Abstract
The surgical management of severe acute pancreatitis (SAP) has evolved rapidly over the past decade. Pancreatitis is a disease that encompasses a range of physiological disturbances that can be mild and transient, or immediately life-threatening and resulting in prolonged critical illness. Importantly, pancreatitis is a dynamic process and can continue to develop in severity for several weeks or even months after the acute insult.
The optimal approach to the patient with SAP first involves prompt recognition of the disease process, identification of onset and duration, and comprehensive evaluation by a multidisciplinary team. The role of the surgeon on this team is central, even for patients who will not necessarily proceed to the OR, as is collaboration with the procedural gastroenterologist, the expert radiologist, and the intensivist.
We attempt to define SAP through the eyes of the surgeon and explore the surgical indications and surgical management of SAP. The major indication for operative intervention here remains the presence of infected pancreatic necrosis, for which there are several operative approaches to source control. In addition, later complications of SAP constitute indications for surgical interventions, such as pancreatic pseudocyst and pancreatic fistula.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute Pancreatitis
- Systemic Inflammatory Response Syndrome
- Pancreatic Fistula
- Severe Acute Pancreatitis
- Abdominal Compartment Syndrome
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Definitions of Pancreatitis
Pancreatitis as a diagnosis encompasses a wide breadth of clinical presentations, ranging from mild abdominal pain that resolves without complication, to a severe, life-threatening illness with devastating long-term complications. See also Chaps. 1 and 2. Given the diversity of this disease, accurate and precise language is necessary to define it. Numerous attempts to define pancreatitis have been made over the years. In 1992, under the leadership of Edward Bradley [1], the Atlanta Classification was developed. This system attempted to unify the vocabulary describing the pancreatic disease process using clinical criteria; however, it was criticized as too vague, unobjective, and confusing. This classification system was revised in 2012, with a goal to provide more objective, clear terms to better classify and define the severity of pancreatitis and its local complications [2]. This modern classification scheme is summarized below.
The clinical diagnosis of acute pancreatitis can be made based on the presence of two of the three following criteria: (1) symptoms of central upper abdominal pain of acute onset, radiating to the back; (2) serum pancreatic enzyme (amylase or lipase) levels greater than three times normal; or (3) characteristic features on cross-sectional abdominal imaging consistent with acute pancreatitis [1, 3–5]. The onset of acute pancreatitis is established with time zero, defined as the time of onset of abdominal pain. Hospital admission times should not be used as time zero as there is often a delay of presentation to the hospital and commonly a need for transfer between hospitals for higher level of care considerations. Following the disease progression from time zero, to time of presentation, through the initial 24–48 h and first weeks of illness is important in stratification of the disease severity. The improvement, worsening, or stagnation of the patient’s condition at these time points have important implications in the patient’s prognosis and can point to increased severity of disease or the development of complications.
Two distinct types of acute pancreatitis are defined in the original and revised Atlanta Classifications [1, 2]: Interstitial edematous pancreatitis (EP), which can be thought of essentially as non-necrotizing pancreatitis, and necrotizing pancreatitis (NP). With EP there is homogeneous enhancement of the pancreas gland and inflammatory changes in the surrounding fat. The defining feature of EP is that there is no evidence of necrosis within the pancreatic parenchyma or surrounding the pancreas on imaging. Fluid collections surrounding the pancreas may or may not be present and are not indicative of necrosis. EP represents 90–95 % of clinical pancreatitis and is often managed outside the ICU, as most such pancreatitis episodes resolve within the first week. Meanwhile, NP constitutes the remaining 5–10 % of acute pancreatitis patients, which usually require ICU management, and often progress to multi-organ system failure with or without sepsis. The defining characteristic of NP is the presence of necrosis either within pancreatic parenchyma or of surrounding tissues. Most commonly, necrosis of both the pancreatic gland and peripancreatic tissues will occur, although either can occur alone. The most rarely seen manifestation is isolated pancreatic parenchymal necrosis. Involvement of the pancreatic parenchyma portends a more ominous clinical journey [1]. Contrast-enhanced CT (CECT) findings of necrosis include non-enhancement of pancreatic parenchyma as well as inflammatory and solid component features of surrounding tissues; however, it is important to recognize that compromise of pancreatic perfusion from necrosis and CT signs of peripancreatic necrosis can evolve over days. Therefore, early CECT imaging (i.e., within the first 7 days) is likely to underestimate the extent of tissue necrosis.
