Abstract
Acute necrotizing pancreatitis is a complication of acute pancreatitis that carries high morbidity and mortality. The majority of patients have sterile necrosis, which can be managed conservatively. However, in contrast, patients with infected necrosis often require drainage or debridement. Two decades ago, open surgical necrosectomy was the drainage modality of choice but this has largely been replaced by minimally invasive techniques. There have been, controverssies over the use of prophylactic antibiotics to prevent infected necrosis and percutaneous aspiration of necrosis to diagnose infection. This chapter provides an overview of the diagnosis and management of necrotizing pancreatitis. The changes in terminology based on the revised Atlanta classification, current guidelines, as well as the evaluation and management of sterile and infected pancreatic necrosis are discussed in detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute Pancreatitis
- Severe Acute Pancreatitis
- Percutaneous Drainage
- Pancreatic Necrosis
- Necrotizing Pancreatitis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Acute pancreatitis has become the most common reason to be hospitalized for a gastrointestinal disease in the USA, with nearly 275,000 admissions reported in 2009 resulting in a total cost of $2.6 billion [1]. While the majority of admissions are for mild acute interstitial pancreatitis, approximately 5–10 % of patients have acute necrotizing pancreatitis [2–6], with rates of 27–42 % reported in other studies [7, 8]. The discrepancy between rates of necrotizing pancreatitis across studies is likely due to the inclusion of transfer patients. Acute necrotizing pancreatitis is associated with significant mortality, ranging from 10–15 % in sterile pancreatic necrosis and approximately 20–30 % in those with infected pancreatic necrosis [9, 10]. However, mortality can be as high as 40 % in patients with concurrent multi-organ failure [6]. The incidence of infected necrosis in patients with necrotizing pancreatitis is approximately 30–45 % but has been decreasing for unclear reasons, which might include more widespread use and earlier administration of enteral nutrition, improved supportive care for patients with concurrent organ failure, and antibiotic treatment for extrapancreatic infections, which may reduce bacterial seeding of pancreatic necrosis [9, 11–13].
The 1992 Atlanta classification defined necrotizing pancreatitis as a diffuse or focal area of nonviable pancreatic tissue on contrast-enhanced imaging, typically associated with extrapancreatic fat necrosis, with non-enhancing pancreatic parenchyma > 3 cm in length or involving > 30 % of the pancreas [14]. However, over the years, small case series have reported on patients with extensive extrapancreatic necrosis but with preserved pancreatic parenchyma [15–17]. Pancreatic necrosis can involve both the pancreatic parenchyma and extrapancreatic tissues (most common), pancreatic parenchyma alone, or extrapancreatic tissue alone (least common). It is important to recognize that extrapancreatic necrosis alone has a lower mortality compared to parenchymal necrosis [17–19] unless the extrapancreatic necrosis becomes infected [18].
Given the deficiencies of the original Atlanta classification in 1992, particularly with regard to the characterization of pancreatic fluid collections, a revision of the Atlanta classification was undertaken in 2007 through the efforts of several expert pancreatologists and pancreatic societies. (See also Chap. 1.) The revised Atlanta classification was published in 2013 [20]. The revised criteria characterize the pancreatic and extrapancreatic collections that can form in necrotizing pancreatitis. In the first 4 weeks from the onset of symptoms, an acute necrotic collection (ANC) can form. This is defined as a non-organized collection that contains variable quantities of fluid and necrotic debris involving the pancreatic parenchyma and/or extrapancreatic tissues. However, solid debris may not be discernable on a CT scan and this can lead to an incorrect diagnosis of an acute fluid collection (AFC), which forms in the context of acute interstitial pancreatitis. Walled-off pancreatic necrosis (WOPN) is a mature, encapsulated collection consisting of variable quantities of solid necrotic tissue. Approximately 1–9 % of patients with acute necrotizing pancreatitis will develop WOPN in 4–6 weeks after the onset of symptoms [20]. On contrast-enhanced CT (CECT), WOPN is defined as a heterogeneous collection with liquid and nonliquid densities, and varying degrees of loculations, some of which can appear homogenous (Fig. 3.1). Both ANC and WOPN can become infected.
Diagnosis
Imaging
Cross-sectional imaging with CECT, or magnetic resonance imaging (MRI), is the imaging modality of choice for diagnosing necrotizing pancreatitis. (See also Chap. 6.) These imaging studies not only determine the presence and extent of necrosis but also local complications, including pseudoaneurysm, duodenal or biliary obstruction, presence of air bubbles indicating infection, and splanchnic thrombosis. CECT remains the gold standard for the diagnosis of pancreatic necrosis (Fig. 3.2). However, it can take several days for pancreatic necrosis to appear on imaging since the pancreas can often appear heterogeneous early in the course of disease. Over the first week, the area(s) of impaired perfusion become more demarcated on CECT. Dynamic CECT is currently recommended after 72–96 h of symptoms if a complication is suspected. Perfusion CT is another imaging modality used to diagnose necrotizing pancreatitis. However, unlike dynamic CTs, smaller amounts of contrast material (40–50 mL) are injected at a higher rate (4–10 mL/s) and at higher concentrations (350–370 mg/kg). Images of the pancreas are then obtained at multiple times. Perfusion CT software is used to calculate perfusion parameters and arterial input function. Perfusion CT has been shown to have a sensitivity of 100 % and specificity of 95.3 % for demonstrating pancreatic necrosis within 72 h of symptom onset but is not in widespread clinical use [21]. The advantage to using CECT includes its widespread availability, rapid scanning, and the ability to detect pancreatic necrosis. Disadvantages include exposure to ionizing radiation, contrast-induced nephrotoxicity, and inability to reliably detect necrotic debris in an ANC or WOPN [22].
