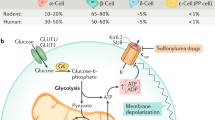
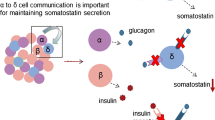
Abstract
The glucagon gene in mammals is a single copy gene that is expressed in a highly tissue-restricted fashion (1). Best known is its expression in a-cells of the pancreatic islets, but it is also expressed in intestinal L-cells, a few neurones in the brain and, at very low levels, the thymus (1-4). Using all six exons of the glucagon gene, a single common transcript and proglucagon prohormone is generated in the pancreas, intestine, and brain (1-3). However, tissue-specific post-translational processing results in the formation of peptides that have distinctly different bioactivities (1). The intestinal L-cells process proglucagon predominantly to glucagon-like peptide 1 (GLP-1), GLP-2, and oxyntomodulin that play important roles in the regulation of insulin secretion, intestinal epithelial proliferation, and intestinal glucose absorption, respectively (2,5). GLP-1 formed in the brain modulates the action of leptin and the central control of feeding (2). In contrast, in pancreatic islets, cleavage of proglucagon results predominantly in the formation of glucagon and, to a lesser extent, GLP-1 (1).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Habener JF, Drucker DJ, Mojsov S, Knepel W, Philippe J. Biosynthesis of glucagon. In: Samols E, ed. The endocrine pancreas. New York: Raven Press, 1991:53–71.
Drucker DJ. Glucagon-like peptides. Diabetes 1998;47:159–69.
Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem 1988; 263:134758.
Throsby M, Homo-Delarche F, Chevenne D, Goya R, Dardenne M, Pleau JM. Pancreatic hormone expression in the murine thymus: localization in dendritic cells and macrophages. Endocrinology 1998;139:2399–406.
Stümpel F, Scholtka B, Hunger A, Jungermann K. Enteric glucagon 37 rather than pancreatic glucagon 29 stimulates glucose absorption in rat intestine. Gastroenterology 1998;115:1–10.
Unger RH, Orci L. Glucagon and the A cell (first part). New Engl J Med 1981;304:1518–24.
Unger RH, Orci L. Glucagon and the A cell (second part). New Engl J Med 1981;304:1575–80.
Lefebvre PJ. Glucagon and its family revisited. Diabetes Care 1995;18:715–30.
Efrat S, Teitelman G, Anwar M, Ruggiero D, Hanahan D. Glucagon gene regulatory region directs oncoprotein expression to neurones and pancreatic a cells. Neuron 1988;1:605–13.
Lee YC, Asa SL, Drucker DJ. Glucagon gene 5’-flanking sequences direct expression of simian virus 40 Tage T antigen to the intestine, producing carcinoma of the large bowel in transgenic mice. J Biol Chem 1992;267:10705–8.
Knepel W. Transcriptional control of pancreatic islet hormones gene expression. Exp Clin Endocrinol 1993;101:39–45.
Drucker DJ, Philippe F, Jepeal L, Habener JF. Glucagon gene 5’-flanking sequences promote islet cell-specific gene transcription. J Biol Chem 1987;262:15659–65.
Philippe J, Drucker DJ, Knepel W, Jepeal L, Misulovin Z, Habener JF. Alpha-cell-specific expression of the glucagon gene is conferred to the glucagon promoter element by the interactions of DNA-binding proteins. Mol Cell Biol 1988;8:4877–88.
Knepel W, Vallejo M, Chafitz JA, Habener JF. The pancreatic islet-specific glucagon G3 transcription factors recognize control elements in the rat somatostatin and insulin-I genes. Mol Endocrinol 1991;5:1457–66.
Morel C, Cordier-Bussat M, Philippe J. The upstream promoter element of the glucagon gene, G1, confers pancreatic alpha cell-specific expression. J Biol Chem 1995;270:3046–55.
Wang M, Drucker DJ. The LIM domain homeobox gene is/-1 is a positive regulator of islet-specific proglucagon gene transcription. J Biol Chem 1995;270:12646–52.
Jin T, Drucker DJ. Activation of proglucagon gene transcription through a novel promoter element by the caudal-related homeodomain protein cdx-2/3. Mol Cell Biol 1996;16:19–28.
Jin T, Trinh DKY, Wang F, Drucker DJ. The caudal homeobox protein cdx-2/3 activates endogenous proglucagon gene expression in InR1–G9 islet cells. Mol Endocrinol 1997;11:203–9.