Necrotizing pancreatitis can be further classified as infected or sterile necrosis; EP does not become infected. Infection of necrotic tissue continues to be associated with a high mortality; therefore, it is essential to recognize its presence. Ongoing sepsis or acute clinical deterioration should raise the suspicion of infected necrosis; however, its presence can be proven by imaging or culture. The pathognomonic radiographic feature is the presence of gas within areas of necrosis on CECT imaging. Diagnosis and management of infected necrosis is further discussed later in the chapter.
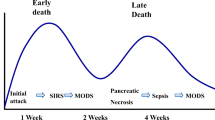
Phases of Acute Pancreatitis
Acute pancreatitis is divided into two disease phases, each with individual risks and associated mortality [1, 2, 6]. During the early phase of acute pancreatitis, which usually lasts the first 1–2 weeks, the pancreatic damage and any systemic complications are a result of the autodigestion of the pancreas as well as the associated cytokine cascade that this elicits and is characterized by the systemic inflammatory response syndrome (SIRS) [7] (Table 16.1). As SIRS persists, the chance of organ failure increases. This early phase can resolve without sequelae, as in mild acute pancreatitis (MAP); however, in the more severe cases, the inflammation continues and leads to further disease processes. This continued systemic inflammation defines the late phase of acute pancreatitis. This phase can last for weeks to months after the initial presentation with pancreatitis, consisting of continued SIRS and/or local or systemic complications, including persistent organ failure.
Stratification of Severity
Stratification of the severity of acute pancreatitis is important, because as stated above, this is a dynamic disease that can manifest with a broad range of physiologic derangements and varying survivability. See also Chap. 2. Early stratification helps to determine patient risk, targets resuscitation, and can help identify patients that require transfer to higher levels of care. Precise and consistent language aids in clear communication between teams and focuses attention to the medical issues that need to be addressed in the treatment plan. The Revised Atlanta Classification of Acute Pancreatitis provides clear clinical characteristics that help to define the degree of pancreatitis that is present [2]. The presence or absence of organ failure, local complications, and/or systemic complications defines three distinct classes of acute pancreatitis: mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP) (Table 16.2).
Mild Acute Pancreatitis
MAP is defined as pancreatitis without the presence of organ failure and no local or systemic complications. Diagnosis is clinical and imaging is usually not required. Enteral feeding is recommended once tolerated, patients are usually discharged within a week of hospitalization, and mortality is rare [2].
Moderately Severe Acute Pancreatitis
MSAP is defined as pancreatitis with transient organ failure (less than 48 h duration) or the existence of local or systemic complications in the absence of persistent organ failure. Examples of local complication include peripancreatic fluid collections, acute necrotic collections, pancreatic pseudocyst, infected necrosis, gastric outlet dysfunction, splenic and portal vein thrombosis, and colonic necrosis. These local complications and their management will be further discussed later in the chapter. Given the breadth of possible associated complications, it follows that the clinical course of MSAP is variable. Transient organ failure and acute fluid collections may resolve without further intervention, whereas other local complications may require debridement or drainage. Mortality in this class of pancreatitis is higher than in acute pancreatitis; however its mortality remains much lower than that of SAP, with rates reported as <8 % [8].
Severe Acute Pancreatitis
SAP is characterized by persistent organ failure (organ failure that does not resolve after 48 h). Persistent organ failure can involve one or multiple organs. While the presence of local complications is not explicitly contained in the diagnosis of SAP, the vast majority of patients with persistent organ failure have local complications as well. Those who develop persistent organ failure during the early phase of pancreatitis have a higher rate of death, with mortality rates reported to range between 36 and 50 % [2].
Development of infected necrosis, in the presence of SAP, is associated with a high mortality rate and should be aggressively managed [2].
