Abstract
Celiac disease (CD) is an enteropathy-induced immune response that occurs on exposure to toxic gluten in the diet and is reversible in most cases once gluten is withdrawn. A gluten-free diet (GFD) is the preferred treatment for CD and leads to reversal of villous atrophy. Individuals with untreated CD may experience significant health complications. Managing CD can potentially prevent or cure some of the associated conditions, such as neurologic complications, nutritional deficiencies, and osteoporosis. Counseling, nutritional support, and follow-up are vital aspects in CD management. GFD remains the mainstay of treatment in CD. Adhering to a strict GFD remains challenging for some individuals, and it has its limitations and other alternatives are called for. There are new and novel treatments emerging, which will be discussed here.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Celiac Disease
- Progressive Multifocal Leukoencephalopathy
- Villous Atrophy
- Celiac Patient
- Gliadin Peptide
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Celiac disease (CD) is a chronic, systemic, autoimmune disorder in genetically predisposed individuals in response to ingestion of toxic gluten. It affects approximately 1 % of the population in Europe and the United States. Gluten proteins belong to the superfamily of prolamins that have diverged among cereals and are unique to the subfamily of Pooideae that include wheat, barley, and rye. They can trigger an autoimmune injury to the gut, skin, liver, joints, uterus, and other organs [1]. Of the individuals with CD, 5–10 % may be sensitive to oats because some people have small-intestinal T cells that react to oat avenins [2]. The resultant lesion in the mucosa of the small intestine is villous atrophy with crypt hyperplasia and intraepithelial lymphocytosis. Villous atrophy is identified on small bowel biopsy, which is considered the gold standard for diagnosing this condition. False negative small bowel histology can be expected due to patchy small bowel mucosal changes [1]. Untreated CD is associated with significant mortality. Undiagnosed CD is associated with a nearly fourfold increased risk of death [3].
Novel Therapies for Celiac Disease
The mainstay of treatment for CD is lifelong avoidance of foods containing gluten. CD, like type 1 diabetes, rheumatoid arthritis, and multiple sclerosis, has a chronic nature where particular HLA alleles are overrepresented among the patients [4]. Most patients go into complete remission when they are put on a gluten-free diet, and they relapse when gluten is reintroduced into the diet. CD is in this respect unique among the chronic inflammatory HLA-associated diseases in that a critical environmental factor has been identified [5].
Although GFD is an effective treatment for CD, it does have its limitations. This is due to its cost, side effects including constipation and weight gain, and the difficulty to maintain a strict GFD. This results in poor dietary compliance. In addition, patients with high-level gluten sensitivity are affected by trace amounts of gluten in foods that are declared gluten-free [6]. These limitations and the insight in the pathogenesis have lead to development of new diagnostics and encouraged investigating into possible novel treatments [7]. Potential therapies involve manipulating the dietary gluten, rendering it less toxic, degrading the enzymes that process gluten, decreasing intestinal permeability, blocking the gluten by inhibiting tissue transglutaminase 2, inhibiting binding of gluten to HLA-DQ with the use of inhibitory peptides, shifting the Th1 to Th2 inflammatory response, proinflammatory cytokine inhibitors, enhancing the immune system, inducing gluten tolerance, gluten vaccines, or preventing or reversing mucosal damage in response to inflammation [8]. Despite the potential treatments that show positive results in theory or ex vivo, the effectiveness, safety, drug delivery, and cost effectiveness of the treatment in vivo need to be taken into account.
See Table 14.1 for a list of experimental therapy, mechanisms of actions, and results.
Avoidance of Toxic Dietary Gluten
Consumption of Gluten with Low Immunogenicity
Selecting products that lack toxic gluten but remain palatable and retains the baking qualities of wheat could be seen as an alternative to the commercially available gluten-free products. Wheat and grains with low immunogenicity have been studied in the management of CD. Certain wheat accessions contain low levels of T-cell stimulatory molecules. By breeding wheat species with low or absent levels of harmful gluten proteins, grains with low or no immunogenicity in celiac patients can be produced. However, a major challenge is the alpha-gliadin gene family, which varies in copy number among wheat cultivars and those that are expressed at different levels (manuscript in preparation). Previously, it has been reported that the immunodominant 33mer encoded by alpha-gliadin genes on wheat chromosome 6D is absent in the diploid einkorn, also known as Triticum monoccum (gene AA), and the tetraploid Triticum turgidum (gene AABB) pasta wheat [9]. On the other hand, hexaploid wheat is needed for bread making. Still, there is a reduction in toxicity observed in vitro with two varieties of bread wheat, one poor in alpha- and beta-gliadins and the other poor in alpha-, beta-, gamma-, and omega-gliadins, which have been tested [10]. Sorghum is a grain that is closely related to maize. Wheat-free sorghum products are safe and palatable in individuals with CD [11].
