Abstract
In this study we examined the role of taurine in mediating increased insulin production and secretion by the pancreas and the functional relevance for plasma glucose homeostasis and brain excitability. One-month-old FVB/NJ males were supplemented with taurine in drinking water (0.05 % w/v) for 4 weeks. At that time, mice were sacrificed and pancreases processed for histology and immunohistochemistry. Additional mice were subjected to a glucose tolerance test (7.5 mg/kg BW) after 12 h fasting. We found that taurine supplementation resulted in a significant increase in the number and size of the islets of Langerhans. Taurine-fed mice were slightly hypoglycemic prior to glucose injection and showed a significantly reduced plasma glucose at 30 and 60 min post-glucose injection when compared to control mice. Concomitant with the increased islets size and glucose tolerance observed in taurine-fed mice there was an increase in insulin, glucagon and somatostatin immunoreactivity in the islets. Previously, we reported that taurine supplementation induces biochemical changes in the GABAergic system in the brain. Those studies show that taurine-fed mice are hyperexcitable, have reduced GABAA receptors expression and increased GAD and somatostatin expression in the brain. In this study, we also found that taurine-fed mice had a significant increase in insulin receptor (IR) immunoreactivity in all brain regions examined. Circulating insulin crosses the blood brain barrier through a saturable mechanism. We propose that increased insulin production and secretion in taurine-fed mice caused increased activation of the central IR and may be partially responsible for the increased neuronal excitability observed in taurine supplemented mice.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Taurine (2-aminoethanesulfonic acid) is a sulfur-containing amino acid. It is one of the most abundant free amino acids in many excitable tissues, including the brain, skeletal and cardiac muscles. Physiological actions of taurine are widespread and include bile acid conjugation, detoxification, membrane stabilization, osmoregulation, neurotransmission, and modulation of cellular calcium levels (Lambardini 1985; Solis et al. 1988; Foos and Wu 2002; Lourenco and Camilo 2002; Saransaari and Oja 2000; Schaffer et al. 2000). Furthermore, taurine plays an important role in modulating glutamate and GABA neurotransmission (Militante and Lombardini 1998; El Idrissi and Trenkner 1999, 2004). We have previously shown that taurine prevents excitotoxicity in vitro primarily through modulation of intracellular calcium homeostasis (El Idrissi and Trenkner 1999). In neurons, calcium plays a key role in mediating glutamate excitotoxicity. Taurine is added to milk formula and in solution for parenteral nutrition of premature babies to prevent retinal degeneration and cholestasis (Huxtable 1992; Lourenco and Camilo 2002). More recently, it has been shown that gestational taurine is able to prevent pancreatic alterations induced by gestational malnutrition, especially a low-protein diet (Dahri et al. 1991; Cherif et al. 1996; Merezak et al. 2001; Boujendar et al. 2002). In addition, taurine administration during gestation delays the mean onset time of diabetes in NOD mice (Arany et al. 2004); whereas taurine supplementation on dams fed with normal diet produces weak glucose intolerance, and increases islet sensitivity to cytokines in offspring (Merezak et al. 2001). Moreover, taurine plays a role in glucose metabolism in adults (Hansen 2001; Franconi et al. 2006).
As a potent anti-oxidant, taurine has a protective effect on the pancreas, presumably by preventing or scavenging free radicals. Previous reports propose the islets from taurine-treated mice had almost double the number of cells positive for proliferating cell nuclear antigen (PCNA). This increase proliferation is accompanied by a reduction in the incidence of apoptosis in islet cells, and also a significant increase in the number of islet cells immunopositive for IGF-II (Arany et al. 2004). Peak of islet cell apoptosis is maximal in the rat pancreas 14 days after birth and is temporally associated with a fall in the islet cell expression of IGF-II (Petrik et al. 1998). IGF-II functions as an islet survival factor in vitro. The induction of islet cell apoptosis in vivo may involve an increased expression of inducible nitric oxide synthase (iNOS) within β cells. Interestingly, taurine is a potent inhibitor of iNOS (Liu et al. 1998). Similarly, Scaglia et al. (1997) have shown decreased replication and an increased incidence of apoptosis in the β cells in the presence of IGF-II.
