Abstract
Taurine plays significant physiological roles, including those involved in neurotransmission. Taurine is a potent γ-aminobutyric acid (GABA) agonist and alters cellular events via GABAA receptors. Alternately, taurine is transported into cells via the high affinity taurine transporter (TauT), where it may also play a regulatory role. We have previously demonstrated that treatment of Hit-T15 cells with 1 mM taurine for 24 h significantly decreases insulin and GABA levels. We have also demonstrated that chronic in vivo administration of taurine results in an up-regulation of glutamic acid decarboxylase (GAD), the key enzyme in GABA synthesis. Here, we wished to test if administration of 1 mM taurine for 24 h may increase release of another β cell neurotransmitter somatostatin (SST) and also directly impact up-regulation of GAD synthesis. Treatment with taurine did not significantly alter levels of SST (p > 0.05) or GAD67 (p > 0.05). This suggests that taurine does not directly affect SST release, nor does it directly affect GAD synthesis. Taken together with our observation that taurine does promote GABA release via large dense-core vesicles, the data suggest that taurine may alter membrane potential, which in turn would affect calcium flux. We show here that 1 mM taurine does not alter intracellular Ca2+ concentrations from 20 to 80 s post treatment (p > 0.05), but does increase Ca2+ flux between 80 and 200 s post-treatment (p < 0.005). This suggests that taurine may induce a biphasic response in β cells. The initial response of taurine via GABAA receptors hyperpolarizes β cell and sequesters Ca2+. Subsequently, taurine may affect Ca2+ flux in long term via interaction with KATP channels.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 The Neuroendocrine Nature of the Pancreas
The pancreas is a dual function organ with both exocrine and endocrine portions. The endocrine portion contains five cell types, including the α- and β-cells responsible respectively for secreting the peptide hormones glucagon and insulin. These are regarded as two master neuroendocrine cells, responsible for maintaining plasma glucose concentrations. Additional endocrine cells include the PP or F cell that secretes pancreatic polypeptide responsible for actions in the gastrointestinal tract, and G-cells that release gastrin to enhance gastric functions. For glucose homeostasis, it is the relative plasma concentrations of glucose that dictates which of the exocytotic mechanisms predominates release of glucagon or insulin. However, the molecular machinery involved in the process is typically activated through changes in membrane potential facilitated by binding of neurotransmitters to their ionotropic receptors. The final cell type is the δ-cell, which appears to be a master regulator of all islet cells through its release of somatostatin (SST).
Along with the release of crucial glucose catabolic and anabolic hormones, the islet cells also secrete several neurotransmitters. Additional neurotransmitters synthesized or released by islet cells are glutamate (α-cells) and GABA (β-cells). Other neurotransmitters participate in pancreatic regulation, but these are likely from neuronal input. Therefore, the three major neurotransmitters regulating the endocrine pancreas are glutamate, GABA, and somatostatin.
In α-cells, glutamate is stored in LDCV where it is co-released with glucagon (Hayashi et al. 2003). Glutamate may bind to ionotropic glutamate receptors (AMPA-type receptor variant GluR4c-flip) on δ-cells (Muroyama et al. 2004) to stimulate release of somatostatin (SST). Alternately, glutamate may bind to AMPA/KA receptors on α-cells, serving as a positive autocrine mechanism to further glucagon release (Cho et al. 2010; Koh et al. 2012).
In β-cells, insulin is stored in large dense-core vesicles (LDCV) along with some of the γ-aminobutyric acid (GABA) synthesized in the β-cell, while a significant pool of GABA remains in synapse-like microvesicles (SLMV) (Sorenson et al. 1991; Nathan et al. 1995; Braun et al. 2004; Braun et al. 2007). In vivo, plasma glucose levels of ≥2.8 mM are sufficient to stimulate the Ca2+-dependent release of insulin from β-cells (Gilon et al. 1991). Any GABA released along with the insulin may be sufficient to bind to GABAA receptors on α cells, initiating a hyperpolarization of α-cells and reduce glucagon release (Rorsman et al. 1989; Suckale and Solimena 2010).