Modified or Pretreated Gluten
Certain lactobacilli added to sourdough for fermentation hydrolyze the gluten peptide and render them less immunotoxic. This process requires prolonged fermentation, resulting in alteration in the size of the dough, so less fermentation time and mixing with 30 % fermented wheat flour was necessary for better baking results as demonstrated in one study [12]. Patients were challenged for 2 days in this pilot study so long-term safety of this method remains unknown.
Removing alpha-gliadin from Triticum aestivum (Chinese Spring) present in chromosome 6 of D-genome (6DS) led to a significant reduction in T-cell stimulatory epitopes but compromised the baking quality of bread. Genetically deleting omega-gliadin, gamma-gliadin, and LMW-GS loci from the short arm of chromosome 1 of the D-genome (1DS) produced a lowering of the immune response to exposure to the wheat with the added benefit of retaining the baking qualities [13]. Another method of detoxifying gluten involves genetically altering the alpha2-gliadin residue by replacing antigenic amino acids with alanine residue, leading to elimination of the T-cell activity [14].
Gluten Detoxification
Oral Enzyme Therapy
Proline residues in some gliadin peptides are resistant to enzymatic degradations in the digestive system leaving them available for abnormal immune response in celiac patients. Enzymatic degradation of this gluten with prolyl endopeptidases (PEP) prevents these peptides from reaching the lamina propria and allows the smaller substrates to be processed by the intestinal brush border enzymes. Microorganisms such as Flavobacterium meningosepticum, Sphingomonas capsulata, and Myxococcus xanthus are able to cleave the immunodominant proline-rich regions [6]. Pyle et al. demonstrated the benefit of PEP in a study when a 2-week gluten challenge with PEP showed no evidence of malabsorption of celiac antibodies [15]. In another study, PEP was reported to require 3 h incubation with the protein in order to degrade it and prevent gluten-related toxicity. This concludes that the ingestion of PEP with diet is unlikely to avoid immune response to gluten [15].
In germinating wheat seeds, gliadin is under the control of intrinsic cystatins. This protease can degrade immunogenic T cell, making it possible to create flour based on germinating wheat safe for celiac individuals but with compromised baking quality.
ALV003 is a mixture of two glutenases, an endoprotease that has the advantage of combining both germinating barley and PEP. Tye-Din et al. tested ALV003-treated wheat flour on celiac patients [17]. The group found no change in symptoms experienced by patients but a reduced IFN-g ELISpot to gliadin in patients consuming the treated flour compared to placebo controls. Further work will help to evaluate the value of this, and a phase IIa trial is currently recruiting in Finland to assess the safety and efficiency of ALV003 in a larger cohort of celiac patients (NCT1255696).
Probiotics
De Angelis et al. showed that VSL#3 predigested gliadins caused a less pronounced reorganization of the intracellular F-actin, which was mirrored by an attenuated effect on intestinal mucosa permeability. The release of zonulin from intestinal epithelial cells treated with gliadins was considerably lower when digested with VSL#3 [18].
Orally ingested IgG is highly resistant to gastric acidity, and approximately 50 % of neutralizing activity survives when reaching the terminal ileum [19]. In view of the low cost and ease of production of cow’s milk antibodies, large-scale production of neutralizing gluten antibodies is potentially easy, safe to use, and cost effective. A clinical phase I trial in the USA is expected [6].
Inhibition of Intestinal Permeability
An important factor contributing to the influx of gluten to the lamina propria is increased intestinal permeability through open epithelial tight junctions. Gluten activates zonulin signaling in tight junctions between epithelial cells of patients with CD, leading to increased intestinal permeability to macromolecules. Larazotide (AT-1001) is an oral tight-junction regulatory peptide that acts locally to inhibit the opening of tight junctions in epithelial cells in the small intestine. The treatment appears to be well tolerated, but it does not prevent small-intestinal epithelial damage upon exposure to gluten [20, 21]. The primary end point of the phase II study on AT1001 has not been reached, and conclusions from other phase I and II studies on the clinical trial register have been performed and are not yet available (NCT362856, NCT492960, NCT 620451, NCT 889473) [22].
