Abstract
Endoscopic ultrasound of the rectum and anus is used for the evaluation of a variety of conditions, most commonly rectal cancer staging and fecal incontinence. This chapter will discuss the indications and technique of endorectal/endoanal ultrasound, in addition to a review of rectal and anal ultrasound anatomy and the interpretation of the images.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Surgeons currently have the ability to use endoscopic ultrasound for diagnostic purposes in the anus and rectum. The most common uses for this technology include rectal cancer staging and fecal incontinence. As treatment options have evolved in the management of rectal cancer, especially after the introduction of neoadjuvant therapy, this data has become even more important. In addition to these two indications, it is less frequently used for identification of occult perianal and perirectal abscesses and fistula tracts. This chapter will discuss the indications for the study, review the anatomy of the rectum and anus, and discuss the technique of endoscopic ultrasound of the anus and rectum and the interpretation of the images acquired.
Endorectal ultrasound (ERUS) is a fairly unique imaging modality in that the study is performed directly by the surgeon. The surgeon, endoscopist, or radiologist has the ability to see the anatomic layers of the rectal wall as well as the layers of the sphincter muscle in the anal canal. This technology was developed in the mid-1980s as a way of advancing both the accuracy and reproducibility of tumor staging as well as giving the ability to stage tumors beyond the reach of the examining finger [1, 2]. Because we can see the various layers of the anatomy with such detail, we are able to use ultrasound data to stage rectal cancer and to describe with very specific detail the presence of sphincter defects in the anal canal. More importantly, as surgeons we are also able to correlate these finding with the still-essential clinical exam and other clinically important data to arrive at the most accurate staging possible. This information is critical in deciding on treatment options for these diseases and is central to the TNM staging system which is currently universally applied for the description and management of rectal cancer [3] (Table 19.1).
Indications
Rectal Cancer
For decades the mainstay of rectal cancer management has been an abdominal perineal resection (APR). With this as the treatment strategy, nuances of the cancer stage are not as important. However, with the increased focus on sphincter preservation and local excision treatment options with transanal endoscopic microsurgery (TEM) or other endoluminal approaches, rectal cancer staging has come to the forefront. Two issues are of central importance in this regard. First, how far does the lesion penetrate the rectal wall? Second, is there lymph node involvement? While hope exists in the future for a better predictor of nodal disease, at present, the best predictor of the N stage for rectal cancer is the T stage [4–7] (Table 19.2). The depth of invasion therefore is the most important predictor of lymph node metastasis and local recurrence in rectal cancer and is essential knowledge in its management.
Endorectal ultrasound is used in the initial evaluation of both rectal polyps as well as invasive rectal cancer. If a lesion is not invasive, management consists of local excision. For invasive lesions, ERUS is performed shortly after initial diagnosis of a tumor prior to initiation of neoadjuvant therapy. It is far less accurate to stage a tumor after the start of neoadjuvant therapy, as ultrasound cannot differentiate between tumor and postradiation fibrosis [8]. Additionally, the exam may be prohibitively uncomfortable as the patient’s radiation therapy progresses. For smaller, polypoid tumors, it is fairly common to be asked to evaluate a patient after an attempt at polypectomy by another physician, and this situation is frequently hard to avoid. However, the accuracy of ERUS in this situation may suffer. Following electrothermal injury from snare cautery or biopsy, the accuracy of the study decreases as the resulting inflammation and scar can cause significant artifact and lead to over-staging of the lesion.
ERUS is the best available means to delineate the depth of invasion of a tumor, and this will assist in decisions regarding the use of neoadjuvant chemoradiation prior to any surgical intervention. Although numbers vary from study to study, the overall accuracy of T stage interpretation in experienced hands is approximately 70 %, and this does vary depending on the skill level of the examiner and the T stage [9, 10]. uT3 lesions are the most accurately diagnosed, whereas uT1 lesions are the least accurate. In addition to the depth of penetration of a lesion, ERUS has the ability to visualize lymph nodes in the perirectal fat. Lymph node accuracy is slightly lower at approximately 65 %. Although the accuracy of this information is slightly poorer compared to tumor depth, it does add significant information to the staging process. Unfortunately, the specifics of decision making based on these results are beyond the scope of this chapter. If the tumor is obstructing in nature, it cannot be examined in a transanal fashion and a high-quality MRI is the preferred option.
