Abstract
Although peritoneal dialysis is a form of portable and wearable dialysis, it requires patients to use fresh dialysate and either perform a number of manual daytime exchanges or use a cycler machine. As such the search has been to develop internal implantable, or external wearable or portable, devices that do not rely on a supply of fresh dialysate, so allowing patients to perform the activities of daily living without restriction, and similarly to be able to drink and eat freely without restriction.
Although the concept of wearable and portable dialysis devices dates back to the pioneering days of the start of dialysis as a long-term treatment for patients with advanced chronic kidney disease, it is only recently with developments in nanotechnology-manufacturing processes, coupled with microcircuit designs and the resurgent interest in sorbent technology that has allowed animal trials and now the first human trials of these devices to take place.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
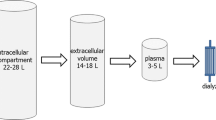
Cellular metabolism leads to the production of nitrogenous waste products which are transported out of cells through active or passive transport systems. These compounds may then circulate freely in plasma water, or bound to proteins and lipids. Although intermittent haemodialysis may effectively clear small water-soluble solutes from plasma water, the clearance of many of the nitrogenous waste products of metabolism is dependent on the rate of passage of solutes from intracellular compartment to extracellular compartment and the dynamic equilibrium between binding to proteins and lipids and the free plasma concentration. As such, a continuous dialysis modality would potentially prove to be a more effective treatment in clearing the waste products of cellular metabolism (Fig. 14.1). In addition, a continuous treatment modality would permit a slower ultrafiltration rate allowing patients to better tolerate fluid removal, so reducing the risk of treatment-associated hypotension and the potentially adverse effects of hypoperfusion on the heart and brain.
2 Historical Developments in Designing a Wearable Dialysis Device
Haemodialysis was originally restricted to patients with acute kidney injury due to the technical problems of obtaining vascular access , the limited availability of haemodialysis machines, clotting within the extracorporeal circuit and providing reliable quality dialysate. It was only when these hurdles were overcome in the mid-to-late 1960s that regular haemodialysis for patients with chronic kidney disease started to become an established treatment. However, it was soon appreciated that life-saving regular thrice-weekly therapy imposed numerous restrictions on patients’ lifestyles, not only in terms of limiting dietary and fluid intake but also limiting the distance that a patient could live from a dialysis centre. As such, the concept of a wearable dialysis device is not new and dates back to the 1970s [1], but the technology at that time did not permit a solution.
The first step in developing a wearable device was to invent a portable haemodialysis machine, which could then allow patients to travel beyond their dialysis centre [2]. The first commercially available device, which could be transported in an automobile, was powered by rechargeable batteries and used a 20-L batch of fresh dialysate, which was then regenerated by passing the spent dialysate through a charcoal module [3]. These early pioneers reported successful treatment outcomes for a small number of patients treated with short daily treatment sessions of less than 2 h. However, the device weighed some 17 kg mainly due to the weight of the large rechargeable batteries and the blood and dialysate pumps, and then with the dialysate weighed around 40 kg [4]. After an initial wave of enthusiasm, the design team abandoned their project as they were unable to significantly reduce the weight and size of the haemodialysis machine [5].
The next major technological advance in the design for a wearable dialysis device followed advances in sorbent technology. Sorbents potentially allowed the regeneration of spent dialysate, so permitting effective dialysis without the constant need for a ready supply of fresh dialysate. Charcoal, although an effective adsorbent for many of the compounds which are retained in patients with chronic kidney disease, including β2 microglobulin, bilirubin, indoxyl sulphate, p-aminohippurate and 3-carboxy-4-methyl-5-propyl-2 furanpropionic acid, does not effectively bind urea. Thus alternative strategies were required to clear urea. One approach was to enzymatically degrade urea using urease which cleaves urea to produce ammonia and carbon dioxide. As ammonia is potentially toxic, any ammonia produced has to be removed before it can circulate back to the patient. This then led to the development of a layered sorbent system, as although activated carbon adsorbs compounds, other sorbents are essentially ionic exchangers, adsorbing some compounds but releasing others in exchange (Fig. 14.2). The Redy® system using this layered sorbent technology was commercially introduced into North America and Western Europe, and so permitted haemodialysis patients greater freedom to travel and dialyse away from home and dialysis centres [6].