MRI can also be used to diagnose pancreatic necrosis. Advantages to MRI include lack of ionizing radiation exposure and the ability to distinguish pancreatic necrosis without the administration of gadolinium using fat-suppressed T1-weighted images, which can be useful in patients with renal insufficiency. In addition, T2-weighted MRI is superior to CT for the evaluation of necrotic debris within pancreatic collections and extrapancreatic fat necrosis. Figure 3.3 shows T2- and T1-weighted MRI images of pancreatic necrosis. Figure 3.4 compares CT and MRI image of WOPN. MRCP also has the ability to delineate a pancreatic ductal disruption and evaluate for bile duct stones. Disadvantages include cost, lack of widespread availability, longer acquisition times, poor patient tolerance, and the contraindication of metallic foreign bodies, which includes coils and pacemakers [22–25].
Development of Infection
Infection of pancreatic necrosis most commonly occurs 2–4 weeks after the onset of acute pancreatitis, or at any point after the development of necrotizing pancreatitis [26, 27]. Pancreatic infection in patients with necrotizing pancreatitis is due to increased intestinal permeability and decreased immunity that occurs during severe acute pancreatitis, termed “gut barrier dysfunction,” which results in the translocation of bacteria. Besselink et al. found that 72 (46.8 %) out of 154 patients with pancreatic parenchymal necrosis developed infected necrosis over a median of 26 days after admission [26]. This high rate of infected necrosis; however, may be partially explained by contamination after fine-needle aspiration (FNA), since all patients with suspected infected necrosis underwent FNA. Bacteremia was shown to be a risk factor in the development of infected necrosis (65 % versus 37.9 %, p = 0.002). In 21 out of 51 patients, who had both bacteremia and infected necrosis, the same pathogen was isolated from both cultures of the blood and pancreatic necrosis. They also reported that patients with extensive necrosis (>30 %) had a higher risk of developing infected necrosis [26]. Other studies have also shown a correlation between the presence and extent of pancreatic necrosis and infection [8, 12, 28].
Diagnosis of Infection
Infected pancreatic necrosis should be suspected if there is progressive clinical deterioration as evidenced by persistent systemic inflammatory response syndrome (SIRS) and/or worsening organ failure [29, 30]. According to the most recent American College of Gastroenterology guidelines on management of acute pancreatitis, infected necrosis should be considered in patients with pancreatic or extrapancreatic necrosis who clinically decline or fail to improve after 7–10 days of hospitalization [31]. In a subset of patients, CT imaging will reveal air bubbles within a collection, which suggests the presence of gas forming organisms or the development of a fistulous tract between a pancreatic collection and the stomach, small bowel, or colon [29]. However, the presence of gas within pancreatic tissue occurs in a minority of patients (Fig. 3.5).
Approximately 25 % of patients presenting with acute pancreatitis develop extrapancreatic infections [26, 32]. Clinical studies have shown that infection of the pancreatic bed is the result of seeding from extrapancreatic infections, most commonly from the bloodstream [26, 33]. These extrapancreatic infections are more often polymicrobial compared to pancreatic infections that are monomicrobial [34]. Gram-negative bacteria are the predominant cultured organisms in pancreatic necrosis. However, the incidence of gram-positive organisms and yeast has been increasing, which is potentially due to the widespread use of broad spectrum antibiotics [33, 35–37]. Candida species are the most common isolated fungus in patients with necrotizing pancreatitis, followed by Torulopsis [38]. Studies have also revealed increased mortality in patients with pancreatic necrosis who develop fungal infection with Candida [37, 39].
There has been controversy with the routine use of FNA for diagnosing infected pancreatic necrosis [40, 41]. CT- or ultrasound-guided FNA has been shown to be a safe, effective, and accurate technique for diagnosing infected necrosis [42–44]. While the detection of infected necrosis can guide therapy and the appropriate use of antibiotics based on a sensitivity profile of organism(s) cultured from the aspirate, some argue that even if the aspirate is positive, the patient should not undergo intervention until 3–4 weeks after onset of disease, as debridement is then preferably delayed. The only widely accepted indication for early debridement is clinical deterioration. In addition, aspiration is not very accurate, with a reported sensitivity of 88 % and specificity of 90 % [43]. If the aspirate is negative for infection but the patient experiences clinical deterioration, then debridement is still indicated.
Management of Sterile and Infected Necrosis
The treatment of necrotizing pancreatitis has changed over the last two decades. Historically, patients with sterile and infected necrosis underwent open surgical necrosectomy at early stages of disease [45]. In recent years, the indication, timing, and approaches towards intervention have changed. With the advent of minimally invasive techniques, the mortality of patients with necrotizing pancreatitis has further decreased [9]. Figure 3.6 displays an algorithm summarizing the approach to intervention in necrotizing pancreatitis based on current evidence.
