briefly a variety of interactions between numbers and other magnitudes. It has been shown that numbers can have a spatial representation organized along a “mental number line” [62, 63] and that numerical processing can interact with saccade performance, shifting of spatial attention, pointing and grasping move ments, and line bisection tasks [64, 65]. On the other hand, numerical processing can be influenced by visuospatial variables. For example, spatial cueing and visual hemifield presentations can produce an influence on numerical comparisons [66, 67]. Interestingly, even eye position can influence both the representation of numbers [68] and the representation of high-level cognitive processes such as non-propositional reasoning [69]. Moreover, physical space perception and the mental number line can be affected similarly in patients with hemineglect [70, 71].
To explain the influence of different magnitudes on each other, a domain-general system has been proposed that would encode abstractly a greater or lesser quantity, independent of the specific metric such as duration, distance, or numerosity [1, 72]. Although several psychophysical effects support the ATOM theory, fewer studies have focused specifically on the interaction between space and time. In one of these, Srinivasan and Carey [73] showed that binding visible lines with tone duration appeared to be easier when their durations were relationally equivalent both in adults and infants.
Even saccadic eye movements can influence and compress magnitude judgments of both space and time [74, 75]. Two bars flashed one hundred ms apart around the time of the saccade are perceived compressed in time (closer in time) much like the spatial compression of a bar flashed around the time of the saccade towards the location of the saccade target [74].
In monkeys, Merritt et al. [61] found symmetrical interactions between temporal and spatial judgments. Other experiments, however, have shown asymmetries in the interference effects, suggesting a less complete overlap between representations of magnitudes. As an example of asymmetry, it has been shown that the duration of a visual stimulus could affect the perception of its length but not the reverse, and that this phenomenon occurs in both adults [58] and children [76]. The same asymmetry has been reported with language. Interestingly in metaphorical language, there are more words describing time in terms of space than describing space in terms of time. In contrast to the results of Merritt et al. [61], asymmetries between space and time have been found by Mendez et al. [77]. They have shown that a previous experience in categorizing distances could affect duration categorization but not the reverse. It is possible that findings about asymmetries may reflect differences in task difficulty for different kinds of magnitudes. Along this line, a recent study by Javadi and Aichelburg [78] has shown that a failure in finding a reciprocal interference between magnitudes may depend on selecting the appropriate range of magnitudes to enable the detection of interfering effects. By using high numerosities and short durations, they found an effect of temporal magnitudes on numerosities, in addition to the opposite direction of interference, which had been previously reported.
Even if the presence of some asymmetries might contradict a strong version of the theory of common magnitude, the interaction between space and time indicates that there is at least a partial overlap between space and time coding, which can affect perceptual decisions.
Further support for a common magnitude system comes also from the results of Stroop-like paradigms, such as the one adopted by Xuan et al. [79]. They have shown that stimuli of four different nontemporal magnitudes such as the number of dots, the numeric value of digits and the luminance and size of squares could affect a duration judgment: stimuli of greater magnitude were judged to last longer. Another study supporting the theory is an old pharmacological study by Meck and Church [80].
Using a psychophysical choice procedure, Meck and Church [80] have shown that methamphetamine shifted the psychophysical functions leftward for both number and duration comparisons in an experiment that used a psychophysical choice procedure.
Other studies goes beyond the ATOM proposal emphasizing that time, like numerosity [62, 63], is represented on a mental time line oriented along a left-to-right dimension that can be accessed through spatial attention mechanisms. The mental time line (MTL) proposal can be considered a more specific hypothesis than ATOM, emphasizing the organization of different magnitudes in a spatial layout. Attention would then operate on the spatial representation of the different magnitudes, probably by using the same parietal areas involved in visuospatial attention. Several studies have tested the MTL hypothesis using similar paradigms to those used for studying numerosity and we will refer here to just a few. In support of the MTL hypothesis it has been found that the presentation of lateralized irrelevant visual distracters can influence temporal perceptions producing underestimation for cues to the left and overestimation for cues to the right [81], the same way as it has been shown for numerical magnitudes. Right hemisphere parietal patients with left neglect show a rightward bias in duration bisection task requiring setting the midpoint of a time interval [82], similarly to what emerged from past studies adopting line bisection tasks. Noteworthy, the same rightward bias has been produced by a TMS over the right parietal cortex [82]. The perception of stimuli duration can be also affected by their location, producing either an underestimation or an overestimation of the duration when presented in the left and in the right hemispaces, respectively [83]. Manipulation of spatial attention by optokinetic stimulation toward the right and left fields produced the same duration distortions [84]. In summary, a variety of psychophysical studies have shown that duration perception can be stretched by other magnitudes, at least to a certain degree. Notwithstanding all this evidence in support of an overlapping between magnitudes, other evidence suggests that the overlapping is only partial. For example, at least for the number and duration domains, it is possible to have dissociations between numerosity and duration functions following different parietal lesions [85], and temporal perception is not affected in adults with developmental dyscalculia [86].
