Abstract
Adequate dialysis requires a measure of the dose, which includes the extent and intensity of each treatment as well as the frequency of treatments. To measure the dose, attention should be directed to the primary goal of dialysis, which is to remove toxins normally eliminated by the patient’s native kidneys, with the ultimate goal of reducing their concentrations in body tissue to sub-toxic levels. Since we have not identified all of the toxins and cannot measure their levels, the focus of measurement should be on clearance of measurable solutes using an easily dialyzed representative such as urea or sodium. Allowances must be made for the self-limiting nature of intermittent hemodialysis, which reduces the treatment efficiency. The dose measurement must include the influences of convection, solute generation, and residual native kidney function, and consideration must be given to the effects of flow and red cell mass within the dialyzer as well as hollow-fiber permeability expressed as a mass transfer coefficient for each solute of interest. Additional attention must be given to sequestration of toxic solutes within the patient, protein binding of toxins, and the residual risk factors that remain after the dialysis treatment has been optimized. This chapter reviews the expressions of hemodialysis dose that have evolved over the past 50 years, such as Kt/V, and provides detailed methods for assessing dialyzer function and for measuring small solute clearances using single- and multi-compartment models of urea kinetics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dialysis dose
- Uremia
- Hemodialysis
- Small solutes
- Diffusion
- Clearance
- Urea
- Kt/V
- Kinetic models
- Hemodialysis adequacy
1 Historical Perspective
Evidence that equilibration of the blood with an isotonic salt solution across a semipermeable membrane as a potential method for removing unwanted substances from the body including drugs and uremic toxins dates back many years [1–3]. However, it was not until Dr. Willem J. Kolff successfully applied hemodialysis (HD) to treat a patient with acute kidney failure that the hypothesized benefit for patients suffering from uremia was proven [4]. This landmark event also confirmed the previous logical hypothesis that the cause of the immediate life-threatening aspect of uremia is from accumulation of small (dialyzable) solutes that normally appear in the urine. The reversal of a previously fatal disease was considered miraculous (patients sometimes awakened from uremic coma during the procedure), so little thought was given to measuring the treatment or determining its adequacy. Perhaps because of its complexity, physicians at the time, including its inventor, also felt that its application should be limited to management of reversible acute kidney disease, serving to allow time for the native kidneys to recover. Not until 1960, with the development of a permanent vascular access device, was management of chronic kidney disease accepted, and a quest for measurement of the dose and its adequacy begun [5, 6].
2 Measuring Diffusion, the Basic Principle of Dialysis
How does the patient and family know that he/she had a good dialysis? Probably after a poor dialysis the patient might feel better, having avoided the symptoms of clinical disequilibrium that often follow significant solute and fluid removal. How does one measure the effect of dialysis? Simply keeping the patient alive is not enough, and one can argue further that even if the patient reports feeling well, the caregiver should not be satisfied. Measuring the dialysis dose and assessment of its adequacy should be anticipatory, identifying inadequacies at an early stage to allow corrections before the symptomatic stage. To answer the patient’s question, the focus should be on the dialysis objective: removal of solute by simple diffusion across a semipermeable membrane.
Since the pioneering work of Thomas Graham [7] and Adolph Fick [8] in the mid to late 1800s, the driving force for diffusion of solutes and gases has been recognized as the concentration of the gas or solute. Most importantly, the rate of diffusion (e.g., bulk movement of solute) is directly proportional to the concentration gradient. Fick’s first law of diffusion has been adapted to dialysis [8]:
Js is the rate of solute movement or flux (e.g., mg/min), Ko is a membrane-specific and solute-specific constant (e.g., cm/min), A is the membrane area (e.g., cm2), ΔC is the solute concentration gradient across the membrane (e.g., mg/mL).
The proportionality constant KoA in Eq. 3.1 is defined as the ratio of flux (Js) to the concentration gradient (ΔC) across the membrane, which is essentially the definition of dialysance: a measurement similar to clearance that takes into consideration solute concentrations on both sides of the membrane. For a hollow-fiber kidney, KoA can be considered the initial clearance at the proximal end of the fibers before any buildup of solute on the dialysate side. When the dialysate concentration is zero, the denominator is simply the blood concentration, and clearance is then equal to dialysance. KoA can also be considered the dialyzer’s maximum clearance at infinite blood and dialysate flow rates. It is a dialyzer-specific measure used to compare the effectiveness of different hollow-fiber dialyzers, but it is also solute-specific (e.g., KoA values for urea and creatinine are different for the same dialyzer). Similar to clearance, which is determined by, but independent of either solute concentrations or flux, KoA is also independent of blood and dialysate flow rates. Its value can be determined by measuring the cross-dialyzer clearance at specified blood and dialysate flow rates [9]:
Qb and Qd are effective blood and dialysate flow rates respectively, and Kd is the dialyzer solute clearance. Equation 3.2, known as the Michael’s equation after its developer [9], is based on an exponential decline in solute concentration along the membrane as blood and dialysate flow in opposite directions for maximum efficiency.
More importantly, once the dialyzer KoA has been determined, a rearrangement of Eq. 3.2 can be used to predict the clearance for any blood and dialysate flow rate:
3 Intermittent Dialysis is Self-Limiting
Despite the constant nature of KoA and the constancy of clearance during a single HD at fixed Qb and Qd, intermittent dialysis is intrinsically self-limiting. For peritoneal dialysis (PD), the clearance (but not the dialysance) gradually falls with time and will eventually extinguish during a single exchange of fluid as solute concentrations in the dialysate completely equilibrate with the patient’s blood concentrations. For intermittent HD, clearances remain constant during the treatment because fresh dialysate is constantly supplied, but the treatment’s effectiveness falls as concentrations in the patient’s blood fall. In the absence of replenishment (G), removal of solute during HD would also extinguish with time (despite a constant Kt/V). This self-limiting feature of dialysis results both from solute buildup on the dialysate side (PD) and from reduction in solute concentrations on the blood side. In other words, for intermittent dialysis, the more one dialyzes the less solute is removed. Fortunately, uremic toxicity is also concentration-dependent, such that dialysis is more effective for the more toxic patient .