Aggressive but conservative supportive therapy is the mainstay treatment for patients with acute necrotizing pancreatitis. Aggressive intravenous fluid resuscitation is required to maintain adequate intravascular volume and end-organ perfusion. (See also Chap. 8.) The controversy lies in what is considered “aggressive” resuscitation. Despite the fact that this is recommended universally in the guidelines of experts and professional societies, there are few randomized trials to guide clinical decision making. One trial utilized a 2 × 2 factorial design where 40 patients were equally randomized to type of fluid administered as well as rate of infusion. The authors demonstrated significantly reduced C-reactive protein levels and prevalence of SIRS at 24 h in those randomized to lactated Ringer’s compared to normal saline but found no difference in the rates of infusion due to a possible crossover effect [46]. Two retrospective studies demonstrated that early aggressive fluid resuscitation is associated with lower rates of SIRS, organ failure, and length of stay [47]. The primary criticism of retrospective studies is the concept of “reverse causation,” where increased fluid was administered to patients with a greater severity of illness [48]. However, caution must be observed since aggressive resuscitation is associated with adverse outcomes due to third spacing of fluid. One study demonstrated that rapid hemodilution can increase the incidence of sepsis within 28 days and inhospital mortality in patients with severe acute pancreatitis [49]. There have not been any studies to date demonstrating that aggressive fluid resuscitation results in a reduced incidence of pancreatic necrosis. Supportive care for the treatment of organ failure should ideally be provided in intensive care units. Early nutritional support with enteral feeding is critical for providing sufficient caloric intake and to maintain the gut barrier, which reduces septic complications, including infected pancreatic necrosis, thereby reducing mortality, multi-organ failure, and the need for surgical intervention [50–52].
Sterile Necrosis
There has been a paradigm shift in the treatment of sterile necrosis. In patients with sterile necrosis and organ failure, surgical debridement was associated with increased mortality [53]. According to a recent consensus conference on necrotizing pancreatitis, sterile ANC do not necessitate early intervention [54]. Sterile asymptomatic WOPN also does not require intervention, as these collections can resolve spontaneously, although the rate of spontaneous resolution is not known. However, the presence of symptoms, including persistent abdominal pain and/or mechanical obstruction, e.g., gastric outlet obstruction or biliary obstruction, development of infection as well as increase in size of WOPN necessitates drainage and the methods used for drainage are similar to those used for infected necrosis.
Use of Prophylactic Antibiotics
The use of prophylactic antibiotics has been controversial. (See also Chap. 9.) Clinical trials in the 1970s did not show improvement in mortality with the use of prophylactic antibiotic use in patients with acute pancreatitis. However, these studies were criticized for the inclusion of patients with mild disease. In the 1990s, the use of prophylactic antibiotics was revisited with the advent of new antibiotics against enteric organisms. Studies showed an improvement in mortality in those presenting with acute necrotizing pancreatitis with the use of prophylactic antibiotics [55, 56]. However, in 2009, a large randomized multicenter trial of prophylactic antibiotics in 276 patients with pancreatic necrosis revealed no difference in the rates of infected necrosis, mortality, and operative necrosectomy [57]. Prior smaller randomized controlled trials revealed similar findings [27, 58]. De Vries et al. [59] evaluated the methodologic quality of randomized controlled trials of antibiotic prophylaxis in patients with severe acute pancreatitis in relation to their outcome. They showed an inverse relationship between the methodological quality and the impact of antibiotic prophylaxis [59]. A Cochrane review of the literature evaluated seven randomized controlled studies consisting of 404 patients found no benefit of antibiotics in preventing infected necrotizing pancreatitis or mortality. However, they reported that there was less mortality and less infected pancreatic necrosis in those receiving beta-lactams antibiotic prophylaxis, although this was not statistically significant [60]. Studies have also revealed that the use of prophylactic antibiotics, which include beta-lactams, have been associated with secondary fungal infections, as well as the selection of multiresistant organisms [35, 36, 61]. In these studies, the prevalence of secondary fungal infection ranged from 11–32 % [37, 61]. Based on the current literature, the use of prophylactic antibiotics in patients with pancreatic necrosis is not recommended. In clinical practice, if infection is suspected, it is reasonable to initiate antibiotics after obtaining blood and urine cultures as well as radiographs and when the results of these investigations become available, the decision to continue or discontinue antibiotics can be made accordingly.
Infected Necrosis
Prior to 1998, surgical management using open necrosectomy was the standard of care for managing infected pancreatic necrosis. In 1998, Freeny et al. [62] reported resolution of sepsis in 47 % of patients with infected necrosis after aggressive percutaneous drainage using multiple catheter(s) and lavage. However, the remaining 53 % of patients required an open necrosectomy and mortality was reported to be 12 % in the total cohort. In the last decade, data have suggested that patients with infected necrosis can be treated conservatively without compromising prognosis [63]. Early management of infected necrosis is similar to conservative approach of sterile necrosis in addition to antibiotics that penetrate the pancreas, e.g., carbapenems, quinolone, metronidazole, and high dose cephalosporins [12, 28]. Amphotericin B and fluconazole are appropriate antifungal agents, although amphotericin is considered first-line.
