Abstract
B lymphocytes are the source of pathogen-specific and neutralizing antibodies (Abs), and they also regulate the development of lymphoid tissue and the activity of T cells and other hematopoietic cell types. Hematopoietic humanized mice (hu-mice) represent a relevant experimental tool to explore basic and translational mechanisms of human B-cell development, B-cell activation, and B-cell function. These animals readily generate human B cells in high proportion relative to other hematopoietic lineages. All stages of B-cell maturation can be found in hu-mice, including naïve, activated, IgM memory, and class-switched B cells, and furthermore these mice produce significant amounts of secreted immunoglobulin. However, the majority of B cells only progress to the immature/transitional stage suggesting that only some of the factors necessary for human B-cell maturation are present and functional in the mouse environment. Thus, in addition to providing a model for understanding human B-cell responses to infections and vaccines and for testing B-cell-specific therapies, the hu-mouse also affords a great opportunity to learn which cell types and molecules are critical for B-cell maturation and function. Here, we review the kinetics, characteristics, and limitations of human B-cell development in hu-mice and summarize what these uniquely translational animal models have taught us about human B-cell biology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- B cell
- B-cell development
- B-cell maturation
- Humanized mice
- Transitional B cell
- Human immunoglobulin
- Antibody response
- Umbilical cord blood
- Xenotransplantation
- BAFF
1 Introduction
B lymphocytes play a versatile role in the immune system’s fight against infections. These cells are the source of pathogen-specific and neutralizing antibodies (Abs) as well as a memory storage of previous pathogen encounters. Some B cells are also “innately” capable of quickly activating a response to a pathogen by secreting low-affinity Abs that weaken the infection before more specific responses are achieved. Furthermore, B cells present antigen and produce cytokines and chemokines that regulate T cells and other blood cell types to coordinate the immune response during the early and late stages of infection. Therefore, B cells are an essential component of an effective and sustained immune response against microbes including viruses such as HIV.
Recent HIV studies have demonstrated that human B cells do have the ability to produce HIV-specific Abs that broadly neutralize most viral clades [1–3]. As an effective HIV vaccine remains elusive, studies aimed at investigating the basis of rare protective anti-HIV Ab response are critical to direct successful immunization strategies. Within this context, hematopoietic humanized mice (hu-mice) represent a relevant experimental tool to explore basic and translational mechanisms of human B-cell development, B-cell activation, and Ab responses. Immunodeficient mice transplanted with human hematopoietic stem cells develop a human immune system that roughly recapitulates the establishment and complexity of the natural system. In this animal model, human B cells, T cells, NK cells, monocytes, and dendritic cells differentiate from the donor hematopoietic stem cells and together coordinate the development and function of the immune system. These chimeric mice are useful, therefore, not only to study how human B cells respond to a vaccine or an infectious agent, but also to discover and characterize the immune factors (cellular or otherwise) that influence the development of these responses. Moreover, since each set of hu-mice is generated from distinct human stem cell donors, these mice also provide an experimental tool to study the effects of natural human genetic variation on B cells.
This chapter will review the kinetics, properties, and limitations of human B-cell development in hu-mice and will discuss how the hu-mouse model can be further improved to support enhanced B-cell numbers and function.
2 Development of Human B Cells in Mice
The bone marrow is the physiological site of B-cell production in mice and humans. Human B cells similarly develop in the bone marrow of hu-mice with clearly identifiable maturational stages [4–8]. Within human B cells developing in the mouse, Ig gene rearrangements display normal kinetics with the light chain following the heavy chain ([8]; and Lang and Pelanda, unpublished observations). Moreover, these rearrangements result in a peripheral B-cell population with a roughly normal lambda:kappa light chain ratio (about 1:1) ([9]; Lang and Pelanda, unpublished observations).