SST from δ-cells inhibits the release of the insulin and glucagon from α- and β-cells (Ahren 2009). These neurotransmitters allow for communication between islet cells that is complex and sophisticated in allowing for the cell communication in response to a rise or fall in glucose levels. Additionally, regulation of insulin and glucagon release (and thus glucose homeostasis) is also facilitated aided by SST secreted from the pancreatic δ cells in response to high levels insulin and glucagon (Goldsmith et al. 1975; Efendic et al. 1979; Taborsky 1983).
The initiation of insulin exocytosis includes multiple signaling events in the β-cells beginning with the Na+-dependent, electrogenic glucose uptake. Once glucose has entered the cytoplasm, glycolysis is initiated, resulting in the cytoplasmic accumulation of ATP; this increase in cytoplasmic ATP inhibits ATP-sensitive K+ (KATP) channels, resulting in a depolarization of β-cells. This depolarization ultimately opens voltage-sensitive Ca2+ channels; ensuing Ca2+ flux stimulates exocytosis of the LDCV containing both insulin and GABA. GABA then binds to the GABAA receptor on α-cells, initiating a depolarization of α-cells and subsequent inhibition of glucagon or to GABAA receptors on β-cells (Gu et al. 1993; Braun et al. 2010).
1.2 Taurine as a Potential Neuromodulator
Taurine is a conditionally essential amino acid whose role as a GABA agonist in the developing brain has been well documented. Taurine concentrations remain high in the neonatal brain for about 6 weeks, and then drops to adult levels and remains as the predominant free amino acid in some tissues (e.g., retina) or second to glutamate (e.g., brain). It is present in high concentrations in excitable cells (neurons, cardiomyocytes and skeletal muscle fibers) and maintains intracellular osmotic balance. Taurine may be synthesized from cysteine (Agrawal et al. 1971) or taken up into cells via the taurine transporter (TauT). In the brain, taurine acts as a GABA agonist, where it binds to GABAA receptors. Taurine activation of GABAA chloride channels alters membrane potential via rapid chloride uptake and subsequent hyperpolarization. Alternately, taurine may also exert a neurotransmitter-like effect through activation of glycine receptors. Although there is speculation of a taurine-specific receptor, to date there is no definitive proof of the existence of such a molecule (Wu and Prentice 2010).
In the pancreas, we and others have shown that release of insulin from β cells may be regulated via the GABAergic signaling system (Kawai and Unger 1983; Satin and Kinard 1998; El Idrissi et al. 2009a, 2010, 2012; Braun et al. 2010; L’Amoreaux et al. 2010). Additionally, we have shown that taurine treatment of β-cell lines is sufficient to induce insulin and GABA release (L’Amoreaux et al. 2010). When plasma glucose levels rise, glucose uptake into β-cells leads to increased exocytosis of LDCV containing insulin and GABA. This calcium-dependent exocytosis relies on the activation of voltage-sensitive calcium channels, which are activated upon depolarization of the membrane potential. The depolarization is driven by inhibition of KATP channels when cytoplasmic ATP levels rise following glycolytic processing of glucose. Taurine also is able to modulate cytoplasmic ATP levels through its interactions with mitochondria (El Idrissi 2008). Additionally, taurine also interacts with the sulfonylurea receptor subunit of KATP channels to inhibit channel activity (Tricarico et al. 2000). Inhibition of LDCV exocytosis may be derived by an autocrine feedback via activation of β-cell GABAA receptors (Braun et al. 2010), GABAB receptors (Gu et al. 1993), or through SST regulation of β-cell activity (McDermott and Sharp 1993; Doyle and Egan 2003). To date, studies have shown taurine’s modulatory effects on cell activities through GABAA receptors, but not GABAB. We have previously demonstrated that taurine can lead to an up-regulation of somatostatin expression in brain (El Idrissi et al. 2009b) and pancreatic islets (El Idrissi et al. 2010). Taurine can stimulate release of SST from neurons (Aguila and McCann 1985), but as yet there are no reports of taurine’s efficacy in stimulating SST release in pancreas.