4 Diffusion in a Flowing Circuit
Figure 3.1 shows what happens inside the dialyzer as blood flows from inlet to outlet and dialysate flows in the countercurrent direction. Solute transfer from blood to dialysate depends on both flow rates and the membrane permeability to each solute. The gradient across the membrane diminishes with time and with distance along the membrane. For solutes with high membrane permeability, the gradient diminished more rapidly with distance as shown in Fig. 3.1a. The downstream dissipation of the gradient is correctable by increasing the blood flow, which explains the flow dependency of clearance. For solutes with low permeability, distance along the membrane has less impact, so solute removal is more dependent on membrane permeability and less dependent on flow as shown in Fig. 3.1b. For patients dialyzed intermittently (e.g., three times weekly) the gradient also diminishes with time and would eventually extinguish in the absence of new solute generation. This accounts in part for the inefficiency of intermittent dialysis as discussed below.
Within the hollow fiber, solutes diffuse across the membrane only from the water fraction of the blood. Because macromolecules like serum lipids and proteins occupy space that excludes water-soluble molecules, they reduce the effective blood flow to about 93 % of the whole blood flow. The role of larger blood components such as erythrocytes depends on the solute. For solutes like urea that diffuse rapidly across red cell membranes the patient’s hematocrit has little influence on clearance, so solute delivery to the membrane is essentially a function of blood water flow, including erythrocyte water [10, 11]. For solutes like creatinine, phosphorus, and uric acid with negligible diffusion from red cells during the 10–20 s transit through the dialyzer, effective flow is restricted to plasma water, which must be used to measure clearances (Table 3.1) [12, 13]. However, red cells contain significant amounts of these solutes that eventually equilibrate with the plasma after leaving the dialyzer. This phenomenon explains in part why creatinine clearances have not been popular as a measure of dialysis adequacy ; the post-dialyzer plasma creatinine concentration is spuriously low and may require several hours to equilibrate with red cells in the same blood sample.
Between dialyses, in addition to solutes, the patient accumulates water. Removal is easily accomplished during dialysis by applying hydrostatic pressure across the dialysis membrane. Since the resulting convective loss of fluid and solute is in the same direction as diffusive solute movement, it adds to the effectiveness of the dialysis. However, the augmenting effect of filtration is less than might be expected because convective transfer of solute across the membrane diminishes the gradient for diffusion, and in contrast to diffusive loss of easily dialyzed solutes like urea, convective losses occur along the entire length of the hollow fiber [14]. At the distal end where urea concentrations may be reduced by 70–80 %, convective transfer of solute is greatly diminished. Equation 3.4 is used to quantify instantaneous solute removal by convection, and illustrates the dilution effect.
Kd is the dialyzer clearance, Qb is the dialyzer blood outflow, Cin and Cout are the inflow and outflow solute concentrations respectively, and Qf is the ultrafiltration flow rate. Note that if Cout is zero, that is, solute removal is complete, Qf adds nothing to dialyzer clearance .
For high-flux dialyzers where filtration rates are typically an order of magnitude greater than for conventional-flux dialyzers, convective fluid removal at the proximal end of the hollow fiber is much greater than at the distal end where oncotic effects may cause filtration to move in the opposite direction, so-called back-filtration [15]. This effect counteracts the negative effect of filtration on diffusion and may contribute to the higher clearances achieved by high-flux dialyzers [16, 17]. For all modes of dialysis, contraction of blood and extracellular fluid volume due to solute-deprived fluid removal helps to maintain the concentration at the blood inlet for a longer time, and thereby increases the effectiveness of the dialysis. This phenomenon highlights the importance of including fluid volume shifts in the mathematical models of dialysis urea kinetics (see below).
5 Origin of Kt/V
The concentration of solute is the driving force for diffusion, and the rate of diffusion is directly proportional to the concentration as noted in Eq. 3.1. Ignoring the effects of volume changes and solute generation, the change in concentration (C) with time (t) can be simplified and expressed mathematically as:
The symbol k is the elimination constant, similar to that of an injected drug, and indicates that the fractional change in concentration (dC/C)/dt is constant during the treatment. When expressed as a fraction of the distribution volume (V), k × V is the clearance (K), which is also constant, since in this overly simplified example we assume that V does not change. Integration of Eq. 3.5 and substituting K/V for k yields:
C0 is the initial concentration and C is the concentration at time (t). Logarithmic transformation of Eq. 3.6 yields:
The left side of the overly simplified Eq. 3.7 (Kt/V) is the fractional clearance expressed per dialysis and normalized to body size (V). The denominator (V) adds value as a correlate to lean body mass, which is usually more desirable than body weight as a normalizing factor for body size. Equation 3.7 helps to illustrate the strong dependence of the clearance (expressed as Kt/V) on the ratio of solute concentrations in two blood samples, one at the beginning (C0), and one at the end of the treatment (C). Note that the ratio is used, not the absolute concentrations, and also note that none of the components of the Kt/V expression need to be measured independently, including the treatment time (t).
If urea is the solute, and its generation (G) and volume changes (ΔV) during the dialysis are included, Eq. 3.8 (see below) must be substituted for Eq. 3.7, but the fundamental strong dependence of Kt/V on pre/post-urea concentrations remains .