Open Necrosectomy
Open surgical necrosectomy, which was routinely performed early in the course of disease in order to remove infected pancreatic necrosis, has been associated with high morbidity and mortality as well as long-term pancreatic exocrine and endocrine insufficiency [64, 65]. (See also Chap. 16.) Throughout the years, the management of infected necrosis has been modified in several ways. First, it is now known that waiting 3–4 weeks after the onset of disease is associated with decreased complications as this allows for the encapsulation of ANCs into WOPN, which will improve conditions for intervention [66, 67]. Second, taking the “less is more approach” has been supported by several studies. A recent study by Garg and colleagues [68] compared conservative therapy to surgical therapy in 80 patients with infected necrotizing pancreatitis. Conservative therapy was defined as the use of antibiotics, enteral nutrition, support of organ failure, and percutaneous drainage of organized or walled-off collections if needed. Surgical therapy was defined as those who were treated with surgical necrosectomy, lavage, and drainage. Patients underwent surgical intervention if they deteriorated despite aggressive conservative therapy. The mortality rates in patients who went to surgery immediately was 43 %, compared to a mortality rate of 28 % in patients whom were treated with conservative approach (p = 0.22) A recent meta-analysis of eight studies comprising 324 patients revealed that 64 % of patients treated with the conservative approach had successful outcomes and a mortality of 12 % [69]. Third, as an alternative to open necrosectomy, minimally invasive approaches have become more accepted. These include percutaneous catheter drainage, minimally invasive retroperitoneal necrosectomies, including video-assisted retroperitoneal debridement (VARD), and endoscopic transluminal necrosectomy.
Percutaneous Drainage
The goal of percutaneous drainage is to drain infected fluid from an ANC or WOPN. Percutaneous drainage of pancreatic and extrapancreatic necrosis involves placement of single or multiple catheters that are typically upsized, irrigated, and manipulated. Freeny et al. [62] were the first to describe the treatment of acute necrotizing pancreatitis in 34 patients with image-guided percutaneous drainage as an alternative to surgical intervention. They used multiple large-bore catheters with vigorous irrigation to achieve successful percutaneous necrosectomy. The authors found that this approach resulted in postponing surgical intervention by median of 4 weeks, and prevented the need for surgery altogether in 47 % of patients. However, their approach required multiple procedures over time to achieve these outcomes. A recent systematic review of 11 studies with 384 patients evaluating percutaneous drainage for treatment of sterile and infected necrotizing pancreatitis found that 56 % of cases were successfully treated with percutaneous drainage and did not require surgical necrosectomy [70]. The size of the percutaneous drains inserted varied from 8 to 28 Fr. Recent prospective studies have confirmed these findings [9].
Percutaneous drainage is a simple procedure. It can be used in situations where a collection or a portion of a collection cannot be accessed endoscopically (e.g., left paracolic gutter extension). It can be used in critically ill patients as a bridge to surgery. It can also be used as a bridge to other minimally invasive surgical procedures. Disadvantages include limited access to the head collections, the necessity for multiple drain exchanges due to drain occlusion and/or repositioning, limited ability to remove necrotic material, and the development of fistulas between the collection and the drain tract exit site [38].
Minimally Invasive Retroperitoneal Necrosectomy
Minimally invasive retroperitoneal necrosectomy includes sinus tract endoscopy, laparoscopic transabdominal necrosectomy, and VARD. (See also Chap. 15.)
Sinus tract endoscopy involves serial dilations of tracts that have formed from previously placed percutaneous catheters under fluoroscopy in the operating room, followed by jet irrigation and lavage using an endoscope or nephroscope. Solid necrotic material is removed with an endoscope. This technique was initially reported by Carter et al. [71] and later by Connor et al. [72]. Mortality has been reported to range from 0 % to 25 % with a median of four procedures performed on each patient with infected necrosis [72].
VARD was initially described by van Santvoort et al. [73]. A percutaneous drain is initially placed in the (peri-) pancreatic collection through the left retroperitoneum. If there is no clinical improvement, then a 5-cm subcostal incision is made near the exit point of the percutaneous drain. The percutaneous drain is followed deeper into the necrotic collection. Under direct videoscopic visualization, further debridement is performed using a laparoscopic forceps. Advantages include the use of both the endoscopic and open approach, as well as removal of larger quantities of necrotic material when compared to the sinus tract endoscopy, thus reducing repeat procedures. However, disadvantages include the exposure to ionizing radiation in the operating room as well as increased costs [73]. A recent prospective multicenter study evaluating the safety and efficacy of VARD reported bleeding and enteric fistulas in 7.5 % and 17.5 %, respectively, and a 30-day mortality of 2.5 % [74]. Since VARD utilizes percutaneous drainage, it carries the potential complication of an external pancreatic fistula. In addition, its use is limited in those with necrosis involving the head of the pancreas, where the application of percutaneous drainage is not amenable through the retroperitoneal approach.
Endoscopic Necrosectomy
In the 1996, Baron et al. [75] described an endoscopic method for draining WOPN employing a transmural approach through the posterior gastric wall or the medial wall of the duodenum. See also Chap. 14. A needle knife sphincterotome was utilized to gain access to the collection. The tract was then dilated using a hydrostatic balloon followed by the insertion of two 10-Fr, 3-cm double pigtail stents into the collection. Saline irrigation was performed in patients who developed infected necrosis using a nasobiliary tube. Over the years, the approach has been modified. Subsequently in 2000, Seifert et al. [76] published a case report of three patients using the direct retroperitoneal endoscopic approach to debride the necrotic pancreas. In the series of the three patients, transmural puncture created a fenetration, which was then dilated with 16-mm balloon, allowing for the advancement of the therapeutic gastroscope into the cavity. Endoscopic debridement was achieved using lavage and electrocautery [76]. The approach of direct entry into the necrotic cavity is known as direct endoscopic necrosectomy [77–79]. When compared to the conventional transmural endoscopic drainage for the treatment of WOPN, direct endoscopic necrosectomy achieved high rates of resolution, shorter length of hospitalization, and reduced rate of cavity recurrence [78].