Incontinence
Endoanal ultrasound (EAUS) is used to evaluate the sphincter mechanism in patients suffering from fecal incontinence. The most common cause of anal sphincter complex injury is obstetrical trauma, in particular third- and fourth-degree perineal injuries. Risk factors for these injuries include forceps deliveries, nulliparity, increasing fetal birth weight, labor length, and performance of a midline episiotomy [11]. Other causes of sphincter injury include iatrogenic injury from previous anorectal surgery (hemorrhoidectomy, fistulotomy, sphincterotomy) and other trauma to the perineum.
Sphincter injuries are very common after vaginal delivery and are frequently occult even in the setting of a perineal tear. EAUS can be used to evaluate for these sphincter defects and is used as a main determinant as to whether someone is a surgical candidate for sphincteroplasty in the treatment of fecal incontinence. EAUS can be used to identify and evaluate both the internal and external anal sphincter. In addition, the puborectalis muscle at the top of the anal canal is seen, as is the superficial external sphincter at the bottom of the anal canal. Pudendal nerve terminal motor latency testing is an adjunct test in addition to ultrasound to evaluate the effectiveness of nerve conduction, as prolonged nerve conduction times can indicate nerve damage as a contributor to fecal incontinence. If the conduction study is abnormal, this may predict poorer outcomes after sphincter reconstructive surgery [12].
Perianal Abscess and Fistula
On occasion, EAUS and ERUS have been used in the diagnosis of perianal abscesses and fistulas. It is generally employed when diagnostic difficulty during an exam under anesthesia is encountered. One can use the technology to identify a fistula tract or internal opening in the anal canal for a presumed perianal fistula, and EAUS can increase the rate of both fistula tract identification and internal opening identification when compared with physical exam alone [13]. The injection of hydrogen peroxide into the fistula tract will cause it to become hyperechoic and has been shown to improve the rate of fistula identification. In the settings of anorectal Crohn’s disease, it can be particularly helpful, as the tracts and abscesses can be quite complex. In addition, it is useful in evaluating rectovaginal and anovaginal fistulas. In this instance, visualized sphincter defects may cause one to consider sphincteroplasty along with an advancement flap procedure as opposed to a simple advancement flap in order to heal an anterior fistula in women.
Anatomy of the Rectum and Anus
The rectum is approximately 12–15 cm in length and descends through the pelvis from the sigmoid colon. There are three semilunar mucosal valves (Houston’s valves) that are viewed endoscopically and must be avoided when entering any instrument. More importantly, the direction of the rectum follows the morphology of the sacrum. After a slight anterior direction in entering the anus, the rectum ascends posteriorly first and then anteriorly to the sacral promontory. When first inserting the rigid sigmoidoscope, the tip is pointed anteriorly at the umbilicus. After inserting it a couple of centimeters to traverse the anal canal, the scope is pointed posteriorly along the sacral hollow and then anteriorly once again. This is essential to understand when entering the rigid sigmoidoscope or ultrasound probe.
The histologic layers of the rectal wall must be understood in order to perform and interpret ERUS. The layers include the mucosa, submucosa, muscularis propria, and perirectal fat. The depth of invasion of a tumor through these layers indicates the T stage of a tumor (Table 19.1).
The anal canal begins at the level of the puborectalis muscle. There are two muscular layers in the canal. The outer muscle begins as the puborectalis, which is really a medial continuation of the levator ani muscles at the pelvic floor. This muscle acts as a sling to control bowel function by creating an angle between the rectum and anal canal. With the relaxation of this muscle, there is posterior straightening of the anorectal junction to allow for defecation. Distal to this is the external sphincter muscle, which is separated into deep, superficial, and subcutaneous layers. This muscle is under voluntary control to aid with continence. Traumatic defects in this muscle can lead to incontinence.