The functional significance of increased insulin production and secretion in response to plasma glucose challenges may be twofold. First, insulin may activate the IGF-II receptors expressed in the islets, which will further promote survival of β cells. Second, insulin, once in the brain, will activate IRs and cause hyperexcitability, which is observed in response to taurine supplementation.
Taurine acts as a partial agonist of GABAA receptors in synaptic membranes (Quinn and Harris 1995), and to activate Cl− influx through GABAA receptors in cerebellar granule cells in vitro (El Idrissi and Trenkner 2004). The interaction of taurine with GABAA receptors can also be shown in vivo. We have previously reported that subcutaneous injections of taurine (43 mg/kg) reduces seizure severity in mice injected with kainic acid (El Idrissi et al. 2003), suggesting that the anti-convulsive effects of taurine might be mediated by direct interaction with the GABAA receptors in vivo. Furthermore, the chronic interaction of taurine with GABAA receptors induces a variety of alterations to the GABAergic system that encompasses key proteins involved in synaptic transmission at the inhibitory synapse. These alterations include increased hippocampal and cortical GAD expression, decreased hippocampal expression of the beta 2/3 subunits of the GABAA receptor (El Idrissi and Trenkner 2004), and an increase in the number of somatostatin-positive neurons (Levinskaya et al. 2006). These biochemical changes to the inhibitory GABAergic system induced by taurine supplementation would affect the efficacy of the inhibitory system within the brain and render neuronal circuits more excitable. Coupled with these changes in the GABAergic system, here we report an increased expression and activation of the insulin receptors will further increase neuronal excitability in taurine-fed mice.
2 Methods
2.1 Animals
All mice used in this study were 2-month-old FVB/NJ males. For taurine-fed mice, taurine was dissolved in water at 0.05 %, and this solution was made available to the mice in place of drinking water for 4 weeks beginning at 4 weeks of age. All mice were housed in groups of three in a pathogen-free room maintained on a 12 h light/dark cycle and given food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the College of Staten Island/CUNY, and were in conformity with National Institutes of Health Guidelines. The number of mice used in these studies was sufficient to provide statistically reliable results.
2.2 Immunohistochemistry
Frozen sections were made as previously described (Levinskaya et al. 2006 and placed onto gelatin-subbed slides. Non-specific binding sites were blocked using 4 % bovine serum albumin (BSA), 10 % normal goat serum (NGS), and 0.05 % Triton X-100 in 0.01 M phosphate-buffered saline (PBS; pH 7.2). Following the blocking step, the slides were rinsed in an antibody dilution cocktail (ABD) consisting of 2 % BSA and 1 % NGS in 0.01 M PBS. Primary antibodies (Chemicon International) employed were directed against insulin receptor (mouse host) diluted 1:500 in ABD. The primary antibody was incubated overnight at 4 °C and then unbound antibodies rinsed with ABD. Secondary antibodies were all raised in goat and directed against appropriate primary antibody type. The anti-mouse IgG was conjugated to Alexa Fluor 488 (Invitrogen/Molecular probes). Images were obtained by confocal microscopy (Leica SP2 AOBS). Nuclei were counterstained with SlowFade with DAPI (Invitrogen). To determine relative changes in protein expression, the gain and offset was identical for all comparisons. The intensity ratios of immunoreactivity were determined by importing the data from the Leica confocal software into Imaris X64 (Bitplane). For each Z stack, the threshold values for insulin receptor immunoreactivity were set for the untreated tissues. When the Z stacks for the taurine-treated tissues were imported, the Z stack were treated the same as the control. Coupling these manipulations with the consistent imaging parameters (same lens, gain and offset for each laser), the data changes are treatment-related. The mean pixel intensity values for each thresholded channel were obtained from the Imaris software and those data imported into InStat statistical software (GraphPad Software Inc.).