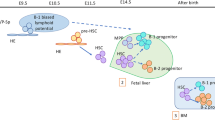
CD19, a marker restricted to the B-cell lineage, is first expressed on pro-B cells and is retained on the surface of B cells throughout maturation before being downmodulated on plasma cells. The expression of the B-cell receptor (BCR) components and isotypes defines the CD19+ bone marrow cells as precursor (pre,H-chain only), immature (H and L chains, IgM), transitional/naive (IgM and IgD), and switched memory (IgA, IgE, or IgG) B cells [10, 11]. Throughout human B-cell development, expression of cell-surface “markers” guides our definition of B-cell stages ([10, 12–17]; Fig. 12.1). For the majority of these markers the absolute expression level varies as the B-cell progresses from an immature through the transitional stage to a mature, naive B cell, with the transitional stage generally presenting an intermediate level of expression [10, 12–14, 18]. The careful selection and measurement of a panel of these markers is required for the proper analysis and definition of B-cell development [13–14, 19]. Using such analyses, we and others have determined that CD34+CD10+ lymphocyte precursors in the bone marrow of hu-mice proceed through normal B-cell development with clearly identifiable pro, pre, immature, and transitional stages of maturation and with a modality that is largely similar to B cells developing in human bone marrow [4–8, 20].
Markers associated with B-cell development stages in humans. The black, green, and violet cell receptors on pre-B cells, immature B cells, and transitional/mature B cells represent the pre-BCR, IgM, and IgD, respectively. The solid line illustrates normal developmental stages of human B-cell maturation and the dashed line illustrates the defective maturation in the hu-mouse
3 B Cell Tolerance in hu-mice
As in the mouse [21–22], B cells developing in human bone marrow undergo a process of selection (central tolerance) that removes many specificities reacting with local self-antigens [23]. By analyzing the B-cell repertoire of hu-mice, two studies have investigated whether B-cell tolerance operates in the context of this model [24–25]. They found that the prevalence of two autoimmune-associated heavy (VH4-34) and light (Vκ4-1) chains progressively decreases from the immature to the more mature B-cell subsets, suggesting some level of tolerance induction. Inconsistent with the above results though, a Hep-2 antinuclear antibody assay indicated that the frequency of autoreactive B cells does not decrease from the immature to the newly emigrant and mature B-cell stages as it does in humans [24 26]. This suggests that B-cell tolerance might be defective in hu-mice. Nonetheless, such conclusion requires a more formal demonstration. Our lab (in collaboration with David Nemazee, The Scripps Institute) has been investigating central B-cell tolerance by using hu-mice that ubiquitously express an anti-human kappa chimeric protein as a synthetic neo self-antigen specific for all human Igκ+ B cells. Our studies indicate that the developing Igκ+ B cells (~ 50 % of all immature B cells) are for the most part excluded from entering the peripheral B-cell population, a phenotype consistent with the induction of tolerance (Lang and Pelanda, unpublished observations). Thus, future studies are needed to precisely define the prevalence and mechanisms of B-cell tolerance in hu-mice.
4 B Cell Maturation Defect in hu-mice
Unlike the normal development of immature B cells in the bone marrow of hu-mice, a defect has been reported in the differentiation of immature/transitional B cells into naive, mature B cells [27]. For instance, while IgM+ B cells in the bone marrow of hu-mice express CD40, HLA-DR, CD5, CD44, and CD268 and high levels of CD10, CD24, and CD38, which are markers of immature/transitional B cells, they most often do not express CD21 and CD22, which label late transitional and mature B cells in humans [7]. This phenotype is consistent with a B-cell developmental block at an early transitional stage (Fig. 12.1). These transitional B cells make up the majority of the B-cell population in the spleen of hu-mice, particularly the younger ones (see below). Although this B cell defect has been clearly established in several hu-mouse models, one should also consider that B cell poiesis is ongoing not only in the bone marrow, but also at detectable levels in the spleen of many hu-mice [8, 28]. Therefore, the skewed representation of transitional B cells in the spleen may in part be due to the continual generation of these cells in situ. Many studies have also reported that most peripheral human B cells in hu-mice atypically express CD5 and have suggested that hu-mice select for the development of a B1 B cell subset [9, 29]. However, it is important to note that in humans this antigen marks B-cell maturation stages and not necessarily a functional subset [12, 15]. Therefore, the expression of CD5 on B cells of hu-mice is more likely related to the fact that most of these cells are immature rather than B1.