At the single cell level, PF neurons encode space, time and number [24, 87–89], and several theories of the PF cortex have emphasized the domain generality processing of PF [90–92]. These considerations led us to investigate timing and spatial representations at the single cell level in PF recording the same neurons in the duration and in the distance discrimination tasks. An additional matching-to-sample (MTS) task served as a control for goal representations. We need to point out that our experiment [43], that we will describe later in this chapter, was not designed to distinguish between the ATOM and the MTL proposals. Its main objective was to investigate the role of PF in decision making within different magnitudes, studied in separate tasks and in absence of any interference between magnitudes.
Common Goal but Separate Decision Signals for Duration and Distance
Figure 2b, c illustrate the MTS and the distance tasks used in addition to the duration task.
We adopted as a control task a particular type of MTS task in which the presentation of the “sample” stimulus was repeated twice (Fig. 2b). In this task, the monkeys were required to choose the same stimulus presented as the sample on that trial (Fig. 2b). We introduced this control task to identify potential neurons sharing a common goal representation. Its main feature was that the goal choice did not depend on any magnitude comparison process. The first and second sample durations were the same as in the duration task but their duration difference was irrelevant to the task. The task was designed to preserve the same task events that characterized the duration task, such as each epoch’s duration and the fixation requirement.
In the distance task on each trial, the monkey viewed two visual stimuli, presented sequentially, on a video screen at different distances from a reference point at screen center. In this task the two stimuli differed in relation to their distance from the reference point and not in their duration. The monkey’s task was to choose the stimulus farther from the central reference point.
We recorded data from 1,209 neurons in the duration discrimination and from 1,671 neurons in the distance discrimination task (Fig. 2c). We recorded 621 neurons from both tasks and 261 neurons from all three tasks, and these subpopulations will be used in the comparison among tasks.
In this part of the chapter, we focus our task comparisons on a decision period immediately after the decision point. The decision point is defined as the moment in time in which the information available would suffice for an ideal observer to reach a decision. The decision point in the distance discrimination task corresponded to the presentation of S2. In the duration discrimination task, to define the decision point we need to distinguish two categories of trials based on which stimulus, either the first or the second, lasted longer. When S2 was shorter than S1, the decision on which stimulus had lasted longer could be made at S2 offset, and this was defined as the decision point for these trials. When S2 lasted longer, the decision point was not “marked” by the S2 offset, but corresponded instead to the moment at which the S2 duration surpassed that of the S1.
As we have already described earlier in this chapter, which focused on the second delay period, for the decision period we classified (two-way ANOVA) the relative duration and the relative distance neurons in two classes, one encoding the relative duration or distance based on the order of the two stimuli and the other based on their features. In the MTS task, for the same task period as in the duration task, we identified goal-selective neurons modulated by the stimulus features (blue and red) of the two samples (one-way ANOVA).
Figure 3a shows an example of a neuron encoding relative duration and relative distance information based on the order of presentation of the two stimuli. This neuron shows a phasic increase of activity in the decision period when the first stimulus was the longest (S1 of greater magnitude) in the duration task and the closest (S2 of greater magnitude) in the distance task, therefore this neuron represents an example of a neuron contributing to different cognitive domains. However, looking at this neuron’s activity, it is apparent that it did not reflect any abstract concept of magnitude. This is because the preference for which stimulus was the longest (higher magnitude) reversed to the smaller magnitude (closer) in the spatial domain. Neurons with a relative duration or distance encoding based on the stimulus order should not be confused with rank-order neurons. Rank-order neurons, in fact, are characterized by differences in activity between the first and the second stimulus. It is likely that the relative encoding signal shown in Fig. 3a might arise from the combination of duration and rank-order information. Figure 3b shows a rank-order neuron with greater activity elicitated by the presentation of S1 compared to that elicitated by the presentation of S2. The rank-order signal was maintained in the first delay, and it is possible that such neurons activated by the S1 presentation could lead later to the activation of other rank-order neurons with a preference for the second stimulus when the second stimulus is turned on. Without this information might not be possible to determine when the duration of the stimulus presented should be compared with the duration of the previous one. Notice that the neuron in Fig. 3b shows a further level of integration that goes beyond a “pure” rank-order signal, consisting of an additional modulation by the color of first stimulus, which was absent during the presentation of the second stimulus. In other words, this cell was specifically tuned to the presentation of a first red stimulus.
To asses at the population level whether the type of encoding based on the stimulus order shown by the neuron in Fig. 3a represented a domain-general signal common to duration and distance computations, we compared the neural selectivity of the same neurons in different tasks.
Figure 4a shows a bar plot for neurons with significant encoding in the duration task only, the distance task only, or in both tasks, based on order of presentation of the two stimuli. We found that the majority of the neurons encoded the decision in each domain independently (two-way ANOVA), as indicated in Fig. 4a by the red and green bars, respectively in the distance and the duration tasks. Only a relatively small percentage of neurons (26 %) indicated by the blue bar participated to the decision process in both domains. We asked whether this last group of neurons could represent abstractly the relative magnitudes in a domain-general way. We found neurons sharing the same preference (dark blue bar), as predicted for neurons representing magnitude in the abstract, but we found them in roughly the same proportion as the neurons with opposite preferences (light blue bar). Therefore, the cell preference in one task appeared independent of that in the other. Although there was no complete dissociation of functions between neurons for distance and duration comparisons and the same neuron could participate to both computations, there was no tendency to share a common preferred magnitude.