Therefore, we sought to examine a system by which taurine may participate in glucose homeostasis via GABA and SST signaling. Here, we used an isolated β cell line (Hit-T15) to investigate the efficacy of taurine in altering SST and GAD expression. Furthermore, we tested the efficacy of taurine in initiating calcium flux in these cells. Together, our data suggests that taurine may participate in glucose homeostasis.
2 Methods
2.1 Taurine’s Effect on Somatostatin and GAD Levels
Pancreatic β-cell lines Hit T-15 from Syrian hamster were grown in Ham’s F12-K medium. For treatments, cells were plated in complete medium on sterile cover glass at 5,000 cells/cm2 until ∼80% confluent. Cells were serum starved for 24 h prior to glucose and taurine treatments. The media were aspirated and replaced with glucose-free Ham’s medium supplemented with 1 mM glucose, 1 mM taurine, or 3 mM glucose for 24 h. Following treatments with the supplemented media, cultures were fixed in 4% paraformaldehyde in PBS. The cells were then prepared for immunohistochemical analysis using the appropriate antibodies diluted 1:400. Primary antibodies included mouse anti-GAD and rabbit anti-somatostatin (Life Technologies/Molecular Probes, Carlsbad, CA). Primary antibodies were detected using goat anti-mouse IgG conjugated with Alexa 633, and goat anti-rabbit IgG conjugated with Alexa 488 (Life Technologies/Molecular Probes, Carlsbad, CA). Following incubation, the cover glass were placed on a drop of antifade (Slow Fade Gold Plus with DAPI) and sealed. The data were collected by confocal microscopy (Leica SP2 AOBS Confocal Microscope, Germany). Gain and offset for the acquisitions were identical for these three treatments.
2.2 Ca2+ Flux
Hit-T15 cells were grown in Hams F12-K medium as described above. For treatments, cells were plated in complete medium on sterile cover glass at 5,000 cells/cm2 until ∼80% confluent. Once confluent cells were incubated for 4 h with a 5 μM/ml solution of the fluorescent Ca2+ indicator Fluo-3 (Life Technologies/Molecular Probes, Carlsbad, CA). Following incubation, β-cells were treated with 1 mM glucose, 3 mM glucose, or 1 mM taurine. Live cell imagining (Zeiss Cell Observer; Carl Zeiss, Thornwood, NY), was used to detect Ca2+ flux every 15 s for a 5 min period following treatment. Images were obtained using both bright field and FITC filters.
2.3 Statistic Analysis
Statistical analyses were performed on intensity values using a one-way ANOVA and Bonferroni post hoc analyses (Prism). Values are expressed as the mean ± SEM. Differences were considered statistically significant when the calculated p value was less than 0.05.
3 Results
3.1 Taurine Does Not Alter Expression of SST or GAD
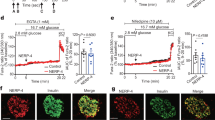
We report here for the first time the presence of SST in the Hit-T15 cell line (Fig. 25.1). Previous studies have confirmed the presence of a SST receptor in this cell line (Thermos et al. 1990; Seaquist et al. 1995; Cheng et al. 2002; Yao et al. 2005), but to date we find no evidence in the literature confirming the expression of the neurotransmitter within these cells. The in vivo role of somatostatin is to modulate exocytosis of LDCV in islets, presumably through the inhibition of Ca2+ flux (Yao et al. 2005). We speculate that these transformed cells may express somatostatin to serve as an autocrine regulator of cell function.