The inner muscular layer of the anal canal is the internal sphincter, which is a continuation of the circular muscle layer of the muscularis propria of the rectum. This is an involuntary muscle that creates a baseline tone in the anal canal for continence of liquid stool and gas. Relaxation of this muscle allows for defecation. Reduced tone in this muscle may lead to fecal soiling or leakage. Lateral to all the muscular layers of the rectum is the ischiorectal fat.
Superficial to the muscle layers of the anal canal is the mucosa and submucosa which contain the hemorrhoidal plexuses. The dentate line is grossly visible and separates two distinct histologic areas of the anus. Above it is the anal transition zone which is a purplish epithelial layer of cuboidal cells which lead to the true columnar epithelium of the rectum. Beneath it is a modified squamous epithelium leading to the true squamous epithelium of the perianal skin. Although histologically and embryologically one may view the dentate line as the end of the rectum, from the surgeon’s perspective, the beginning of the anal canal should be viewed as the anorectal ring. This ring is the most proximal part of the muscular anus at the puborectalis. The importance of this lies in the ability of the surgeon to measure on digital exam the level of a palpable lesion above or below this point so he can both describe the location of a lesion without ambiguity as well as make surgical decisions regarding sphincter preservation surgery. Conversely, some may use the anal verge in describing the location of anorectal lesions. This is a poor substitute, as the length of the anal canal varies significantly, and this value will not be able to guide further management.
Technique
Instrument Setup
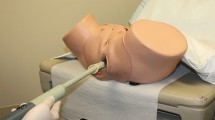
The BK Medical ultrasound probe (BK Medical, Herlev, Denmark) is the most widely utilized, and although there are other systems, this section will describe the assembly and use of this instrument (Fig. 19.1a, b). The ultrasound probe rotates a transducer continually to provide a 360° cross-sectional image of the rectum and anus. The setup for ERUS and EAUS is similar; however, a different probe tip is utilized. The probe contains a wire at its base which is attached to the computer and monitor console. A metal shaft is placed over the inner ultrasound shaft and secured at its base. An ultrasound crystal is then inserted at the top of the probe.
The two most common crystals are the 7 and 10 MHz frequency crystals. Newer instruments have a crystal contained within the shaft of the probe that is able to transmit differing frequencies using the console to select them. In general the higher the frequency of the probe, the higher the resolution of the image, but the lower the depth of tissue penetration and what ultimately can be seen. Typically the 7 MHz crystal is used for imaging of the rectum and the 10 MHz crystal is used for imaging of the anus. The reasoning behind this relates to the focal length. Because the focal length of the 7 MHz crystal is between 2 and 5 cm versus only 1 and 4 cm with the 10 MHz crystal, there is a better opportunity to evaluate the perirectal fat for pathologic lymphadenopathy. This being said, one can use increasing frequencies to enhance clarity of the image at the expense of focal distance. It is particularly easy to change between frequencies as desired with newer machine models, which is done by selecting frequencies via the console as opposed to replacing the crystal at the tip of the instrument in older models.
Endorectal Ultrasound
For ERUS, a balloon is placed over the 7 MHz crystal and secured with a rubber ring to the probe. This is further secured with a metal ring. Insufflation of the balloon is necessary to gain contact with the rectal wall, and this is accomplished with sterile water or saline solution. The water is injected through the probe at its side port. Initially, it is necessary to inject water, rotate the probe vertically downward, and withdraw air from the balloon. Water is used as an acoustic window, similar to the “standoff” technique used in abdominal ultrasound (see Chap. 4). Air or bubbles in the fluid of the balloon cause significant artifact and must be removed as fully as possible. This process may need to be repeated several times to ensure no artifacts in the image.
After enema preparation, the patient is placed in a left lateral decubitus position. Digital rectal exam is performed and is ideally followed by rigid sigmoidoscopy to identify the location of the lesion. If the lesion being evaluated is in the very lower rectum (e.g., beneath the first rectal value), rigid sigmoidoscopy can be omitted at the operator’s discretion. Although some may not employ rigid sigmoidoscopy and use a blind insertion technique, sigmoidoscopy is extremely useful as it allows one to accurately and safely place the probe proximal to the lesion. This gives one assurance that the entirety of the lesion has been evaluated. In addition, visible lymph nodes are often found just proximal to the lesion as opposed to at the level of the lesion, so one should begin the evaluation proximal to the lesion if possible.