5 Mature B Cells Accumulate in Lymph Nodes (LNs) and Spleens of Older hu-Mice
Unlike mouse bone marrow chimeras that, within a few weeks, achieve a steady-state engraftment that is maintained for months to even years, the engraftment of the human hematopoietic system within the mouse is dynamic: it takes longer to appear and changes continually over time. This dynamic engraftment has unique characteristics in the various lymphatic tissues: it establishes early in the bone marrow and thymus, is slightly delayed in the blood and spleen, and is greatly delayed in the LNs, where engraftment of mesenteric LNs precedes that of peripheral LNs by weeks (Fig. 12.2, and [7]). Furthermore, chimerism gradually wanes in the bone marrow, and then in blood and spleen, eventually extinguishing the engrafted human immune system.
By studying the kinetics of human hematopoietic engraftment, our group has found that the defect in B-cell maturation in hu-mice is not an absolute inherent inability to progress beyond the transitional stage [7]. The B cells that populate the LNs of hu-mice, in fact, are CD10lo, CD21hi, CD22hi, and CD44hi, which are characteristics of mature B cells. In addition, the LN B cells can be activated, as evident by the presence of IgG class-switched cells and B cells that express CD27 and CD11c memory antigens, as well as CD80, CD86, CD25, and CD69 activation markers.
In contrast to the LN, B cells in the spleen of hu-mice display a range of maturational stages from the predominant transitional to the less frequent mature, activated, and memory class-switched types [7]. This range is well demonstrated by the broad distribution of CD10, CD24, and CD38 expression within the splenic B-cell population of an individual hu-mouse and among multiple hu-mice. Importantly, our studies with BALB/c/Rag2null/Il2rγnull (BRG) hu-mice have demonstrated that the frequency of mature B cells in the spleen increases with time, such that 20 weeks after transplantation more than 75 % of hu-mice have a mature B-cell population (Fig. 12.3a, and [7]). LNs, which harbor mainly mature B cells, are also more frequent in older hu-mice [7] and the increased numbers of mature B cells in these older animals drives higher IgM and IgG concentrations in their sera (Fig. 12.3b). Thus, there exists a “goldilocks” time period for mature B cells in hu-mice, between 16 and 24 weeks after transplant, during which a significant population of mature B cells is present in LNs and spleen, and human hematopoiesis is still ongoing in the bone marrow. Notably, the dynamic characteristic of B-cell maturation appears to be common to hu-mice whether they are generated on a BRG, [5 7, 27] or nonobese diabetic, NOD (NSG or NOG, [4 8, 24, 28–30]; Lang and Pelanda, unpublished observations) genetic background. However, when compared to age-matched BRG mice, NOD recipients have a slightly higher proportion of mature B cells in the spleen (Lang and Pelanda, unpublished observations), most likely as a consequence of higher human chimerism and, therefore, cell numbers [31–34]. Nonetheless, the B cells in the NOD recipients secrete significantly lower human Ig, particularly IgG, suggesting inferior function relative to B cells in BRG mice ([4, 7, 8, 24, 30, 35]; Lang and Pelanda, unpublished observations).