6 Modeling Urea Kinetics
Regardless of what we think is going on within the hollow-fiber membranes during dialysis, it is possible to precisely model solute flux, including the effect of ultrafiltration , using a mass balance approach where input equals output. Figure 3.2 depicts the elements contributing to urea mass balance within the patient during and between dialyses. Equation 3.8 is the solution to the mass balance equations in Fig. 3.2 and provides a practical estimate of fluctuating serum urea concentrations while the patient’s urea volume varies usually by several kilograms during and between treatments. Equation 3.8 also incorporates residual native kidney function and urea generation, and is used as the fundamental tool for modeling urea kinetics.
Single-compartment model of urea mass balance. Equation 3.8 is the explicit solution to the differential equation in this figure, which is used to resolve Kt/V and G from a single pre-dialysis BUN and a single post-dialysis BUN. V is the urea distribution volume, C is the urea concentration, Kd is the dialyzer clearance, Kr is the kidney clearance, and G is the urea generation rate. K is the sum of Kd and Kr during dialysis, and is equal to Kr between dialyses
C is the solute concentration at any time (t), C0 is the initial concentration, V is the solute distribution volume, ΔV is the rate of fluid removal, G is the solute generation rate, Kr is the patient’s native kidney solute clearance, and Kd is the dialyzer clearance.
Urea modeling uses Eq. 3.8 in a reverse manner. The modeler measures C and C0 (analogous to Eq. 3.7) then solves for G and Kt/V using computerized iterations of Eq. 3.8. The modeler must also have knowledge of volume fluxes (ΔV), Kr, and t, although these are less critical. Equation 3.8 yields a profile of the BUN during and between treatments and repeats itself weekly because the interdialysis treatment intervals are asymmetric during the week. Each treatment is assumed to be identical, but the patient begins the treatment differently because of the time asymmetry. For example, if dialysis is performed three times per week, the patient will have accumulated solute for 2 or 3 days depending on the day of the week. Equation 3.8 is solved (by iteration) twice, once during dialysis , and again between dialyses when Kd is zero. Note that the results are expressed in relative terms, as a fraction of the patient’s urea volume. For example, to resolve V, knowledge of Kd is necessary and vice versa. Ordinarily, the user provides an estimate of Kd, which is assumed to be constant throughout the treatment as noted above; Kd and KoA can be measured using samples collected simultaneously from the blood inflow and outflow ports or estimated using Eqs. 3.2 and 3.3.
During dialysis, Kd has the major influence; between dialyses G dominates. This means that Kt/V is primarily determined by the pre-dialysis and post-dialysis BUN values (see Eq. 3.7), and G is determined by the post-dialysis and subsequent pre-dialysis BUN values. Because the primary modeling outcome is Kt/V, an independent measure of Kd is not required, and errors in estimates of Kd have little influence on the resulting Kt/V dose measurement. Similar to Eq. 3.7, the ratio of post- to pre-BUN values determines Kt/V; absolute values are not considered. Absolute values, however, can be used to measure G using an iterative method as depicted in Fig. 3.3, eliminating the need to sample blood again at the next dialysis [18]. Since urea is an end product of protein metabolism, G can be converted to a protein equivalent, a net protein catabolic rate normalized to V (PCRn), as shown in Eq. 3.9 [19]. PCRn can be useful as an adjunct to dietary counseling :
Measuring G with only 2 BUN values. The upper graph shows a weekly BUN profile generated by Eq. 3.8 that uses an excessively high value for G. In the middle graph the value is too low. By repeated iteration, a value for G is found (lower graph) that matches the pre-dialysis BUN with the end-week BUN. [18]
7 More Refined Modeling
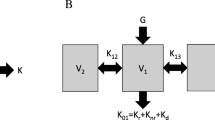
The single-compartment (single V) model diagramed in Fig. 3.2 predicts BUN concentrations during and between dialyses, but the results do not coincide precisely with measured values, especially for short intense dialysis as shown in Fig. 3.4. BUN values are overestimated during dialysis and underestimated between dialyses, especially in the immediate post-dialysis period. The cause of these discrepancies is delayed diffusion among the patient’s body compartments, most notably intracellular versus extracellular, which reduces the effective volume of distribution during dialysis and causes a rebound in concentration as the two compartments re-equilibrate post dialysis. Despite the unique and rapid diffusibility of urea across red cell membranes as noted above, urea kinetics in the remainder of the body are better described by a two-compartment model, as shown in Fig. 3.5. This model is similar to the single-compartment model depicted in Fig. 3.2 with an added remote compartment volume (V2) and concentration (C2). Unfortunately, the addition of a second compartment complicates the mathematics such that the equations depicted in Fig. 3.5 are not easily resolved explicitly and require more complex mathematical manipulations for a solution [20]. A method using numerical analysis has been implemented and made available on an Internet site devoted to dialysis dosing [21].