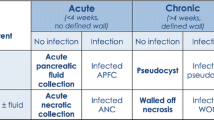
Defining Pancreatic Collections
CT or MRI imaging is helpful in identifying and classifying local complications which typically present as peripancreatic collections; however, the term “peripancreatic collections” encompasses a heterogeneous group of entities. Therefore, the Revised Atlanta Classification divides these into four distinct groups, defined by their contents and architecture. Correct diagnosis is important, as management and potential complications of these collections can differ significantly. Collections containing only fluid are defined as either acute peripancreatic fluid collections or pancreatic pseudocysts, whereas acute necrotic collections or walled-off necrosis are collections of necrotic tissue with or without a fluid component.
Acute peripancreatic fluid collections are fluid collections that develop during the early phase of edematous pancreatitis in the fascial planes of the retroperitoneum. They do not have any defining wall, are homogenous-appearing on imaging, and are sterile. They may be single or multiple and tend to resolve without intervention.
Pancreatic pseudocysts are peripancreatic collections composed solely of fluid, with no solid components, that have a well-defined, circumscribing wall. The fluid is usually high in amylase and results from disruption of a pancreatic duct with persistent leakage. Pseudocysts can also develop following parenchymal necrosis of the pancreatic gland that isolates a viable, functioning distal pancreas, leading to localized leakage from the separated duct. These pseudocysts often develop after necrosectomy, as fluid accumulates within the necrosectomy space.
Acute necrotic collections are defined as a collection of variable amounts of necrotic tissue with or without fluid, which occurs within 4 weeks of an episode of pancreatitis. These collections may be loculated and may be difficult to differentiate from acute pancreatic fluid collections when imaging performed during the first week of the disease process; therefore, sequential imaging is often helpful to fully define the collection. MRI and ultrasound may help to better define the solid components of these collections.
Walled-off necrosis is a collection of necrotic tissue with an enhancing wall that implies maturity and encapsulation of acute necrotic collections. These usually require greater than 4 weeks to develop. These may be single or multiple, near to the gland or located at sites distant from the pancreas. These may be sterile or infected. Similarly to acute necrotic collections, these may be misdiagnosed as pancreatic pseudocysts due to CT imaging limitations, which is why additional imaging such as MRI and ultrasound techniques is useful to correctly identify these collections.
Prognostic Measures of Acute Pancreatitis
Given the wide spectrum of pancreatitis, early identification of those patients at risk for severe disease, complications, and mortality is imperative. See also Chaps. 4 and 6. Patients with obvious organ dysfunction or severe disease warrant intensive care monitoring; however, predicting patients who will develop severe disease on admission is not always straightforward. Multiple scoring systems have been proposed to attempt to identify patients at risk. One of the earliest prognostic scores was developed by Dr. JH Ranson in 1974 using clinical criteria at admission and 48 h to evaluate the severity and mortality risk of acute pancreatitis based on clinical data. Several other prognostication systems have been proposed. The CT Severity Index (CTSI), which grades pancreatitis severity based on radiographic findings of necrosis and fluid collection, has been shown to correlate with statistical significance with mortality of pancreatitis [9]. APACHE II score [10] uses physiologic variables to calculate risk. Although it can be calculated at 24 h, the score at this time has a poor predictive value for severe disease [5]; however, because it can be calculated daily, following the trend can be very useful. Increasing APACHE II scores during the first 48 h are associated with development of severe pancreatitis, whereas decreases point toward mild, resolving disease. SIRS [7] (see Table 16.1) has also been used to predict mortality. In one study, patients with acute pancreatitis and the absence of SIRS on admission had a mortality rate of 0.7 %, patients with SIRS on admission that resolved after 48 h had a mortality rate of 8 %, whereas patients with persistent SIRS at 48 h had a mortality rate of 25 % [11]. None of these scoring systems have been conclusively proven to most accurately predict severe disease and mortality, rather they should be used to triage patients and identify those at risk for more severe disease.
Surgical Management of Severe Acute Pancreatitis
In current practice, surgical interventions in acute pancreatitis are aimed at the management of complications of SAP and the ensuing inflammatory process as well as at prevention of recurrent pancreatitis, as in cases of gallstone pancreatitis. Surgical intervention during the early phase of acute pancreatitis is extremely difficult given the severe inflammation and should be limited to life-threatening complications.