Hussain MA, Lee J, Miller CP, Habener JF. POU domain transcription factor brain 4 confers pancreatic a-cell-specific expression of the proglucagon gene through interaction with a novel proximal promoter GI element. Mol Cell Biol 1997;17:7186–94.
Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature 1997;385:257–60.
Andersen FG, Heller RS, Petersen HV, Jensen J, Madsen OD, Serup P. Pax6 and Cdx2/3 form a functional complex on the rat glucagon gene promoter Gl-element. FEBS Lett 1999;445:306–10.
Ritz-Laser B, Estreicher A, Klages N, Saule S, Philippe J. Pax-6 and Cdx-2/3 interact to activate glucagon gene expression on the G, control element. J Biol Chem 1999;274:4124–32.
Andersen FG, Jensen J, Heller RS, Petersen HV, Lasson LI, Madsen OD, Serup P. Pax6 and Pdxl form a functional complex on the rat somatostatin gene upstream enhancer. FEBS Lett 1999;445:315–20.
Kruse F, Rose SD, Swift GH, Hammer RE, MacDonald R.I. An endocrine-specific element is an integral component of an exocrine-specific pancreatic enhancer. Genes Dev 1993;7:774–86.
Swift GH, Liu Y, Rose SD, Bischof LJ, Steelman S, Buchberg AM, Wright CVE, MacDonald RJ. An endocrine-exocrine switch in the activity of the pancreatic homeodomain protein PDX1 through formation of a trimeric complex with PBX1b and MRG1 (MEIS2). Mol Cell Biol 1998;18:5109–20.
Habener JF, Stoffers DA. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Assoc Am Physicians 1998;110:1221.
Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/NeuroD-deficient mice. Genes Dev 1997;11:2323–34.
Dumonteil E, Laser B, Constant I, Philippe J. Differential regulation of the glucagon and insulin I gene promoters by the basic helix-loop-helix transcription factors E47 and BETA2. J Biol Chem 1998;273:19945–54.
Lai E, Clark KL, Burley SK, Darnell JE. Hepatocyte nuclear factor 3/fork head or “winged helix” proteins: A family of transcription factors of diverse biologic function. Proc. Natl Acad Sci USA 1993;90:10421–3.
Philippe J, Morel C, Prezioso VR. Glucagon gene expression is negatively regulated by hepatocyte nuclear factor 36. Mol Cell Biol 1994;14:3514–23.
Cockell M, Stolarczyk D, Frutiger S, Hughes GJ, Hagenbüchle O, Wellauer PK. Binding sites for hepatocyte nuclear factor 36 or 3_ and pancreas transcription factor 1 are required for efficient expression of the gene encoding pancreatic _ -amylase. Mol Cell Biol 1995;15:1933–41.
Vaisse C, Kim J, Espinosa R, Le Beau MM, Stoffel M. Pancreatic islet expression studies and polymorphic DNA markers in the genes encoding hepatocyte nuclear factor-3a, -313, -3’y, -4’y, and -6. Diabetes 1997;46:1364–7.
Fürstenau U, Schwaninger M, Blume R, Kennerknecht I, Knepel W. Characterization of novel protein kinase C response element in the glucagon gene. Mol Cell Biol 1997;17:1805–16.
Kaestner KH, Katz J, Liu Y, Drucker DJ, Schütz G. Inactivation of the winged helix transcription factor HNF3a affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev 1999;13:495–504.
Overdier DG, Porcella A, Costa RH. The DNA-binding specificity of the hepatocyte nuclear factor 3/forkhead domain is influenced by amino acid residues adjacent to the recognition helix. Mol Cell Biol 1994;14:2755–66.
Janknecht R, Nordheim A. Gene regulation by Ets proteins. Biochim Biophys Acta 1993;1155:346–56.
Wasylyk B, Hahn SL, Giovane A. The Eta family of transcription factors. Eur J Biochem 1993;211:7–18.
Ang SL, Rossant J. HNF-313 is essential for node and notochord formation in mouse development. Cell 1994;78:561–74.
Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE Jr. The winged-helix transcription factor HNF-38 is required for notochord development in the mouse embryo. Cell 1994;78:575–88.
Knepel W, Jepeal L, Habener JF. A pancreatic islet cell-specific enhancer-like element in the glucagon gene contains two domains binding distinct cellular proteins. J Biol Chem 1990;265:872–535.
Epstein J, Cai J, Glaser T, Jepeal L, Maas R. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J Biol Chem 1994;269:8355–61.