Two-compartment diffusion model. Fast iterative resolution of the two differential equations shown in this figure yield values for V1, V2, KC, and G. V1 is the dialyzed compartment volume, V2 is the remote compartment volume, and KC is the inter-compartment mass transfer coefficient. Other symbols are the same as defined in Fig. 3.2
The single-compartment assumption causes the errors in predicted concentrations as shown in Fig. 3.4 but when used to calculate the dialysis dose as Kt/V, the two errors during and after the end of dialysis tend to cancel each other; the resulting values for Kt/V (and Kd) calculated by each model are similar, justifying clinical use of the simpler model [22]. Despite this minimization of the single-compartment error, some authorities have objected to using the immediate post-dialysis BUN as an indicator of the dialysis dose, since it is falsely low if compared to the equilibrated value shown in Fig. 3.6. The latter is determined by extrapolating the late inter-dialysis concentration curve back to the immediate post-dialysis time, which essentially converts the patient’s urea kinetics to a single compartment but with an equilibrated clearance (eK). The resulting eK and eKt/V are always lower than the dialyzer instantaneous clearance and single pool Kt/V (spKt/V). The lowered clearance is an effective whole body clearance defined as the removal rate divided by the average urea concentration in the patient’s body compartments at the time the removal rate is measured. eKt/V was used in the HEMO Study (see below) as the target for randomization [23], and by the European Best Practice Guideline Expert Group as a target for HD adequacy in general [24]. Fortunately, a two-compartment model is not needed to calculate eKt/V; approximations based on the intensity of dialysis have been developed [25–27], one of which is shown here [26]:
Source of eKt/V. The equilibrated post-dialysis BUN shown here as the large circle is obtained by extrapolating measured post-dialysis BUN values. It is always higher than the BUN measured immediately post dialysis (shown just below it). Whole body eKt/V, which is derived from the equilibrated BUN, is always lower than spKt/V, which is derived from the immediate post-dialysis BUN
To complicate the model further, the immediate rebound in urea concentration post dialysis is not entirely due to delayed diffusion. Disequilibrium within the blood compartment is caused by multiple parallel circuits with markedly different blood flow rates [28]. The most rapidly flowing circuit is the route through the patient’s arteriovenous fistula , heart and lungs, and back [29]; this cardiopulmonary (CP) circuit has a round-trip circulation time of 5–15 s depending on the patency of the fistula and the patient’s cardiac output. The CP circuit also happens to be the dialyzed circuit, all others feeding into it from venous return. As a result the urea concentration falls to a lower level in the CP circuit during dialysis, as much as 20 mg/dl lower than in the periphery, and it rebounds within about 2 min when the blood pump is stopped [28]. This flow-related disequilibrium differs from the diffusion -related disequilibrium (Fig. 3.6) with respect to several factors listed in Table 3.2.
In addition to reducing solute clearance, disequilibrium has a significant impact on the method for drawing the post-dialysis BUN. A method that yields a modeled dialyzer clearance equivalent to the actual cross-dialyzer clearance is shown in Table 3.3. If the sample is drawn too soon, before potential access recirculation has dissipated, the dialysis dose , expressed as a delivered clearance, will be overestimated, putting the patient in jeopardy from under-dialysis. If drawn too late, the dose will be inconsistent from treatment to treatment.
8 Intermittent Versus Continuous Dialysis
Solute disequilibrium is a consequence of high clearances applied intermittently. This phenomenon together with the self-limiting nature of intermittent dialysis as described above reduces the treatment efficiency, which means that more dialysis (clearance × time) must be applied to achieve the same concentration-lowering effect as continuous dialysis . When dialysis is applied continuously (e.g., continuous PD) or for native kidney function, constant replenishment of solute on the blood side (G) eliminates this inefficiency, and solute disequilibrium is essentially nonexistent. When minimum standards for PD and HD are compared, it appears that patients maintained with continuous PD require approximately half of the weekly clearance × time required by HD patients. Two theories have been put forth to explain this observation. One is based on peak urea concentrations, claiming that peak concentrations correlate better with overall uremic toxicity than mean levels, and the other is based on solute disequilibrium, claiming that toxic solutes are sequestered in remote compartments that equilibrate more slowly with the dialyzed compartment, essentially preventing the dialyzer from completing its job. Slow continuous treatments eliminate peaks and allow time for equilibration. Both theories have a basis in mathematical modeling and both produce similar solute concentration profiles under a variety of conditions as discussed below under “Dosing Frequent Dialysis” [30].
Although less efficient than continuous treatment, intermittent treatments are much easier to measure. Continuous clearances such as PD or native kidney function require collections of urine and/or dialysate during a defined time period. Intermittent hemodialysis clearances only require measuring the change in blood concentrations from beginning to end of the treatment and applying a model of solute kinetics as described above. Blood sampling alone is required; collection of dialysate is not necessary.
9 Practical Differences Between Hemodialysis Kt/V and Native Kidney Glomerular Filtration Rate (GFR)
In comparison with the native kidney, the clearance concept and definition are the same but the methods for measuring and expressing clearance differ, as shown in Table 3.4. For HD, urea is the preferred marker solute instead of creatinine because of the red cell creatinine disequilibrium discussed above, the additional patient-specific information obtained about protein nutrition, and the sensitivity of urea clearances to dialyzer effectiveness. Urea is not favored as a measure of native kidney clearance because tubular urea reabsorption is variable and unpredictable. Most current incenter dialysis treatments are intermittent, so the expression of dose must take into account the time during which the patient is not dialyzed. Expressing the dose as a clearance per dialysis satisfies this requirement as long as the frequency is specified as part of the dose. Instead of body surface area, the denominator for the dialysis dose is the volume of urea distribution, an automatic result of urea kinetic modeling as noted above and a mathematical convenience. Lastly, the fluctuations in urea concentration between and during intermittent dialyses allow measuring the dose by mathematical modeling without need for dialysate collection.
10 Dosing Frequent Dialysis
As noted above, the efficiency of HD depends on the frequency, increasing with more frequent treatments and eventually reaching maximum efficiency with continuous treatment. To include frequency in the dose, the peak concentration hypothesis [31] has been applied, which redefines the clearance as the removal rate divided by the average peak concentration [32]. This newly defined continuous equivalent clearance , called “standard Kt/V” (stdKt/V) is expressed as a fractional clearance similar to spKt/V, but as a weekly clearance similar to PD. The target is slightly higher than the target for continuous PD (2.0 per week) and is independent of dialysis frequency. Figure 3.7 shows the relationship between spKt/V and stdKt/V for different frequencies of dialysis. Of note, the current minimum standard for spKt/V is 1.2 per dialysis 3×/week, which corresponds to a stdKt/V of 2.0/week as shown in Fig. 3.7.
