Abstract
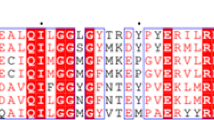
Human Class 1 and Class 2 aldehyde dehydrogenases have been sequenced at both the protein (Hempel et al., 1984, 1985) and DNA level (Hsu et al., 1988, 1989). Studies on the tertiary structure of aldehyde dehydrogenase are in progress (Baker et al., 1995, sheep Class 1; Hurley and Weiner, 1992, beef Class 2), but are not sufficiently advanced to suggest which amino acid residues are important in catalysis. Cys 302 is the only completely conserved cysteine in all known forms of the enzyme (Hempel et al., 1993), and labelling by various substrates and substrate analogues (von Bahr-Lindstrom et al., 1985; Kitson et al., 1991; Pietruszko et al., 1993) has implicated this residue as the probable active site nucleophile. This has been confirmed for the Class 2 enzyme by site-directed mutagenesis (Weiner et al., 1991). In order to establish whether Cys 302 is also the active site nucleophile for Class 1 aldehyde dehydrogenase we decided to carry out mutagenesis at Cys 302. A separate mutant was constructed in which Cys 301, the adjacent residue, was changed to alanine while Cys 302 was left unchanged.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Baker, H.M., Brown, R.L., Dobbs, A.J., Kitson, K.E., Kitson, T.M. and Baker, E.N., 1995, Crystallisation of sheep liver cytosolic aldehyde dehydrogenase in a form suitable for high resolution X-ray structural analysis, These Proceedings.
Dickinson, F.M., Hart, G.J. and Kitson, T.M., 1981, The use of pH-gradient ion-exchange chromatography to separate sheep liver cytoplasmic aldehyde dehydrogenase from mitochondrial enzyme contamination, and observations on the interaction between the pure cytoplasmic enzyme and disulfiram, Biochem. J., 199: 573–579.
Ghenbot, G. and Weiner, H., 1992, Purification of liver aldehyde dehydrogenase by p-hydroxyacetophenone-Sepharose affinity matrix and the coelution of chloramphenicol acetyl transferase from the same matrix with recombinantly expressed aldehyde dehydrogenase, Protein Expression Purif. 3: 470–478.
Hempel, J., von Bahr-Lindström, H. and Jörnvall, H., 1984, Aldehyde dehydrogenase from human liver: primary structure of the cytoplasmic isoenzyme, Eur. J. Biochem., 141: 21–35.
Hempel, J., Kaiser, R. and Jörnvall, H., 1985, Mitochondrial aldehyde dehydrogenase from human liver: primary structure, differences in relation to the cytosolic enzyme, and functional correlations, Eur. J. Biochem., 153: 13–28.
Hempel, J., Nicholas, H. and Lindahl, R., 1993, Aldehyde dehydrogenases: widespread structural and functional diversity within a shared framework, Protein Science, 2: 1890–1900.
Hsu, L.C., Bendel, R.E. and Yoshida, A., 1988, Genomic structure of the human mitochondrial aldehyde dehydrogenase gene, Genomics, 2: 57–65.
Hsu, L.C., Chang, W.-C. and Yoshida, A., 1989, Genomic structure of the human cytosolic aldehyde dehydrogenase gene, Genomics, 5: 857–865.
Hurley, T.D. and Weiner, H., 1992, Crystallisation and preliminary X-ray investigation of bovine liver mitochondrial aldehyde dehydrogenase, J. Mol. Biol., 227: 1255–1257.
Kao, P.N. and Karlin, A., 1986, Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues, J. Biol. Chem., 261: 8085–8088.
Kitson, T.M., 1982a, Further studies of the action of disulfiram and 2,2′-dithiodipyridine on the dehydrogenase and esterase activities of sheep liver cytoplasmic aldehyde dehydrogenase, Biochem. J., 203: 743–754.
Kitson, T.M., 1982b, The activation of aldehyde dehydrogenase by diethylstilboestrol and 2,2′-dithiodipyridine, Biochem. J., 207: 81–89.
Kitson, T.M. and Loomes, K.M., 1985, Modification of thiol groups in cytoplasmic aldehyde dehydrogenase, Alcohol, 2: 97–101.
Kitson, T.M., Hill, J.P. and Midwinter, G.G., 1991, Identification of a catalytically essential nucleophilic residue in sheep liver cytoplasmic aldehyde dehydrogenase, Biochem. J., 275: 207–210.
Kunkel, T.A., Roberts, J.D. and Zakour, R.A., 1987, Rapid and efficient mutagenesis without phenotypic selection, Methods Enzymol., 154: 367–382.
Loomes, K.M. and Kitson, T.M., 1989, Studies of the action of symmetrical and mixed disulphide thiol-modi-fying reagents on the esterase activity of cytoplasmic aldehyde dehydrogenase, Biochim. Biophys. Acta, 998: 1–6.
Pietruszko, R., Abriola, D.P., Blatter, E.E. and Mukerjee, N., 1993, Aldehyde dehydrogenase: aldehyde dehydrogenation and ester hydrolysis, In Enzymology and Molecular Biology of Carbonyl Metabolism (Weiner, H., Crabb, D.W. and Flynn, T.G., eds), pp. 221–232, Plenum Press, New York and London.
Stayner, C.K. and Tweedie, J.W., 1995, Cloning and characterisation of the cDNA for sheep liver cytosolic aldehyde dehydrogenase, These Proceedings.
Tabor, S. and Richardson, C.C., 1985, A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes, Proc. Natl. Acad. Sci. U.S.A., 82: 1074–1078.
von Bahr-Lindström, H., Jeck, R., Woenckhaus, C., Sohn, S., Hempel, J. and Jörnvall, H., 1985, Characterization of the coenzyme binding site of liver aldehyde dehydrogenase: differential reactivity of coenzyme analogues, Biochemistry, 24: 5847–5851.
Weiner, H., Farres, J., Wang, T.T.Y., Cunningham, S.J., Zheng, C.-F. and Ghenbot, G., 1991, Probing the active site of aldehyde dehydrogenase by site directed mutagenesis, in Enzymology and Molecular Biology of Carbonyl Metabolism 3 (Weiner, H., Wermuth, B. and Crabb, D.W., Eds.), pp. 13–17, Plenum Press, New York.
Yoshida, A., Hsu, L.C. and Davé, V., 1992, Retinal oxidation activity and biological role of human cytosolic aldehyde dehydrogenase, Enzyme 46: 239–244.
Zheng, C.-F., Wang, T.T.Y. and Weiner, H., Cloning and expression of the full-length cDNAs encoding human liver class 1 and class 2 aldehyde dehydrogenase, Alcohol. Clin. Exp. Res., 17: 828-8. Clin. Exp. Res., 17: 828-831.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1995 Springer Science+Business Media New York
About this chapter
Cite this chapter
Jones, K.M., Kitson, T.M., Kitson, K.E., Hardman, M.J., Tweedie, J.W. (1995). Human Class 1 Aldehyde Dehydrogenase. In: Weiner, H., Holmes, R.S., Wermuth, B. (eds) Enzymology and Molecular Biology of Carbonyl Metabolism 5. Advances in Experimental Medicine and Biology, vol 372. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-1965-2_3
Download citation
DOI: https://doi.org/10.1007/978-1-4615-1965-2_3
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-5808-4
Online ISBN: 978-1-4615-1965-2
eBook Packages: Springer Book Archive