Bar plot counting the neurons specific to one (red or green) or to both tasks (blue), with neurons having the same preference summed in the dark-colored bar and neurons with different preferences summed in the light-colored bar. (a) Neurons encoding relative information based on order. (b) Neurons encoding relative information based on the stimulus features in the format of a. (c) Subpopulation of cells in (b) recorded in all three tasks. The Venn diagram shows the number of cells with significant effects in all the various combinations of the tasks. Percentages refer to the cells showing the same preference (red or blue) in either two or three overlapping tasks
In addition to the relative duration and distance encoding signals based on the order of presentation of the two stimuli, we also compared the relative encoding based on the stimulus features between tasks. We will examine now the proprieties of the neurons encoding which stimulus had the greatest magnitude in the duration and distance tasks based on the stimuli features, but first we start by examining the different type of neurons that could be expected.
Figure 5 shows the activity of four ideal neurons characterized by different proprieties. In this Fig. 5, all the four ideal neurons are chosen for having the greatest activity for the red longer stimuli. Examining the activity of the neuron of Fig. 5a in the distance task it appears that it shows an opposite preference in the two tasks and for this reason it could not represent any common magnitude. The neuron of Fig. 5b is not involved in distance comparisons because is a pure duration neuron. The neurons of Fig. 5c, d show the same preference for red stimuli of greater magnitude, making them potential candidates for representing a domain-general signal. However, after examining the activity of these neurons in the MTS task, it is apparent that only the neuron of Fig. 5c shows a true domain-general signal, while the neuron of Fig. 5c represents the red goal. In fact, only for the neuron of Fig. 5c the preference for red longer and farther stimuli cannot not be accounted in terms of red goal encoding. Now we will examine two example PF neurons in relationship to the categories defined for the ideal neurons. Figure 3c shows a neuron that encoded the relative duration based on the stimulus features during the decision period of the duration task. The neuron preferred the longest blue stimulus, but did not show any selectivity for which stimulus was the farthest in the distance task, resembling the ideal neuron shown in Fig. 5b.
Four ideal neurons modulated by the relative duration based on the stimulus features in the duration task. All the four ideal neurons show the highest activity for the red longer stimulus (a). This neuron shows an opposite preference in the distance discrimination task, with higher activity for the blue farthest stimulus, therefore, this neuron could not encode which stimulus had greater magnitude in a domain-general way. (b) Similarly to the neuron in (a), this ideal neuron cannot not encode domain-general information, but in this case it is because it is not modulated in the distance discrimination task. (c) Neuron showing a domain-general coding of relative magnitude. For this neuron the preference for the red stimulus of greater magnitude in both tasks could not be accounted in terms of goal encoding, because this neuron does not show higher activity for the red goal in the MTS task. (d) Neuron encoding the red goal. For this neuron, the red goal encoding in the MTS sample task can account for the preference for red longer and farther stimuli in the duration and distance tasks. On the top an example trial for each task, blue lines indicate trials with blue longer stimuli, blue farther stimuli, and blue samples, respectively in the duration, distance, and MTS tasks
Figure 3d shows a different type of neuron with a preference for red longer trials. The same neuron, when tested in the distance discrimination task, showed a similar relative distance encoding, with a preference for red farther stimuli. Therefore, not only was this neuron part of the comparison of both magnitudes, but it also shared the same preference. That is, it seemed to encode for “red-greater” in both tasks. To distinguish between the two possibilities exemplified by the neurons of Fig. 5c, d, we need to examine the activity in the MTS task. Was the neuron of Fig. 3d encoding “red greater” as a domain-general abstract signal, independent of goal encoding, or was it just encoding the red goal? Examining the activity of this neuron in the MTS task helps answering this question. Figure 3d (bottom) in fact shows that the same neuron maintained its preference in the MTS task. That is, it encoded the red goal, a finding that supports the second interpretation: this cell encoded a goal signal as exemplified by the ideal neuron in Fig. 5d.
Then we asked what proportion of neurons encoded goals, similarly to the ideal neuron represented in Fig. 5d and to the neuron of Fig. 3d, rather than abstract magnitude as for the ideal neuron of Fig. 5c. Figure 4b shows the relative encoding in the duration and distance tasks based on the stimulus features. As with the duration encoding based on the order of presentation, the majority of neurons encoded relative magnitude in one domain only, either for space (red bar) or for time (green bar), with only a third of neurons (blue bar) encoding it in both tasks (Fig. 4b). However, in contrast to the neurons of Fig. 4a, all of the neurons modulated in both tasks (blue bar in Fig. 4b), with rare exceptions, shared the preference for the same stimulus as the stimulus of greater magnitude (dark blue bar). We then tested the subpopulation of these neurons studied also in the MTS task for goal effects. We found that all these neurons, with rare exceptions, when studied in the MTS task, shared the same preference as in the duration task (see Fig. 4c) supporting the idea that neurons encoding which stimulus was farther and longer based on the stimulus features in both tasks encoded the goal chosen by the monkey. Note that without the MTS control task, we might have interpreted the activity of these neurons as an example of common magnitude encoding.