Single pool versus continuous equivalent (standard) Kt/V. The single-pool dose per dialysis on the horizontal axis is compared to the equivalent (standard) weekly dose on the vertical axis. When given three times weekly, the currently accepted minimum dose is 1.2 per dialysis, which closely matches the minimum dose in the USA for continuous PD (large circle)
An explicit simplified equation, based on a fixed volume urea kinetic model has been developed for converting eKt/V to stdKt/V [27]:
A recent modification of Eq. 3.11 allows variations in urea volume and Kr [33]:
S is the patient’s stdKt/V from Eq. 3.11; Ufw is the patient’s weekly fluid removal in ml; F is the dialysis weekly frequency; V is the patient’s urea distribution volume in ml; Kr is the patient’s native kidney urea clearance in ml/min; 10080 is the number of minutes in a week.
11 Adequacy of the Dose
The question of adequacy relates to native kidney function as well as dialysis. We have a vague sense that GFRs > 20 ml/min are adequate, but some patients are able to tolerate GFRs as low as 5–10 ml/min for sustained periods of time [34]. The established minimum dose for PD patients is a weekly urea clearance index (Kt/V) of 1.7 [35]. The latter translates, for an average size patient with a urea volume of 30 L, to about 5 ml/min. Recall that GFR overestimates urea clearance because urea is reabsorbed by the native kidney, and it underestimates creatinine clearance because creatinine is secreted. Since the dialyzer has neither reabsorptive nor secretive functions, urea clearance should correspond to native kidney GFR on average. This reasoning leads to a conclusion that the minimum level of dialysis for continuous PD is equivalent to a barely acceptable level of native kidney function; hence the word “minimum” should be emphasized. For HD patients, standard Kt/V (see above) has been introduced to allow comparisons among more frequent and continuous clearances, including native kidney function. Published USA guidelines specify a minimum stdKt/V 2.0/week, which translates to about 7 ml/min for an average size patient. These surprisingly low levels of replacement function are based on outcomes studies such as the HEMO and ADEMEX studies that failed to show improvement in mortality and various secondary outcomes including hospitalization rates when the dose was increased [23, 35].
Reports of improved outcomes in patients dialyzed more frequently led investigators to suggest that intermittent treatments have intrinsic limitations that can only be overcome by increasing the frequency of treatments to 4–6 sessions per week. Solute kinetic analysis also suggested that increasing the treatment time would be more effective when applied more than 3×/week (see Fig. 3.7). In keeping with these theoretical considerations and marked benefits reported from uncontrolled studies, controlled clinical trials showed significant improvements in patient outcomes but somewhat less impressive than anticipated. The US National Institutes of Health-sponsored Frequent Hemodialysis Network study found that short daily incenter dialysis for 1 year improved the primary composite outcome of survival + reduction in left ventricular (LV) mass [36], the latter mainly in patients with ventricular hypertrophy. Quality of life was also improved. A similar improvement in LV mass was noted in a smaller Canadian study that compared frequent nocturnal HD with standard treatments given three times per week [37]. Together with findings of a significant reduction in pre-dialysis blood pressure, the data suggest that accumulation of fluid between dialyses is detrimental, but correctable by an increase in dialysis frequency. Phosphorus control was also improved as evidenced by lower pre-dialysis serum concentrations and a reduced requirement for oral phosphate binders. Whether the predictable increase in removal of other small solutes contributed to the clinical improvements is not possible to dissect from the data. For the present, more frequent dialysis is recommended for patients who prefer it and for patients with poor control of BP, volume, or serum phosphorus.
It is important to distinguish between adequate dialysis and adequate care of the patient. These distinct concepts are sometimes confused. Dialysis is the major focus of the nephrologist, but it is only a subset of the latter. Care certainly would be considered inadequate if it consisted only of dialysis and assessment of the dialysis dose . Measures of the adequacy of care in other spheres are also required. Patients approaching the need for dialysis usually bring with them a legacy of medical problems some of which may have contributed to the decline in kidney function. These problems are not necessarily alleviated or even improved by dialysis, and usually require attention, sometimes more attention than the dialysis itself.
12 Influence of Native Kidney Function on the Dose
Considered precious and frequently measured in the months and years prior to starting dialysis, residual native kidney function (Kr) is largely ignored once dialysis has begun. Perhaps use of terminology such as “replacement therapy” gives the impression that it no longer matters. The fallacy of this concept was well shown by the Netherlands Cooperative study where the mortality rate in patients with no Kr exceeded that of patients even with a Kr of 1–3 ml/min by an order of magnitude [38, 39]. For patients managed with PD, Kr is measured with each assessment of dialysis adequacy but the practice of collecting the patient’s urine to measure Kr in HD patients is unusual. Several factors may explain this seemingly strange behavior: (1) PD patients are schooled in self-care and tend to be more self-directed. (2) Adequacy of dialysis is more difficult to measure in PD patients so it is done only 3 or 4 times/year instead of monthly in HD patients. (3) Combining Kr with Kd is conceptually easier in PD patients where simple addition suffices (see below).
Once the dialysis dose is reduced, Kr must be monitored carefully to guard against under-dialysis when kidney function deteriorates further. Opponents of Kr measurements point to the negative psychological impact on patients whose treatment time requires an increase when Kr diminishes or is lost. Caregivers must then struggle to convince the patient that a higher dose of dialysis is necessary. Financial providers might also object to equal pay for reduced and full (anuric) doses of dialysis (Table 3.5) [38, 40–48].