The next wave of enthusiasm for developing wearable haemodialysis devices came following the introduction of haemofiltration. Improvements in dialyzer design and biomaterials now permitted patients to be treated by continuous ultrafiltration [7]. These early pioneering devices used the patient’s native arterial pulse pressure to provide the hydrostatic pressure required for ultrafiltration, using either a Scribner shunt at the wrist (radial artery) or a Thomas shunt in the groin (femoral artery), with ultrafiltration simply controlled by manually adjusting a gate clamp. These simple experimental devices had many hurdles to overcome not only regulation of blood flow and ultrafiltration rates but also patient safety and as such were never commercially developed [7]. Although these wearable haemofiltration devices could successfully control fluid balance they could not satisfactorily control uraemia, as large volumes of ultrafiltrate were needed to obtain sufficient uraemic solute clearance, and this would then require the reinfusion of a corresponding amount of fresh replacement fluid to prevent volume depletion. Some designers tried to overcome this problem by recycling the spent ultrafiltrate through a sorbent cartridge and then returning the regenerated fluid back to the patient [8]. However, these designs then increased device complexity by adding a number of pumps, as blood flow had to be regulated to provide a controlled ultrafiltrate flow through the sorbents, and a continuous heparin infusion was required to prevent clotting in the extracorporeal circuit [8]. Although described as a wearable haemofiltration device by the inventors, this prototype was only used to treat two hospital inpatients. The sorbent cartridges based on the sorbent system used by the Redy® system quickly became saturated and had to be changed up to three or four times each day [9]. As such, this device was never commercially developed.
Newer dialysis machine developments based on a batch dialysate system either made from domestic water or using sterile bags of dialysate have been recently designed for the home haemodialysis market, such as the NxStage System 1 (NxStage Medical Inc, Lawrence, Massachusetts, USA) [10]. These machines allow patients to travel and dialyse in hotels and houses, but again patients essentially still require an automobile to pack the dialysis machine and bags of sterile dialysate.
Due to the technological challenges in developing a truly wearable hemodialysis device, there has been little progress until recently.
3 Current Approaches to Developing a Wearable Dialysis Device
3.1 Continuous Wearable Peritoneal Dialysis Devices
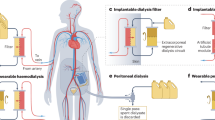
It could be argued that peritoneal dialysis (PD) is both a wearable and portable dialysis therapy. However, continuous ambulatory peritoneal dialysis (CAPD) typically requires three or four exchanges with fresh dialysate each day, and although automated cycler peritoneal dialysis (APD) typically allows the patient daytime freedom, the patient has to be connected to the machine overnight, and treatment requires the use and storage of relatively large volumes of fresh dialysate and then the disposal of spent dialysate and used consumables. As such, a number of designs have been proposed to develop a wearable PD system based on sorbent technology to regenerate spent dialysate effluent to limit additional fresh dialysate fluid exchanges.
The Vicenza group proposed a wearable artificial kidney (ViWAK) [11] which utilised a novel dual lumen peritoneal catheter so allowing a continuous flow of peritoneal dialysate into and out of the peritoneum driven by a small light weight battery powered mini-pump. The ViWAK was a relatively simple device, as it required the patient to start the day by instilling a fresh standard glucose-based dialysate, which was then left to dwell for 2 h. Thereafter the dialysate pump would start and peritoneal dialysate then continuously recycled with spent dialysate pumped first through a filter to remove proteins and prevent them coating the sorbents, and then a series of sorbent cartridges containing a mixture of microporous activated carbon and polystyrene resin. Then in the evening, the spent dialysate was to be drained out and a fresh 7.5 % icodextrin dialysate instilled for overnight dialysis. The system relied on residual renal function and the icodextrin exchange to achieve volume control. The inventors proposed that the device could potentially be modified to include a small pouch of glucose, which could then be added to the recycled dialysate to improve ultrafiltration [11]. As such, the proposed ViWAK required two standard CAPD exchanges each day compared to the conventional four.