With the advent of newer endoscopic techniques and modalities [74], the EUS-guided approach has been adopted to localize a site from the posterior gastric wall or medial wall of the duodenum to reduce the risk of complications and improve success rates [80, 81]. Endoscopic placement of transmural stents have been used to create a temporary fistula for drainage of pancreatic collections. Over the years, these stents have been modified. Prior studies used plastic stents for drainage of WOPN [78]. However, these stents are susceptible to obstruction, migration [82], and ineffective drainage, particularly for WOPN [83]. Belle et al. [84] reported a case report on their experience of using a partially covered self-expanding metal stent (SEMS) in patients with WOPN. The SEMS creates a wide diameter outflow tract for the drainage of solid debris and provides a port of access for further endoscopic interventions. Since then, several case reports detailing the use of fully covered SEMS in patients with WOPN have been promulgated [85, 86]. A novel fully-covered metal stent, named AXIOS, with bilateral flanges and a wider diameter, has been developed [87]. It has been reported to have easy deployment, and its large diameter permits faster drainage and allows for therapeutic interventions [88], which may include direct endoscopic necrosectomies.
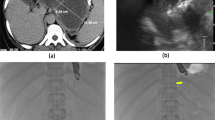
The GEPARD study [81] was the first study to report the long-term outcomes of patients who undergo direct endoscopic necrosectomy. This retrospective study included 93 patients in six centers in Germany with mean follow-up of 6 years. These patients had infected WOPN and underwent endoscopic transmural necrosectomy every 1–4 days until the removal of all necrotic material. The authors reported an initial clinical success in 80 % of patients, and of these patients, 84 % had sustained clinical improvement after mean follow-up period of 43 months, and 10 % needing further endoscopic intervention. Major complications were seen in 26 % of cases, which included bleeding in 14 %, perforation in 6 %, air embolism in 2 %, and mortality in 7.5 % at 30 days. Gardner et al. [79] reported the results of the largest multicenter study evaluating direct endoscopic necrosectomy. A total of 104 patients underwent direct endoscopic necrosectomy for WOPN with the insertion of an endoscope across the cystgastrostomy or cystduodenostomy tract and removal of the necrotic debris. The overall success was reported to be 91.3 % in resolution of the WON and the mean duration to cavity resolution after initial resolution was 4.1 months. Complications included bacteremia/ infection (27 %), bleeding (20 %), perforation (13 %), and pneumoperitoneum (20 %). This large study showed that direct endoscopic necrosectomy is a successful alternative to surgical or percutaneous debridement for the management of WOPN. The disadvantages of this approach include the need for several procedures for successful debridement, the time-consuming nature of the procedure, and the need for specialized endoscopic expertise. Figure 3.7 demonstrates endoscopic drainage and necrosectomy. Figure 3.8 demonstrates endoscopic resolution of the necrotic collection.
Endoscopic drainage and necrosectomy. (a) Transmural puncture is performed under EUS guidance and a guidewire is advanced into the pancreatic cavity with the use of fluoroscopy. (b) Fluoroscopic evaluation of the guidewire into the fluid collection. (c, d) Balloon dilation of the tract with a 12- to 15-mm CRE balloon advanced over guidewire under fluoroscopic guidance in a 54-year-old male with acute necrotizing pancreatitis of unclear etiology. The waist of the balloon (arrows) defines the site of the cystgastrostomy. (e) Fully covered 22-mm diameter × 60-mm long esophageal metal (Tae Woong Medical) stent placed across the cystgastrostomy into the necrotic collection
The PENGUIN (Pancreatitis, Endoscopic Transgastric vs Primary Necrosectomy in Patients with Infected Necrosis) trial was a prospective, randomized trial evaluating 22 patients with infected WOPN who underwent percutaneous catheter drainage. If this failed, patients were randomized to endoscopic transgastric or surgical necrosectomy. The surgical necrosectomy consisted of VARD, or if not feasible, then laparotomy. They found that endoscopic transgastric necrosectomy was associated with significantly reduced IL6 levels, multi-organ failure, and external pancreatic fistulas when compared to the surgical necrosectomy [89].
Combined percutaneous and endoscopic drainage of WOPN was described by Ross et al. [90]. Patients in this study initially underwent a CT-guided placement of a percutaneous drainage catheter into the WOPN to remove necrotic debris. The catheters were irrigated three times a day. The patients were immediately transferred to the endoscopic suite where an endoscopic transmural drainage was performed with the utilization of two transenteric double-pigtail stents. The cystgastrostomy fistula redirects the pancreatic secretions into the small bowel. The combined drainage technique avoids the utilization of large-diameter balloon dilation of the cystenterostomy, thereby reducing the risk of hemorrhage and free perforation into the peritoneum. The authors also reported a low rate of endoscopic reintervention with this approach, as well as absence of chronic pancreaticocutaneous fistula formation, which has been shown in patients with central gland necrosis and percutaneous drains [90]. This approach is associated with reduced length of hospitalization, radiological procedures, and number of ERCPs when compared to those who underwent percutaneous drainage only [91, 92]. However this approach may be limited to only a few centers nationwide, given that the coordination of percutaneous drainage through interventional radiology and endoscopic drainage immediately after may be difficult to arrange.
