Abstract
This chapter describes properties of the skin which endow it with the ability to function as an immune response organ. Cellular constituents of the innate (nonspecific) and secondary (specific) immune responses in the skin are discussed. Lymphocyte trafficking which enables the immune response by interaction of lymphocyte surface molecules (addressins) and their cognate receptors in the skin and lymph node is presented. Helper T-cell subsets, the cytokines they produce, and cytokine receptors are outlined and illustrated. The significance of cytokines as therapeutic targets for cutaneous lymphomas and inflammatory diseases is emphasized. Topographical localization and interaction of specific cellular components of the immune response in inflammatory and neoplastic disorders are outlined. Finally, a table summarizing antibodies currently in use for the diagnosis and classification of cutaneous lymphomas and leukemias is included.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Atopic Dermatitis
- Anaplastic Large Cell Lymphoma
- Mycosis Fungoides
- Immune Effector Cell
- Lymphoid Infiltrate
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction to Cutaneous Lymphoid Tissue
Skin Meets Environment
The skin is the body’s major interface with the environment where it is exposed to a diverse repertoire of foreign antigens. It is also a highly susceptible portal of entry for infectious organisms. For its protective role, the skin has therefore developed highly effective immediate and long-term immune responses. The patterns of inflammatory responses may set the stage for the development of subsequent malignant lymphomas. Accordingly, malignant lymphocytes that traffic to and through the skin share many immunologic and genotypic features of their benign counterparts involved in cutaneous immune surveillance.
Innate Immune Response
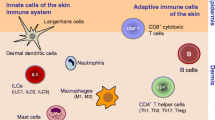
The innate immune system, also known as nonspecific immune system and first line of defense, comprises the cells and mechanisms that defend the host from infection by other organisms in a nonspecific manner. This “innate” response is focused on early (minutes to hours) response to pathogens. Triggers of the innate immune response include microbial and foreign glycolipids, glycoproteins, and DNA complexes (Beetz et al. 2008; Holtmeier and Kabelitz 2005; Kabelitz 2011; Kabelitz et al. 2000). Cellular components of the innate immune system include neutrophils, mast cells, macrophages, dendritic cells, and effector lymphocytes. The immediate effector lymphocytes include natural killer (NK) cells and gamma-delta T cells (γ/δ T cells) (Kabelitz 1992; Kabelitz et al. 2005; Kim et al. 2005). The γ/δ T cells, which comprise 10 % of skin T lymphocytes, along with B cells and NK cells, are largely localized in the dermis and subcutaneous tissue and, for their role as immune effector cells, are also predisposed to the basal layer of the epidermis and outer root sheaths of hair follicles (Kabelitz 2011; Kabelitz et al. 2000).
Secondary Immune Response
The secondary immune responses require the regulation of circulating and peripheral memory T cells, and the skin is postulated to be an active peripheral lymphoid organ, hosting 20 billion T cells, twice that in circulation (Egawa and Kabashima 2011). The adaptive or long-term component of the immune response is composed mainly of T cells bearing α/β T-cell antigen receptors (alpha-beta T cells). These so-called α/β T cells, which comprise 90 % of the cutaneous T cells, acquire homing capacity to the epidermis through the activation of surface addressins enabling them to interact with epidermal Langerhans cells for microbial recognition. Differential expression of surface receptors appears to determine T-cell localization. More than 90 % of these cells express cutaneous lymphocyte antigen (CLA), CD45RO and CCR4 receptors, which are characteristic surface antigens of effector memory T cells (Fig. 2.1). Unlike memory T cells common in the blood circulation and in lymph nodes (which additionally express CCR7 chemokine receptor), memory T cells resident in the skin lack the central memory T-cell marker CCR7 (Egawa and Kabashima 2011). The circulating central memory T-cell pool transiently expresses lymph node homing CCR7 and CD62L (L-selectin). Upon encountering its cognate antigens within the lymph nodes, these central memory T cells downregulate these markers and upregulate CLA + and other skin-specific homing addressins necessary for homing to the skin.