Regardless of efforts to measure Kr, efforts to preserve native kidney function in patients prior to initiating dialysis should be continued after dialysis is started. Table 3.6 lists recommended precautions and practices to preserve Kr.
Combining native kidney urea clearance with continuous dialysis clearance is a simple matter of addition, but combining with intermittent (HD) urea clearance requires manipulation of the data to account for their non-simultaneous occurrences. As noted above, intermittent dialysis is less efficient than continuous dialysis, so adjustments for differences in efficiency must be made as well. The first method listed in Table 3.7 was also the first used and continues to be applied:
Kd is the dialyzer clearance , Td is the treatment time, Kr is the patient’s native kidney clearance, Tr is the inter-dialysis interval, and V is the urea distribution volume. Since Kr has its major impact between dialyses, it is reasonable to use the inter-dialysis time interval (first column in Table 3.8) as a multiplier when calculating Kr × Tr. To account for differences in efficiency, Tr can be inflated, as shown in the second column of Table 3.8.
The second method listed in Table 3.7 involves reducing the dialyzer component to a continuous equivalent clearance (e.g., standard K or stdKt/V as described above), followed by simple addition. Care must be taken to avoid including Kr in the method for downsizing Kd [33].
Alternatives to Urea Modeling
The urea reduction ratio (URR), defined as (C0 − C)/C0 where C0 is the pre-dialysis BUN and C is the post-dialysis BUN, is a crude measure of urea extraction during a single dialysis. Its strength is simplicity, and it involves little or no manipulation of the raw data, two advantages that are perhaps the reasons it was chosen by the US Centers for Medicare and Medicaid Services (CMS) for monitoring its constituent dialysis clinics. The URR cannot be used to measure continuous clearances, does not include residual kidney function , and fails to incorporate the additional clearance afforded by ultrafiltration, sometimes as much as 20–30 % of the total Kt/V . Urea generation during dialysis is also not accounted for, an especially important factor during prolonged dialysis sessions.
Simplified formulas for estimating Kt/V from formal urea modeling are available as well. The most popular was developed by Daugirdas and recently upgraded to include more frequent dialyses [49, 50]:
R is the ratio of post-dialysis BUN to pre-dialysis BUN. This measure is especially helpful in population studies where the opportunity for modeling individual patients is not available.
Cross-dialyzer solute clearance can be measured as a change in conductivity in response to a pulsed change in the inlet dialysate concentration [51, 52]. Most dialysis delivery systems monitor dilution of a dialysate concentrate using conductivity meters, so the machine is already poised to measure “conductivity clearance,” better termed “ionic dialysance.” Since sodium and its accompanying anion are responsible for > 90 % of the dialysate conductivity, conductivity changes simply reflect sodium dialysance, which is nearly identical to the clearance of urea (and other small solutes). Figure 3.8 shows the pulsed change in conductivity induced on the dialysate inlet side (ΔCin) and the response (ΔCout) on the outlet side recorded by conductivity electrodes placed in the inflow and outflow dialysate lines. The ionic dialysance is calculated as [51, 53]:
Equation 3.15 provides an instantaneous measure of small solute clearance, equivalent to cross-dialyzer urea clearance. It must be measured several times during the dialysis to obtain an average for the entire treatment to generate a measure equivalent to urea Kt/V. Advantages to this method include real-time monitoring, no blood sampling or analysis, no disposables, and ready use of body surface area as the denominator. Disadvantages include the need for multiple measurements during each dialysis, and need for an independent measure of V to meet current standards, which are measured as Kt/V.
Some authorities have argued that urea is a poor surrogate for uremic toxins, suggesting that Kt/V urea is inappropriate as a measure of dose [54, 55]. This argument fails to consider that absolute levels of urea are not part of Kt/V and that urea is simply a marker for small solute clearance, as noted above. Comparison with PD, however, and the development of standard Kt/V suggest that a sequestered solute might be a better marker [56, 57]. Other solutes too, such as larger (or middle) molecules and protein-bound toxins might be more representative [58–60], especially for the residual syndrome. A comparison among these marker solutes is presented in Table 3.9.
Removal of salt and water has been highlighted as an essential part of the dose or prescription [61]. Fluid accumulation between dialyses must be limited by dietary restriction, and the excess must be removed during dialysis to prevent states of fluid overload and its consequences, including hypertension, pulmonary edema, and death. Rapid removal of fluid, however, has been associated with hypotension and adverse cardiac consequences including arrhythmias and myocardial stunning [62]. Uncontrolled studies have shown that these adverse consequences are correlated with the treatment time, leading some to recommend that the patient’s treatment time be extended to a minimum of 4 h, regardless of Kt/V, and/or that a maximum rate of fluid removal be set at 10–15 ml/kg body weight [63–65]. These recommendations seem reasonable although they require more of the patient’s time, and their validity has not been established in controlled clinical studies.
Although fluid removal by ultrafiltration during dialysis is an essential requirement for most patients, it is not essential for some. In contrast to solute removal, some uremic patients require no fluid removal and conversely, removal of fluid alone will never reverse uremia. Fluid accumulation is therefore not an essential part of the uremic syndrome, and the ultrafiltration component of the dialysis dose must be considered adjunctive therapy.