Examining the time course of the population activity averages of the three classes of neurons, it appeared that both the population of neurons encoding the perceptual decision on the relative magnitude only in one task based on either the order of presentation or their stimulus features showed a signal that dissipated earlier than the goal encoding neurons (see Fig. 3 in Genovesio et al. [43]) supporting a role in the initial decision process that lead to the goal selection.
Modality-Specific and Modality-Generality in a Strategy Task
The difference between the domain-specific activities and the domain-general goal neural activities and their time course, with the first leading to the second in the PF cortex, in some respects can be considered analogous to the difference between modality-specific and modality-general activities described in a previous study in monkeys [93], which used a strategy task to study PFdl, the orbitofrontal cortex, and the frontal pole cortex. In that task, a cue instructed one of two strategies: stay with the previous response or shift to the alternative. The cue could be either one visual stimulus or a specific reward amount. We compared the activity in two version of the task using different reward amounts and different visual stimuli as strategy cues.
We found that the spatial goal coding during the period of the strategy cue presentation was modality-specific, with the spatial goal preference (right or left) independent of the cue modality. Later in the delay period, the neurons transitioned from a modality-specific response to a modality-general response, one sharing the preference for the same position.
In contrast to the goal encoding, we did not find any correlation between the preferred strategies in the two tasks with different cue modalities. Therefore, strategy encoding appeared modality-specific in PFdl (and also in orbitofrontal cortex), in contrast to the modality-general goal encoding found after an initial modality-specific encoding.
The role of the PFdl in the generation of goals has been emphasized by several neurophysiological studies in monkeys [88, 94–96] as mentioned earlier. Furthermore, several brain-imaging studies in humans have confirmed goal encoding in PFdl. For example, Rowe et al. [97] have shown that self generated finger movements as opposed to externally dictated movements activated PFdl, and Jahanshahi et al. [98] have shown that the generation of more random numbers produced more activation in PFdl.
Conclusions
Several brain-imaging studies have implicated a parietal-frontal network in a domain-general representation of magnitudes [1, 8, 9, 62, 99, 100], and, as we have already discussed, cross-modal interference has been shown between several domains, such as spatial and temporal [1, 58, 61, 72, 74, 79, 84, 101].
Notwithstanding these studies, our findings suggest that considering the representation and the comparison of magnitudes as a unitary process can be an oversimplification. In our experiment, we have focused on the relative encoding of magnitudes such as “greater than” and “less than” rather than on the absolute magnitude codes, such as “large” and “small”. We asked whether a neuron that encoded “greater than” in the duration task also encoded “farther than” in the distance task. We have not yet examined whether a neuron that encodes a “long” duration encodes also a “far” stimulus. To our knowledge, no other neurophysiological study has addressed the study of common magnitude in terms of relative coding. Tudusciuc and Nieder [102] have addressed the coding of absolute magnitude in monkeys in the context of numerosity and spatial length. Adopting a delayed matching-to-sample task design, they have described neurons that encoded absolute magnitudes either in only one domain or in both domains in both PF and in the ventral intraparietal area (VIP). However, it is not clear whether the neurons encoding both magnitudes shared the same preference for numerosity and line length. Moreover, their task did not require relative magnitude comparisons, such as “greater than” and “less than”. Being domain-specific instead of domain-general, however, does not contradict with the role of PF in generating other, more abstract representations within each magnitude. For example, we have described for the same experiment a highly abstract coding of the relative distance [103], which was independent of the location of the two stimuli presented (above or below the reference point). In our experiment, we have identified domain-specific perceptual processing at the single-cell level. These neurons were located in the same PF location with no clear separation [43]. Therefore, it is not surprising that brain-imaging studies would detect common activations for different magnitudes because the activity of different classes of neurons overlaps within the same voxels. The differences between imaging data and ours might also be reconciled by assuming that the neurons encoding goals were shared by the duration and the distance domains. The presence of a goal representation was not surprising in view of the many previous studies that have reported such representations in PF [88, 94, 95, 104] and it is possible that the psychophysical interaction across cognitive domains occurs at the level of goal choices, rather than at the level of perceptual decisions.
Moreover, we have shown that spatial and temporal computations tended to share also a common representation in terms of left/right goal [43] or response and the level of action can represent another source of interactions among magnitudes. Our results are in line with the original idea of the ATOM proposal that suggests that the development of magnitude processing originates from the interaction with the external world through action to which it is strictly associated [1]. Several past studies have supported this proposal [105, 106]. In accordance with this idea, it has been shown that semantic information labelled on target objects such as “LARGE” and “SMALL” can affect the grip opening [105]. The grip aperture was larger when the objects were labelled with “LARGE” than when they were labelled with “SMALL”. Numbers can similarly affect action: large numbers speed up the grip opening and small numbers speed up the grip closing [106]. To summarize, the neuronal population that encodes spatial goals and responses identified in PF might generate interference between different magnitudes and actions by serving as a shared resource for choosing among different options.