Wrege A, Diedrich T, Hochhuth C, Knepel W. Transcriptional activity of domain A of the rat glucagon G3 element conferred by an islet-specific nuclear protein that also binds to similar pancreatic islet cell-specific enhancer sequences (PISCES). Gene Expr 1995;4:205–16.
Yildiz N, Diedrich T, Knepel W. Nuclear protein binding and functional activity of a variant insulin gene found in non-insulin-dependent diabetes mellitus. Exp Clin Endocrinol Diabetes 1996;104:218–27.
Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that Pax6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev 1997;11:1662–73.
Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 1991;113:1435–49.
Mansouri A, Hallonet M, Gruss P. Pax genes and their roles in cell differentiation and development. Curr Opin Cell Biol 1996;8:851–7.
Epstein JA, Glaser T, Cai J, Jepeal L, Walton DS, Maas RL. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev 1994;8:2022–34.
Xu W, Rould MA, Jun S, Desplan C, Pabo CO. Crystal structure of a paired domain-DNA complex at 2.5 A resolution reveals structural basis for Pax developmental mutations. Cell 1995;80:639–50.
Beimesche S, Neubauer A, Herzig S, Grzeskowiak R, Diedrich T, Cierny I, Scholz D, Alejel T, Knepel W. Tissue-specific transcriptional activity of a pancreatic islet cell-specific enhancer sequence/Pax6-binding site determined in normal adult tisues in vivo using transgenic mice. Mol Endocrinol 1999;13:718–28.
Goudet G, Delhalle S, Biemar F, Martial JA, Peers B. Functional and cooperative interactions between the homeodomain PDX1, Pbx, and Prep1 factors on the somatostatin promoter. J Biol Chem 1999;274:4067–73.
Diedrich T, Fürstenau U, Knepel W. Glucagon gene G3 enhancer: evidence that activity depends on combination of an islet-specific factor and a winged helix protein. Biol Chem 1997;378:89–98.
Hochhuth C, Neubauer A, Knepel W. Transactivation of the rat glucagon gene promoter by the CCAAT/enhancer-binding protein. Endocrine 1994;2:833–9.
St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing a-cells in mouse pancreas. Nature 1997; 387:406–9.
Kiefer TJ, Heller RS, Unson CG, Weir GC, Habener JF. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology 1996;137:5119–25.
Heller RS, Kieffer TJ, Habener JF. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing a-cells of the rat endocrine pancreas. Diabetes 1997;46:785–91.
Rorsman P, Hellman B. Voltage-activated currents in guinea pig pancreatic _ 2 cells. J Gen Physiol 1988;91:223–42.
Rorsman P. Two types of CaZ* currents with different sensitivities to organic Cat* channel antagonists in guinea pig pancreatic a2 cells. J Gen Physiol 1988;91:243–54.
Van der Zee EA, Buwalda B, Strubbe JH, Strosberg AD, Luiten PGM. Immunocytochemical localization of muscarinic acetylcholine receptors in the rat endocrine pancreas. Cell Tissue Res 1992;269:99–106.
Verspohl EJ, Tacke R, Mutschler E, Lambrecht G. Muscarinic receptor subtypes in rat pancreatic islets: binding and functional studies. Eur J Pharmacol 1990;178:303–11.
Knepel W, Chafitz J, Habener JF. Transcriptional activation of the rat glucagon gene by the cyclic AMP-responsive element in pancreatic islet cells. Mol Cell Biol 1990;10:6799–6804.
Oetjen E, Diedrich T, Eggers A, Eckert B, Knepel W. Distinct properties of the cAMP-responsive element of the rat insulin I gene. J Biol Chem 1994;269:27036–44.
Drucker DJ, Campos R, Reynolds R, Stobie K, Brubaker PL. The rat glucagon gene is regulated by a protein kinase A-dependent pathway in pancreatic islet cells. Endocrinology 1991;128:394–400.
Miller CP, Lin JC, Habener JF. Transcription of the rat glucagon gene by the cyclic AMP response element-binding protein CREB is modulated by adjacent CREB-associated proteins. Mol Cell Biol 1993;13:7080–90.
Meyer TE, Habener JF. Cyclic adenosine 3’, 5’-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev 1993;14:269–90.
Ellenberger TE, Brandl Cl, Struhl K, Harrison SC. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted a helices: crystal structure of the protein-DNA complex. Cell 1992;71:1223–37.
Gonzalez G, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 1989;59:675–80.