13 The Future of Dosing
In view of continued high morbidity and mortality rates and failed attempts to improve the outcomes of dialysis patients including improved biocompatibility of dialyzer membranes, higher clearances, high-flux dialysis, increases in thrice weekly Kt/V, and more frequent or prolonged treatments, it is reasonable to look elsewhere for an explanation and question current methods for measuring the dialysis dose. Contributions of the native kidney to personal health may be subtle and yet to be discovered, perhaps analogous to erythropoietin support of red cell mass. Patient comorbidities, independent of the kidney failure, may contribute to the high mortality. Poorly dialyzed solutes such as those listed in Table 3.9 may be responsible. However, one must not lose sight of the remarkable ability of dialysis to prolong life that would end within a few days in an anuric patient. The prolongation of life is surely due to removal of small dialyzable (urinary) solutes, reducing their concentrations in the patient to sub-lethal levels. Dialysis does nothing more than remove small solutes by diffusion across a relatively tight semipermeable membrane. There is nothing complex or mysterious about therapeutic dialysis. Therefore, first and foremost in our responsibilities to the patient should be a measure of small solute clearance. After that, the field is open to further exploration and treatment of the residual syndrome, which should be encouraged.
References
Abel JJ, Rowntree LG, Turner BB. On the removal of diffusible substances from the circulating blood by means of dialysis. Trans Assoc Am Physicians. 1913;28:51–73.
Haas G. Dialysieren des stromenden Blutes am Lebenden: Bemerkung zu der Arbeit von Necheles in dieser Wochenschrift. Klin Wochenschr. 1923;2(Suppl):1888–92.
Haas G. Versuche der Blutauswaschung am Lebenden mit Hilfe der Dialyse. Klin Wochenschr. 1925;4:1888–95.
Kolff WJ, Berk HTJ, ter Welle M, van der Ley AJW, van Dijk EC, van Noordwijk J. The artificial kidney, a dialyzer with a great area. Acta Med Scand. 1944;117:121–8.
Scribner BH, Caner JEZ, Buri R, Hegstrom RM, Burnell JM. The treatment of chronic uremia by means of intermittent dialysis: a preliminary report. Trans Am Soc Artif Intern Organs. 1960;6:114–9.
Gotch FA, Sargent JA, Keen ML. Individualized, quantified dialysis therapy of uremia. Proc Clin Dial Transpl Forum. 1974;1:27–37.
Graham T. Liquid diffusion applied to analysis. Phil Trans Royal Soc London. 1861;151:183–93.
Fick A. Ueber diffusion. Annln Phys. 1855;94:59–74.
Michaels AS. Operating parameters and performance criteria for hemodialyzers and other membrane-separation devices. Trans Am Soc Artif Intern Organs. 1966;12:387–92.
Cheung AK, Alford MF, Wilson MM, Leypoldt JK, Henderson LW. Urea movement across erythrocyte membrane during artificial kidney treatment. Kidney Int. 1983;23(6):866–9.
Sands JM, Timmer RT, Gunn RB. Urea transporters in kidney and erythrocytes. Am J Physiol. 1997;273(3 Pt 2):F321–39.
Descombes E, Perriard F, Fellay G. Diffusion kinetics of urea, creatinine and uric acid in blood during hemodialysis. Clinical implications. Clin Nephrol. 1993;40(5):286–95.
Schneditz D, Yang Y, Christopoulos G, Kellner J. Rate of creatinine equilibration in whole blood. Hemodial Int. 2009;13(2):215–21.
Husted FC, Nolph KD, Vitale FC, Maher JF. Detrimental effects of ultrafiltration on diffusion in coils. J Lab Clin Med. 1976;87:435–42.
Baurmeister U, Travers M, Vienken J, Harding G, Million C, Klein E, et al. Dialysate contamination and back filtration may limit the use of high-flux dialysis membranes. ASAIO Trans. 1989;35(3):519–22.
Dellanna F, Wuepper A, Baldamus CA. Internal filtration–advantage in haemodialysis? Nephrol Dial Transplant. 1996;11(Suppl 2):83–6.
Santoro A, Conz PA, De Cristofaro V, Acquistapace I, Gaggi R, Ferramosca E, et al. Mid-dilution: the perfect balance between convection and diffusion. Contrib Nephrol. 2005;149:107–14.
Depner TA, Cheer A. Modeling urea kinetics with two vs. three BUN measurements. A critical comparison. ASAIO J. 1989;35:499–502.
Borah MF, Schoenfeld PY, Gotch FA, Sargent JA, Wolfson M, Humphreys MH. Nitrogen balance during intermittent dialysis therapy of uremia. Kidney Int. 1978;14:491–500.
Schneditz D, Daugirdas JT. Formal analytical solution to a regional blood flow and diffusion-based urea kinetic model. ASAIO J. 1994;40:M667–73.
Daugirdas JT, Depner TA, Greene T, Silisteanu P. Solute-solver: a web-based tool for modeling urea kinetics for a broad range of hemodialysis schedules in multiple patients. Am J Kidney Dis. 2009;54(5):798–809.
Depner TA. Multicompartment models. Prescribing hemodialysis: a guide to urea modeling. Boston: Kluwer Academic Publishers; 1991. pp. 91–126.
Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–9.
European Best Practice Guidelines Expert Group on Hemodialysis ERA. Section II. Haemodialysis adequacy. Nephrol Dial Transplant. 2002;17(Suppl 7):16–31.
Daugirdas JT. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther. 1995;2:295–304.
Tattersall JE, DeTakats D, Chamney P, Greenwood RN, Farrington K. The post-hemodialysis rebound: predicting and quantifying its effect on Kt/V. Kidney Int. 1996;50:2094–102.
Leypoldt JK, Jaber BL, Zimmerman DL. Predicting treatment dose for novel therapies using urea standard Kt/V. Semin Dial. 2004;17(2):142–5.