Historical Approach: Surgical Indications
Early in the twentieth century, the mainstay of treatment of SAP was early debridement. Lord Moynihan, a prominent British surgeon in the 1920s went so far as to say that “… recovery from this disease, apart from operation, is so rare that no case should be left untreated” [12]. Surgical interventions ranged from debridement with gauze drainage, marsupialization of the gland, to complete resection, with the main goal of treatment to remove all necrotic tissue early in the disease process. Surgical practice in the 1970s and 1980s, however, shifted to emphasis on conservative management, with teaching suggesting that surgical intervention was futile and associated with high mortality. Identification of patients likely to benefit from surgical intervention and surgical techniques promoting safe removal of infected, necrotic tissue were pioneered by Bradley et al. [13]. This landmark study demonstrated a significantly improved survival with pancreatic debridement in those patients with infected necrosis. The optimal timing of surgical intervention was investigated over the ensuing decade, with evidence suggesting later surgical intervention preferable to early intervention in most patients. This was proven via a randomized clinical study by Mier et al. that was ultimately stopped prior to completion given the extremely high mortality rate in patients who underwent early debridement (58 %) compared to those who underwent late debridement (27 %) [14].
Indications for Surgery: Acute Complications
During the early phase of pancreatitis, the main tenet of current therapy is conservative and supportive management. Adequate and early fluid resuscitation is critically important in the care of these patients and may help reduce the incidence of SIRS and organ failure [15]. Early enteral feeding can be accomplished in most patients, with the benefit of decreased infectious complications and mortality [16]. Enteral feeding has most often been accomplished via nasojejunal tube placement, to decrease stimulation of the pancreas; however, multiple studies have demonstrated that nasogastric or nasoduodenal feeding is safe and of similar benefit compared to jejunal feeding [17, 18]. Any abdominal interventions should be limited in this acute phase of active inflammation, with the main recommendation to only to treat severe, catastrophic conditions, such as hemorrhage, perforation of a hollow viscus organ, and abdominal compartment syndrome (ACS).
Catastrophic Abdomen
Pancreatitis is primarily a destructive inflammatory process, which not only destroys its own parenchyma, but can erode into adjacent structures and lead to injury and compromise of surrounding structures, with devastating complications. Rare abdominal catastrophes, such as bowel ischemia, ACS, and uncontrolled hemorrhage, require emergent surgical intervention, even in the early phases of pancreatitis.
Bowel ischemia can develop due to ACS and occasionally needs to be treated emergently. ACS by itself, without bowel ischemia, is also a surgical emergency regardless of stage of pancreatitis. ACS is defined as sustained intra-abdominal pressure >20 mmHg that is associated with the onset of new organ failure. This can occur due to the massive fluid resuscitation required during the treatment of the early phase of pancreatitis. Emergent decompressive laparotomy for relief of ACS is imperative, with removal of any non-viable bowel occasionally warranted, although complications of this intervention are high.
Additionally, bowel ischemia can be due to inflammation from pancreatitis surrounding the mesenteric vessels, resulting in compromise of the small bowel and occasionally the colon. The most common presentation in this case is a patient who fails to respond appropriately to apparently adequate resuscitative measures. Diagnosis is difficult and is typically made at exploration (Fig. 16.1).
Intraoperative findings in during exploratory laparotomy performed in a 65-year-old male who initially presented to an outside hospital with acute abdominal pain found to be due to gallstone pancreatitis. His clinical condition worsened overnight, during which time he required 6 L fluid for the treatment of oliguria and hypotension. He was transferred to our hospital and received 18 h of aggressive resuscitation, but continued to have worsening lactate and subsequent development of intra-abdominal compartment syndrome. Upon exploration he was found to have a large section of ischemic and necrotic bowel
Intra-abdominal hemorrhage associated with acute pancreatitis is most often due to bleeding from a pseudoaneurysm. Pseudoaneurysms develop due to weakening of the vessel wall after exposure to proteolytic enzymes and other inflammatory mediators of pancreatitis. Fortunately, this complication occurs with relative infrequency, affecting only 1–3 % of acute pancreatitis patients; however, it is associated with high mortality [19, 20]. Acute catastrophic hemorrhage from pseudoaneurysmal bleeding has been increasingly managed by angiographic and interventional techniques and is the preferred initial management. If noninvasive techniques fail or are unavailable, surgical intervention becomes necessary. Immediate laparotomy followed by packing to control the bleeding is the first step. If feasible, repair and exclusion of the pseudoaneurysm is performed, however, given the massive inflammation in the area surrounding the pseudoaneurysm it is often not possible. At this time, packing of the wound cavity is the next step, most often in the context of damage control surgery. Definitive repair of the pseudoaneurysm is undertaken once the patient can tolerate further surgical intervention and the early phase of pancreatitis is past.
