Abstract
Introduction
A significant proportion of patients with Crohn’s disease (CD) lose response to antibodies directed against tumor necrosis factor α (TNF). Prior TNF-antagonist failure is associated with lower rates of response to subsequent TNF-antagonist therapy. In patients failing two anti-TNF agents, a choice exists between using a third-anti-TNF therapy or natalizumab (NAT), an α-4 integrin inhibitor. A cost-effectiveness analysis comparing these competing strategies has not been performed.
Methods
A decision analytic model was constructed to compare the performance of certolizumab pegol (CZP) versus NAT in patients with moderate to severe CD. Previously published estimates of efficacy of third-line anti-TNF therapy and NAT were used to inform the model. Costs were expressed in 2010 US dollars. A 1-year time frame was used for the analysis.
Results
In the base case estimate, use of NAT was only marginally more effective [0.71 vs. 0.70 quality adjusted life-years (QALYs)] than CZP but was expensive with an incremental cost-effectiveness ratio (ICER) of $381,678 per QALY gained. For CZP 2 months response rate of at least 24%, NAT had an ICER above the willingness-to-pay (WTP) threshold. The model was sensitive to the costs of both therapies; for all CZP costs below $2,300 per dose, NAT had higher ICER than the WTP threshold. Substituting adalimumab for CZP resulted in similar ICER estimates and thresholds for NAT use.
Conclusions
In patients with moderate to severe CD failing two TNF-antagonists, using a third TNF-antagonist therapy appears to be a cost-effective strategy without significantly compromising treatment efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The past decade has seen remarkable advances in the treatment of patients with Crohn’s disease (CD). Antibodies against tumor-necrosis factor α (anti-TNF) have emerged as an important tool in the treatment of moderate to severe CD. Initially, infliximab (IFX) demonstrated efficacy in inducing and maintaining remission in luminal and fistulizing CD [1, 2]. Subsequently, two other anti-TNF agents, adalimumab (ADA) [3] and certolizumab pegol (CZP) [4], have shown similar efficacy in CD. Though open-label studies and single-center registries confirm long-term durability of these agents [5, 6], between 10 and 20% of patients lose response annually [7].
Randomized control trials for ADA (GAIN) [8] and CZP (WELCOME) [9] determined that a substantial proportion of patients who lose response to one agent can regain response with a second anti-TNF. However, this rate of response is lower in patients with prior TNF-antagonist exposure compared to those who are TNF-antagonist naive [10, 11]. Limited published experience details the potential benefit of a third TNF-antagonist in patients with loss of response or intolerance to two such therapies. The only published report included 67 patients from a multi-center study from Europe among whom 45% were able to continue treatment through 9 months [12]. Natalizumab (NAT) is an α-4 integrin inhibitor that has demonstrated efficacy in treatment of CD and is available as a treatment option in CD patients failing anti-TNF therapy [13]. However, concern regarding the risk of progressive multifocal leukoencephalopathy (PML) [14] has limited more widespread use. In the clinical trials, the response rate to NAT does not appear to be significantly influenced by prior TNF-antagonist failures [13].
Large-scale randomized controlled trials of competing treatment strategies are unlikely to be conducted in this select group of patients failing two anti-TNF agents due to the large sample size required and the associated costs. In the absence of such clinical trial data, decision analysis models approximating a virtual cohort of patients serve as a valuable tool for informing clinical practice and also in providing information regarding the cost-effectiveness of each of the treatment strategies. The aim of our study was to develop a decision model examining the comparative effectiveness and cost of third TNF-antagonist therapy (CZP) to NAT in patients with two prior anti-TNF failures.