Currently, the ViWAK has only been tested in short-term in vitro experiments and awaits animal and human clinical trials to determine the adsorptive capacity of the sorbents .
An alternative design based on peritoneal dialysis is the automated wearable artificial kidney (AWAK) which differs from the ViWAK in having a traditional single lumen peritoneal dialysis catheter . As dialysate flow is discontinuous with the AWAK, a reservoir is required to store regenerated dialysate before it is pumped back into the patient [12] (Fig. 14.3 and 14.4). In contrast to the ViWAK, the AWAK device is designed to continuously regenerate dialysate from a single standard glucose containing peritoneal dialysate for up to 1 month. As such the dialysate not only has to be recycled through a series of sorbents but also refreshed by adding glucose, bicarbonate and other electrolytes. Thus, the design is more complex than the ViWAK requiring an additional chamber containing electrolytes, lactate and glucose to refresh the regenerated dialysate. The sorbents contain urease to enzymatically degrade urea, then as ammonia is released the device requires a sensor to check that no ammonia is returned to the patient, and alarm to inform the patient that the sorbents have become saturated and need changing.
The AWAK differs from conventional peritoneal dialysis in that it is designed around a high-flow tidal peritoneal dialysis prescription , with an initial fresh glucose containing peritoneal dialysate fill of approximately 750 ml which is then recirculated in a tidal manner at 4.0 L/h [13] powered by a rechargeable battery driven pump, with any ultrafiltrate generated over an 8–10-h period drained into a separate bag attached to the daily exchangeable module (Fig. 14.3). As the battery requires recharging, the patient needs to connect the device to mains electricity overnight. In addition, the AWAK is designed to have both daily and monthly disposable sections, designed for ease of replacement (Fig. 14.4), with the daily replacement section containing the sorbents, reservoir, degassing unit and refreshing electrolyte/bicarbonate/glucose pouch. To change this section the patient would have to perform a standard drain out and then once re-inserted, start again instilling a fresh glucose-based dialysate. As such, if the sorbents had to be replaced twice or three times a day, then not only would there be the financial costs of replacing items, but in essence the patient would be performing three manual daytime exchanges and then overnight recharging the battery using mains electricity.
To put this into context, a healthy 70 kg dialysis patient would be expected to generate around 9–10 g of urea nitrogen daily when eating a recommended dietary protein intake of 1 g/kg [14]. Although urease and 250 g of zirconium can readily catalyze 2 g urea/h and adsorb ammonia released, this projected urea clearance would exhaust the sorbent combination within a day. As such, this would require either more sorbent with an increased weight, or more than one daily cartridge exchange. Thus the AWAK has been designed with two sorbent cartridges of different size: the smaller one designed to extract 3.5 g urea nitrogen and the larger one to remove 10 g urea nitrogen [15].
The concept behind the AWAK in terms of high-flow tidal peritoneal dialysis has been tried in patients for up to 5 h as a proof of concept study, with an extrapolated weekly Kt/Vurea of 3.4–4.5 depending upon patient transporter characteristics. Although the AWAK has been trialed in animal experiments, the first clinical trial remains awaited.
3.2 Continuous Wearable Haemodialysis Devices
The challenges to design and manufacture a wearable continuous haemodialysis device are somewhat similar to those for a peritoneal dialysis device but have two more complications due to the need for continuous blood access and prevention of clotting in the extracorporeal circuit. In the intensive care setting, continuous renal replacement therapy (CRRT) using slower blood and dialysate flows than standard intermittent haemodialysis can deliver both significantly higher small and middle molecular weight solute clearances.