B-cell maturation and function depend on the presence of T cells. a Dynamic engraftment of human lymphocytes in mice. The schematic illustrates the frequency of T and B cells in the spleen following stem cell transplantation. The functional engraftment is also portrayed as the presence of mature B cells and human IgG. b Correlation of T cells with mature B cells in the spleen of hu-mice. Each symbol represents data from an individual mouse. c Human IgM and IgG in sera of individual hu-mice relative to the presence of engrafted LNs in the same mouse. (Panels b and c courtesy of ref. [7]; pp. 2090–210. Copyright 2013. The American Association of Immunologists)
In spite of their defective maturation, B cells are over-represented relative to other hematopoietic cell types, particularly during the first 16 weeks after transplant. This phenotype is thought to be largely contingent on the inefficient function of mouse cytokines on some human hematopoietic cell types [36–39]. B-cell activating factor (BAFF or BlyS) is considered the most relevant cytokine for B cells [40]. In mice, it not only promotes the survival of mature B cells, but it also contributes to Ig class switch and to the differentiation of immature B cells into transitional and mature B cells [41–42]. Mirroring the stages during which BAFF functions, the expression of CD268 (BAFF-R) on B cells begins at the immature stage and progressively increases throughout differentiation into a mature B cell. Similar to B cells in their native hosts, those that develop in hu-mice begin to express CD268 at the immature cell stage, but the expression remains low on all splenic and LN B cells [7]. Schmidt and colleagues have shown that mouse BAFF is abundant in hu-mice and, although it binds human CD268, it fails to properly signal [43], The low CD268 expression on human B cells likely represents internalization of the receptor upon binding large amounts of mouse BAFF. We have detected human BAFF mRNA in tissue of hu-mice at levels that positively correlate with numbers of mature splenic B cells (Lang and Pelanda, unpublished observations). In addition, treatment of hu-PBL mice with human BAFF significantly increases the survival of mature B cells [43], and BAFF injections into hu-PBL and hu-mice result in higher human Ig serum concentrations ([35]; and Lang and Pelanda, unpublished observations). Overall, these data suggest that ectopic expression of human BAFF may improve the survival and perhaps the generation of mature B cells in hu-mice. Efforts are ongoing to develop new recipient mouse strains that express human BAFF with the goal of enhancing B-cell maturation and function.
6 Necessity of T Cells for the Maturation of Human B Cells
The rise of mature B cells in the spleen of hu-mice over time and their unique presence in LNs suggest that the specific human factors that are responsible for this phenotype are produced in a tissue and time-specific manner. T cells were found to gradually accumulate in peripheral lymphoid tissue of hu-mice starting around 12 weeks after transplant (Fig. 12.3a, [7, 44–45]). These T cells slowly accumulate in the spleen and readily populate the LNs along with mature B cells. Notably, we have observed a highly significant correlation between T-cell frequency and mature B cells in the spleen (Fig. 12.3b). Supporting a model in which T cells mediate B-cell maturation, addition of syngeneic, exogenous T cells to hu-mice expedites B-cell maturation, while in vivo depletion of T cells retards this process [7]. Furthermore, addition of T cells to transitional B-cell cultures also mediates B-cell maturation [15].
While detailed reports of B-cell maturation studies in T-cell-deficient mice and humans are not available to our knowledge, some studies have shown that patients with T-cell immunodeficiency display a transitional (cord blood-like) and/or functionally impaired B-cell phenotype [13, 46–47]. The precise mechanism by which T cells contribute to B-cell maturation is an active area of investigation and studies with hu-mice will be particularly useful in this context. Some data suggest that activation of T cells might play a role in the B-cell maturation process [7, 48]. CD40, MHC, and T-cell cytokines are initial relevant candidates, although it remains possible that the effect of T cells is indirect and B-cell maturation requires another cell population that is directly modulated by T cells.
Presently, the correlation between T cell and mature B-cell numbers has practical implications for the use of hu-mice: the frequency of T cells in the peripheral blood can be used to infer the maturation state of B cells and, thus, help select proper animals for experimentation [7]. These findings, moreover, may have important connotations for cord blood transplantation, which now represents more than 25 % of all human hematopoietic transplants due to their increased availability and reduced HLA-match requirements. The reconstitution kinetics of human cord blood transplantation largely mirrors the development of the human immune system in mice and include the delayed T cell appearance that is likely responsible for the unfortunate high-mortality rate associated with infections. The state of B-cell maturation in these patients has not been studied although the B-cell function is known to be reduced [49]. Moreover, a report of improved early B-cell function when cord blood is not depleted of T cells [50] is consistent with a role of T cells in B-cell maturation. Therefore, the hu-mouse can serve as a model system to characterize and test improvements in cord blood transplantations.