After advancing the sigmoidoscope proximal to the lesion, the lens of the scope is removed and the ultrasound probe lubricated and gently placed through the sigmoidoscope until resistance is met. The sigmoidoscope is then withdrawn over the probe to its base as low as possible and the light source of the sigmoidoscope turned off. The balloon is insufflated with between 30 and 60 ml of water. The total amount relates to the diameter of the rectum and can be adjusted during the procedure. The goal is clear visualization of the layers of the rectum. Underdistention may create an artifact if there is no contact between the balloon and the mucosa, whereas overdistention may not allow one to see the delineation between layers.
The button on the probe is pressed to begin rotating the crystal, and the syringe on the side port is oriented toward the patient’s right shoulder. This positioning will result in proper orientation of the lesions on the monitor, with anterior lesions at 12 o’clock, posterior lesions at 6 o’clock, left-sided lesions at 3 o’clock, and right-sided lesions at 9 o’clock. Orientation can be confirmed with visualization of the prostate in men and vagina in women. The prostate and seminal vesicles are very straightforward to identify, whereas the vagina can be identified both visually as well as with concurrent digital exam.
The probe is then slowly withdrawn through the tumor. One attempts to visualize any lymph nodes in the perirectal fat as well as the tumor itself. Lymph nodes can be distinguished from vessels as they disappear over a distance whereas vessels are continuous. After the tumor is fully passed, the process can be repeated. Typically we repeat this process three times to be confident in our reading. This often necessitates the removal of the probe and reinsertion of the rigid sigmoidoscope to be sure one is once again above the lesion prior to reinsertion of the probe. Prior to removal of the probe, the balloon is always fully desufflated.
With regard to fine tuning of the imaging, some adjustments should be mentioned. One can increase and decrease the size of the image on the monitor, and this can be helpful. Additionally, the gain knob can be adjusted as necessary for clarity of image. As mentioned previously, the balloon itself can be insufflated or desufflated further if one cannot see all layers of the rectum on the ultrasound image. Lastly, the angle at which the probe is held will influence the quality of the image. Ideally, it is held perpendicular to the rectum at all levels. One must strive to have the probe centered in the ultrasound image by moving the probe handle in different directions to accomplish this.
Endoanal Ultrasound
Endoanal ultrasound is less technically challenging to perform than ERUS. The initial setup is similar; however, a 10 MHz frequency is used instead of the 7 MHz frequency. Instead of placing a balloon over the crystal, a solid plastic cap is placed over the crystal and attached to the probe (Fig. 19.1). The cap is then filled with sterile water through the same stopcock used for ERUS. Care must once again be taken to avoid air bubbling in the cap, although the cap has a pinhole in the tip so once the cap is filled, air usually has been displaced by the water.
The patient is placed in the same left lateral decubitus position and digital exam is performed. There is no need for sigmoidoscopy in EAUS. The probe is lubricated and placed in the distal rectum with the syringe in line with the right shoulder and the button on the probe pressed to begin rotating the crystal. Several passes are taken to evaluate the internal and external sphincter. There will be artifact if the tip of the probe is either in the rectum or out of the canal, as either situation results in loss of contact of the probe with a surface. In women it can be particularly helpful to place a finger in the vagina. This will confirm orientation as well as give the ability to measure the perineal body on the monitor if desired. Pressing down on the posterior wall of the vagina with a gloved finger will be seen by morphologic change in the image as well as a curvilinear hyperechoic line.
Gain and image size can be adjusted similarly to TRUS. If evaluating a fistula, peroxide can be injected into the fistula tract to identify it and highlight its course. During either EAUS or ERUS, images can be captured and printed utilizing controls on the probe.