(a) Hit-T15 cells treated with 1 mM glucose. Staining for SST (green) and GAD67 (red). (b) Hit-T15 cells treated with 3 mM glucose. SST levels are decreased and GAD67 increased. (c) Treatment with 1 mM taurine has no effect on the release of SST or expression of GAD67. (d) Only treatment with 3 mM glucose significantly impacted SST levels (p < 0.001); neither 3 mM glucose nor 1 mM taurine affected GAD67 expression
We also demonstrated here taurine’s lack of efficacy in promoting SST release from these cells. Somatostatin levels were significantly decreased (p < 0.001) in the presence of 3 mM glucose (Fig. 25.1b, d), but SST levels in the taurine-treated were identical to those cells treated with 1 mM glucose (Fig. 25.1c, d). We also examined the role of taurine in eliciting an increase in GAD67 expression, which we have demonstrated in vivo with taurine treatment (El Idrissi et al. 2009a). We demonstrated that while 3 mM glucose significantly decreased somatostatin and increased GAD levels (p < 0.05) compared to those cells treated with 1 mM glucose 1 mM taurine was insufficient in eliciting these responses (Fig. 25.1c, d).
3.2 Taurine May Induce a Latent Ca2+ Flux
We hypothesized that the exocytosis of LDCV in response to taurine was dependent on the flux of Ca2+. To test the efficacy of taurine in eliciting calcium flux, we preloaded Hit-T15 cells with the fluorescent indicator Fluo-3, and then treated cells with subthreshold (1 mM) glucose, suprathreshold (3 mM) glucose, or 1 mM taurine. The 1 mM glucose was insufficient to induce calcium flux, yet 3 mM glucose did cause a significant increase in calcium flux (p < 0.001; Fig. 25.2). There were no significant differences in Ca2+ flux between 1 mM glucose and 1 mM taurine in the first 60 s following treatment (p > 0.05; Fig. 25.2). Following the initial 60 s, there was a significant increase (p < 0.005) in Ca2+ levels in the taurine-treated cells that persisted for an additional 120 s (Fig. 25.2).
These data suggests that taurine may initially initiate calcium sequestration, followed by calcium flux via a second mechanism. The data are similar to findings of taurine’s role in calcium sequestration in neurons (El Idrissi and Trenkner 2003; El Idrissi 2008). Taurine increases cytoplasmic ATP levels as a consequence of mitochondrial calcium buffering (Han et al. 2004; El Idrissi 2008). Taurine likely increases cytoplasmic ATP levels by increasing mitochondrial Ca2+ influx via the Ca2+ uniporter, which increases mitochondrial metabolic function (Han et al. 2004). This strongly suggests that in these studies using a β-cell line, taurine is most likely acting via GABAA receptors initially. Because β-cells in vivo express GABAA receptors, we hypothesize that taurine’s initial action in intact islets is to bind to GABAA receptors on β cells, causing both a hyperpolarization of the membrane and calcium sequestration. Following prolonged exposure to taurine, taurine may enter the cell via TauT where we hypothesize that intracellular taurine may bind to the KATP channel, polarizing the membrane and initiating Ca2+ flux that subsequently leads to prolonged exocytosis of the LDCV.
3.3 Proposed Model for Taurine’s Influence on Insulin Release
We propose that taurine may act through two independent mechanisms, one short term and the other long term (Fig. 25.3). Initially, taurine binds to GABAA receptors (Step 1), initiating rapid Cl− influx and hyperpolarization of β cells (Step 2). This hyperpolarization triggers calcium sequestration (Step 3), initially buffering the cell and increasing cytoplasmic ATP levels (Step 4). The timing of these initial four step must be further investigated. It is possible that the initial phase (Steps 1–4) will permit exocytosis of insulin by increasing cytoplasmic ATP levels and inhibiting KATP channels. Alternately, this may be a short-term (2 min) mechanism for β cells to retain intracellular insulin. During the subsequent 2 min interval, Ca2+ might be sufficient to premit limited release of insulin (Fig. 25.2).