7 Human Ab Responses in Mice
Analyses of the Ig heavy- and light-chain usage among the human B cells residing in the mouse have confirmed the presence of a diverse heavy- and light-chain repertoire, a repertoire that is grossly indistinguishable from that of B cells in humans [24]. Nevertheless, Ab responses in hu-mice have been reported to be weak and sporadic [7, 44, 48, 51, 52]. This defect appears to be partly due to the inefficient maturation of B cells because enhanced immunization responses have been observed in older hu-mice, which display higher numbers of mature B cells. This improvement was significant for IgM and IgG responses to a T-independent antigen (e.g., NP-Ficoll) and for IgM responses to a T-dependent antigen (e.g., DTaP) [7, 44]. T-cell-dependent IgG responses were also slightly improved [7], suggesting the presence of both a population of competent B cells capable of Ig class-switch and a productive cognate B–T cell collaboration. However, even in the presence of T cells and mature B cells, the Ab responses to immunogenic challenges in hu-mice remain inferior to those observed in humans and mice. Thus, important factors necessary for normal B-cell function and T–B collaboration are still lacking in hu-mice. Our histological studies noted that although the lymphoid tissue of animals bearing significant numbers of mature B cells displays an increased colocalization of T and B cells, the lymphoid architecture remains abnormal [7], potentially still limiting Ab responses. Another issue relates to the possibility that human thymocytes are educated on mouse MHC instead of human HLA antigens, thus affecting B–T cell cognate interaction. Indeed, supplying a human MHC class II allele [53] or cotransplanting a human thymus [29, 54, 55] enhances T-dependent Ab responses in hu-mice. Nevertheless, these responses remain inferior to those in intact mice and humans leaving this issue only partly resolved.
In humans and mice, preimmune serum is often used as a control for immunization responses, which is appropriate given the steady state of their immune systems. In hu-mice, however, similar to changing B- and T-cell frequencies and function, human Ig levels increase with age and differ greatly among individual chimeras (Fig. 12.3c, and [56]), providing a challenge for the determination of antigen-specific responses. Comparing Ab responses among hu-mice is complicated by two major factors both correlating with sera Ig concentrations: (1) a nonspecific background that is measured even in enzyme-linked immunosorbent assay (ELISA) plates that are not coated with antigen; and (2) a polyreactive response to antigen, most notably of the IgM isotype that is detected in both unimmunized and immunized mice [7]. Thus, the exact measure of Ab responses remains a challenge, emphasizing the need of a well-controlled, standardized assay to allow comparisons of Ab responses in hu-mice among different laboratories.
8 Concluding Remarks
The past decade has witnessed an extensive characterization of human B-cell development in immunodeficient BALB/c and NOD mice. In these models, the human B cells are able to mature, class-switch , and produce Ig to both “natural” antigens and immunological challenges. The development of human B cells in the mouse follows a similar progression as is observed in human bone marrow, albeit with reduced efficiency to mature past the transitional B cell stage. This immature state along with defective T-B collaboration and localization within secondary lymphoid organs are likely responsible for the inferior Ab responses of B cells in hu-mice. Nonetheless, there are already many practical applications of the current hu-mice for B cell studies, including those of Epstein-Barr virus (EBV) infection [57–59], HIV infection [60–61], B-cell depleting therapies [62–63], and production of humanized monoclonal Abs [56].
Our current knowledge of human B-cell development and function in the mouse is useful for both experimental strategies in this system and for practical applications in humans. The hu-mouse model is an excellent system to dissect mechanisms of human B-cell development, including selection and tolerance. Hu-mice generated with fetal or cord blood stem cells may be representative of emerging immune systems, such as the case for newborns, cord blood transplantations, and patients recovering from immunodepletion therapies (i.e., rituximab). It is well established that immunizations in these circumstances are not as effective as in a stable, adult immune system. Thus, this model may be useful to help guide childhood vaccination strategies and treat patients with immunodeficiency, autoimmunity, and transplantation.
References
Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, et al. A blueprint for HIV vaccine discovery. Cell Host Microbe. 2012;12(4):396–407.
Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37(3):412–25.
Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–42.
Choi B, Chun E, Kim M, Kim ST, Yoon K, Lee KY, et al. Human B cell development and antibody production in humanized NOD/SCID/IL-2Rgamma(null) (NSG) mice conditioned by busulfan. J Clin Immunol. 2011;31(2):253–64.
Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004;104(13):3886–93.
Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102(3):873–80.