Tissue Transglutaminase Blockade
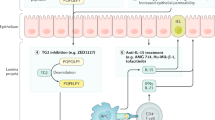
Tissue transglutaminase 2 (TG2) stimulates the process of gliadin binding to HLA-DQ2/DQ8 leading to T-cell activation. TG2 inhibition could possibly prevent the selective deamidation of gluten peptides and blocking the binding to the HLA molecules and preventing or reversing the process of T-cell activation and cell damage [5, 6].
Preclinical tests in vitro on small-intestinal samples from a celiac patient demonstrated inhibition of TG2 by cystamine, a competitive inhibitor, and 2-[(2-oxopropyl)thio]imidazolium inhibitors (L682777 or R283). The consequences of TG2 inhibitors in vivo and the effect of inhibiting all transglutaminase action are unknown [6]. Pasternak et al. demonstrated that TG inhibitors based on a high-affinity thiol binding group displayed a very high specificity for TG2 in vitro, which is very promising [6] (Pasternack R, Hils M, Zedira Company, Darmstadt, Germany, personal communication, September 2009).
Th1 to Th2 Shift
In CD, dietary gluten triggers Th1-type immune response leading to enteropathy. A decapeptide (sequence QQPQDAVQPF) isolated from durum wheat prevents the activation of peripheral lymphocytes in CD. This 10mer is isolated by affinity chromatography and gel filtration from alcohol-soluble protein fraction of durum wheat [23, 24]. Silano et al. [25] demonstrated the antagonist effect of this decamer and its ability to inhibit the abnormal immune response triggered by gliadin. The intestinal T lymphocytes derived from eight children with CD were incubated with deamidated gliadin peptide alone and simultaneously with the 10mer. The results revealed that the incubation of celiac intestinal T cells with deamidated gliadin peptides resulted in a significant increase in cell proliferation and IFN-gamma release. The 10mer caused a downregulation of IFN-gamma and upregulation of IL-10, which has an immunomodulatory role, leading to a shift from Th1 to Th2 lymphocyte response [26].
HLA-DQ Groove Blockade
Inhibitors of HLA-DQ2 that present gliadin peptides have been studied to prevent the activation of the inflammatory cascade in CD following exposure to toxic gluten. Attempts to block immune activation were investigated in other immune-mediated conditions such as rheumatoid arthritis, multiple sclerosis, and type 1 diabetes mellitus. Part of the reason for the lack of success in these experiments was achieving effective drug delivery [27, 28]. However, in CD the DQ2 inhibitor will need to reach the small intestine directly via the oral route either before or with gluten ingestion.
Amino acid substitution of gliadin can convert the epitope to an agonist or antagonist and affects in turn the inflammatory process [29]. Alanine amino acid substitution at key positions (3, 8, and 10) in the immunodominant peptide in residues 62–75 of a-2-gliadin in wheat abolishes the immunogenicity of the peptide when tested against T-cell clones [14]. The neutral alanine amino acid present in the peptide affected the capability of the peptide itself to lie within the cleft of the HLA-DQ molecules. Anderson et al. substituted an alanine or lysine amino acid in the immunodominant a-gliadin peptide sequence p57–73 QE65. The substitution to the gliadin peptides could abolish their capacity to stimulate IFN-g production from CD4 T cells and also have anti-inflammatory or protective effects in HLA-DQ2+ CD [30].
Proinflammatory Cytokines Inhibition
Various cytokine therapies are being developed for chronic inflammatory conditions. The advances in the field of CD are limited due to the low acceptability of side effects from these drugs.
IL-10 from regulatory T cells suppresses Th1 cells and likely acts as a mildly counter-regulatory cytokine [31]. IL-10 ex vivo suppresses gluten-dependent T-cell activation in cultured celiac small-intestinal mucosa [32]. But another study tested recombinant IL-10 in Crohn’s disease was discontinued due to lack of effect [33].