In addition to pseudoaneurysm formation, pancreatitis can cause diffuse bleeding from tissue necrosis, and bleeding can occur from hemorrhagic pseudocysts, which can lead to uncontrolled hemorrhage in the event of pseudocyst rupture. Similarly to pseudoaneurysms, these complications present more often during the late phase of pancreatitis, but can occur during the early phase as well. Typically, selective mesenteric angiography can identify the site of bleeding [19]. Initial management remains the same, control of bleeding, hopefully via noninvasive angiography or via abdominal packing. Further management, such as removal of necrotic tissue and management of pseudocysts, is discussed later in this chapter and should be attempted once the patient can tolerate surgery and the active inflammatory phase is over. This further management is important, as without removal of the necrotic tissue, intra-abdominal hemorrhage has a very high rate of recurrence [20].
Indications for Surgery: Later Complications
Infected Necrosis
Infection of necrotic tissue during SAP is an important determinant of mortality; therefore, it is essential to differentiate between sterile and infected necrosis. Ongoing sepsis or acute clinical deterioration should raise suspicion of infected necrosis and its presence can be proven by imaging or culture. The presence of the pathognomic finding of gas in necrotic tissue spaces on cross-sectional imaging confirms the diagnosis. In our experience, the presence of gas is frequently associated with duodenal or enteric fistulae. Without gas within the necrotic area on imaging, infected necrosis can be diagnosed via image-guided fine-needle aspiration (FNA) sent for gram stain and culture. It should be noted that current recommendations of the International and American Pancreatic Associations (APA) state that FNA should not routinely be performed, in part due to the risk of false negative results (12–25 %) [4]. Infection can develop de novo in previously sterile necrotic tissue via bacterial translocation from the gastrointestinal tract; however, it is important to recognize that secondary infection can occur after instrumentation, via FNA, endoscopy, and ERCP. These procedures should be performed only when necessary, and fever or worsening of the patient’s condition following these interventions should prompt concern for infection. Clinical scenarios that should arouse suspicion of the presence of infected pancreatic necrosis include patients with severe pancreatitis whose severe SIRS now progresses to severe sepsis, or in patients with sepsis who continues to decline clinically despite targeted antibiotic therapy. In the critically ill pancreatitis patient, all other sources for infection must be thoroughly searched for and either ruled out or treated promptly, such as pneumonia, urinary tract infection, line infection, sinusitis, and cholecystitis.
Once infected necrosis is diagnosed, timely intervention must be undertaken with the goal of surgical treatment being debridement and removal of the infected tissue, thereby controlling the infection and halting the release of proinflammatory mediators. If patient condition permits, removal of the necrotic tissue should be postponed until 3–4 weeks after the onset of pancreatitis. This leads to safer operating conditions, as decreased inflammation leads to decreased operative bleeding and better delineation of necrotic tissue, which allows the surgeon to minimize the amount of viable tissue that is removed, thereby reducing the exocrine and endocrine complications with pancreatic insufficiency [20]. Surgical removal of necrotic tissue is also indicated if the necrotic tissue is, or has previously been, hemorrhagic, if the necrotic tissue leads to ongoing gastric, intestinal, or biliary obstruction continuing >4 to 8 weeks after pancreatitis, or the patient continues to have ongoing organ failure after several weeks of acute pancreatitis without improvement.