Lang J, Kelly M, Freed BM, McCarter MD, Kedl RM, Torres RM, et al. Studies of lymphocyte reconstitution in a humanized mouse model reveal a requirement of T cells for human B cell maturation. J Immunol. 2013;190(5):2090–101.
Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice). Int Immunol. 2009;21(7):843–58.
Matsumura T, Kametani Y, Ando K, Hirano Y, Katano I, Ito R, et al. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gammac(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp Hematol. 2003;31(9):789–97.
Perez-Andres M, Paiva B, Nieto WG, Caraux A, Schmitz A, Almeida J, et al. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78(Suppl 1):S47–60.
Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320.
Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–91.
Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176(3):1506–16.
Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105(11):4390–8.
Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5 + pre-naive B cell population. J Immunol. 2009;182(7):4116–26.
Hoffkes HG, Schmidtke G, Schmucker U, Uppenkamp M, Brittinger G. Immunophenotyping of B lymphocytes by multiparametric flow cytometry in bone marrow aspirates of healthy adults. Ann Hematol. 1995;71(3):123–8.
Veneri D, Ortolani R, Franchini M, Tridente G, Pizzolo G, Vella A. Expression of CD27 and CD23 on peripheral blood B lymphocytes in humans of different ages. Blood Transfus. 2009;7(1):29–34.
Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182(10):5982–93.
Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, et al. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal CD138- and CD138 + plasma cells. Haematologica. 2010;95(6):1016–20.
Lang J, Weiss N, Freed BM, Torres RM, Pelanda R. Generation of hematopoietic humanized mice in the newborn BALB/c-Rag2null Il2rgammanull mouse model: a multivariable optimization approach. Clin Immunol. 2011;140(1):102–16.
Pelanda R, Torres RM. Central B-cell tolerance: where selection begins. Cold Spring Harb Perspect Biol. 2012;4(4):a007146.
Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol. 2009;21(6):626–33.
Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632–8.
Chang H, Biswas S, Tallarico AS, Sarkis PT, Geng S, Panditrao MM, et al. Human B-cell ontogeny in humanized NOD/SCID gammac(null) mice generates a diverse yet auto/poly- and HIV-1-reactive antibody repertoire. Genes Immun. 2012;13(5):399–410.
Ippolito GC, Hoi KH, Reddy ST, Carroll SM, Ge X, Rogosch T, et al. Antibody repertoires in humanized NOD-scid-IL2Rgamma(null) mice and human B cells reveals human-like diversification and tolerance checkpoints in the mouse. PLoS ONE. 2012;7(4):e35497.
Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–7.
Vuyyuru R, Patton J, Manser T. Human immune system mice: current potential and limitations for translational research on human antibody responses. Immunol Res. 2011;51(2–3):257–66.
Kim M, Choi B, Kim SY, Yang JH, Roh CR, Lee KY, et al. Co-transplantation of fetal bone tissue facilitates the development and reconstitution in human B cells in humanized NOD/SCID/IL-2Rgammanull (NSG) mice. J Clin Immunol. 2011;31(4):699–709.
Biswas S, Chang H, Sarkis PT, Fikrig E, Zhu Q, Marasco WA. Humoral immune responses in humanized BLT mice immunized with West Nile virus and HIV-1 envelope proteins are largely mediated via human CD5 + B cells. Immunology. 2011;134(4):419–33.
Wang X, Qi Z, Wei H, Tian Z, Sun R. Characterization of human B cells in umbilical cord blood-transplanted NOD/SCID mice. Transpl Immunol. 2012;26(2–3):156–62.
Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, et al. Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011;108(32):13218–23.
Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135(1):84–98.
Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac−/−, Balb/c-Rag1−/−gammac−/−, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70(10):790–802.
Ito M, Kobayashi K, Nakahata T. NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr Top Microbiol Immunol. 2008;324:53–76.
Giassi LJ, Pearson T, Shultz LD, Laning J, Biber K, Kraus M, et al. Expanded CD34 + human umbilical cord blood cells generate multiple lymphohematopoietic lineages in NOD-scid IL2rgamma(null) mice. Exp Biol Med (Maywood). 2008;233(8):997–1012.