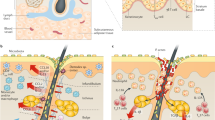
The role of effector T cells and memory T cells in recall immune responses (Modified from Clark 2010). Memory immune responses can be divided into three stages. First, dendritic cells (DC) take up antigen following pathogen reexposure and present it to effector T resident locally within the skin. These cells proliferate and effect clearance of the pathogen. Second, DC carry endocytosed antigen to the skin-draining lymph nodes where it is presented to central memory T cells. These cells then give rise to new populations of skin homing T effector cells that migrate to the skin and clear the infection; third, inflammation leads to endothelial activation and nonspecific recruitment of T cells from the blood
Cutaneous Trafficking of Lymphocytes
A subset of T lymphocytes migrate to the skin via dermal postcapillary venules and recirculate to regional lymph nodes that drain the skin, creating a local network of immune surveillance. For a detailed pictorial review, see (Clark 2010), (Fig. 2.1). This decades-old hypothesis by Streilein (Streilein 1978, 1983, 1985, 1989; Streilein and Bergstresser 1984) is supported by in vivo and in vitro observations and is validated in live mammalian studies by two-photon microscopy which visualize this regional network and its dynamic stepwise development (Egawa et al. 2011). In addition, live animal skin color changes and microscopy of skin-associated lymphocytes trafficking from the lymph nodes is visualized using a technique relying on new fluorescent-tagged cells (Tomura 2012) which changes from fluorescent green to red upon exposure to skin-directed UV (ultraviolet) light (Kaplan et al. 2005, 2008). Accumulating evidence indicates that skin is a crucial modulator of the global immune response and is considered to be a crucial peripheral lymphoid organ akin to the lymph nodes (Egawa and Kabashima 2011).
An orchestrated set of ligand-receptor and chemokine exchanges between immune effector cells, epithelia, and dermal high endothelial venules lead to cutaneous lymphoid infiltrates. These lymphoid infiltrates generate a pattern of reaction that may appear more or less concentrated within the epidermis, dermis, and/or subcutaneous tissue depending on the main participating cells and the signals they receive. Remarkable inroads into the origin and function of the normal components of the immune response provide insights into the histologic architecture, phenotype, and behavior of malignant lymphoma/leukemia counterparts.
Cytokines Define Functional T Helper Subsets
Cytokines are the soluble hormonelike messengers active in mediating the immune response. Deregulation or overproduction of select cytokines is known to play a major role in the pathogenesis of common cutaneous inflammatory diseases, e.g., psoriasis (Schaerli et al. 2004; Wolk et al. 2009 ) and atopic dermatitis (Asarch et al. 2008; Koga et al. 2008). Ongoing research indicates that cytokines also are involved in the pathogenesis of cutaneous lymphomas (Saed et al. 1994; Vowels et al. 1994a, b). Clinical trials targeting cytokines and their receptors have shown promise for the treatment of cutaneous inflammatory and autoimmune diseases (Asarch et al. 2008; Miossec et al. 2009). The interaction between proinflammatory T cells and regulatory T cells also determines cutaneous manifestations of graft-versus-host disease (Beres and Drobyski 2013). Serum cytokine/receptor levels are useful markers of disease activity and quantification of tumor burden (Wasik et al. 1996; Kadin et al. 2012). Therefore, we feel it is essential for our readers to have a basic understanding of cytokines and the cells that produce them.