Two different approaches are currently at the design stage and undergoing trials. Firstly, a European consortium based in Holland using a simple modification of the CRRT circuit in which the ultrafiltrate is passed through a nanoporous biopolymer made from clay designed to absorb albumin bound toxins, and the ‘cleaned’ ultrafiltrate is then returned to the patient after a further mini-filter to ensure no sorbent microparticles pass back into the patient [16]. Potentially, some of the ultrafiltrate can be discarded to regulate hydration status. So far, this device has been used in a goat model of uraemia with short-term experimental results reporting a urea removal 10–15 mmol/h, creatinine 0.6 mmol/h, potassium 2.0 mmol/h and phosphate 0.75 mmol/h. Although effective, these preliminary data should be interpreted with caution, as removal rates are dependent upon serum concentrations, and protein caking of the dialyzer capillary fibres, and may also fall with time as sorbents become saturated. However, as this design has no dialysate and there is no replacement solution containing electrolytes or bicarbonate, then patients treated by such a device will most likely require additional bicarbonate supplementation to correct the metabolic acidosis of chronic kidney disease, and in addition may develop electrolyte imbalances due to differences in adsorption and release of cations by the sorbents. The current prototype does not have any separate ultrafiltration module, although this is anticipated in later designs. As yet this device has had limited testing in an animal model as a proof of concept trial, and will require additional animal model testing and require the addition of safety control features prior to human trials.
The other device, termed as wearable artificial kidney (WAK), was developed in Los Angeles and differred in a number of key issues. First, it employs a small, lightweight battery-powered pump that contains two chambers, so that when one is full the other is empty. This changes the pressure either side of the capillary fibre (Fig. 14.5), whereas with the conventional haemodialysis machine, roller blood pump design pressure is constant, and as such this novel pump design reduces protein deposition on the capillary surface so maintaining solute clearances over time [17]. Second, as the risk of clotting in the extracorporeal circuit is increased at blood–air interfaces, the arterial and venous reservoir and air-detector have been removed from the circuit and replaced by water impermeable but gas permeable plastic tubing to allow for the removal of carbon dioxide microbubbles, formed by the reversible reaction between bicarbonate and hydrogen ions and water and carbon dioxide, with the reaction equilibrium depending upon the pressure and temperature within the extracorporeal circuit. Similarly as urease is also used in the sorbent system, gas permeable plastic tubing is also used in the dialysate circuit again to remove carbon dioxide microbubbles. As the dialysate needs to be regenerated, then after passage through a series of sorbents the small volume of recirculating dialysate is ‘refreshed’ by the infusion of bicarbonate and electrolytes to correct metabolic acidosis and maintain electrolyte balance due to ion exchange with the sorbents (Fig. 14.6).
This device has been tested in the laboratory and in both animal experiments of acute kidney injury and also in dialysis dependent chronic kidney disease patients for up to 8 h [18]. These studies have shown that the small solute, urea and creatinine clearances were similar to those achieved by CRRT; however, the relative clearance of phosphate and beta 2 microglobulin were higher than anticipated, most likely due to the action of the pulsatile pump reducing protein deposition of the capillary dialyzer membrane [19]. Safety studies did not show any significant haemolysis with the novel blood/dialysate pump design. Similarly, the sorbents were not exhausted after 72 h in animals and after 8 h in humans. Unfractionated heparin was used to anticoagulated the WAK circuit and provided the systemic activated partial thromboplastin time was maintained > 60 s, no extracorporeal clotting was observed. However, it must be recognised that these are preliminary studies, and these encouraging results need to be confirmed in further longer duration clinical trials to determine the life expectancy of the sorbents and the frequency of sorbent exchange. In the long-term oral anticoagulants, either factor Xa or thrombin inhibitors may well be a better option than heparin, due to the individual sensitivity to unfractionated heparin and the potential side effects of long-term heparin exposure including osteoporosis and heparin induced thrombocytopaenia.