In CD, the main cytokine produced by the gliadin-specific T-cell clones is IFN-gamma [34]. IFN-g blocking antibody can prevent histologic damage to healthy mucosa in celiac patients [35]. Studies in Crohn’s disease revealed disappointing results of phase I/II trials. The drop in Crohn’s disease activity index (CDAI) in a small cohort did not reach statistical significance due to an unusually high drop in CDAI in the placebo group [36]. Whereas Reinisch et al. found in a larger cohort of Crohn’s disease patients, there was a significant decrease in CRP levels. However, this failed to translate into a clinical response [37]. Such studies may encourage research on testing anti-TNF in CD based on the studies undertaken so far in other inflammatory conditions.
Interleukin-15 (IL-15) is a key proinflammatory, innate response cytokine that plays an important role in several autoimmune diseases. IL-15 inhibitors could be used as a promising therapeutic strategy in CD. Baslund et al. demonstrated that HuMax-IL-15, which is a human IgG1 anti-IL-15 monoclonal antibody, had acceptable side effects in rheumatoid arthritis [38]. Another study investigated the effects of treatment with an IL-15 antagonist (CRB-15) that decreased the incidence and severity of collagen-induced arthritis [39]. More studies need to be conducted to observe the effects in CD in clinical practice.
Anti-TNF-a treatment has been instigated in studies on treatment of refractory CD [40]. Reports demonstrate slow mucosal recovery following treatment with regular anti-TNF-a infusions at 8 weekly intervals [41], or a single dose TNF-a followed by azathioprine maintenance [40]. These studies were on a small group with selection bias so more studies need to be undertaken for accurate conclusions to be drawn.
Induction of Gluten Tolerance
CD is an immune-mediated response to ingested gluten. Induction of immune tolerance to gluten, if successful, could prevent this process from occurring. Intranasal administration of gliadin peptides in transgenic DQ8 mice resulted in lowering the T-cell proliferative response to gliadin and dampening of the inflammatory cascade [42–44]. However, there could be enormous variation in the response by individual patients, making this approach less robust.
Gluten Peptide Vaccine
Another strategy used a gluten vaccination containing three select immunogenic 16mer peptides derived from alpha-gliadin, omega-gliadin, and hordein that account for 60 % of the overall gluten T-cell response. The vaccination was given subcutaneously to gliadin-specific TCR/DQ2 transgenic mice. The result was a suppression of CD4 T-cell proliferation and IL-2 and IFN-g production and increased the expression of T-reg by splenic CD4 cells in response to a gluten challenge. Tye-Din et al. [45] demonstrated that the same 16mer peptides are recognized by the majority of HLA-DQ2-positive, gluten-positive peripheral blood T cells. A patented vaccine continuing the 16mer has finished recruiting for a phase I clinical trial. HLA-DQ8, on the other hand, has a different immunodominant epitope and will not respond to the vaccine. In addition, as the innate immune system plays a role in activating the immune system, celiac patients have different response to the same antigen stimulus [45].
Pathogenic CD4+ Th Cells Inhibition
Gliadin-specific type 1 regulatory T (Tr1) cells are found in the intestinal mucosa of individuals with CD. Gianfrani et al. [46] reported that gliadin-specific Tr1 cell clones suppressed proliferation of pathogenic Th0 cells. Methods to boost the numbers of Tr1 to offer a therapeutic measure need to be sought.
Anti-adhesion Therapy
Chronic inflammatory diseases exhibit leukocyte migration and retention. Adhesion molecules regulate the influx of leukocytes in normal and inflamed gut, local lymphocyte stimulation, and antigen presentation in intestinal mucosal cells. MadCAM-1 is an adhesion molecule specific to the gut. In inflammatory bowel disease (IBD), most of the adhesion molecules are upregulated in inflammatory bowel disease [47]. Inhibiting leukocyte adhesion will prevent the migration of leukocytes into inflamed tissue, which could be a promising treatment for CD. These inhibitory molecules are being studied in IBD so far. There are two humanized antibodies under evaluation for IBD. The first is INTEGRIN-a4 antagonist and includes natalizumab, which is an antibody used in multiple sclerosis and IBD [48, 49], and alemtuzumab, which has been studied in the treatment of refractory CD with conflicting efficacy data [50]. A trail in IBD patients failing anti-TNF-a treatment reported improvement with natalizumab infusion (clinical trial NCT00801125). However, Ananthakrishnan et al. [51] demonstrated that in patients with moderate to severe CD failing two TNF-antagonists, using a third TNF-antagonist therapy appears to be a cost-effective strategy compared to using natalizumab as a third-line therapy without significantly compromising treatment efficacy. Natalizumab is associated with 0.1 % risk of developing progressive multifocal leukoencephalopathy (PML) [52].