Step-up Approach
A landmark RCT performed by the Dutch Acute Pancreatitis Study Group [93] compared a minimally invasive technique with open necrosectomy. The inclusion criteria for this study were stringent. After screening 378 patients, 88 patients with confirmed or suspected infected pancreatic necrosis were randomized to percutaneous drainage versus open necrosectomy. For those randomized to PD, if there was no clinical improvement in 72 h and if the position of the drains were inadequate, then a second drainage procedure would take place. If there was no clinical improvement in 72 h, then a “step-up” approach would include VARD with postoperative lavage or endoscopic drainage. The patients randomized to the step-up approach had lower rates of multisystem organ failure, major complications such as diabetes and need for pancreatic enzyme supplementation when compared to the open necrosectomy group. Mortality was not different between the two groups; however, the study was not powered to demonstrate a difference in mortality rates. This study has shifted the treatment paradigm away from invasive surgery and towards a minimally invasive approach for patients with infected ANCs [93].
Conclusion
There have been great advances in the diagnosis and management of necrotizing pancreatitis over the last decade. The first was the revised Atlanta classification, which refines the characterization of ANCs and WOPN. The second are the various modalities for the diagnosis of infected and sterile necrosis, which include dynamic CECT and MR imaging. The third is the shift in the management paradigm from surgical to conservative and minimally invasive approaches with the goal of delaying intervention until a collection becomes organized. Conservative management with intravenous fluids and enteral feedings continue to be the mainstay of therapy for patients with sterile and infected necrosis. Prophylactic antibiotics are not recommended in patients with pancreatic necrosis. In patients with sterile necrosis who remain asymptomatic, no intervention is required. However, patients who develop symptoms or infection, warrant intervention. The decision as to which approach to intervention to pursue should be guided by the presence of the adequate surgical, endoscopic, and/or radiological expertise. The optimal management of an infected ANC requires the minimally invasive step-up approach, which consists of percutaneous drainage initially followed by endoscopy and/or minimally invasive retroperitoneal necrosectomy if necessary. This has been associated with reduced rates of complications. Endoscopic therapy alone using direct necrosectomy or large-bore transmural metal stents has largely become the mainstay of therapy for patients with symptomatic and/or infected WOPN.
Conflict of interest. Vikesh Singh is a consultant for Abbvie, Santarus, D-Pharm, Novo Nordisk and Boston Scientific. The other authors have no disclosures.
References
Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87.
Wilson C, McArdle CS, Carter DC, Imrie CW. Surgical treatment of acute necrotizing pancreatitis. Br J Surg. 1988;75:1119–23.
Wall I, Badalov N, Baradaria R, Iswara K, Li JJ, Tenner S. Decreased mortality in acute pancreatitis related to early aggressive hydration. Pancreas. 2011;40:547–50.
De Campos T, Cequeira C, Kuryura L, Parreira JG, Solda S, Perlingeiro JA, et al. Morbimortality indicators in severe acute pancreatitis. JOP. 2008;3:690–7.
de Beaux AC, Palmer KR, Carter DC. Factors influencing morbidity and mortality in acute pancreatitis: an analysis of 279 cases. Gut. 1995;37:121–6.
Perez A, Whang EE, Brooks DC, Moore Jr FD, Hughes MD, Sica GT, et al. Is severity of necrotizing pancreatitis increased in extended necrosis and infected necrosis? Pancreas. 2002;25:229–33.
Mier J, Leon E, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173:71–5.
Buchler MW, Gloor B, Muller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–25.
van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmen Ali U, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254–63.
Petrov MS, Shanbhaq S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–20.
Isenmann R, Rau B, Heger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg. 1999;86:1020–4.
Garg PK, Khanna S, Bohidar NP, Kapil A, Tandon RK. Incidence, spectrum, and antibiotic sensitivity pattern of bacterial infections among patients with acute pancreatitis. J Gastroenterol Hepatol. 2001;16:1055–9.
Ashley SW, Perez A, Pierce EA, Brooks DC, Moore Jr FD, Whang EE, et al. Necrotizing pancreatitis: contemporary analysis of 99 consecutive cases. Ann Surg. 2001;234:572–9.
Bradley 3rd EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg. 1993;128:586–90.
Howard JM, Wagner SM. Pancreatography after recovery from massive pancreatic necrosis. Ann Surg. 1989;209:31–5.
Madry S, Fromm D. Infected retroperitoneal fat necrosis associated with acute pancreatitis. J Am Coll Surg. 1994;178:277–82.
Sakorafas GH, Tsiotos GG, Sarr MG. Extrapancreatic necrotizing pancreatitis with viable pancreas: a previously under-appreciated entity. J Am Coll Surg. 1999;188:643–8.
Bakker OJ, van Santvoort H, Besselink MG, Boermeester MA, van Eijck C, Dejong K, et al. Extrapancreatic necrosis without pancreatic parenchymal necrosis: a separate entity in necrotizing pancreatitis. Gut. 2013;62:1475–80.
Singh VK, Bollen TL, Wu BU, Repas K, Maurer R, Yu S, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol. 2011;9:1098–103.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11.
Tsuji Y, Tamamoto H, Yazumi S, Watanabe Y, Matsuda K, Yamamoto H, Chiba T. Perfusion computerized tomography can predict pancreatic necrosis in early stages of severe acute pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1484–92.