We cannot rule out the possibility that there is a domain-general representation in other parts of the brain, and the parietal cortex might be a candidate [1] and it has been shown [107] that some parietal cortex neurons represent the same rule in both spatial and numerical domains. Parietal cortex has also been proposed as an important node of a timing network [20, 52, 108, 109]. In support of a parietal representation of magnitudes, the parietal cortex has been found to be activated in brain-imaging studies by tasks that require orienting attention to spatial locations and time intervals [110] and by collision tasks that required the integration of spatial and temporal information to predict the collision [111]. However, as we have shown in PF, these brain-imaging studies in parietal cortex also cannot determine whether or not different networks of neurons participate in coding different magnitudes.
To better understand what is shared among magnitude representations, we should consider the distributed representation of time, where different computations can share resources with other magnitudes with different degree of overlapping. Following this idea, it is useful to identify the different stages in the processing of magnitude information. First, there could be a partial overlap of computational resources for different magnitudes in the parietal cortex, in which different magnitudes have been hypothesized to share a common representational format along a common mental, spatially organized line. Second, based on our results, we can assume that there is an additional level of resource sharing for goal and response representations in PF. In between the two stages, we have identified a dissociation of functions between neurons comparing magnitudes based on the order and on the features of stimuli for distances and durations.
Our results suggest that future experiments should address the study of magnitudes and their interplay by fractioning magnitude comparison in different computational steps, as we have started to do with our study. Considering a common magnitude representation as a unique system, although distributed through several brain areas, is apparently an oversimplification.
At the same time, we cannot rule out the possibility that domain-general representations in the comparison process might vary flexibly, based on whatever task demands happen to prevail at any given time, and especially when subjects are required to formulate cross-domain judgments.
In summary, in the context of the scalar timing theory [112], which postulates a model with different clock, memory and comparison modules, we have shown that the “comparison timing module” hypothesized by the model appears to be specific to durations.
In a comprehensive theory of the PF cortex, it has been proposed by Wise and Passingham [113] that the main role of PF is generating goals or sequence of goals based on the current context and the current needs. The current context can include information relative to different magnitudes, such as duration, distance, number and the order of the events. In a recent extension of the theory that includes the posterior parietal cortex, Genovesio et al. [114] have proposed that new prefrontal- posterior parietal cortex networks evolved in anthropoids as a specialization for rapid learning of what foraging goals to choose based on relative metrics, with the information about the relative metric provided by the parietal cortex. According to this view, this network that evolved in anthropoid primates to improve foraging choices served as a pre-adaptation for the development of human reasoning and intelligence.
References
Walsh VA. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn Sci. 2003;7(11):483–8.
Lejeune H, Maquet P, Bonnet M, Casini L, Ferrara A, Macar F, et al. The basic pattern of activation in motor and sensory temporal tasks: positron emission tomography data. Neurosci Lett. 1997;235(1–2):21–4.
Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, et al. Brain activation induced by estimation of duration: a PET study. Neuroimage. 1996;3(2):119–26.
Nenadic I, Gaser C, Volz HP, Rammsayer T, Hager F, Sauer H. Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp Brain Res. 2003;148(2):238–46.
Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci. 2013;36:313–36.
Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. 1998;18(3):1085–95.
Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res. 1998;7(1):15–39.
Onoe H, Komori M, Onoe K, Takechi H, Tsukada H, Watanabe Y. Cortical networks recruited for time perception: a monkey positron emission tomography (PET) study. Neuroimage. 2001;13(1):37–45.
Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4(3):317–23.
Danckert J, Ferber S, Pun C, Broderick C, Striemer C, Rock S, et al. Neglected time: impaired temporal perception of multisecond intervals in unilateral neglect. J Cogn Neurosci. 2007;19(10):1706–20.
Kagerer FA, Wittmann M, Szelag E, Steinbüchel N. Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia. 2002;40(3):357–66.
Koch G, Oliveri M, Carlesimo GA, Caltagirone C. Selective deficit of time perception in a patient with right prefrontal cortex lesion. Neurology. 2002;59(10):1658–9.
Jones CR, Rosenkranz K, Rothwell JC, Jahanshahi M. The right dorsolaterale prefrontal cortex is essential in time reproduction: an investigation with repetitive transcranial magnetic stimulation. Exp Brain Res. 2004;158(3):366–72.
Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003;60(11):1844–6.
Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, et al. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43(3):396–417.
Trivino M, Correa A, Arnedo M, Lupianez J. Temporal orienting deficit after prefrontal damage. Brain. 2010;133(4):1173–85.
Vallesi A, Mussoni A, Mondani M, Budai R, Skrap M, Shallice T. The neural basis of temporal preparation: insights from brain tumor patients. Neuropsychologia. 2007;45(12):2755–63.
Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb Cortex. 2007;17(2):466–74.
Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 1979;171(2):213–24.
Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci. 2005;8(2):234–41.
Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38(2):317–27.
Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci. 2006;9(7):48–55.
