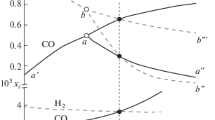
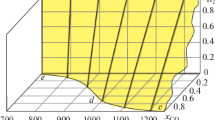
Abstract
The properties of iron oxides and chlorides allow the construction of many thermochemical cycles for water-splitting.
There are three fundamental steps in every cycle. Evaluations of these steps for different paths are given.
The state of the knowledge of the chemical reactions is examined and the critical items that need further development are indicated.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Knoche, K. F. and J. Schubert, VDI-Forsch., Heft 549, 25–32 (1972).
Hardy-Grena, C., Decomposition thermique de l’eau à travers des cycles chimiques de la famille Fe-Cl2, Report EUR-4958f (1973).
Hydrogen production from water using nuclear heat, Progress Report No. 3, EUR-5059e (1973).
Wentorf, Jr., R. H. and R. H. Hanneman, Thermochemical hydrogen generation, General Electric Company, Report No. 73CRD222 (1973).
Knoche, K. F. and J. Schubert, European Community Study Agreement, No. 045-72-7-ECID (F) (1973).
Jonin, M. V., A. A. Kozhakova, V. G. Nikitina, Zh. Prikl. Khim. 35., 900–902 (1962).
Wilson, L. E. and N. W. Gregory, Vapor-solid equilibria in the iron-chlorine system, J. Phys. Chem., 62, 433–437 (1958).
Hydrogen production from water using nuclear heat, Progress Report No. 4 (in press).
Daradimos, G. and U. Kuxmann, Erzmetall, 24 (4), 163–172 (1971).
Funk, J. E. and R. M. Reinstrom, Final Report Energy Depot Electrolysis Systems Study, Allison Division, General Motors, EDR-3714, Vol. II, Supplement A (1964).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1975 Plenum Press, New York
About this chapter
Cite this chapter
De Beni, G. (1975). Considerations on Iron-Chloride-Oxygen Reactions in Relation to Thermochemical Water-Splitting. In: Veziroğlu, T.N. (eds) Hydrogen Energy. Springer, Boston, MA. https://doi.org/10.1007/978-1-4684-2607-6_33
Download citation
DOI: https://doi.org/10.1007/978-1-4684-2607-6_33
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4684-2609-0
Online ISBN: 978-1-4684-2607-6
eBook Packages: Springer Book Archive