Of the two main subsets of T lymphocytes, CD4 and CD8, the CD4 T helper subset (Th) is the main cytokine producer. Initially two main Th subset groups were defined by the cytokines they secrete. Th1 cells, regulated by transcription factors Tbet and STAT4, secrete IFN-gamma and induce inflammatory responses to intracellular pathogens. Th2 cells, regulated by GATA3 and STAT5, are represented by interleukins 4, 5, and 13, respond to extracellular parasites, and are associated with the promotion of IgE and eosinophilia. Th9 cells are closely related to Th2 cells but have the capacity to produce IL-9 and IL-10. IL-9 is produced by tumor cells in some anaplastic large cell lymphomas and Hodgkin lymphomas (Merz et al. 1991). More recently described Th17 cells, which recruit neutrophils and macrophages to sites of inflammation, are regulated by ROR and STAT3, convey autoimmune responses, and promote antimicrobial immunity at the body’s borders, e.g., linings of the respiratory and gastrointestinal tracts and the skin. Th17 cells also can produce interleukin 22 which induces epithelial and mucosal cells to secrete antimicrobial peptides active against fungi and bacteria. The proinflammatory and autoimmune effects of Th17 cells are counterbalanced by natural and induced regulatory T cells (Tregs), regulated by the transcription factor FoxP3 and the secretion of immunosuppressive cytokines TGF-beta and IL-10. T cells associated with follicular center differentiation employ transcription factor Bcl-6 and appear to be the source of neoplastic cells in some primary cutaneous CD4+ small-/medium-sized pleomorphic T-cell lymphomas (Rodriguez Pinilla et al. 2009) and mycosis fungoides/Sezary syndrome cases (Meyerson et al. 2013).
CD4+ T cells demonstrate remarkable plasticity (Zhou et al. 2009). It appears that expression of Foxp3 by induced Treg cells (iTregs) or IL-17 by Th17 cells may not be stable. TGF-beta promotes Th17 cell differentiation in a concentration-dependent manner. At low concentrations, TGF-beta synergizes with interleukin IL-6 and IL-21 to promote IL-23 receptor (IL23R) expression, favoring Th17 cell differentiation. High concentrations of TGF-beta repress IL23R expression and favor the development of Foxp3+ Treg cells (Zhou et al. 2008). See Fig. 2.2 on the Th subset cytokines and transcription factors.
Duhen characterized a population of human skin-homing memory CD4+ T cells that express the chemokine receptors CCR10, CCR6, and CCR4 and produce IL-22 but neither IL-17 nor IFN-gamma. The differentiation of T cells producing only IL-22 is efficiently induced in naive T cells by plasmacytoid dendritic cells in an IL-6- and tumor necrosis factor-dependent manner (Duhen et al. 2009). Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor (Alam et al. 2010).
An overview of Th subsets and the cytokines they produce is presented in Fig. 2.2 (and Table 2.1). For more detailed descriptions, readers are referred to several review articles (Maddur et al. 2012; Miossec et al. 2009; Zhou et al. 2009).
Functional Compartments
Epidermis: Generation of Lichenoid Infiltrate
Following antigen stimulation or injury to the skin, there is epidermal keratinocyte activation with lymphokine release. In addition, Toll-like receptors of dendritic antigen-presenting cells (APC) located in the epidermis and dermis are activated with antigenic epitopes from microbes or foreign bodies. Following antigen activation, Langerhans cells and plasmacytoid dendritic cells migrate to the lymph nodes draining the skin to recruit more lymphocytes.
Antigen-Presenting Cells
A skin-associated lymphoid tissue (SALT) develops (Fig. 2.3). This pattern resembles the distribution of neoplastic skin lymphoid infiltrates. SALT initially are composed of T cells that are attracted to Langerhans cells, the epidermal sentinel immune cells, which are also epidermal antigen-presenting cells. It is postulated that Langerhans cells contribute or perhaps initiate the pathogenesis and recruitment of the malignant T cells in the skin, particularly in mycosis fungoides (Edelson 2001; Berger et al. 2002).