Image Interpretation
Endorectal Ultrasound
In evaluating the rectum, there are a series of circular hyperechoic bright lines and surrounding circular hypoechoic dark areas with a most peripherally outer bright area (Fig. 19.2a, b). These will be described from the center outward. The probe is a small bright circular lucency visualized in the center of the image. This is surrounded by a circular dark area which represents the water-filled balloon. The inner bright line surrounding this dark area depicts the interface between the balloon and the rectal mucosa. The inner dark area beyond this represents the mucosa and muscularis mucosa. The middle bright line represents the submucosa. The outer dark area beyond this represents the muscularis propria. The outer bright area beyond this last dark layer represents the perirectal fat.
(a) Diagram of the visualized layers of the rectal wall. The dark center represents the ultrasound balloon. The inner bright line is interface between balloon and mucosa. The inner dark area is mucosa and submucosa. The middle bright line is submucosa. The outer dark area is muscularis propria. The outer bright area is perirectal fat. (b) Portion of a normal ultrasound of the rectal wall. If you look from the center outward to the left, you can see a similar progression of bright lines and dark areas
A rectal mass is typically hypoechoic in nature. When evaluating the depth of a rectal mass, one attempts to visualize which layer this hypoechoic structure reaches in the above pattern. Beyond focusing on the mass itself, one uses the uniform, irregular, or broken nature of the middle bright line and outer bright area to determine if their boundaries have been broken. For uT0 lesions (e.g., polyps), the mass can be seen within the inner dark area (Fig. 19.3a, b). For uT1 lesions (submucosal invasion), the mass is seen in the inner dark area extending to the middle bright line causing irregular stippling of the line but with no clear break (Fig. 19.4). This is perhaps the most subtle diagnosis to make. For uT2 lesions (muscularis propria invasion), the mass is seen extending to the outer dark area with a clear break in the middle bright line (Fig. 19.5a, b). The border between the outer dark area and outer bright area is still uniform in appearance. For uT3 lesions (perirectal fat invasion), one can see an extension of the mass into the outer bright area or significant scalloping of the border between the outer dark area and outer bright area (Fig. 19.6a, b). In the case of uT4 lesions, there is invasion into surrounding structures such as the prostate, vagina, cervix, uterus, bladder, or bony structure. In these instances, the hypoechoic mass is seen extending to the outer bright area and the hyperechoic signals of the outer bright area are lost between the mass and the structure.
uT2 lesions invade the muscularis propria. In (a) the arrowhead shows the tumor extending to the outer dark area (muscularis propria), and the arrow shows the interface between the outer dark area and outer bright area to be uniform without penetration or stippling by the tumor. In (b) the arrows depict the interface between the tumor and the outer bright area to be uniform. In this example, we see a circumferential tumor in the muscularis propria, and all hypoechoic areas beyond the interface of the balloon and mucosa (the inner bright line) represent a tumor
uT3 lesions invade into the perirectal fat. In (a) the arrow shows the edge of the tumor extending into the outer bright area (perirectal fat). Compare the irregularity of this interface between the outer dark area and outer bright area to elsewhere in the image where it is uniform and normal. In (b), the arrow shows scalloping of the outer dark area and outer bright area interface, also indicative of a uT3 tumor with invasion into the perirectal fat. The prostate is visualized anteriorly as a dark structure (labeled P). In this instance one can still see a thin bright plane between the tumor and prostate. If there is no visualized bright plane between these two hypoechoic structures, it would be consistent with a uT4 lesion
As stated previously, the evaluation of lymph nodes can be inaccurate. To begin with, evidence that one is visualizing a node must be proven by moving the probe above and below it. Vasculature and lymphatic channels will be continuous, whereas lymph nodes will appear and disappear with movement of the probe. Also as stated previously, lymphadenopathy often occurs above a lesion so one should be sure to begin the study above it. The typical appearance of a malignant lymph node is a hypoechoic structure with regular borders (Fig. 19.7). Hyperechoic structures or those with irregular borders are more often inflammatory in nature. Many feel that the presence of a lymph node on ultrasound imaging denotes some form of pathology, and it has been shown that the larger the size of the node (e.g., greater than 5 mm), the more often the node contains metastatic disease [14].