The most current recommendations from the International Association of Pancreatology (IPA) and APA state that the optimal interventional strategy for suspected or confirmed infected necrosis is initial management with image-guided percutaneous catheter drainage or endoscopic transluminal drainage, followed by endoscopic or surgical debridement only if necessary [4]. This is following the results of the PANTER trail, a randomized controlled trial comparing open surgical necrosectomy to a minimally invasive approach [21]. This study compared 88 patients randomized to either open necrosectomy or a minimally-invasive “step-up” approach involving percutaneous drainage and post-procedural irrigation of the drained space. If necessary, this was followed by definitive tissue debridement via a video-assisted retroperitoneal debridement (VARD) (Fig. 16.2) and continued postoperative irrigation and drainage. These patients were followed through 6 months after discharge. The primary endpoint was a composite of either “death” or the occurrence of “major complications” comprised of: new-onset organ failure (parameters defined for pulmonary, circulatory, and renal failure), any system complications such as DIC, severe metabolic disturbances or GI bleeding, or visceral organ perforation, ECF, or intra-abdominal hemorrhage. The secondary endpoints were the individual components of the primary endpoint. This study demonstrated that there was no difference in mortality between the groups, and the minimally invasive step-up approach was associated with significantly lower rates of new-onset organ failure, as well as fewer longer-term complications such as pancreatic insufficiency. In addition, health care resource utilization and ICU readmission rates were significantly lower in the minimally invasive step-up group. The medical costs, both direct and indirect, per admission and at 6-month follow-up were shown to be lower by 12 % in the step-up group.
An image from a video-assisted retroperitoneal debridement for infected necrosis of the pancreas. This is a view of the retroperitoneal approach in a patient with infected necrosis tracking down left gutter. A stent has been placed through stomach in left upper field and the guidewire from retroperitoneal approach in right camera field
Endoscopic procedures have also been performed in conjunction with percutaneous or VARD procedures to remove necrotic pancreatic tissue. They can be performed via transluminal or transgastric approach. The benefit of these approaches is that pancreatic fistulas will not develop, as all pancreatic fluid produced will be drained into the stomach or intestine. However, a significant disadvantage is that multiple procedures are needed to remove sufficient necrotic tissues [22].
Open Techniques
If open necrostomy is performed, a variety of techniques have been employed. The mortality rates for the following techniques have been shown to be equivalent in experienced hands, with rates less than 15 % for any of the listed techniques [20]. Thus, surgeon preference dictates the approach, although the distinct advantages and disadvantages of each are worth mentioning. All four of these methods have in common initial debridement, which can often be completed during the initial visit, and these methods then vary by the manner in which they establish continued debridement or lavage of the necrosectomy space to facilitate continued egress of stubbornly attached necrotic tissue.
-
Transperitoneal laparotomy with open packing—open midline laparotomy, surgical necrosectomy, packing the retroperitoneal space with the abdomen left open, requiring multiple re-laparotomies.
-
Transperitoneal laparotomy with staged re-laparotomy—open midline laparotomy, surgical necrosectomy, no packing left within, open abdomen requiring multiple re-laparotomies.
-
Closed lavage of the retroperitoneum—open laparotomy, surgical necrosectomy, drains left within the retroperitoneum, closure of lesser sac, postoperative continuous irrigation.
-
Closed packing—open laparotomy, surgical necrosectomy, packing left within the retroperitoneum, return to OR for removal of packing, and closure of the abdomen.
Complications of the open procedures above include extensive bleeding in the necrosectomy space and increased cumulative blood loss, fistula formations to the GI tract, gastric outlet obstruction, and incisional hernia [20].
A few comments regarding the technical approach to the open debridement of pancreatic necrosis seem appropriate. It is the habit of this author to perform a transverse incision in the upper abdomen and to remove the gallbladder at the first operation. Typically, a surgical jejunostomy tube is placed, then the transverse colonic mesentery is divided, opening the lesser sac. At this point, pancreatic sequestrum is usually easily entered. It is important to gently debride the pancreatic tissue, as bleeding may ensue with more vigorous debridement. Irrigation typically frees additional tissue. The inferior transverse colonic omentum is tacked to the peritoneum at the inferior margin of the incision, to keep purulence in the lesser sac from spreading to the lower abdomen. The lesser sac is packed with laps or kerlex gauze, and the wound is temporarily closed with a vacuum-assisted dressing, with planned reoperation every 48 h until no further necrotic tissue is encountered. At this point the fascia can be closed with drains placed in the lesser sac.
Pancreatic Abscess
Pancreatic abscess is the most common complication of pancreatitis that mandates re-intervention after necrosectomy [20]. These generally occur after 5 weeks of the onset of pancreatitis. Pancreatic abscesses usually remain contained and are less destructive than infected pancreatic necrosis, and thus can be managed typically with percutaneous drainage. Failure of percutaneous approach would mandate operative intervention for drainage.