Scheme proposing the dual roles of taurine in eliciting exocytosis of LDCV containing insulin and GABA. In Steps 1–4, taurine elicits sequestration of Ca2+; in Steps 5–7 either interaction with KATP channels or cytoplasmic ATP increase via the GABAA receptor or both activates voltage-sensitive Ca2+ channels for Ca2+ flux required for exocytosis
With long-term (24 h) exposure to taurine, TauT takes up the taurine (Step 5), which may then interact with the KATP channel’s sulfonylurea receptor subunit (Step 6) to inhibit the channel (Tricarico et al. 2000; Schaffer et al. 2010). Through either or both mechanisms, taurine treatment increases cytoplasmic ATP levels, inhibiting KATP channels and triggering a depolarization of β cells (Step 7), which in turn activates voltage-sensitive calcium channels (Step 8) and thus calcium flux and exocytosis of the LDCV (Step 9). Through our work and the work of others, the hypothesis of the modes of action of taurine is certainly plausible. Our laboratory will continue to examine the roles of taurine in eliciting release of insulin from the β cells.
4 Discussion
In the in vivo environment, when interstitial fluid levels reach ∼3 mM glucose pancreatic β-cells respond by releasing insulin and inhibiting glucagon release. In our in vitro studies, we used 3 mM glucose as our minimal dose required to initiate insulin release. Conversely, 1 mM glucose served as a control dose in which insulin should not be released. When examining the relative fluorescence intensity of insulin and GABA in the Hit cells, abundant signals for both markers were observed (L’Amoreaux et al. 2010). In those studies, we demonstrated that 1 mM taurine alone is effective in lowering plasma insulin and glucose as the values observed were significantly lower than those cells treated with the subthreshold glucose concentration of 1 mM.
Since taurine can affect release of insulin and GABA from LDCV, we demonstrated here that the mechanism by which taurine promotes exocytosis of these vesicles is different from the mechanism by which SST is released. Further, we provided evidence that the taurine-dependent up-regulation of GAD expression requires a feedback mechanism from α cells. We believe that in chronic administration of taurine during early pancreatic development, that a feedback mechanism between α and β cells provide a mechanism through which taurine alters expression of the β2 subunits of GABAA receptors, which leads to a requirement for increased GABA and thus increased GAD expression. As in vivo β cells also express GABAA receptors (Braun et al. 2010), an evaluation of GABAA receptor expression on this cell line is warranted and is forthcoming.
Taurine likely interacts with pancreatic β cells through two mechanisms. Initially, taurine binds to GABAA receptors to inhibit exocytosis of LDCV. Taurine is a potent GABA agonist and, as such, is likely playing a role in the feedback mechanism to inhibit further release of insulin via LDCV exocytosis. During a prolonged exposure to taurine, the amino acid is transported into β cells via the TauT transporter. Once in the cytoplasm, taurine may bind to the sulfonylurea receptor (SUR) of the ATP-dependent potassium channels. There is strong evidence that taurine can interact with SUR in muscle cells (Tricarico et al. 2000; Schaffer et al. 2010). Use of glibenclamide, a sulfonylurea, in perfused isolated pancreases moderately increases insulin release (Efendic et al. 1979) and also enhances the release of arginine-dependent release of SST (Efendic et al. 1980). Based upon the chemical similarities of the two molecules (Fig. 25.4), it is plausible that taurine may also serve as an antidiabetic agent and work via inhibition of KATP channels.
5 Conclusion
Taurine may serve as a cost-effective treatment for diabetes in that it promotes insulin release from β cells. We present evidence that short-term administration of taurine (such as in the diet) may elicit a response via GABAA receptors and restrict insulin release. With chronic administration of taurine, binding of taurine to the ATP-dependent potassium channels may elicit insulin release. Further studies are needed to confirm these observations and to determine the role of TauT in regulating insulin release from β cells.