Chrivia JC, Kwok RPS, Lamp N, Hagiwara M, Montminy MR, Goodmann RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 1993;365:855–9.
Kwok RPS, Lundblad JR, Chrivia JC, Richards JP, Bächinger HP, Brennan RG, Roberts SGE, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 1994;370:223–6.
Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature 1994;370:226–8.
Parker D, Ferreri K, Nakajima T, LaMorte VJ, Evans R, Koerber SC, Hoeger C, Montminy MR. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol Cell Biol 1996;16:694–703.
Lundblad JR, Kwok RPS, Laurance ME, Harter ML, Goodman RH. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature 1995;374:85–8.
Eckner R. p300 and CBP as transcriptional regulators and targets of oncogenic events. Biol Chem 1996;377:685–8.
Parker D, Jhala US, Radhakrishnan I, Yaffe MB, Reyes C, Shulman AI, Cantley LC, Wright PE, Montminy M. Analysis of an activator:coactivator complex reveals an essential role for secondary structure in transcriptional activation. Mol Cell 1998;2:353–9.
Hua QX, Jia WH, Bullock BP, Habener JF, Weiss MA. Transcriptional activator-coactivator recognition: nascent folding of a kinase-inducible transactivation domain predicts its structure on coactivator binding. Biochemistry 1998;37:5858–66.
Korzus E, Torchia J, Rose DW, Xu L, Kurokawa R, McInerney EM, Mullen TM, Glass CK, Rosenfeld MG. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science 1998;279:703–7.
Mymryk JS, Smith MM. Influence of the adenovirus 5 E1A oncogene on chromatin remodelling. Biochem Cell Biol 1997;75:95–102.
Nakajima T, Uchida C, Anderson SF, Lee CG, Hurwitz J, Parvin JD, Montminy M. RNA helicase A mediates association of CBP with RNA polymerise II. Cell 1997;90:1107–12.
Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem 1996;271:9009–13.
Dallas PB, Yaciuk P, Moran E. Characterization of monoclonal antibodies raised against p300: both p300 and CBP are present in intracellular TBP complexes. J Virol 1997;71:1726–31.
Flint J, Shenk T. Viral transactivating proteins. Annu Rev Genet 1997;31:177–212.
Arany Z, Newsome D, Oldread E, Livingston DM, Eckner R. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature 1995;374:81–4.
Schwaninger M, Lux G, Blume R, Oetjen E, Hidaka H, Knepel W. Membrane depolarization and calcium influx induce glucagon gene transcription in pancreatic islet cells through the cyclic AMP-responsive element. J Biol Chem 1993;268:5168–77.
Schwaninger M, Blume R, Oetjen E, Lux G, Knepel W. Inhibition of cAMP-responsive element-mediated gene transcription by cyclosporin A and FK506 after membrane depolarization. J Biol Chem 1993;268:23111–5.
Ghosh A, Greenberg ME. Calcium signalling in neurones: molecular mechanisms and cellular consequences. Science 1995;268:239–47.
Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science 1998;281:1505–9.
Schwaninger M, Blume R, Oetjen E, Knepel W. The immunosuppressive drugs cyclosporin A and FK506 inhibit calcineurin phosphatase activity and gene transcription mediated through the cAMPresponsive element in a nonimmune cell line. Naunyn-Schmiedeberg’s Arch Pharmacol 1993;348:541–5.
Schwaninger M, Blume R, Krüger M, Lux G, Oetjen E, Knepel W. Involvement of the Cat+-dependent phosphatase calcineurin in gene transcription that is stimulated by cAMP trough cAMP response elements. J Biol Chem 1995;270:8860–6.
Krüger M, Schwaninger M, Blume R, Oetjen E, Knepel W. Inhibition of CREB- and cAMP response element-mediated gene transcription by the immunosuppressive drugs cyclosporin A and FK506 in T cells. Naunyn-Schmiedeberg’s Arch Pharmacol 1997; 356:433–40.
Barton K, Muthusamy N, Chanyangam M, Fischer C, Clendenin C, Leiden JM. Defective thymocyte proliferation and IL-2 production in transgenic mice expressing a dominant-negative form of CREB. Nature 1996;379:81–5.
Rudolph D, Tafuri A, Gass P, Hämmerling GJ, Arnold B, Schütz G. Impaired foetal T cells development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc Natl Acad Sci USA 1998;95:4481–6.
Siemann G, Blume R, Grapentin D, Oetjen E, Schwaninger M, Knepel W. Inhibition of CREB/CRE-mediated transcription by the immunosuppressive drugs cyclosporin A and FK506 depends on the promoter context. Mol Pharmacol 1999;1094–1100.