AJM300, which is an orally active small molecule, also antagonizes INTEGRIN-a4. Studies in a small cohort of Crohn’s disease patients demonstrated reduction in CDAI but no difference compared to placebo [53].
The second humanized antibody targets the adhesion molecule INTEGRIN-a4b7 expressed by gut T cells. Molecules in this group include vedolizumab (MLN02), which demonstrated in phase II trials the capacity to induce remission in ulcerative colitis [54, 55], and etrolizumab, which appears to be well tolerated in moderate to severe ulcerative colitis, but phase II studies are warranted to observe clinical improvement [56].
Overall, large cohort studies are required to conclude the potential safety and efficacy of these drugs in CD.
Intestinotrophic Mitogens
Intestinotrophic mitogens prevent intestinal damage. R-spondin 1 (Rspo1) is a novel epithelial mitogen that stimulates the growth of small and large bowel mucosa. Zhao et al. [57] demonstrated in mouse models of colitis that Rspo1 is able to stimulate the growth of crypt cells, which will hasten mucosal regeneration. This in turn restores the intestinal architecture. The effect of Rspo1 in humans is unknown and studies are yet to be conducted.
Conclusion
While there have been a number of new approaches developed over the years to prevent the onset of the disease in CD patients, they do not have reached a level that would permit abolishing a gluten-free diet for them. Furthermore, most studies rely on the use of synthetic peptides to analyze the immune response rather than intact proteins to predict what is toxic or not. A new approach is therefore needed where intact proteins can be tested in the future.
References
Rewers MJ. Epidemiology of celiac disease: what are the prevalence, incidence, and progression of celiac disease? Bethesda, MD: National Institutes of Health; 2004.
Lundin KE, Nilsen EM, Scott HG, Løberg EM, Gjøen A, Bratlie J, et al. Oats induced villous atrophy in celiac disease. Gut. 2003;52:1649–52.
Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137(1):88–93.
Thorsby E. Invited anniversary review: HLA associated diseases. Hum Immunol. 1997;53:1–11.
Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81.
Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–33.
Sollid LM, Lundin KE. Diagnosis and treatment of celiac disease. Mucosal Immunol. 2009;2:3–7.
Lerner A. New therapeutic strategies for celiac disease. Autoimmun Rev. 2010;9(3):144–7.
Molberg O, Uhlen AK, Jensen T, Flaete NS, Fleckenstein B, Arentz-Hansen H, et al. Mapping of gluten T-cell epitopes in the bread wheat ancestors: implications for celiac disease. Gastroenterology. 2005;128(2):393–401.
Frisoni M, Corazza GR, Lafiandra D, De Ambrogio E, Filipponi C, Bonvicini F, et al. Wheat deficient in gliadins: promising tool for treatment of celiac disease. Gut. 1995;36:375–8.
Ciacci C, Maiuri L, Caporaso N, Bucci C, Del Giudice L, Rita Massardo D, et al. Celiac disease: in vitro and in vivo safety and palatability of wheat-free sorghum food products. Clin Nutr. 2007;26:799–805.
Di Cagno R, De Angelis M, Lavermicocca P, De Vincenzi M, Giovannini C, Faccia M, et al. Proteolysis by sourdough lactic acid bacteria: effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl Environ Microbiol. 2002;68:623–33.
van den Broeck HC, van Herpen TW, Schuit C, Salentijn EM, Dekking L, Bosch D, et al. Removing celiac disease-related gluten proteins from bread wheat while retaining technological properties: a study with Chinese Spring deletion lines. BMC Plant Biol. 2009;9:41.
Ellis HJ, Pollock EL, Engel W, Fraser JS, Rosen-Bronson S, Wieser H, et al. Investigation of the putative immunodominant T cell epitopes in celiac disease. Gut. 2003;52:212–17.
Pyle GC, Paaso B, Anderson BE, Allen DD, Marti T, Li Q, et al. Effect of pre-treatment of food gluten with prolyl endopeptidase on gluten-induced malabsorption in celiac sprue. Cell Mol Life Sci. 2007;64:345–55.