Depner TA, Rizwan S, Cheer AY, Wagner JM, Eder LA. High venous urea concentrations in the opposite arm. A consequence of hemodialysis-induced compartment disequilibrium. ASAIO J. 1991;37:141–43.
Schneditz D, Kaufman AM, Polaschegg HD, Levin NW, Daugirdas JT. Cardiopulmonary recirculation during hemodialysis. Kidney Int. 1992;42:1450–6.
Depner TA. Benefits of more frequent dialysis: lower TAC at the same Kt/V. Nephrol Dial Transpl. 1998;13:20–4.
Keshaviah PR, Nolph KD, Van Stone JC. The peak concentration hypothesis: a urea kinetic approach to comparing the adequacy of continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis. Peritoneal Dial Int. 1989;9:257–60.
Gotch FA. The current place of urea kinetic modelling with respect to different dialysis modalities. Nephrol Dial Transpl. 1998;13(Suppl 6):10–4.
Daugirdas JT, Depner TA, Greene T, Levin NW, Chertow GM, Rocco MV. Standard Kt/Vurea: a method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 2010;77(7):637–44.
Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–19.
Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307–20.
Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–300.
Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298(11):1291–9.
Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–70.
Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726–32.
Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, et al. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients. A longitudinal study. Nephrol Dial Transplant. 1995;10:2295–305.
Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12(10):2158–62.
Brown PH, Kalra PA, Turney JH, Cooper EH. Serum low-molecular-weight proteins in haemodialysis patients: effect of residual renal function. Nephrol Dial Transplant. 1988;3(2):169–73.
Amici G, Virga G, Rin G D, Grandesso S, Vianello A, Gatti P, et al. Serum beta-2-microglobulin level and residual renal function in peritoneal dialysis. Nephron. 1993;65(3):469–71.
Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int. 2003;64(6):2238–43.
Marquez IO, Tambra S, Luo FY, Li Y, Plummer NS, Hostetter TH, et al. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol. 2011;6(2):290–6.
Wang AY, Wang M, Woo J, Law MC, Chow KM, Li PK, et al. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Int. 2002;62(2):639–47.
Wang AY, Woo J, Wang M, Sea MM, Sanderson JE, Lui SF, et al. Important differentiation of factors that predict outcome in peritoneal dialysis patients with different degrees of residual renal function. Nephrol Dial Transplant. 2005;20(2):396–403.
Wang AY, Woo J, Sea MM, Law MC, Lui SF, Li PK. Hyperphosphatemia in Chinese peritoneal dialysis patients with and without residual kidney function: what are the implications? Am J Kidney Dis. 2004;43(4):712–20.
Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–13.
Daugirdas JT, Leypoldt JK, Akonur A, Greene T, Depner TA. Improved equation for estimating single-pool Kt/V at higher dialysis frequencies. Nephrol Dial Transplant. 2013;28(8):2156–60.
Petitclerc T, Bene B, Jacobs C, Jaudon MC, Goux N. Non-invasive monitoring of effective dialysis dose delivered to the haemodialysis patient. Nephrol Dial Transplant. 1995;10:212–6.
Gotch FA, Panlilio FM, Buyaki RA, Wang EX, Folden TI, Levin NW. Mechanisms determining the ratio of conductivity clearance to urea clearance. Kidney Int Suppl. 2004;89:S3–24.
Filippo S D, Manzoni C, Andrulli S, Pontoriero G, Dell’Oro C, La Milia V, et al. How to determine ionic dialysance for the online assessment of delivered dialysis dose. Kidney Int. 2001;59(2):774–82.
Vanholder R, DeSmet R, Lesaffer G. Dissociation between dialysis adequacy and Kt/V. Semin Dial. 2002;15(1):3–7.
Eloot S, Van Biesen W, Glorieux G, Neirynck N, Dhondt A, Vanholder R. Does the adequacy parameter Kt/V(urea) reflect uremic toxin concentrations in hemodialysis patients? PLoS ONE. 2013;8(11):e76838.
Depner TA. Uremic toxicity: urea and beyond. Semin Dial. 2001;14(4):246–51.
Ishizaki M, Matsunaga T, Itagaki I. What is a surrogate marker for optimal dialysis? Hemodial Int. 2007;11(4):478–84.
Eloot S, Torremans A, De SR, Marescau B, De WD, De Deyn PP, et al. Kinetic behavior of urea is different from that of other water-soluble compounds: the case of the guanidino compounds. Kidney Int. 2005;67(4):1566–75.
Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008;73(10):1174–80.
Meyer TW, Sirich TL, Hostetter TH. Dialysis cannot be dosed. Semin Dial. 2011;24(5):471–9.
Foundation NK. K/DOQI clinical practice guidelines and clinical practice recommendations for 2006 Updates: hemodialysis adequacy, peritoneal dialysis adequacy, and vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S1–322.
McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3(1):19–26.
Kurella M, Chertow GM. Dialysis session length (“t”) as a determinant of the adequacy of dialysis. Semin Nephrol. 2005;25(2):90–5.
Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79(2):250–7.
Flythe JE, Curhan GC, Brunelli SM. Disentangling the ultrafiltration rate-mortality association: the respective roles of session length and weight gain. Clin J Am Soc Nephrol. 2013;8(7):1151–61.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Depner, T. (2016). Hemodialysis Dose. In: Magee, C., Tucker, J., Singh, A. (eds) Core Concepts in Dialysis and Continuous Therapies. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-7657-4_3
Download citation
DOI: https://doi.org/10.1007/978-1-4899-7657-4_3
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-7655-0
Online ISBN: 978-1-4899-7657-4
eBook Packages: MedicineMedicine (R0)