Methods
Model Structure
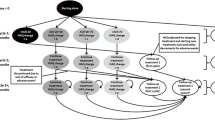
Our base case consisted of a hypothetical cohort of 35-year-old patients with moderate to severe luminal CD with loss of response (LOR) to two prior TNF-antagonists (secondary failures). The two treatment strategies available were (1) initiation of a third TNF-antagonist; or (2) NAT (Fig. 1). We chose CZP as the third TNF-antagonist because of the earlier availability of IFX and ADA, thus increasing the probability of their use as the first- and second-line agents. Moreover, in the single published report on the efficacy of third anti-TNF therapy [12], two-thirds of the patients were treated with CZP. Patients in the CZP arm were started on 400 mg subcutaneously at weeks 0, 2, and 4 and continued on monthly maintenance therapy. Patients in the NAT arm were administered 300 mg intravenously every month. Response was assessed at 2 months. Patients with clinical response at this time point were continued on maintenance therapy through month 12. Patients with no response at 2 months discontinued therapy and were managed with non-TNF therapy. Patients with an initial response could achieve one of the three health states—remission, partial response, or loss of response (active disease). Patients who were in remission or partial response continued maintenance therapy with CZP or NAT while those with LOR were switched to non-TNF therapy. A 1-year time horizon was used for the analysis, which was performed using TreeAge Pro 2011 software (TreeAge Software Inc., Williamstown, MA, USA). The analysis was from a third-party payer perspective and included all treatment and health states costs but did not include indirect costs (e.g., time missed from work) incurred by the patient.
Model Inputs and Assumptions
Probabilities: Efficacy
The response rate for CZP as the third TNF-antagonist therapy was based on a multi-center report by Allez et al. [12] (Table 1). The clinical response at 6 weeks reported in that study was used as our 2-month response estimate as the closest available time point. The probability of remaining under treatment with CZP at 1 year was used as the overall 1-year response rate as the best available data though it is possible that some patients were maintained on treatment despite inadequate response. Among responders, the proportion of patients achieving remission at 1 year was not available from that study. Consequently, we extrapolated this estimate based on the proportion of patients in remission at week 20 among the subset of patients with clinical response. Given the small sample size of this retrospective study, we also used data from the WELCOME trial [9] as an alternate short-term (2 months) efficacy estimate. The WELCOME trial examined the performance of CZP as a second anti-TNF agent in patients with secondary failure to infliximab [9]. Efficacy estimates for NAT were obtained from the ENACT trials [13]. Response rate at 2 months, as well as proportion of patients in response or remission at 1 year was obtained from the published figures from the trial. The response rate to non-TNF therapy was based on a Markov model by Silverstein et al. [15] from the pre-TNF antagonist time period. The likelihood of remission and/or response in this model is similar to that obtained in uncontrolled trials of other potential therapies in this setting (such as tacrolimus, mycophenolate) [16]. However, recognizing that the Silverstein study estimates represent an inception cohort that may have a higher likelihood of response, we also performed a sensitivity analysis varying the rates of non-response and/or need for surgery among patients failing CZP or NAT and electing to try non-TNF therapies. All events or change in health states were assumed to occur at the mid-point of the time interval.
Probabilities: Safety
Mortality estimates at 1 year were assumed to be equivalent to that of a 35-year-old obtained from the National Center for Health Statistics life table. The risk of PML with NAT at 1 year was assumed to be 1:1,000 [17] though this risk is likely lower in patients who receive fewer than 12 infusions. Owing to the high mortality associated with PML, the 1-year mortality rate for NAT users incorporated the PML incidence and was considered to be 0.0023 compared to 0.0013 for CZP. There is a lack of significant data on the safety of third-line TNF-antagonist therapy. Consequently, the rate of serious infections with third anti-TNF were obtained from the WELCOME and CHOICE trials; the ENACT trials provided this data for NAT users. Though there is a variation in the type of serious infections, we assumed, as a worst-case scenario, that the cost of a serious adverse effect would approximate the cost of sepsis [18].