Cytology
A small population of reactive T cells, upon stimulation with certain antigens, demonstrate small cell cerebriform nuclear morphology. Anti-CD3 stimulation is one such trigger (Reinhold et al. 1994). Ultrastructural and monoclonal antibody analysis shows that a normal subset of peripheral blood cells have a cerebriform appearance (Matutes et al. 1983). In practice, less than 3 % of epidermal or dermal T cells have cerebriform nuclei. Although genetic lesions are known to be correlated with blastic transformation of these small cells, certain mitogens/antigen triggers in vitro also appear to cause blastic cytologic transformation of neoplastic cerebriform cells (Kadin et al. 1984).
Dermis: Generation of Nodular Infiltrate
Subsequently, lymphocytes primed within the epidermis accumulate in the dermis around dermal postcapillary venules and dendritic cells, likely leading to nodular and sometimes diffuse dermal lymphoid infiltrates. Recruitment and help from circulating B cells and Thf cells also come into play. B cells are seen early around capillaries and venules, then later form follicles containing germinal centers composed of B cells, with scattered follicular T helper (Tfh) cells. These cells accumulate in the T-cell ridge between the mantle and light zone. These cells, unlike the interfollicular T cells, coexpress several follicle center associated markers including CD10, Bcl-6, PD1, and CXCL13. Lymphomas arising from this subset tend to form nodules in the dermis with the expression of CD3 plus the aforementioned Tfh subset markers. Angioimmunoblastic T-cell lymphomas, primary cutaneous small- and medium-sized T-cell lymphomas, and some PTCL not otherwise specified (NOS) have been reported to express these markers. See Chap. 10 for further discussion.
NK T Cells and Gamma-Delta T Cells
Gamma-delta T and NK cells respond to stress-associated factors and are present in the normal epithelial tissues as well as in normal human dermis. They are well-known effectors of the immediate immune response but also have other unique surveillance abilities. These cells express receptors for homing to non-inflamed skin as well as surface receptors for the recognition of allogeneic tumor cells and are reported to be part of the immune surveillance for the elimination of cutaneous tumors and distressed cells (Ebert et al. 2006).
T-Cell Reaction Followed by Accumulation of B Cells
Memory T cells expressing alpha-beta appear to be a major component of skin tropic cells with immune surveillance against previously encountered antigens (Schaerli et al. 2004). Hence, repeated stimulation of skin may induce accumulation of alpha-beta T cells along with NK and gamma-delta T cells.
In vivo findings support this accumulative cellular migration sequence with a dominant CD4+ effector T-cell subset reaction (Fuhlbrigge et al. 1997; Kupper and Edelson 1987; Hoeller et al. 2009) in the epidermis and into the dermis. These CD4+ T cells migrate from dermal venules and infiltrate between bundles of dermal collagen fibers to form nodules within the dermis (Matheu et al. 2008). Two-photon microscopy studies, which allow imaging of movement of live cells, indicate a defined sequence of cell populations trafficking to the skin from draining lymph nodes. This trafficking is more conspicuous among CD4+ T cells than CD8 T cells (Gebhardt et al. 2011). B-cell transition is slower than T-cell migration (Gebhardt et al. 2009; Mackay et al. 1988; Wakim et al. 2008; Tomura et al. 2008).
Pathogens and Dendritic Cells
The expansion of T helper subsets into defined Th1, Th2, or Th17 profiles appears to be induced by specific types of pathogens. Th1 cells function in autoimmunity and immune response to intracellular pathogens. They secrete IFN-gamma. Th2 cells, which deal with extracellular parasites and are also active in allergy and asthma. Th17 cells provide immunity against a variety of extracellular pathogens, including bacteria such as Klebsiella pneumoniae and fungi such as Cryptococcus neoformans and Candida (Pelletier et al. 2010). The Th17 axis is active in the pathogenesis of a range of dermatological diseases including allergic contact dermatitis, atopic dermatitis, psoriasis, and scleroderma (Asarch et al. 2008).