Morgan DE. Imaging of acute pancreatitis and its complications. Clin Gastroenterol Hepatol. 2008;6:1077–85.
Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatoography with outcome. Radiology. 1999;211:727–35.
Pamuklar E, Semelka RC. MR imaging of the pancreas. Magn Reson Imaging Clin N Am. 2005;13:313–30.
Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715–23.
Besselink MG, van Santvoort HC, Boermeester MA, et al. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267–73.
Dellinger EP, Tollado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674–83.
Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis a perspective clinical study. Gastroenterology. 1986;91:433–8.
Rau BM, Bothe A, Kron M, Beger HG. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol. 2006;4:1053–61.
van Brunschot S, Bakker OJ, Besselink MG, Bollen TL, Fockens P, Gooszen HG, et al. Treatment of necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2012;10:1190–201.
Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15.
Bourgaux JF, Defez C, Muller L, Vivancos J, Prudhomme M, Navarro F, et al. Infectious complications, prognostic factors and assessment of anti-infectious management of 212 consecutive patients with acute pancreatitis. Gastroenterol Clin Biol. 2007;31:431–5.
Behrman SW, Bahr MH, Dickson PV, et al. The microbiology of secondary and postoperative pancreatic infections: implications for antimicrobial management. Arch Surg. 2011;146:613–9.
Noor MT, Radhakrishna Y, Kocchar R, Kocchar R, Wig JD, Sinha SK, et al. Bacteriology of infection in severe acute pancreatitis. JOP. 2011;12:19–25.
Kocchar R, Ahammed SKM, Chakrabarti A, Ray P, Sinha SK, Dutta U, et al. Prevalence and outcome of fungal infection in patients with severe acute pancreatitis. J Gastroenterol Hepatol. 2009;24:743–7.
Gloor B, Muller CA, Worni M, Stahel PF, Redaelli C, Uhl W, et al. Pancreatic infection in severe pancreatitis: the role of fungus and multiresistent organisms. Arch Surg. 2001;136:592–6.
Connor S, Alexakis N, Neal T, Raraty M, Ghaneh P, Evans J, et al. Fungal infections but not type of bacterial infection is associated with a high mortality in primary and secondary infected pancreatic necrosis. Dig Surg. 2004;21:297–304.
Trikudanathan G, Navaneethan U, Vege SS. Intra-abdominal fungal infections complicating acute pancreatitis: a review. Am J Gastroenterol. 2011;106:1188–92.
Isenmann R, Schwarz M, Rau B, Trautmann M, Schober W, Beger HG. Characteristics of infection with Candida species in patients with necrotizing pancreatitis. World J Surg. 2002;26:372–6.
Banks PA. Pro: computerized tomographic fine needle aspiration (CT-FNA) is valuable in the management of infected pancreatic necrosis. Am J Gastroenterol. 2005;100:2371–2.
Pappas TN. Con: computerized tomographic aspiration of infected pancreatic necrosis: the opinion against its routine use. Am J Gastroenterol. 2005;100:2373–4.
Gerzog SG, Banks PA, Robbins AH, Johnson WC, Spechler SJ, Wetzner SM, et al. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology. 1987;93:1315–20.
Rau B, Pralle U, Mayer JM, Beger HG. Role of ultrasonographically guided fine-need aspiration cytology in the diagnosis of infected pancreatic necrosis. Br J Surg. 1998;85:179–84.
Banks PA, Gerzog SG, Langevin RE, Silverman SG, Sica GT, Hughes MD. CT-guided aspiration of suspected pancreatic infection: bacteriology and clinical outcome. Int J Pancreatol. 1995;18:265–70.
Beger HG, Buchler M, Bittner R, Oettinger W, Block S, Nevalaien T. Necrosectomy and postoperative local lavage in patients with necrotizing pancreatitis: results of a prospective clinical trial. World J Surg. 1988;12:255–62.
Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactates Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–7.
Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705–9.
De Madaria E, Soler-Sala G, Sanchez-Paya J, Lopez-Font I, Martinez J, Gonez-Escolar L, et al. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843–50.
Mao EQ, Fei J, Peng YB, Tang YQ, Zhang SD. Rapid hemodilution is associated with increases sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl). 2010;123:1639–44.
Olah A, Padavi G, Belagyi T, Nagy A, Issekutz A, Mohamed GE. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition. 2002;18:259–62.
Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg. 2006;23:336–45.
Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev 2010;1:CD002837.
Hartwig W, Maksan S-M, Foitzik T, Schmidt J, Herfarth C, Klar E. Reduction in mortality with delayed surgical therapy of severe pancreatitis. J Gastrointest Surg. 2002;6:481–7.
Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176–94.
Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480–3.
Saino V, Kemppaninen E, Puolakkainen P, Taavitsainen M, Kivisaari L, Valtonen V, et al. Early antibiotics treatment in acute necrotizing pancreatitis. Lancet. 1995;346:663–7.
Xue P, Deng LH, Zhang ZD, Yang XN, Wan MH, Song B, et al. Effect of antibiotic prophylaxis on acute necrotizing pancreatitis results of a randomized controlled trial. J Gastroenterol Hepatol. 2009;24:736–42.
Isenmann R, Runzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, et al. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo controlled, double-blind trial. Gastroenterology. 2004;126:997–1004.
De Vries AC, Besselink MGH, Buskens E, Ridwan BU, Schipper M, van Erpecum KJ, et al. Randomized controlled trials of antibiotics prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome. Pancreatology. 2007;7:531–8.