Schneider B, Ghose GM. Temporal production signals in parietal cortex. PLoS Biol. 2012;10(10):e1001413.
Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. J Neurophysiol. 2006;95(5):3281–5.
Lebedev MA, O’Doherty JE, Nicolelis MA. Decoding of temporal intervals from cortical ensemble activity. J Neurophysiol. 2008;99(1):166–86.
Ohmae S, Lu X, Takahashi T, Uchida Y, Kitazawa S. Neuronal activity related to anticipated and elapsed time in macaque supplementary eye field. Exp Brain Res. 2008;184(4):593–8.
Oshio K, Chiba A, Inase M. Delay period activity of monkey prefrontal neurones during duration-discrimination task. Eur J Neurosci. 2006;23(10):2779–90.
Oshio K, Chiba A, Inase M. Temporal filtering by prefrontal neurons in duration discrimination. Eur J Neurosci. 2008;28(11):2333–43.
Sakurai Y, Takahashi S, Inoue M. Stimulus duration in working memory is represented by neuronal activity in the monkey prefrontal cortex. Eur J Neurosci. 2004;20(4):1069–80.
Tsujimoto S, Sawaguchi T. Neuronal activity representing temporal prediction of reward in the primate prefrontal cortex. J Neurophysiol. 2005;93(6):3687–92.
Brody CD, Hernandez A, Zainos A, Romo R. Timing and neural encoding of somatosensory parametric working memory in macaque prefrontal cortex. Cereb Cortex. 2003;13(11):1196–207.
Renoult L, Roux S, Riehle A. Time is a rubberband: neuronal activity in monkey motor cortex in relation to time estimation. Eur J Neurosci. 2006;23(11):3098–108.
Mita A, Mushiake H, Shima K, Matsuzaka Y, Tanji J. Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat Neurosci. 2009;12(4):502–7.
Lucchetti C, Bon L. Time-modulated neuronal activity in the premotor cortex of macaque monkeys. Exp Brain Res. 2001;141(2):254–60.
Merchant H, Zarco W, Pérez O, Prado L, Bartolo R. Measuring time with different neural chronometers during a synchronization-continuation task. Proc Natl Acad Sci U S A. 2011;108(49):19784–9.
Merchant H, Pérez O, Zarco W, Gámez J. Interval tuning in the primate medial premotor cortex as a general timing mechanism. J Neurosci. 2013;33(21):9082–96.
Kilavik BE, Confais J, Ponce-Alvarez A, Diesmann M, Riehle A. Evoked potentials in motor cortical local field potentials reflect task timing and behavioral performance. J Neurophysiol. 2010;104(5):2338–51.
Confais J, Kilavik BE, Ponce-Alvarez A, Riehle A. On the anticipatory pre-cue activity in motor cortex. J Neurosci. 2012;32(15):15359–68.
Chiba A, Oshio K, Inase M. Striatal neurons encoded temporal information in duration discrimination task. Exp Brain Res. 2008;186(4):671–6.
Matell MS, Meck WH, Nicolelis MA. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci. 2003;117(4):760–73.
Portugal GS, Wilson AG, Matell MS. Behavioral sensitivity of temporally modulated striatal neurons. Front Integr Neurosci. 2011;5:30.
Genovesio A, Tsujimoto S, Wise SP. Feature- and order-based timing representations in the frontal cortex. Neuron. 2009;63(2):254–66.
Genovesio A, Tsujimoto S, Wise SP. Encoding goals but not abstract magnitude in the primate prefrontal cortex. Neuron. 2012;74(4):656–62.
Roesch MR, Olson CR. Neuronal activity dependent on anticipated and elapsed delay in macaque prefrontal cortex, frontal and supplementary eye fields, and premotor cortex. J Neurophysiol. 2005;94(2):1469–97.
Tsujimoto S, Sawaguchi T. Neuronal representation of response-outcome in the primate prefrontal cortex. Cereb Cortex. 2004;14(1):47–55.
Tsujimoto S, Genovesio A, Wise SP. Comparison of strategy signals in the dorsolateral and orbital prefrontal cortex. J Neurosci. 2011;31(12):4583–92.
Genovesio A, Brasted PJ, Mitz AR, Wise SP. Prefrontal cortex activity related to abstract response strategies. Neuron. 2005;47(2):307–20.
Genovesio A, Tsujimoto S, Wise SP. Encoding problem-solving strategies in prefrontal cortex: activity during strategic errors. Eur J Neurosci. 2008;27(4):984–90.
Hoshi E, Tanji J. Area-selective neuronal activity in the dorsolateral prefrontal cortex for information retrieval and action planning. J Neurophysiol. 2004;91(6):2707–22.
Tsujimoto S, Genovesio A, Wise SP. Monkey orbitofrontal cortex encodes response choices near feedback time. J Neurosci. 2009;29(8):2569–74.
Yumoto N, Lu X, Henry TR, Miyachi S, Nambu A, Fukai T, et al. A neural correlate of the processing of multi-second time intervals in primate prefrontal cortex. PLoS One. 2011;6(4):e19168.