Matysiak-Budnik T, Candalh C, Cellier C, Dugave C, Namane A, Vidal-Martinez T, et al. Limited efficiency of prolyl-endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology. 2005;129:786–96.
Tye-Din JA, Anderson RP, French RA, Brown GJ, Hodsman P, Siegel M, et al. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin Immunol. 2010;134:289–95.
De Angelis M, Rizzello CG, Fasano A, Clemente MG, De Simone C, Silano M, et al. VSL#3 probiotic preparation has the capacity to hydrolyse gliadin polypeptides responsible for celiac sprue. Biochim Biophys Acta. 2006;1762:80–93.
Warny M, Fatimi A, Bostwick EF, Laine DC, Lebel F, LaMont JT, et al. Bovine immunoglobulin concentrate-clostridium difficile retains C difficile toxin neutralizing activity after passage through the human stomach and small intestine. Gut. 1999;44:212–17.
Paterson BM, Lammers KM, Arrienta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in celiac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–66.
Kelly CP, Green PH, Murray JA, et al. Intestinal permeability of larazotide acetate in celiac disease: results of a phase IIB 6-week gluten-challenge clinical trial (abstr). Gastroenterology. 2009;136 Suppl 1:M2048.
Donnelly SC, Ellis HJ, Ciclitira PJ. Pharmacotherapy and management strategies for celiac disease. Expert Opin Pharmacother. 2011;12(11):1731–44.
De Vincenzi M, Gasbarrini G, Silano V. A small peptide from durum wheat gliadin prevents cell agglutination induced by prolamin-peptides toxic in celiac disease. Toxicology. 1997;120:207–13.
De Vincenzi M, Luchetti R, Giovannini C, Pogna NE, Saponaro C, Galterio G, et al. In vitro toxicity testing of alcohol-soluble proteins from diploid wheat Triticum monococcum in celiac disease. Biochem Toxicol. 1996;11:313–8.
Silano M, Di Benedetto R, Maialetti F, De Vincenzi A, Calcaterra R, Trecca A, et al. A 10-residue peptide from durum wheat promotes a shift from a Th1-type response toward a Th2-type response in celiac disease. Am J Clin Nutr. 2008;87(2):415–23.
Silano M, Di Benedetto R, Trecca A, Arrabito G, Leonardi F, De Vincenzi M. A decapeptide from durum wheat prevents celiac peripheral blood lymphocytes from activation by gliadin peptides. Pediatr Res. 2007;61:67–71.
Falcioni F, Ito K, Vidovic D, Belunis C, Campbell R, Berthel SJ, et al. Peptidomimetic compounds that inhibit antigen presentation by autoimmune disease-associated class II major histocompatibility molecules. Nat Biotechnol. 1999;17:562–7.
Ishioka GY, Adorini L, Guery JC, Gaeta FC, LaFond R, Alexander J, et al. Failure to demonstrate long-lived MHC saturation both in vitro and in vivo. Implications for therapeutic potential of MHC-blocking peptides. J Immunol. 1994;152:4310–19.
Silano M, Vincentini O, Iapello A, Mancini E, De Vincenzi M. Antagonist peptides of the gliadin T-cell stimulatory sequences: a therapeutic strategy for celiac disease. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S191–2.
Anderson RP, van Heel DA, Tye-Din JA, Jewell DP, Hill AV, et al. Antagonists and non toxic variants of the dominant wheat gliadin T cell epitope in celiac disease. Gut. 2006;55:485–91.
Forsberg G, Hernell O, Melgar S, Israelsson A, Hammarström S, Hammarström ML. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology. 2002;123:667–78.
Salvati VM, Mazzarella G, Gianfrani C, Levings MK, Stefanile R, De Giulio B, et al. Recombinant human IL-10 suppresses gliadin dependant T-cell activation in ex vivo cultured celiac intestinal mucosa. Gut. 2005;54:46–53.
Colombel JF, Rutgeerts P, Malchow H, Jacyna M, Nielsen OH, Rask-Madsen J, et al. Interleukin-10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut. 2001;49:42–6.
Nissen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from celiac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995;37:766–76.
Przemioslo RT, Lundin KE, Sollid LM, Nelufer J, Ciclitira PJ. Histological changes in small bowel mucosa induced by gliadin sensitive T lymphocytes can be blocked by anti-interferon gamma antibody. Gut. 1995;36:874–9.