One problem with sorbents for wearable devices is balancing the amount and weight of sorbent against the duration of activity. More recently activated mesoporous carbon has been introduced as a sorbent. This differs from the traditional activated microporous carbon in that the pores are larger, and as such this allows proteins including albumin to permeate through the structure which not only increases the effective absorptive capacity by increasing relative surface area but also increases removal of protein bound azotaemic toxins (Fig. 14.7). Due to the rekindling of interest in sorbents [20], this has led to the design and creation of sorbents specifically targeting different solutes (Table 14.1). Traditional sorbents do not clear urea, and as such many devices have used enzymatic clearance. Although activated microporous carbons do not bind urea, some of the newer mesoporous carbons can bind urea, although this may be due to the mixer agents used to create the three-dimensional structure of the mesocarbon monoliths. Other approaches to remove urea include electro-oxidisation using carbon electrodes, although this process releases both carbon dioxide and chlorine, so requiring not only a degassing unit to remove carbon dioxide but also an additional carbon filter to remove chlorine.
4 Alternative Strategies to Wearable Dialysis Devices—Implantable Devices
4.1 The Implantable Artificial Kidney
A totally different approach is to try and design an artificial dialysis device based on the normal human kidney, with a filtration unit and then a tubular unit to process the ultrafiltrate. If such a device could be implanted inside a patient then potentially it could operate without the patient having to change sorbent cartridges, juggle with anticoagulant dosing and manage connection and disconnection problems and avoid the potential infection risks associated with central venous access catheters used by the external wearable haemodialysis devices and changing modules. Such a device could also be offered to a wider spectrum of patients than the wearable devices. However, to be successful not only has the device to provide adequate solute clearances and allow regulation of hydration status but also the lifespan of any such implantable artificial kidney device anastomosed to the iliac vessels and connected to the bladder would have to be of sufficient duration to overcome the disadvantages of replacement or subsequent removal of the whole system or parts of the device.
As an implantable device would use the patient’s arterial pulse pressure to drive ultrafiltration, it requires no external pump or electrical supply. However, designing the vascular access then becomes the first hurdle in developing any implantable device. Whereas, synthetic arterial grafts have been a major clinical advance, both synthetic and vein grafts used for arterio-venous vascular access have had limited success in dialysis patients, due to venous stenoses and thrombosis. Preliminary in vivo animal studies using synthetic silicon darts inserted into pig femoral veins for vascular access darts led to the development of mural thrombi and adherent clot. However, this could be reduced by modifying the silicon surface of the dart with polyethylene glycol [21]. However, further extensive work is required before these vascular access devices could be used on humans.
The traditional dialyzer design would not be able to provide the ultrafiltration volume required to be an effective artificial glomerulus. As such the proposed artificial glomerulus has been designed with a nanotechnology produced silicon-based parallel multi-slit membrane, similar to street storm drain designed to remove flood water (Fig. 14.8) [22]. Nanotechnology manufacturing can reliably produce flat-sheet silicon wafer membranes with elongated 5–10 nm slit-shaped pores that in vitro and in vivo testing have matched predicted hydraulic permeability and steric and electrostatic hindrances [23].
However, this design, although very efficient at removing ultrafiltrate, poses the problem of haemoconcentration and deposition of plasma proteins at the dialyzer membrane surface, as such increasing protein deposition. Protein fouling of a biomaterial is a complex sequence of events resulting in soluble proteins being irreversibly deposited on the dialyzer membrane [24]. Although short-term in vitro studies have shown that modification of the silicon membrane with polyethylene glycol retained hydraulic permeability and sieving curves over 4 days, additional longer animal studies are required before this technology could be tried on humans, and it may well be that a different approach is required to achieve the longevity required of an implantable device.