Pancreatic Pseudocyst
The management of pancreatic pseudocysts is a continually evolving paradigm. Previous dogma recommending drainage of pseudocysts that persisted greater than 6 weeks no longer holds true. The majority will resolve on their own, follow a benign course, and can be managed with no further intervention [20]; however, if they become symptomatic or are noted to grow during a period of observation, intervention becomes necessary. A diameter of >6 cm is often quoted as an indication for intervention; however, this remains controversial. Treatment can be performed in many ways, and the management is best decided via interdisciplinary team discussions. Percutaneous drainage is currently indicated only for emergency drainage of infected cysts, especially early in the course of pancreatitis, since recurrence and fistula development occur with high rates in this approach [23]. Endoscopic drainage, either via transpapillary or transmural approach, has a high success rate in experienced hands, and lower risk of fistula formation, as the drainage of pancreatic secretions can be directed enterally [23]. Surgical interventions that can be used to drain pseudocysts cysts enterally include cystgastrostomy or a Roux loop cystojejunostomy. Pseudocysts located in the pancreatic tail are difficult to drain enterally and may be best treated by pancreatic resection. However, the mortality and morbidity following resection are higher than after surgical drainage [20].
Pancreatic Fistula
Treatment of pancreatic fistulas due to acute pancreatitis is managed in a similar fashion to fistulas of other etiologies. Conservative management is usually attempted first (e.g., jejunal tube feeds, bowel rest, TPN, octreotide); however, if this fails, further interventions are necessary. Endoscopic transpapillary stenting has also been proposed as an intervention to treat pancreatic fistulas, as the stenting decreases the intraductal pressure and helps shunt the pancreatic secretions into the duodenum instead of the fistula [24]. Surgical management is reserved for those patients who are not responding to the above measures. In this setting, depending on the location of the fistula, the patient may undergo Whipple procedure (Fig. 16.3), Roux-en Y pancreaticojejunostomy, cystojejunostomy, or distal pancreatic resection [25].
Special Considerations with Biliary Pancreatitis
The two leading causes of acute pancreatitis in the United States are gallstones and alcohol consumption. Although it may seem intuitive to perform ERCP in patients with gallstone pancreatitis, early ERCP has not been routinely recommended for patients with mild or severe gallstone pancreatitis. The only group that has been demonstrated in prospective, randomized clinical trials to benefit from early ERCP with stone extraction and sphincterotomy has been the subset of patients with gallstone pancreatitis who have obstructive jaundice and/or cholangitis. Without these features, early ERCP has been shown to lead to high complication rates with no observable benefit [26, 27].
In patients with biliary pancreatitis, patients discharged after resolution of pancreatitis have a high recurrence rate if the causative factor is not controlled. One review reported that 18 % of patients who had an interval cholecystectomy performed a median of 40 days after the initial pancreatitis admission were readmitted prior to cholecystectomy for biliary-related complications [28]. Current recommendations are that patients with MAP undergo laparoscopic cholecystectomy during their index admission [4]. In elderly or unfit patients with biliary pancreatitis who are unable to tolerate same-admission cholecystectomy, an alternative or bridge to surgery is elective ERCP with sphincterotomy to lower the risk of recurrent SAP. For patients with concurrent cholecystitis, if the patient is at high risk for cholecystectomy, a cholecystostomy tube can be placed [29]. For severe biliary pancreatitis with peripancreatic collections, cholecystectomy should be delayed until the collections resolve, typically 6 weeks after the onset of pancreatitis [4]. Cholecystectomy is advised in all patients that can tolerate the procedure, as the risk of recurrent pancreatitis is decreased following ERCP with sphincterotomy, but has no effect on the risk of acute cholecystitis and other gallstone-related gallbladder disease [28]. It is important for the surgeon to recognize the fact that these procedures are frequently difficult and may require an open approach. This requires appropriate counseling of the patient and appropriate preoperative planning.
Conclusion
Acute pancreatitis and its complications make up a diverse and nuanced disease, whose management is characterized by complex issues and many subtleties. Multidisciplinary management is best for the patient and provides a greater breadth of treatment options. Surgical management continues to play an important role in the care of patients with acute pancreatitis and its sequelae.