Abbreviations
- GABA:
-
γ-Aminobutyric acid
- Tau:
-
Taurine
- GAD:
-
Glutamic acid decarboxylase
- TauT:
-
Taurine transporter
- LDCV:
-
Large dense-core vesicles
- SLMV:
-
Synapse-like microvesicles
- SST:
-
Somatostatin
References
Agrawal HC, Davison AN, Kaczmarek LK (1971) Subcellular distribution of taurine and cysteinesulphinate decarboxylase in developing rat brain. Biochem J 122:759–763
Aguila MC, McCann SM (1985) Stimulation of somatostatin release from median eminence tissue incubated in vitro by taurine and related amino acids. Endocrinology 116:1158–1162
Ahren B (2009) Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8:369–385
Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, Johnson PR, Rorsman P (2010) Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 59:1694–1701
Braun M, Wendt A, Birnir B, Broman J, Eliasson L, Galvanovskis J, Gromada J, Mulder H, Rorsman P (2004) Regulated exocytosis of GABA-containing synaptic-like microvesicles in pancreatic beta-cells. J Gen Physiol 123:191–204
Braun M, Wendt A, Karanauskaite J, Galvanovskis J, Clark A, MacDonald PE, Rorsman P (2007) Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells. J Gen Physiol 129:221–231
Cheng H, Yibchok-anun S, Coy DH, Hsu WH (2002) SSTR2 mediates the somatostatin-induced increase in intracellular Ca(2+) concentration and insulin secretion in the presence of arginine vasopressin in clonal beta-cell HIT-T15. Life Sci 71:927–936
Cho JH, Chen L, Kim MH, Chow RH, Hille B, Koh DS (2010) Characteristics and functions of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors expressed in mouse pancreatic {alpha}-cells. Endocrinology 151:1541–1550
Doyle ME, Egan JM (2003) Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev 55:105–131
Efendic S, Enzmann F, Nylen A, Uvnas-Wallensten K, Luft R (1979) Effect of glucose/sulfonylurea interaction on release of insulin, glucagon, and somatostatin from isolated perfused rat pancreas. Proc Natl Acad Sci U S A 76:5901–5904
Efendic S, Enzmann F, Nylen A, Uvnas-Wallensten K, Luft R (1980) Sulphonylurea (glubenclamide) enhances somatostatin and inhibits glucagon release induced by arginine. Acta Physiol Scand 108:231–233
El Idrissi A (2008) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34:321–328
El Idrissi A, Boukarrou L, L’Amoreaux W (2009a) Taurine supplementation and pancreatic remodeling. Adv Exp Med Biol 643:353–358
El Idrissi A, Boukarrou L, Splavnyk K, Zavyalova E, Meehan EF, L’Amoreaux W (2009b) Functional implication of taurine in aging. Adv Exp Med Biol 643:199–206
El Idrissi A, Trenkner E (2003) Taurine regulates mitochondrial calcium homeostasis. Adv Exp Med Biol 526:527–536
El Idrissi A, Yan X, L’Amoreaux W, Brown WT, Dobkin C (2012) Neuroendocrine alterations in the fragile X mouse. Results Probl Cell Differ 54:201–221
El Idrissi A, Yan X, Sidime F, L’Amoreaux W (2010) Neuro-endocrine basis for altered plasma glucose homeostasis in the Fragile X mouse. J Biomed Sci 17 Suppl 1:S8
Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC (1991) The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 129:2521–2529
Goldsmith PC, Rose JC, Arimura A, Ganong WF (1975) Ultrastructural localization of somatostatin in pancreatic islets of the rat. Endocrinology 97:1061–1064
Gu XH, Kurose T, Kato S, Masuda K, Tsuda K, Ishida H, Seino Y (1993) Suppressive effect of GABA on insulin secretion from the pancreatic beta-cells in the rat. Life Sci 52:687–694
Han J, Bae JH, Kim SY, Lee HY, Jang BC, Lee IK, Cho CH, Lim JG, Suh SI, Kwon TK, Park JW, Ryu SY, Ho WK, Earm YE, Song DK (2004) Taurine increases glucose sensitivity of UCP2-overexpressing beta-cells by ameliorating mitochondrial metabolism. Am J Physiol Endocrinol Metab 287:E1008–E1018
Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y (2003) Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem 278: 1966–1974
Kawai, Unger RH (1983) Effects of gamma-aminobutyric acid on insulin, glucagon, and somatostatin release from isolated perfused dog pancreas. Endocrinology 113:111–113
Koh DS, Cho JH, Chen L (2012) Paracrine interactions within islets of Langerhans. J Mol Neurosci 48(2):429–440
L’Amoreaux WJ, Cuttitta C, Santora A, Blaize JF, Tachjadi J, El Idrissi A (2010) Taurine regulates insulin release from pancreatic beta cell lines. J Biomed Sci 17(Suppl 1):S11
McDermott AM, Sharp GW (1993) Inhibition of insulin secretion: a fail-safe system. Cell Signal 5:229–234
Muroyama A, Uehara S, Yatsushiro S, Echigo N, Morimoto R, Morita M, Hayashi M, Yamamoto A, Koh DS, Moriyama Y (2004) A novel variant of ionotropic glutamate receptor regulates somatostatin secretion from delta-cells of islets of Langerhans. Diabetes 53:1743–1753
Nathan B, Floor E, Kuo CY, Wu JY (1995) Synaptic vesicle-associated glutamate decarboxylase: identification and relationship to insulin-dependent diabetes mellitus. J Neurosci Res 40:134–137
Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, Smith PA (1989) Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341:233–236
Satin LS, Kinard TA (1998) Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: what messages do acetylcholine, glutamate, and GABA transmit? Endocrine 8:213–223
Schaffer SW, Jong CJ, Ramila KC, Azuma J (2010) Physiological roles of taurine in heart and muscle. J Biomed Sci 17 Suppl 1:S2
Seaquist ER, Armstrong MB, Gettys TW, Walseth TF (1995) Somatostatin selectively couples to G(o) alpha in HIT-T15 cells. Diabetes 44:85–89
Sorenson RL, Garry DG, Brelje TC (1991) Structural and functional considerations of GABA in islets of Langerhans. Beta-cells and nerves. Diabetes 40:1365–1374
Suckale J, Solimena M (2010) The insulin secretory granule as a signaling hub. Trends Endocrinol Metab 21:599–609
Taborsky GJ Jr (1983) Evidence of a paracrine role for pancreatic somatostatin in vivo. Am J Physiol 245:E598–E603
Thermos K, Meglasson MD, Nelson J, Lounsbury KM, Reisine T (1990) Pancreatic beta-cell somatostatin receptors. Am J Physiol 259:E216–E224
Tricarico D, Barbieri M, Camerino DC (2000) Taurine blocks ATP-sensitive potassium channels of rat skeletal muscle fibres interfering with the sulphonylurea receptor. Br J Pharmacol 130:827–834
Wu JY, Prentice H (2010) Role of taurine in the central nervous system. J Biomed Sci 17 Suppl 1:S1
Yao CY, Gill M, Martens CA, Coy DH, Hsu WH (2005) Somatostatin inhibits insulin release via SSTR2 in hamster clonal beta-cells and pancreatic islets. Regul Pept 129:79–84
Acknowledgments
We thank Jonathan Blaize and Janto Tachjadi for assistance with the confocal microscopy. Support for the confocal microscope comes from the National Science Foundation (DBI 0421046). Support also from the Professional Staff Congress of the City University of New York. We would also like to thank the Organizing Committee of the 18th International Taurine Meeting for the scientific and social programs and the participants that made this a memorable conference.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Cuttitta, C.M., Guariglia, S.R., Idrissi, A.E., L’Amoreaux, W.J. (2013). Taurine’s Effects on the Neuroendocrine Functions of Pancreatic β Cells. In: El Idrissi, A., L'Amoreaux, W. (eds) Taurine 8. Advances in Experimental Medicine and Biology, vol 775. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6130-2_25
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6130-2_25
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6129-6
Online ISBN: 978-1-4614-6130-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)