Fürstenau U, Schwaninger M, Blume R, Jendrusch EM, Knepel W. Characterization of a novel calcium response element in the glucagon gene. J Biol Chem 1999;274:5851–60.
Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 1997;15:707–47.
Crabtree GR. Generic signals and specific outcomes: signalling through Ca’, calcineurin, and NF-AT. Cell 1999;96:611–4.
Wolfe SA, Zhou P, Dötsch V, Chen L, You A, Ho SN, Crabtree GR, Wagner G, Verdine GL. Unusual rel-like architecture in the DNA-binding domain of the transcription factor NFATc. Nature 1997;385:172–6.
Peterson BR, Sun LJ, Verdine GL. A critical arginine residue mediates cooperativity in the contact interface between transcription factors NFAT and AP-1. Proc Natl Acad Sci USA 1996;93:136–716.
Garcia-Rodriguez C, Rao A. Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP). J Exp Med 1998;187:2031–6.
Philippe J, Drucker DJ, Habener JF. Glucagon gene transcription in an islet cell line is regulated via a protein kinase C-activated pathway. J Biol Chem 1987;262:1823–8.
Nitsch D, Boshart M, Schütz G. Activation of the tyrosine aminotransferase gene is dependent on synergy betweeen liver-specific and hormone-responsive elements. Proc Natl Acad Sci USA 1993;90:5479–83.
Boyle WJ, Smeal T, Defize LHK, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell 1991;64:573–84.
Thanos D, Maniatis T. NF-_B: A lesson in family values. Cell 1995;80:529–32.
Burgering BMT, Bos JL. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci 1995;20:18–22.
Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci 1995;20:117–22.
Nishizuka Y. Intracellular signalling by hydrolysis of phospholipids and activation of protein kinase C. Science 1992;258:607–14.
Olson EN, Burgess R, Staudinger J. Protein kinase C as a transducer of nuclear signals. Cell Growth Diff 1993;4:699–705.
Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Mariné D, Rapp UR. Protein kinase C_ activates RAF-1 by direct phosphorylation. Nature 1993;364:249–52.
Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science 1995;269:403–7.
Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 1995;81:1159–70.
Chen L, Komiya I, Inman L, McCorkle K, Alam T, Unger RH. Molecular and cellular responses of islets during perturbations of glucose homeostasis determined by in situ hybriditation histochemistry. Proc Natl Acad Sci USA 1989;86:1367–71.
Chen L, Komiya I, Inman L, O’Neil J, Appel M, Alam T, Unger RH. Effects of hypoglycaemia and prolonged fasting on insulin and glucagon gene expression. J Clin Invest 1989;84:711–14.
Shi ZQ, Rastogi KS, Lekas M, Efendic S, Drucker DJ, Vranic M. Glucagon response to hypoglycaemia is improved by insulin-independent restoration of normoglycaemia in diabetic rats. Endocrinology 1996;137:3193–9.
Dumonteil E, Magnan C, Ritz-Laser B, Meda P, Dussoix P, Gilbert M, Ktorza A, Philippe J. Insulin, but not glucose lowering corrects the hyperglucagonemia and increased proglucagon messenger ribonucleic acid levels observed in insulinopenic diabetes. Endocrinology 1998;139:4540–6.
Philippe J. Glucagon gene transcription is negatively regulated by insulin in a hamster islet cell line. J Clin Invest 1989;84:672–7.
Cohen P, Alessi DR, Cross DAE. PDK1, one of the missing links in insulin signal transduction? FEBS Lett 1997;410:3–10.
Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Cuff Opin Genet Dev 1998;8:55–62.
Shepherd PR, Whithers DJ, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. J Biochem 1998;333:471–90.
Philippe J. Insulin regulation of the glucagon gene is mediated by an insulin-responsive DNA element. Proc Natl Acad Sci USA 1991;88:7224–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2001 Springer Science+Business Media New York
About this chapter
Cite this chapter
Knepel, W. (2001). The α-Cell and Regulation of Glucagon Gene Transcription. In: Habener, J.F., Hussain, M.A. (eds) Molecular Basis of Pancreas Development and Function. Endocrine Updates, vol 11. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-1669-9_5
Download citation
DOI: https://doi.org/10.1007/978-1-4615-1669-9_5
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-5669-1
Online ISBN: 978-1-4615-1669-9
eBook Packages: Springer Book Archive