Villatoro E, Mulla M, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;12:CD002941.
Berzin TM, Rocha FG, Whang EE, Mortele KJ, Ashley SW, Banks PA. Prevalence of primary fungal infections in necrotizing pancreatitis. Pancreatology. 2007;7:63–6.
Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. Am J Roentgenol. 1998;170:969–75.
Runzi M, Niebel W, Goebell H, Gerken G, Layer P. Severe acute pancreatitis: nonsurgical treatment of infected necroses. Pancreas. 2005;30:195–9.
van Goor H, Sluiter WJ, Bleichrodt RP. Early and long term results of necrosectomy and planned re-exploration for infected necrosis. Eur J Surg. 1997;163:611–8.
Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, et al. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505.
Uhl W, Warshaw A, Imrie C, Bassi C, McKay CJ, Lankisch PG, et al. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2:565–73.
Besselink MG, Verwer TJ, Schoenmaeckers EJ, Buskens E, Ridwan BU, Visser MR, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142:1194–201.
Garg PK, Sharma M, Madan K, Sahni P, Banerjee D, Goyal R. Primary conservative treatment results in mortality comparable to surgery in patients with infected pancreatic necrosis. Clin Gastroenterol Hepatol. 2010;8:1089–94.
Molli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology. 2013;144:333–40.
van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG, et al. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98:18–27.
Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175–80.
Connor S, Ghaneh P, Raraty M, Sutton R, Rosso E, Garvey CJ, et al. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270–7.
van Santvoort HC, Besselink MG, Horvath KD, Sinanan MN, Bollen TL, van Ramshorst B, et al. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB. 2007;9:156–9.
Horvath K, Freeny P, Escallon J, Heagerty P, Comstock B, Glickerman DJ, et al. Safety and efficacy of video-assisted retroperitoneal debridement for infected pancreatic collections: a multicenter, prospective, single-arm phase 2 study. Arch Surg. 2010;145:817–25.
Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111:755–64.
Seifert H, Wehrmann T, Schmitt T, Zeuzem S, Caspary WF. Retroperitoneal endoscopic debridement for infected peripancreatic necrosis. Lancet. 2000;356:653–5.
Papachristou GI, Takahashi N, Chahal P, Sarr MG, Baron TH. Peroral endoscopic drainage/debridement of walled off pancreatic necrosis. Ann Surg. 2007;245:943–51.
Gardner TB, Chahal P, Papachristou GI, Vege SS, Petersen BT, et al. A comparison of direct endoscopic necrosectomy with transmural drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085–94.
Gardner TB, Coelho-Prabhu N, Gordon SR, Gelrud A, Maple JT, Papachristou GI, et al. Direct endoscopic necrosectomy for treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73:718–26.
Gardner TB. Endoscopic management of necrotizing pancreatitis. Gastrointest Endosc. 2012;76:1214–23.
Seifert H, Biermer M, Schmitt W, Jurgensen C, Will U, Gerlach R, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicenter study with long-term follow-up (the GEPARD study). BMJ. 2009;58:1260–6.
Binmoeller KF. EUS-guided drainage of pancreatic fluid collections using fully covered self-expandable metal stents. Gastroenterol Hepatol. 2013;9:442–4.
Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echoendoscope. Endoscopy. 2001;33:473–7.
Belle S, Collet P, Post S, Kaehler G. Temporary cystgastrostomy with self-expending metallic stents for pancreatic necrosis. Endoscopy. 2010;42:493–5.
Itoi T, Nageshwar Reddy D, Yasuda I. New fully-covered self-expandable metal stent for endoscopic ultrasonography-guided intervention in infectious walled-off pancreatic necrosis (with video). J Hepatobiliary Pancreat Sci. 2013;20:403–6.
Fabbri C, Luigiano C, Cennamo V, Polifemo AM, Barresi L, Jovine E, et al. Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy. 2012;44:429–33.
Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011;43:337–42.
Gornals JB, De la Serna-Higuera C, Sanchez-Yague A, Loras C, Sanchez-Cantos AM, Perez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013;27:1428–34.
Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Bollen TL, van Eijck CH, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053–61.
Ross A, Gluck M, Irani S, Hauptmann E, Fotoohi M, Siegal J, et al. Combines endoscopic and percutaneous drainage of organized pancreatic necrosis. Gastrointest Endosc. 2010;71:79–84.
Gluck M, Ross A, Irani S, Lin O, Gan SI, Fotoohi M, et al. Dual modality drainage for symptomatic walled-off pancreatic necrosis reduced length of hospitalization, radiological procedures, and number of endoscopies compared to standard percutaneous drainage. J Gastrointest Surg. 2012;16:248–56.
Gluck M, Ross A, Irani S, Lin O, Hauptmann E, Siegal J, et al. Endoscopic and percutaneous drainage of symptomatic walled-off pancreatic necrosis reduces hospital stay and radiographic resources. Clin Gastroenterol Hepatol. 2010;8:1083–8.
van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–502.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Afghani, E., Singh, V.K. (2015). Sterile and Infected Pancreatic Necrosis. In: Forsmark, C., Gardner, T. (eds) Prediction and Management of Severe Acute Pancreatitis. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0971-1_3
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0971-1_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0970-4
Online ISBN: 978-1-4939-0971-1
eBook Packages: MedicineMedicine (R0)