Costs and Utilities
All costs were calculated in 2010 US dollars. Average wholesale drug prices were obtained from the 2010 Drug Topics Red book (Table 2) [19]. Per-treatment costs were estimated for a 400-mg monthly dose of CZP and 300-mg dose of NAT. Infusion costs were estimated from a prior decision model [20] and inflation adjusted to 2010 dollars using the healthcare component of the consumer price index. The monthly cost of each disease state was obtained from a recent Markov model of health costs associated with CD [21]. Mean total costs for ‘mild disease’ was used to represent cost for response while the costs for severe disease represented the costs associated with active disease. Surgical costs were obtained from a prior Markov model [15] and adjusted to 2010 dollars. The utility estimates for each health state were based on a prior estimate [15]. The values for mild disease were used for patients with clinical response not meeting the definition of remission (Table 2).
Sensitivity Analyses
We varied the costs of CZP and NAT over a range of estimates. We also varied the effectiveness of CZP and NAT to identify potential thresholds for cost-effectiveness. A willingness-to-pay (WTP) threshold of $80,000 per quality adjusted life-year (QALY) gained was considered acceptable for the incremental cost-effectiveness ratio (ICER) [22]. We used the results of the PRECiSE 4 trial to examine the impact of re-induction with CZP in patients losing response [23]. We substituted ADA for CZP to examine its cost-effectiveness as a third TNF-antagonist therapy. Two-way sensitivity analyses were performed simultaneously varying the effectiveness or costs of both CZP and NAT.
Results
In the base-case analysis, NAT was marginally more effective (0.71 QALY) than CZP (0.70 QALY) with a higher average cost ($51,842 vs. $46,314) for an incremental cost-effectiveness ratio (ICER) of $381,678 per QALY gained (Table 3). In a virtual cohort of 100,000 patients, NAT use resulted in a larger number of patients in remission (36,513 vs. 25,147), and fewer patients with active disease or requiring surgery (Fig. 2). However, the number of patients who died in the NAT arm was marginally higher (320 vs. 234).
These results were sensitive to the costs of both drugs. If the CZP cost per dose was reduced to $500, the mean annual cost decreased to $33,298, resulting in a significantly greater ICER for NAT ($1,024,408). However, increasing the per dose CZP cost to $2,500 resulted in an ICER of only $137/QALY for NAT. For all CZP costs below $2,350 per dose, NAT had higher ICER than the WTP threshold. On the other hand, reduction of the NAT costs to $2,625 or less resulted in an acceptable ICER costs for this therapy compared to CZP. For NAT cost per dose of $2,450 or lower, this strategy dominated CZP by being more effective and less expensive. Figure 3 presents the two-way sensitivity analysis at varying costs of NAT and CZP use.
The overall effectiveness and costs associated with each therapy were also sensitive to the ability to achieve clinical response at 2 months (Table 3). A 2-month response rate to CZP of less than 24% resulted in an acceptable ICER for NAT. In other words, if at least 24% of the patients with two anti-TNF failures achieve a clinical response to CZP at 2 months and are able to maintain this response at rates identified in prior publications, it remains a more cost-effective option than NAT at traditionally accepted WTP thresholds. For example, a CZP response rate of 20% resulted in an ICER of $69,012 per QALY gained for NAT. At response rates of greater than 80%, CZP use dominated NAT. On the other hand, given the significantly higher costs associated with NAT therapy, even a response rate of 90% at 2 months resulted in an ICER of $194,655 per QALY gained compared to CZP (Table 3). Figure 4 presents the two-way sensitivity analysis demonstrating the relationship between the effectiveness of both therapies.
Though there is no data specifically on the efficacy of re-induction with CZP in patients losing response in the setting of two prior TNF-antagonist failures, we extrapolated data from the PRECiSE 4 trial where patients who relapsed on CZP maintenance therapy were re-induced with an additional dose of CZP. In this analysis, CZP dominated NAT in the base-case with a marginally greater effectiveness (+0.001 QALY) and lower costs (−$7,766). Above a threshold of 15% response rate at 2 months, CZP was the more cost-effective strategy, and it was the dominant strategy at response rates greater than 59%.