Hommes DW, Mikhajlova TL, Stoinov S, Stimac D, Vucelic B, Lonovics J, et al. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut. 2006;55:1131–7.
Reinisch W, de Villiers W, Bene L, Simon L, Rácz I, Katz S, et al. Fontolizumab in moderate to severe Crohn’s disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm Bowel Dis. 2010;16:233–42.
Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–92.
Ferrari-Lacraz S, Zanelli E, Neuberg M, Donskoy E, Kim YS, Zheng XX, et al. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol. 2004;173:5818–26.
Gillet HR, Arnot ID, McIntyre M, Campbell S, Dahele A, Priest M, et al. Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology. 2002;122:800–5.
Costantino G, della Torre A, Lo Presti MA, Caruso R, Mazzon E, Fries W. Treatment of life-threatening type 1 refractory celiac disease with long-term infliximab. Dig Liver Dis. 2008;40:74–7.
Maurano F, Siciliano RA, De Giulio B, Luongo D, Mazzeo MF, Troncone R, et al. Intranasal administration of one alpha gliadin can downregulate the immune response to whole gliadin in mice. Scand J Immunol. 2001;53:290–5.
Rossi M, Maurano F, Caputo N, Auricchio S, Sette A, Capparelli R, et al. Intravenous or intranasal administration of gliadin is able to down-regulate the specific immune response in mice. Scand J Immunol. 1999;50:177–82.
Senger S, Luongo D, Maurano F, Mazzeo MF, Siciliano RA, Gianfrani C, et al. Intranasal administration of a recombinant alpha-gliadin down-regulates the immune response to wheat gliadin in DQ8 transgenic mice. Immunol Lett. 2003;88:127–34.
Tye-Din JA, Stewart JA, Dromey JA, Beissbarth T, van Heel DA, Tatham A, et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010;2:41ra51.
Gianfranci C, Levings MK, Sartirana C, Mazzarella G, Barba G, Zanzi D, et al. Gliadin-specific type 1 regulatory cells from the intestinal mucosa of treated celiac patients inhibit pathogenic T cells. J Immunol. 2006;177:4178–86.
Van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2002;8(4):291–300.
Arulanandam T. Biological characteristics of anti-alpha4 integrin monoclonal antibody (natalizumab) a selective adhesion molecule (SAM) inhibitor for the treatment of multiple sclerosis and Crohn’s disease [SA23]. Inflamm Res. 2004;53:3.
Ghosh S, Panaccione R. Anti-adhesion molecule therapy for inflammatory bowel disease. Ther Adv Gastroenterol. 2010;3:239–58.
Verbeek WH, Mulder CJ, Zweegman S. Alemtuzumab for refractory celiac disease. N Engl J Med. 2006;355:1396–7.
Ananthakrishnan AN, Hur C, Korzenik JR. Certolizumab pegol compared to natalizumab in patients with moderate to severe Crohn’s disease: results of a decision analysis. Dig Dis Sci. 2012 Feb;57(2):472–80.
Ransohoff RM. Natalizumab and PML. Nat Neurosci. 2005;8:1275.
Takazoe M, Watanabe M, Kawaguchi T, et al. Oral alpha-4 integrin inhibitor (AJM300) in patients with active Crohn’s disease: a randomised double-blind, placebo-controlled trial. Gastroenterology. 2009;136(Suppl):S1066.
Behm BW, Bickston SJ. Humanized antibody to the alpha4beta7 integrin for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2009;1, CD007571. doi:10.1002/14651858.CD007571.
Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–507.
Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, et al. A randomised phase I study of etrolizumab (rhuMAb β7) in moderate to severe ulcerative colitis. Gut. 2012;61:918–32.
Zhao J, de Vera J, Narushima S, Beck EX, Palencia S, Shinkawa P, et al. R-spondin1, a novel intestinotropic mitogen, ameliorates experimental colitis in mice. Gastroenterology. 2007;132:1331–43.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nasr, I., Messing, J., Ciclitira, P.J. (2014). Novel and Experimental Therapies on the Horizon. In: Rampertab, S., Mullin, G. (eds) Celiac Disease. Clinical Gastroenterology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-8560-5_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8560-5_14
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-8559-9
Online ISBN: 978-1-4614-8560-5
eBook Packages: MedicineMedicine (R0)