Endoanal Ultrasound
The appearance of the anal canal on ultrasound is very straightforward. They will be described from the center outward as was done in the ERUS section (Fig. 19.8). The probe is at the center and appears the same in both ERUS and EAUS image types as a small bright circle. After a large dark area which depicts the water-filled probe tip used in EAUS, there is an inner bright line which represents the interface between the probe tip and the anal mucosa. Beneath this is an inner bright area which represents the mucosal, hemorrhoidal plexuses and submucosal tissues. The internal sphincter is seen as a middle dark area and the external sphincter is seen as an outer bright area surrounding it. On a normal study, the internal sphincter appears uniform throughout the anal canal. The external sphincter begins most proximally at the puborectalis as a u-shaped structure (Fig. 19.9a, b). As one moves distally, this outer bright area becomes circular in the mid-anal canal (Fig. 19.10). Distally as one moves beyond the internal sphincter in the anal canal, the middle dark area is lost and the remaining bright area beyond the inner bright line represents the superficial external sphincter (Fig. 19.11).
Diagram of the normal anatomy of the middle anal canal. After the inner dark area which represents the water-filled cap is an inner bright line. This represents the interface between the probe and anal mucosa. This is followed by an inner bright area which depicts the mucosa, hemorrhoidal plexuses and submucosa of the anal canal. The middle dark area represents the internal sphincter muscle, and the outer bright area represents the external sphincter muscle. Compare this with Fig. 19.10 as an example
Normal anatomy of the proximal anal canal. In (a) and (b), the outer bright structure depicts puborectalis. This is a U-shaped structure at this level, with arrows at the ends of the structure. Incorrectly interpreted, one might see this as a sphincter defect, but this is a normal anatomic finding at this most proximal level of the canal
Using the above as the normal, one can evaluate for defects in the musculature from prior trauma. This is normally seen as hypoechoic defects in the outer bright area of the external sphincter and loss of uniformity with thinning or destruction of the middle dark area which represents the internal sphincter (Fig. 19.12a, b). These defects are often seen anteriorly in women as a result of obstetrical trauma. They can also be seen from iatrogenic injury due to surgical management of hemorrhoids, fistulas, and fissures anywhere in the anal canal.
Anterior sphincter disruption. In both (a) and (b), we see loss of the outer bright area anteriorly (arrows point to the edges of normal outer bright areas) and loss of the internal sphincter (middle dark area) as well (arrowhead). In (a) in particular, note the mixed echogenicity of the area between the two arrows at the top of the image. This depicts external sphincter scar from prior trauma
Hypoechoic defects can be seen in the case of fistulas (Fig. 19.13). With the addition of hydrogen peroxide, fistula tracts can be elucidated and made hyperechoic for ease of identification. Hydrogen peroxide is commonly used as an adjunct technique in the identification of an internal opening of a perianal fistula that is difficult to find with easy passage of a fistula probe. Injection of hydrogen peroxide using an angiocath through the external opening in the perianal skin can result in bubbles at the internal opening along the mucosa of the anal canal (often at the level of the dentate line). A similar injection technique, when used in combination with EAUS, can elucidate the fistula tract for identification and ultimately safe passage of a fistula probe or draining seton.
Three-Dimensional Endorectal Ultrasound
Three-dimensional endorectal ultrasound is based on the same principles as two-dimensional ultrasound and newer ultrasound machines carry the capability to produce these images. In addition to cross-sectional images, coronal and sagittal reconstructions can also be produced and may help in image interpretation. In these machines the ultrasound crystal is contained within the probe itself, and this allows for movement of the crystal back and forth by depressing buttons on the probe handle. This allows for easier image acquisition and is a more comfortable exam for the patient, as the probe usually only needs to be inserted once. As stated above, the frequency can be adjusted on the console depending on the circumstances in these newer models which can be helpful in certain circumstances. Although the three-dimensional images acquired are visually appealing, studies attempting to show increased accuracy in rectal cancer staging have been equivocal [15, 16].