References
Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the international symposium on acute pancreatitis, Atlanta, GA, September 11-13, 1992. Arch Surg. 1993;128(5):586–90.
Sarr MB, Bollen P, Dervenis T, Gooszen C, Johnson H, Tsiotos C, et al. The new revised classification of acute pancreatitis 2012. Surg Clin North Am. 2013;93(3):549–62.
American Gastroenterological Association (AGA) Institute on “Management of Acute Pancreatits” Clinical Practice and Economics Committee, AGA Institute Governing Board. AGA Institute medical position statement on acute pancreatitis. Gastroenterology. 2007;132(5):2019–21.
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–15.
Banks PF, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–400.
Brisinda GV, Crocco S, Mazzari A, Tomaiuolo A, Santullo P, Grossi F, et al. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23(7):541–51.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
Vege SS, Gardner TB, Chari ST, Munukuti P, Pearson RK, Clain JE, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis”. Am J Gastroenterol. 2009;104(3):710–5.
Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156(3):767–72.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93(6):738–44.
Moynihan B. Acute pancreatitis. Ann Surg. 1925;81:132–42.
Bradley III EL, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg. 1991;161(1):19–24.
Mier J, León EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173(2):71–5.
Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9(8):705–9.
Petrov M, van Santvoort H, Besselink MGH, van der Heijden Geert JMG, Windsor J, Gooszen H. Enteral nutrition and the risk of mortality and infectious complications in patients with severe acute pancreatitis: a meta-analysis of randomized trials. Arch Surg. 2008;143(11):1111–7.
Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, Imrie CW. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. Am J Gastroenterol. 2005;100(2):432–9.
Joshi YK. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. J Clin Gastroenterol. 2006;40(5):431–4.
Balthazar EJ, Fisher LA. Hemorrhagic complications of pancreatitis: radiologic evaluation with emphasis on CT imaging. Pancreatology. 2001;1(4):306–13.
Werner J, Feuerbach S, Uhl W, Bachler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54(3): 426–36.
Besselink MG, van Santvoort HC, Nieuwenhuijs VB, Boermeester MA, Bollen TL, Buskens E, et al. Minimally invasive ‘step-up approach’ versus maximal necrosectomy in patients with acute necrotising pancreatitis (PANTER trial): design and rationale of a randomised controlled multicenter trial [ISRCTN13975868]. BMC Surg. 2006;6:6.
Voermans RP, Bruno MJ, van Berge Henegouwen MI, Fockens P. Review article: translumenal endoscopic debridement of organized pancreatic necrosis–the first step towards natural orifice translumenal endoscopic surgery. Aliment Pharmacol Ther. 2007;26 Suppl 2:233–9.
Lerch MM, Stier A, Wahnschaffe U, Mayerle J. Pancreatic pseudocysts: observation, endoscopic drainage, or resection? Dtsch Ãrztebl Int. 2009;106(38):614–21.
Bakker OJ, van Baal MC, van Santvoort HC, Besselink MG, Poley JW, Heisterkamp J, Bollen TL. Endoscopic transpapillary stenting or conservative treatment for pancreatic fistulas in necrotizing pancreatitis: multicenter series and literature review. Ann Surg. 2011;253(5):961–7.
Alexakis N, Sutton R, Neoptolemos J. Surgical treatment of pancreatic fistula. Dig Surg. 2004;21:262.
Folsch UR. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1996;336(4):237–42.
Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328(4):228–32.
van Baal MC, Besselink MG, Bakker OJ, van Santvoort HC, Schaapherder AF, Nieuwenhuijs VB. Timing of cholecystectomy after mild biliary pancreatitis: a systematic review. Ann Surg. 2012;255(5):860–6.
Spira RM, Nissan A, Zamir O, Cohen T, Fields SI, Freund HR. Percutaneous transhepatic cholecystostomy and delayed laparoscopic cholecystectomy in critically ill patients with acute calculus cholecystitis. Am J Surg. 2002;183(1):62–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Venugopal, R., Pokorney-Colling, K., Beilman, G.J. (2015). Surgical Approaches. In: Forsmark, C., Gardner, T. (eds) Prediction and Management of Severe Acute Pancreatitis. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0971-1_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0971-1_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0970-4
Online ISBN: 978-1-4939-0971-1
eBook Packages: MedicineMedicine (R0)