Th17 and MF
There is limited evidence of Th17 differentiation of tumor cells in mycosis fungoides, particularly in cases with accumulating neutrophils (Ciree et al. 2004) or with a higher rate of progression to advanced stages (Litvinov et al. 2010).
Epithelioid Histiocytes and Granulomas
Epithelioid histiocytes and tissue macrophages aggregate as discrete clusters within the dermis or subcutaneous tissue as granulomas. These arise as an immune system response to an antigen, in which epithelioid macrophages and inflammatory and immune effector cells accumulate. Chronic granulomas are often surrounded by fibrosis or a lymphocyte cuff, but early formation appears as microgranulomas or lymphohistiocytic clusters. Presence of granulomas is often equated with infections, but a variety of noninfectious triggers should also be kept in mind such as autoimmune disorders including rheumatoid skin nodules, Churg-Strauss syndrome, or iatrogenic injections, vaccinations, and lymphoproliferative disorders. Among the latter is a granulomatous form of mycosis fungoides, which can be associated with slack skin.
Histiocytosis arising from tumors of antigen-presenting cells as well as those common in children should also be considered in the proper setting. See discussion in Chap. 14 of histiocytic lesions of the skin.
Subcutaneous Tissue
The subcutaneous tissue or hypodermis is composed of fat and blood vessels. Commonly involved by transformed and diffuse lymphomas, it is seldom inflamed except in certain types of panniculitis. See Chap. 11 for discussion.
The deep vascular plexus is located just above this layer and seldom is involved by perivascular lymphoid infiltrates. In contrast, the superficial vascular plexus just beneath the epidermis is often the site of inflammatory T-cell reactions in dermatitis and psoriasis (Kunstfeld et al. 1997; Lechleitner et al. 1999). Changes in endothelial structure and acquisition of addressins facilitate skin homing above the subcutis (Lechleitner et al. 1999). Gamma-delta T cells and their malignant counterparts can be partially localized to the panniculus (Magro and Wang 2012). The less frequent skin epithelial localization of these cells and their relative lower density compared to alpha-beta T cells are well recognized (Hocker et al. 2012).
Immunohistochemistry Panel for Evaluation of Lymphohemotoporetic Cutareous Diseases
An immunohistochemical panel is often used in the evaluation of lymphohematopoietic cutaneous diseases. Here we list the commonly used antibodies and show their normal tissue cross-reactions in addition to the usual targets. See list of commonly used antibodies in Table 2.2 and illustrations demonstrating their usefulness in cutaneous hematopathology (Figs. 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 2.10, 2.11, 2.12, 2.13, 2.14, 2.15, 2.16, 2.17, 2.18, 2.19, 2.20, 2.21, 2.22, 2.23, 2.24, 2.25, 2.26, 2.27, 2.28, 2.29, 2.30, 2.31, 2.32, 2.33, and 2.34).
References
Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2010;107:5943–8.
Asarch A, Barak O, Loo DS, Gottlieb AB. Th17 cells: a new therapeutic target in inflammatory dermatoses. J Dermatolog Treat. 2008;19(6):318–26.
Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D. Innate immune functions of human gamma delta T cells. Immunobiology. 2008;213(3–4):173–82.
Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol. 2013;4:163.
Berger CL, Hanlon D, Kanada D, Dhodapkar M, Lombillo V, Wang N, Christensen I, Howe G, Crouch J, El-Fishawy P, Edelson R. The growth of cutaneous T-cell lymphoma is stimulated by immature dendritic cells. Blood. 2002;99(8):2929–39.
Ciree A, Michel L, Camilleri-Broet S, Jean LF, Oster M, Flageul B, Senet P, Fossiez F, Fridman WH, Bachelez H, Tartour E. Expression and activity of IL-17 in cutaneous T-cell lymphomas (mycosis fungoides and Sezary syndrome). Int J Cancer. 2004;112(1):113–20.
Clark RA. Skin-resident T, cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130(2):362–70.
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63.