For patients with no response or loss of response, we assumed a natural history with distribution of remission, response, and active states with non-TNF therapy based on a prior model. In a sensitivity analysis, we eliminated the remission state from that model and utilized only states of response (moderately active), no response (severe), and need for repeat surgery. Such an analysis marginally decreased the ICER at the base-case ($342,731/QALY) and increased the threshold for cost-effectiveness for CZP (34%). Figure 5 presents a one-way sensitivity analysis for the ICER for NAT compared to CZP at varying expected rates of response to non-TNF therapies (range 0–0.5). As demonstrated in the figure, as the likelihood of response to non-TNF therapies increased, the ICER for NAT compared to CZP also increased. For an expected response rate of 0%, the ICER for NAT was $261,627/QALY compared to an ICER of $394,063/QALY for a response rate of 50%.
There were two deaths (2/67) in the study by Allez et al. While this is likely an over-estimate of mortality with third anti-TNF strategy, we performed a sensitivity analysis where the mortality in the CZP arm was doubled (to 0.0026). This did not significantly change the ICER for NAT or the cost-effectiveness thresholds. Availability of a JC virus antibody assay may allow for better stratification of PML risk. Assuming a significantly greater incidence of PML [and consequently mortality (2–5 x)] in the NAT arm also did not significantly change our estimates.
Substituting ADA for CZP resulted in a similar cost-effectiveness estimate for NAT for the base-case with an ICER of $340,014 per QALY gained. Response rates of 80% or higher resulted in ADA being the dominant strategy. For NAT use to be cost-effective compared to ADA, the response rate to ADA at 2 months would have to be lower than 27%. For sensitivity analyses related to treatment safety, the ICER for NAT remained above the WTP threshold even for a rate of serious infection with CZP of 20%. Doubling the incidence of PML to 2:1,000 or eliminating its incidence also did not affect the ICER as it remained an uncommon event.
Discussion
In this decision analysis, we compared the performance of a third-line TNF-antagonist (CZP) to NAT (an α-4 integrin inhibitor) in patients with two prior anti-TNF failures. We found that switching treatment class to NAT may be marginally more effective than CZP. However, due to the higher treatment costs associated with NAT, routine trial of a third TNF-antagonist in such patients is more cost-effective for a predicted rate of response at 2 months of at least 24%.
Despite achieving initial response, between 10 and 20% of patients lose response to TNF-antagonist therapy every year [5, 7]. Among such patients, the GAIN [8] and WELCOME [9] trials showed that ADA and CZP use in patients who failed IFX therapy may be successful in achieving response in a significant proportion of patients. However, in the CHARM trial evaluating ADA as maintenance therapy in moderate to severe CD, 47% of TNF-antagonist-naive patients achieved remission at week 26 compared to 32% of patients with exposure to IFX, though both were significantly greater than placebo [3]. A similar difference in response rates was noted with CZP in the PRECiSE 2 trial [11]. In the single published report on third line TNF-antagonist therapy, Allez et al. reported their experience with 67 patients treatment with either CZP (n = 40) or ADA (n = 27) [12]. They found that nearly two-thirds of patients responded by week 6 (61%) with 45% continuing therapy at 9 months.
At the individual patient level, the decision regarding choice of therapy in patients with two prior TNF-antagonist failures rests on treatment efficacy and safety. Our decision analysis demonstrates that NAT use resulted in a marginal gain in QALY over CZP. In a virtual cohort of 100,000 patients, the NAT strategy had a greater proportion of patients achieving response and fewer with active disease or requiring surgery. However, if it is possible to re-induce a subset of patients losing response with an additional dose of CZP, then CZP becomes the dominant treatment strategy.