Jin DZ, Fujui N, Graybiel AM. Neural representation of time in cortico-basal ganglia circuits. Proc Natl Acad Sci U S A. 2009;106(45):19156–61.
Ninokura Y, Mushiake H, Tanji J. Integration of temporal order and object information in the monkey lateral prefrontal cortex. J Neurophysiol. 2004;91(1):555–60.
Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behav Brain Res. 1997;84(1–2):203–23.
Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol. 2008;18(2):145–52.
Hanakawa T, Honda M, Sawamoto N, Okad T, Yonekura Y, Fukuyama H, et al. The role of rostral Brodmann area 6 in mental operation tasks: an integrative neuroimaging approach. Cereb Cortex. 2002;12(11):1157–70.
Lebedev MA, Wise SP. Tuning for the orientation of spatial attention in dorsal premotor cortex. Eur J Neurosci. 2001;13(5):1002–8.
Casasanto D, Boroditsky L. Time in the mind: using space to think about time. Cognition. 2008;106(2):579–93.
Mitchell CT, Davis R. The perception of time in scale model environments. Perception. 1987;16(1):5–16.
Basso G, Nichelli P, Frassinetti F, Di Pellegrino G. Time perception in a neglected space. Neuroreport. 1996;7(13):2111–4.
Merritt DJ, Casasanto D, Brannon EM. Do monkeys think in metaphors? Representations of space and time in monkeys and humans. Cognition. 2010;117(2):191–202.
Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol. 2003;20(3):487–506.
Hubbard EM, Piazza M, Pinel P, Dehaene S. Interactions between number and space in parietal cortex. Nat Rev Neurosci. 2005;6(6):435–48.
Fischer MH, Castel AD, Dodd MD, Pratt J. Perceiving numbers causes spatial shifts of attention. Nat Neurosci. 2003;6(6):555–6.
Perrone G, de Hevia MD, Bricolo E, Girelli L. Numbers can move our hands: a spatial representation effect in digits handwriting. Exp Brain Res. 2010;205(4):479–87.
Nicholls ME, McIlroy AM. Spatial cues affect mental number line bisections. Exp Psychol. 2010;57(4):315–9.
Lavidor M, Brinksman V, Göbel SM. Hemispheric asymmetry and the mental number line: comparison of double-digit numbers. Neuropsychologia. 2004;42(14):1927–33.
Brunamonti E, Falcone R, Genovesio A, Costa S, Ferraina S. Gaze orientation interferes with mental numerical representation. Cogn Process. 2012;13(4):375–9.
Brunamonti E, Genovesio A, Carbè K, Ferraina S. Gaze modulates non-propositional reasoning: further evidence for spatial representation of reasoning premises. Neuroscience. 2011;173:110–5.
Zorzi M, Priftis K, Umiltà C. Brain damage: neglect disrupts the mental number line. Nature. 2002;417(6885):138–9.
Doricchi F, Guariglia P, Gasparini M, Tomaiuolo F. Dissociation between physical and mental number line bisection in right hemisphere brain damage. Nat Neurosci. 2005;8(12):1663–5.
Gallistel CR, Gelman II. Non-verbal numerical cognition: from reals to integers. Trends Cogn Sci. 2000;4(2):59–65.
Srinivasan M, Carey S. The long and the short of it: on the nature and origin of functional overlap between representations of space and time. Cognition. 2010;116(2):217–41.
Morrone MC, Ross J, Burr D. Saccadic eye movements cause compression of time as well as space. Nat Neurosci. 2005;8(7):950–4.
Burr DC, Ross J, Binda P, Morrone MC. Saccades compress space, time and number. Trends Cogn Sci. 2010;14(12):528–33.
Casasanto D, Fotakopoulou O, Boroditsky L. Space and time in the child’s mind: evidence for a cross-dimensional asymmetry. Cogn Sci. 2010;34(3):387–405.
Mendez JC, Prado L, Mendoza G, Merchant H. Temporal and spatial categorization in human and non-human primates. Front Integr Neurosci. 2011;5:50.
Javadi AH, Aichelburg C. When time and numerosity interfere: the longer the more, and the more the longer. PLoS One. 2012;7(7):e41496.
Xuan B, Zhang D, He S, Chen X. Larger stimuli are judged to last longer. J Vis. 2007;7(10):2.1-5.
Meck WH, Church RM. A mode control model of counting and timing processes. J Exp Psychol Anim Behav Process. 1983;9(3):320–34.
Di Bono M, Casarotti K, Priftis L, Gava C, Umiltà M, Zorzi M. Priming the mental time line. J Exp Psychol Hum Percept Perform. 2012;38(4):838–42.
Oliveri M, Koch G, Salerno S, Torriero S, Gerfo EL, Caltagirone C. Representation of time intervals in the right posterior parietal cortex: implications for a mental time line. Neuroimage. 2009;46(4):1173–9.
Vicario CM, Pecoraro P, Turriziani P, Koch G, Caltagirone C, Oliveri M. Relativistic compression and expansion of experiential time in the left and right space. PLoS One. 2008;3(3):e1716.