Conclusion
Endorectal and endoanal ultrasound are useful diagnostic studies in the evaluation of rectal cancer, fistulas, and incontinence. One can visualize the anatomic layers of the rectal wall and anal canal in great detail. This is essential in the initial staging of rectal cancer and guides subsequent management, whether it is neoadjuvant chemoradiation or surgery. Endoanal ultrasound accurately identifies and delineates anal sphincter defects. This information is essential in the planning of the surgical management of fecal incontinence. ERUS can also be helpful in the identification of perianal abscesses and fistulas that are difficult to diagnose clinically. By placing the ultrasound in the hands of the surgeon, critical imaging for the optimal surgical management of the patient can be obtained.
References
Hildebrandt U, Feifel G. Preoperative staging of rectal cancer by intrarectal ultrasound. Dis Colon Rectum. 1985;28(1):42–6.
Benyon J, Roe AM, Foy DMA, Channer JL, Virjee J, Mortensen NJ. Preoperative staging of local invasion in rectal cancer using endoluminal ultrasound. J R Soc Med. 1987;80:23–4.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Colon and rectum. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. p. 143–64.
Brodsky JT, Richard GK, Cohen AM, Minsky BD. Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer. 1992;69(2):322–6.
Killingback M. Local excision of carcinoma of the rectum: indications. World J Surg. 1992;16(3):437–46.
Blumberg D, Paty PB, Guillem JG, Picon AI, Minsky BD, Wong WD, et al. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum. 1999;42(7): 881–5.
Sitzler PJ, Seow-Choen F, Ho YH, Leong APK. Lymph node involvement and tumor depth in rectal cancers: an analysis of 805 patients. Dis Colon Rectum. 1997;40(12):1472–6.
Gavioli M, Bagni A, Piccagli I, Fundaro S, Natalini G. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer: comparison between sonographic and histopathologic changes. Dis Colon Rectum. 2000;43(8):1075–83.
Garcia-Aguilar J, Pollack J, Lee S, Hernandez de Anda E, Mellgren A, Wong WD, et al. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45(1):10–5.
Kauer WKH, Prantl L, Dittler HJ, Siewert JR. The value of endosonographic rectal carcinoma staging in routine diagnostics: a 10-year analysis. Surg Endosc. 2004;18:1075–8.
Christianson LM, Bovbjerg VE, McDavitt EC, Hullfish KL. Risk factors for perineal injury during delivery. Am J Obstet Gynecol. 2003;189(1):255–60.
Pla-Martí V, Moro-Valdezate D, Alos-Company R, Solana-Bueno A, Roig-Vila JV. The effect of surgery on quality of life in patients with faecal incontinence of obstetric origin. Colorectal Dis. 2007;9:90–5.
Toyonaga T, Tanaka Y, Song JF, Katori R, Sogawa N, Kanayama H, et al. Comparison of accuracy of physical examination and endoanal ultrasonography for preoperative assessment in patients with acute and chronic anal fistula. Tech Coloproctol. 2008;12:217–23.
Katsura Y, Yamada K, Ishizawa T, Yoshinaka H, Shimazu H. Endorectal ultrasonography for the assessment of wall invasion and lymph node metastasis in rectal cancer. Dis Colon Rectum. 1992;35(4):362–8.
Kim JC, Kim HC, Yu CS, Han KR, Kim JR, Lee KH, et al. Efficacy of 3-dimentional endorectal ultrasonography compared with conventional ultrasonography and computed tomography in preoperative rectal cancer staging. Am J Surg. 2006;192:89–97.
Kim JC, Cho YK, Kim SY, Park SK, Lee MG. Comparative study of three-dimensional and conventional endorectal ultrasonography used in rectal cancer staging. Surg Endosc. 2001;16:1280–5.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Frenkel, J.L., Marks, J.H. (2014). Endoluminal Ultrasound: Anatomy, Technique, and Intervention of the Anorectum. In: Hagopian, E., Machi, J. (eds) Abdominal Ultrasound for Surgeons. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9599-4_19
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9599-4_19
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9598-7
Online ISBN: 978-1-4614-9599-4
eBook Packages: MedicineMedicine (R0)