Ebert LM, Meuter S, Moser B. Homing and function of human skin gamma delta T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176(7):4331–6.
Edelson RL. Cutaneous T, cell lymphoma: the helping hand of dendritic cells. Ann N Y Acad Sci. 2001;941:1–11.
Egawa G, Honda T, Tanizaki H, Doi H, Miyachi Y, Kabashima K. In vivo imaging of T-cell motility in the elicitation phase of contact hypersensitivity using two-photon microscopy. J Invest Dermatol. 2011;131(4):977–9.
Egawa G, Kabashima K. Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J Invest Dermatol. 2011;131(11):2178–85.
Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389(6654):978–81.
Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10(5):524–30.
Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477(7363):216–9.
Hocker TL, Wada DA, El-Azhary R, Gibson LE. Expression of T-cell receptor-gamma delta in normal human skin, inflammatory dermatoses and mycosis fungoides. J Cutan Pathol. 2012;39(4):419–24.
Hoeller C, Richardson SK, Ng LG, Valero T, Wysocka M, Rook AH, Weninger W. In vivo imaging of cutaneous T-cell lymphoma migration to the skin. Cancer Res. 2009;69(7):2704–8.
Holtmeier W, Kabelitz D. gamma delta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151–83.
Kabelitz D. Function and specificity of human gamma/delta-positive T cells. Crit Rev Immunol. 1992;11(5):281–303.
Kabelitz D. gamma delta T-cells: cross-talk between innate and adaptive immunity. Cell Mol Life Sci. 2011;68(14):2331–3.
Kabelitz D, Glatzel A, Wesch D. Antigen recognition by human gamma delta T lymphocytes. Int Arch Allergy Immunol. 2000;122(1):1–7.
Kabelitz D, Marischen L, Oberg HH, Holtmeier W, Wesch D. Epithelial defence by gamma delta T cells. Int Arch Allergy Immunol. 2005;137(1):73–81.
Kadin ME, Nasu K, Sako D, Su IJ. Distinctive phorbol ester-induced morphological and surface antigen changes in mycosis fungoides, the Sezary syndrome, and adult T-cell leukemia. Cancer Res. 1984;44(8):3383–7.
Kadin ME, Pavlov IY, Delgado JC, Vonderheid EC. High soluble CD30, CD25, and IL-6 may identify patients with worse survival in CD30+ cutaneous lymphomas and early mycosis fungoides. J Invest Dermatol. 2012;132(3 Pt 1):703–10.
Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23(6):611–20.
Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38(9):2369–76.
Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, Ubriani R, Vittorio CC, Junkins-Hopkins JM, Wysocka M, Rook AH. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115(4):798–812.
Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128(11):2625–30.
Kunstfeld R, Lechleitner S, Groger M, Wolff K, Petzelbauer P. HECA-452+ T cells migrate through superficial vascular plexus but not through deep vascular plexus endothelium. J Invest Dermatol. 1997;108(3):343–8.
Kupper TS, Edelson RL. The role of cytokines in the pathophysiology of T cell mediated skin disease. J Dermatol. 1987;14(6):517–23.
Lechleitner S, Kunstfeld R, Messeritsch-Fanta C, Wolff K, Petzelbauer P. Peripheral lymph node addressins are expressed on skin endothelial cells. J Invest Dermatol. 1999;113(3):410–4.
Litvinov IV, Jones DA, Sasseville D, Kupper TS. Transcriptional profiles predict disease outcome in patients with cutaneous T-cell lymphoma. Clin Cancer Res. 2010;16(7):2106–14.
Mackay CR, Kimpton WG, Brandon MR, Cahill RN. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J Exp Med. 1988;167(6):1755–65.
Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181(1):8–18.
Magro CM, Wang X. Indolent primary cutaneous gamma/delta T-cell lymphoma localized to the subcutaneous panniculus and its association with atypical lymphocytic lobular panniculitis. Am J Clin Pathol. 2012;138(1):50–6.
Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, De la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29(4):602–14.
Matutes E, Robinson D, O’Brien M, Haynes BF, Zola H, Catovsky D. Candidate counterparts of Sezary cells and adult T-cell lymphoma-leukaemia cells in normal peripheral blood: an ultrastructural study with the immunogold method and monoclonal antibodies. Leuk Res. 1983;7(6):787–801.
Merz H, Fliedner A, Orscheschek K, Binder T, Sebald W, Muller-Hermelink HK, et al. Cytokine expression in T-cell lymphomas and Hodgkin’s disease. Its possible implication in autocrine or paracrine production as a potential basis for neoplastic growth. Am J Pathol. 1991;139:1173–80.
Meyerson HJ, Awadallah A, Pavlidakey P, Cooper K, Honda K, Miedler J. Follicular center helper T-cell (TFH) marker positive mycosis fungoides/Sezary syndrome. Mod Pathol. 2013;26:32–43.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–98.
Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–43.
Reinhold U, Herpertz M, Kukel S, Oltermann I, Uerlich M, Kreysel HW. Induction of nuclear contour irregularity during T-cell activation via the T-cell receptor/CD3 complex and CD2 antigens in the presence of phorbol esters. Blood. 1994;83(3):703–6.
Rodriguez Pinilla SM, Roncador G, Rodriguez-Peralto JL, Mollejo M, Garcia JF, Montes-Moreno S, et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am J Surg Pathol. 2009;33:81–90.
Saed G, Fivenson DP, Naidu Y, Nickoloff BJ. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas Sezary syndrome expresses a Th2-type profile. J Invest Dermatol. 1994;103(1):29–33.
Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, Moser B. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199(9):1265–75.
Streilein JW. Lymphocyte traffic, T-cell malignancies and the skin. J Invest Dermatol. 1978;71(3):167–71.
Streilein JW. Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol. 1983;80(Suppl):12s–616.
Streilein JW. Circuits and signals of the skin-associated lymphoid tissues (SALT). J Invest Dermatol. 1985;85(1 Suppl):10s–313.
Streilein JW. Skin-associated lymphoid tissue. Immunol Ser. 1989;46:73–96.
Streilein JW, Bergstresser PR. Langerhans cells: antigen presenting cells of the epidermis. Immunobiology. 1984;168(3–5):285–300.
Tomura M. Visualization of cellular dynamics in the entire body using the photoconvertible protein “Kaede” of transgenic mice. Seikagaku. 2012;84(3):195–202.
Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, Kanagawa O. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc Natl Acad Sci U S A. 2008;105(31):10871–6.
Vowels BR, Lessin SR, Cassin M, Jaworsky C, Benoit B, Wolfe JT, Rook AH. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994a;103(5):669–73.
Vowels MR, Tiedemann K, Lam-Po-Tang R, Tucker DP. Use of granulocyte-macrophage colony-stimulating factor in two children treated with cord blood transplantation. Blood Cells. 1994b;20(2–3):249–54.
Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol. 2008;181(9):5837–41.
Wasik MA, Vonderheid EC, Bigler RD, Marti R, Lessin SR, Polansky M, Kadin ME. Increased serum concentration of the soluble interleukin-2 receptor in cutaneous T-cell lymphoma. Clinical and prognostic implications. Arch Dermatol. 1996;132(1):42–7.
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom BE, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, Sabat R. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl). 2009;87(5):523–36.
Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–40.
Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kadin, M.E., Cualing, H.D. (2014). Functional Organization of the Skin as an Immune Response Organ: Skin, Immune Response, and Useful Immunohistochemistry. In: Cualing, H., Kadin, M., Hoang, M., Morgan, M. (eds) Cutaneous Hematopathology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-0950-6_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-0950-6_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-0949-0
Online ISBN: 978-1-4939-0950-6
eBook Packages: MedicineMedicine (R0)