The normal kidney produces an ultrafiltrate of around 140 l/day, and as such any implantable artificial ‘glomerular’ device has to be linked to a ‘tubule’ device designed to reabsorb large volumes of ultrafiltrate, so that patients only pass 1–2 L of urine per day, yet excrete sufficient waste products to remain healthy. The reverse osmosis membrane used in conventional haemodialysis is designed to treat large volumes of fluid, and produce a much smaller volume or purified water. As such an artificial tubule could potentially be developed based on this design of a coiled membrane. However compared to the native renal tubule the key to any artificial device is whether it could differentiate between which solutes to reabsorb and which to excrete. Unfortunately such a design remains to be created. Another possibility for the future would be to design a renal tubule cell bioreactor, but this is some years away, as current devices are somewhat large and bulky and could not be implanted as yet [25]. As such a renal tubular-based bioreactor linked to a synthetic ‘glomerular’ membrane could potentially provide both the reabsorptive capacity for the ultrafiltrate as well as providing some additional tubular metabolic control.
5 Summary
Although haemodialysis has moved from an experimental treatment restricted to patients with acute kidney injury to one providing routine outpatient treatments to more than 2 million patients worldwide, the mortality of patients with chronic kidney disease remains unacceptably high. Daily and continuous dialysis treatments allow the potential for greater solute removal and better control of hydration and as such would be expected to provide not only improved patient survival but also better quality of life by allowing more liberal fluid and dietary intake.
The advent of nanotechnology manufacturing has allowed the development of wearable artificial kidney prototypes for the treatment of patients with CKD5d, with a number of devices based on current haemodialysis and peritoneal dialysis paradigms, and three devices currently planning clinical trials. Success of these devices will depend not only upon solute removal but also their ability to control electrolyte, acid–base and volume status on one hand, but equally important success will be judged on patient acceptance and in the current financial climate the cost per treatment. These newer treatment paradigms may well not be suitable for all chronic kidney disease patients, but could potentially offer more patients the advantage of both more frequent and longer dialysis treatments than current in-centre or satellite dialysis centre-based haemodialysis programs. On the other hand, looking further into the future implantable devices could potentially offer a treatment solution for the majority, and over the next few years, proof-of-concept experiments need to be performed to show the feasibility of such innovative renal replacement devices mirroring the native kidney.
As such, we are potentially at the start of a new era in the treatment of chronic kidney disease patients with new treatment paradigms on the horizon based on wearable and implantable devices that could not only potentially improve patient survival but also quality of life.
References
Lande AJ, Roberts M, Pecker EA (1977) In search of a 24 hours per day artificial kidney. J Dialysis. 1:805–23.
Jacobsen SC, Stephen RL, Bulloch EC, Luntz RD, Kolf WJ (1975) A wearable artificial kidney: functional description of hardware and clinical results. Proc Clin Dial Transplant Forum. 5:65–71.
Stephens RL, Jacobsen SC, Atkin-Thor E, Kolf WJ (1976) A portable wearable artificial kidney (WAK): initial evaluation. Proc Euro Dial Transplant Assoc. 12:511–18.
Kolff WJ, Jacobsen S, Stephen RL, Rose D (1976) Towards a wearable artificial kidney. Kidney Int. 7(suppl):S300–4.
Stephen RL, Kablitz C, Jacobsen S, Kolff WJ (1978) Combined technological clinical approach to wearable dialysis. Kidney Int suppl. 8:S125–32.
Blumenkrantz MJ, Gordon A, Roberts M, Lewin AJ, Pecker EA, Moran JK, Coburn JW, Maxwell MH (1979) Applications of the Redy sorbent system to hemodialysis and peritoneal dialysis. Artif Organs. 3(3):230–6.
Shaldon S, Beau MC, Deschodt G, Lysaght MJ, Ramperez P, Mion C (1980) Continuous ambulatory hemofiltration. Trans Am Soc Artif Intern Organs. 26:210–23.