Rongvaux A, Willinger T, Takizawa H, Rathinam C, Auerbach W, Murphy AJ, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci U S A. 2011;108(6):2378–83.
Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol. 2011;32(7):321–7.
Rathinam C, Poueymirou WT, Rojas J, Murphy AJ, Valenzuela DM, Yancopoulos GD, et al. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118(11):3119–28.
van Lent AU, Dontje W, Nagasawa M, Siamari R, Bakker AQ, Pouw SM, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2−/−IL-2Rgammac−/− mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183(12):7645–55.
Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237(1):205–25.
Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2(7):465–75.
Rowland SL, Leahy KF, Halverson R, Torres RM, Pelanda R. BAFF receptor signaling aids the differentiation of immature B cells into transitional B cells following tonic BCR signaling. J Immunol. 2010;185(8):4570–81.
Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS ONE. 2008;3(9):e3192.
Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7.
Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, et al. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2−/−gammac−/− mice. J Virol. 2007;81(6):2700–12.
Small TN, Keever C, Collins N, Dupont B, O’Reilly RJ, Flomenberg N. Characterization of B cells in severe combined immunodeficiency disease. Hum Immunol. 1989;25(3):181–93.
Roifman CM, Zhang J, Chitayat D, Sharfe N. A partial deficiency of interleukin-7R alpha is sufficient to abrogate T-cell development and cause severe combined immunodeficiency. Blood. 2000;96(8):2803–7.
Chen Q, He F, Kwang J, Chan JK, Chen J. GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J Immunol. 2012;189(11):5223–9.
Bunin N, Small T, Szabolcs P, Baker KS, Pulsipher MA, Torgerson T. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: persistent immune deficiency in pediatric transplant survivors. Biol Blood Marrow Transplant. 2012;18(1):6–15.
Chiesa R, Gilmour K, Qasim W, Adams S, Worth AJ, Zhan H, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naive CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156(5):656–66.
Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–30.
Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176(4):2053–8.
Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, et al. Expression of HLA class II molecules in humanized NOD. Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS ONE. 2011;6(5):e19826.
Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111(8):4293–6.
Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, et al. A model for personalized in vivo analysis of human immune responsiveness. Sci Transl Med. 2012;4(125):125ra30.
Becker PD, Legrand N, van Geelen CM, Noerder M, Huntington ND, Lim A, et al. Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS ONE. 2010;5(10):e13137.
Sato K, Misawa N, Nie C, Satou Y, Iwakiri D, Matsuoka M, et al. A novel animal model of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in humanized mice. Blood. 2011;117(21):5663–73.
Wahl A, Linnstaedt SD, Esoda C, Krisko JF, Martinez-Torres F, Delecluse HJ, et al. A cluster of virus-encoded microRNAs accelerates acute systemic Epstein-Barr virus infection but does not significantly enhance virus-induced oncogenesis in vivo. J Virol. 2013;87(10):5437–46.
White RE, Ramer PC, Naresh KN, Meixlsperger S, Pinaud L, Rooney C, et al. EBNA3B-deficient EBV promotes B cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest. 2012;122(4):1487–502.
Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol. 2013;25(3):403–9.
Hur EM, Patel SN, Shimizu S, Rao DS, Gnanapragasam PN, An DS, et al. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood. 2012;120(23):4571–82.
Glorius P, Baerenwaldt A, Kellner C, Staudinger M, Dechant M, Stauch M, et al. The novel tribody [(CD20)(2)xCD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia. 2013;27(1):190–201.
Heider KH, Kiefer K, Zenz T, Volden M, Stilgenbauer S, Ostermann E, et al. A novel Fc-engineered monoclonal antibody to CD37 with enhanced ADCC and high proapoptotic activity for treatment of B-cell malignancies. Blood. 2011;118(15):4159–68.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lang, J., Pelanda, R. (2014). Human B-Cell Development in a Mouse Environment. In: Poluektova, L., Garcia, J., Koyanagi, Y., Manz, M., Tager, A. (eds) Humanized Mice for HIV Research. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1655-9_12
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1655-9_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1654-2
Online ISBN: 978-1-4939-1655-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)