The reason for prior TNF-antagonist failure is important in deciding subsequent therapy. Response to subsequent anti-TNF therapy in patients who are primary non-responders (PNR) to one agent is significantly lower than in those with secondary loss of response [10, 24]. In the study by Allez et al., only one patient had experienced PNR to the prior anti-TNF therapies [12]. He failed to respond to the third TNF-antagonist as well, suggesting that NAT or other treatments from an alternate treatment class may be more appropriate in such patients. Our sensitivity analysis varying the short-term response rate to CZP mirrors this as well. If less than 24% of patients achieve an initial treatment response at 2 months, then NAT appears to be the more cost-effective therapy and results in a greater incremental gain in QALYs.
In a comparison of the safety of both therapies, the risk of PML with a high mortality rate resulted in a larger number of deaths with that strategy. However, given the rarity of occurrence of PML, this finding did not significantly influence the cost-effectiveness analyses. We saw a similar rate of serious infections with both strategies as noted in the clinical trials. However, there is limited safety data regarding the use of a third anti-TNF and to obtain reliable estimates, we had to extrapolate from two prior trials of second anti-TNF agents (WELCOME and CHOICE trials) [9, 25]. Doubling the rate of occurrence of PML to 2:1,000 also did not significantly affect the ICER, suggesting that while safety remains an important concern for patients and physicians in making treatment decisions, serious adverse events, including death, fortunately remain very uncommon and thereby have a limited impact of cost-effectiveness of therapy.
On a societal level, the choice of therapy is additionally determined by costs associated with the therapy and the health states. The significantly higher costs associated with NAT makes the TNF-antagonist strategy more appealing at base-case estimates. For response rates of at least 30% or higher at 2 months with CZP, the ICER for NAT exceeded the traditional WTP threshold. Even at high initial response rates for NAT (>90%), the costs associated led to the ICER being greater than $80,000 per QALY gained. A 25% reduction in NAT costs to $2,500 per infusion yielded an acceptable mean annual cost with an ICER below the WTP threshold.
Clinical predictors of response to TNF-antagonist therapy have been hard to define as the results of various studies have been inconsistent [10]. Duration of disease, younger age of onset, and smoking status have been shown to be predictors of TNF-antagonist therapy [10, 26, 27]. Serologic and genetic predictors of response to TNF-antagonists have also been explored in a few studies with some promising results [28, 29]. However, given the difference in clinical response rates between TNF-naive and TNF-experienced patients and potentially different dominant biological pathways of inflammation, such predictors may not be applicable equally to both cohorts.
Our study has a few limitations. There is only a single published report on the efficacy of third-line TNF-antagonist therapy [12]. Also, criteria for response and remission in a retrospective study may be different from the more rigorous criteria used in clinical trials. As a result, we performed the sensitivity analyses using efficacy data from the WELCOME trial, which is more comparable to the ENACT trials. As more information on the natural history of patients with severe disease becomes available, the results of our model need to be updated. Similarly, there is limited data on the safety of anti-TNF therapy in this setting to accurately model attributable costs. Our decision analysis is restricted to a time horizon of 1 year due to lack of information on the performance on the natural history of refractory disease beyond this time period. Given the lifelong nature of CD, future models will need to include a longer treatment horizon. Also, while economic analyses are important to inform treatment algorithms on a societal scale, treatment decisions for individual patients are based on perceived efficacy and risk, rather than cost of treatment. Consequently, decision analyses models such as ours are only one component of the decision-making process. While our model addressed secondary loss of response, the treatment choice between a second anti-TNF or NAT in the setting of primary non-response is an equally important one. However, lack of data on efficacy of 2nd or 3rd anti-TNF agents in this setting precluded our being able to examine that question in our model.
There are several implications to our findings. To our knowledge, ours is the first decision analysis comparing the performance of two possible treatment strategies in patients with two prior TNF-antagonist failures; one prior decision analysis has examined treatment strategies in patients failing the first TNF-antagonist therapy [20]. Given a potential shift towards the use of biologic agents early in the disease course, patients with prior anti-TNF exposure and treatment failures are likely to comprise a substantial part of the IBD practice, particularly in referral centers. With the availability of effective agents (such as NAT) belonging to an alternative treatment class, treatment decisions gain increasing complexity influenced by efficacy, safety, and cost. Randomized controlled trials between the potential treatment strategies in this setting are unlikely due to the large sample size needed to demonstrate a difference and the high associated costs. In the absence of such trials, decision analysis models approximating virtual cohorts of patients are a useful source of comparative effectiveness data. More nuanced decision-making will need to incorporate individual patient clinical and biological factors. Further research on such predictive tools in the setting of prior TNF-antagonist failure will be helpful to personalize the therapy for each individual patient.