Vicari CM, Caltagirone C, Oliveri M. Optokinetic stimulation affects temporal estimation in healthy humans. Brain Cogn. 2007;64(1):68–73.
Cappelletti M, Freeman ED, Cipolotti L. Numbers and time doubly dissociate. Neuropsychologia. 2011;49(11):3078–92.
Cappelletti M, Freeman ED, Butterworth B. Time processing in dyscalculia. Front Psychol. 2011;2:364.
Nieder A, Freedman DJ, Miller EK. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297(5587):1708–11.
Genovesio A, Brasted PJ, Wise SP. Representation of future and previous spatial goals by separate neural populations in prefrontal cortex. J Neurosci. 2006;26(27):7305–16.
Lebedev MA, Messinger A, Kralik JD, Wise SP. Representation of attended versus remembered locations in prefrontal cortex. PLoS Biol. 2004;2(11):1919–35.
Baars BJ, Ramsøy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26(12):671–5.
Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14(4):172–9.
Wilson CR, Gaffan D, Browning PG, Baxter MG. Functional localization within the prefrontal cortex: missing the forest for the trees? Trends Neurosci. 2010;33(12):533–40.
Tsujimoto S, Genovesio A, Wise SP. Neuronal activity during a cued strategy task: comparison of dorsolateral, orbital, and polar prefrontal cortex. J Neurosci. 2012;32(32):11017–31.
Rainer G, Rao SC, Miller EK. Prospective coding for objects in primate prefrontal cortex. J Neurosci. 1999;19(13):5493–505.
Tsujimoto S, Genovesio A, Wise SP. Transient neuronal correlations underlying goal selection and maintenance in prefrontal cortex. Cereb Cortex. 2008;18(12):2748–61.
Kusunoki M, Sigala N, Gaffan D, Duncan J. Detection of fixed and variable targets in the monkey prefrontal cortex. Cereb Cortex. 2009;19(11):2522–34.
Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex. 2005;15(1):85–95.
Jahanshahi M, Dirnberger G, Fuller R, Frith CD. The role of the dorsolateral prefrontal cortex in random number generation: a study with positron emission tomography. Neuroimage. 2000;12(6):713–25.
Fias W, Lammertyn J, Reynvoet B, Dupont P, Orban GA. Parietal representation of symbolic and nonsymbolic magnitude. J Cogn Neurosci. 2003;15(1):47–56.
Pinel P, Piazza M, Le Bihan D, Dehaene S. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41(6):983–93.
Magnani B, Oliveri M, Mancuso G, Galante E, Frassinetti F. Time and spatial attention: effects of prism adaptation on temporal deficits in brain damaged patients. Neuropsychologia. 2011;49(5):1016–23.
Tudusciuc O, Nieder A. Contributions of primate prefrontal and posterior parietal cortices to length and numerosity representation. J Neurophysiol. 2009;101(6):2984–94.
Genovesio A, Tsujimoto S, Wise SP. Prefrontal cortex activity during the discrimination of relative distance. J Neurosci. 2011;31(11):3968–80.
Saito N, Mushiake H, Sakamoto K, Itoyama Y, Tanji J. Representation of immediate and final behavioral goals in the monkey prefrontal cortex during an instructed delay period. Cereb Cortex. 2005;15(10):1535–46.
Glover S, Rosenbaum DA, Graham J, Dixon P. Grasping the meaning of words. Exp Brain Res. 2004;154(1):103–8.
Andres M, Davare M, Pesenti E, Olivier X, Seron X. Number magnitude and grip aperture interaction. Neuroreport. 2004;15(18):2773–7.
Eiselt AK, Nieder A. Representation of abstract quantitative rules applied to spatial and numerical magnitudes in primate prefrontal cortex. J Neurosci. 2013;33(17):7526–34.
Battelli L, Walsh V, Pascual-Leone A, Cavanagh P. The ‘when’ parietal pathway explored by lesion studies. Curr Opin Neurobiol. 2008;18(2):120–6.
Bueti D, Walsh V. The parietal cortex and the representation of time, space, number and other magnitudes. Philos Trans R Soc Lond B Biol Sci. 2009;364(1525):1831–40.
Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18(18):7426–35.
Assmus A, Marshall JC, Ritzl A, Noth J, Zilles K, Fink GR. Left inferior parietal cortex integrates time and space during collision judgments. Neuroimage. 2003;20(1):S82–8.
Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann N Y Acad Sci. 1984;423:52–77.
Wise SP, Passingham RE. The neurobiology of the prefrontal cortex: anatomy, evolution, and the origin of insight (Oxford psychology series). Oxford: Oxford University Press; 2012.
Genovesio A, Wise SP, Passingham RE. Prefrontal-parietal function: from foraging to foresight. Trends Cogn Sci. 2014;18(2):72–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Genovesio, A., Tsujimoto, S. (2014). From Duration and Distance Comparisons to Goal Encoding in Prefrontal Cortex. In: Merchant, H., de Lafuente, V. (eds) Neurobiology of Interval Timing. Advances in Experimental Medicine and Biology, vol 829. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1782-2_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1782-2_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1781-5
Online ISBN: 978-1-4939-1782-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)