Murisasco A, Baz M, Boobes Y, Bertocchio P, el Mehdi M, Durand C, Reynier JP, Ragon A (1986) A continuous hemofiltration system, using sorbents for hemofiltrate regeneration. Clin Nephrol. 26(Suppl. 1):S53–7.
Murisasco A, Reynier JP, Ragon A, Boobes Y, Baz M, Durand C, Bertocchio P, Agenet C, el Mehdi M (1986) Continuous arterio-venous hemofiltration in a wearable device to treat end-stage renal disease. Trans Am Soc Artif Intern Organs. 32:567–71.
Takahashi S (2012) Future home hemodialysis—advantages of the NxStage system one. Contrib Nephrol. 177:117–26.
Ronco C, Fecondini L (2007) The Vicenza wearable artificial kidney for peritoneal dialysis (ViWAK PD). Blood Purif. 25:383–8.
Roberts M, Ash SR, Lee DB (1999) Innovative peritoneal dialysis: flow-thru and dialysate regeneration. ASAIO J. 45(5):372–8.
Lee DBN, Roberts M (2008) A peritoneal based automated wearable artificial kidney. Clin Exper Nephrol. 12:171–80.
Maroni BJ, Steinman TI, Mitch WE (1985) A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 27:58–65.
Roberts M, Lee DBN (2006) Wearable artificial kidneys. A peritoneal dialysis approach. Dial Transplant. 36:780–2.
Wester M, Simonis F, Gerritsen KG, Boer WH, Wodzig WK, Kooman JP, Joles JA (2013) A regenerable potassium and phosphate sorbent system to enhance dialysis efficacy and device portability: an in vitro study. Nephrol Dial Transplant. 28(9):2364–71.
Gura V, Davenport A, Beizai M, Ezon C, Ronco C (2009) Beta 2-microglobulin and phosphate clearances using a wearable artificial kidney: a pilot study. Am J Kidney Dis. 54:104–11.
Davenport A, Gura V, Ronco C, Beizai M, Ezon C, Rambod E (2007) A wearable hemodialysis device for patients with end-stage renal failure: a pilot study. Lancet. 370:2005–10.
Gura V, Macy AS, Beizai M, Ezon C, Golper TA (2009) Technical breakthroughs in the wearable artificial kidney (WAK). Clin J Am Soc Nephrol. 4(9):1441–8.
Ash SR (2008) The Allient dialysis system. Semin Dial. 17:164–6.
Melvin ME, Fissell WH, Roy S, Brown DL (2010) Silicon induces minimal thrombo-inflammatory response during 28-Day intravascular implant testing. ASAIO J. 56:344–8.
Hofmann CL, Fissell WH (2010) Middle-molecule clearance at 20 and 35 ml/kg/h in continuous veno-venous haemodiafiltration. Blood Purif. 29:259–63.
Fissell WH, Dubnisheva A, Eldridge AN, Fleischman AJ, Zydney AL, Roy S (2009) High-performance silicon nanopore hemofiltration membranes. J Memb Sci. 326:58–63.
Holland NB, Qiu Y, Ruegsegger M, Marchant RE (1998) Biomimetic engineering of non-adhesive glycocalyx-like surfaces using oligosaccharide surfactant polymers. Nature. 392:799–801.
Fissell WH, Kimball J, MacKay SM, Funke A, Humes HD (2001) The role of a bioengineered artificial kidney in renal failure. Annals New York Acad Sci. 944:284–95.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Davenport, A. (2016). Wearable Dialysis Devices. In: Magee, C., Tucker, J., Singh, A. (eds) Core Concepts in Dialysis and Continuous Therapies. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-7657-4_14
Download citation
DOI: https://doi.org/10.1007/978-1-4899-7657-4_14
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-7655-0
Online ISBN: 978-1-4899-7657-4
eBook Packages: MedicineMedicine (R0)