In conclusion, in patients with loss of response to two TNF-antagonists, both CZP and NAT yielded similar estimates of effectiveness. The higher treatment costs with NAT resulted in CZP being the preferred strategy for all predicted response rates in excess of 30%. Larger studies on clinical and biological predictors of response to 2nd- and 3rd-line TNF antagonist therapy are urgently needed to accurately guide selection of therapy to improve patient outcomes.
References
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250.
Gonzaga JE, Ananthakrishnan AN, Issa M, et al. Durability of infliximab in Crohn’s disease: a single-center experience. Inflamm Bowel Dis. 2009;15:1837–1843.
Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500.
Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838.
Sandborn WJ, Abreu MT, D’Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol. 2010;8:688–695 e2.
D’Haens GR, Panaccione R, Higgins PD, et al. The London position statement of the world congress of gastroenterology on biological therapy for IBD with the European Crohn’s and colitis organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212.
Hanauer SB, Panes J, Colombel JF, Bloomfield R, Schreiber S, Sandborn WJ. Clinical trial: impact of prior infliximab therapy on the clinical response to certolizumab pegol maintenance therapy for Crohn’s disease. Aliment Pharmacol Ther. 2010;32:384–393.
Allez M, Vermeire S, Mozziconacci N, et al. The efficacy and safety of a third anti-TNF monoclonal antibody in Crohn’s disease after failure of two other anti-TNF antibodies. Aliment Pharmacol Ther. 2010;31:92–101.
Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925.
Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446.
Silverstein MD, Loftus EV, Sandborn WJ, et al. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57.
Ng SC, Chan FK, Sung JJ. Review article: the role of non-biological drugs in refractory inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:417–427.
Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933.
Dubinsky MC, Reyes E, Ofman J, Chiou CF, Wade S, Sandborn WJ. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005;100:2239–2247.
2010 Drug Topics Red Book. PDR Network, LLC, 2010.
Kaplan GG, Hur C, Korzenik J, Sands BE. Infliximab dose escalation versus initiation of adalimumab for loss of response in Crohn’s disease: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2007;26:1509–1520.
Malone DC, Waters HC, Van Den Bos J, Popp J, Draaghtel K, Rahman MI. A claims-based Markov model for Crohn’s disease. Aliment Pharmacol Ther. 2010;32:448–458.
Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Mak. 2002;22:417–430.
Sandborn WJ, Schreiber S, Hanauer SB, Colombel JF, Bloomfield R, Lichtenstein GR. Reinduction with certolizumab pegol in patients with relapsed Crohn’s disease: results from the PRECiSE 4 Study. Clin Gastroenterol Hepatol. 2010;8:696–702 e1.
Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4:355–366.
Lichtiger S, Binion DG, Wolf DC, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–1239.
Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol. 2010;105:1574–1582.
Kugathasan S, Werlin SL, Martinez A, Rivera MT, Heikenen JB, Binion DG. Prolonged duration of response to infliximab in early but not late pediatric Crohn’s disease. Am J Gastroenterol. 2000;95:3189–3194.
Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58:1612–1619.
Arijs I, Quintens R, Lommel LV, et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2010;16:2090–2098.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ananthakrishnan, A.N., Hur, C. & Korzenik, J.R. Certolizumab Pegol Compared to Natalizumab in Patients with Moderate to Severe Crohn’s Disease: Results of a Decision Analysis. Dig Dis Sci 57, 472–480 (2012). https://doi.org/10.1007/s10620-011-1896-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1896-3