2.3 Intraperitoneal Glucose Tolerance Test
Mice from both groups were fasted overnight (12 h) and then injected intraperitoneally with 0.02 ml/g of body weight d-glucose (7.5 % stock solution in saline). Blood samples were taken by tail venesection at 0 min (just before glucose injection) and at 30-, 60-, and 120-min intervals after the glucose load. Glucose was measured with Ascensia Breeze portable glucose meter (Bayer, Germany). Mice were given only water during the test.
2.4 Statistic Analysis
Statistical significance was determined by Student's t-test. Each value was expressed as the mean ± SEM. Differences were considered statistically significant when the calculated P value was less than 0.05.
3 Results
3.1 Taurine-Fed Mice Exhibit Hyperinsulinemia and Glucose Tolerance
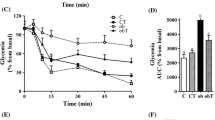
We have previously reported that taurine-supplemented mice have increase islets size and number. To determine the functional significance of these histological changes, we tested the tolerance of mice to glucose injection as an indicator of the pancreas efficiency to regulate plasma glucose homeostasis. As expected, control mice showed a drastic increase in plasma glucose concentration 30 min after challenge with a gradual decrease over through 120 min. By the end of the experiment, mice were slightly hypoglycemic relative to baseline (Fig. 1). In contrast, mice fed taurine showed a significant tolerance to glucose injection. Baseline plasma glucose levels indicated that these mice were slightly, but not significantly, hypoglycemic compared to controls. However, the response to glucose injection was drastically reduced (p < 0.001) at 30 min compared to controls. At 60 min following the challenge, the plasma glucose level in the taurine-fed mice rose slightly but was still significantly reduced over the controls at this time. Not until at 120 min post-challenge that the plasma glucose levels were similar in both groups.
Effect of taurine supplementation on glucose homeostasis. Intraperitoneal glucose tolerance test on overnight fasted control mice (n = 12) and taurine supplemented mice (n = 12). Values are expressed as means ± S.E.M obtained from three experiments. **p < 0.01, *p < 0.05 when compared with taurine group
3.2 Taurine-Fed Mice Have Increased Expression of Insulin Receptors in the Brain
To further investigate the functional significance of the histological changes occurring in the pancreas and the increased insulin production and secretion in response to glucose challenge, we examined the expression of the insulin receptor in the brain. Insulin is primarily a metabolic hormone functioning on muscle, fat and liver via activation of its cognate receptor, though it also functions on tissues that are not considered classically metabolic, such as the vasculature and the brain. Once insulin is secreted it crosses the blood-brain barrier by a transporter-mediated saturable mechanism. The IR is widely expressed in the brain but demonstrates denser expression in certain regions (Unger et al. 1991). A higher level of expression is found in the olfactory regions, amygdaloid complex, hippocampus, pyriform cortex and thalamus. This regional specify implicates insulin, through activation of its receptor, in various brain function that are mediated by these brain structures. In this study, we examined the levels of IR expression in the brain and found that taurine-fed mice have a significant increase in IR expression in all brain regions compared to controls (Fig. 2).
Effect of taurine supplementation on insulin receptor expression in the hippocampus. Upper panel representative confocal images showing insulin receptor (green) immunoreactivity in CA3 region of the hippocampus from control and taurine-fed mouse, respectively. Lower panel depicts the Imaris reconstruction of the z-stacks. Hippocampi from taurine-fed mice show a significant increase in immunoreactivity for insulin receptor. Scale bar = μm
4 Discussion
We have previously shown that taurine-fed mice have a significant increase in the size of islets of Langerhans when compared to controls (El Idrissi et al. 2009). Concomitant with these histological changes, taurine-fed mice were tolerant to glucose challenges. When mice were injected with a glucose solution, taurine-fed mice handled plasma glucose load significantly better that controls (Fig. 1). Maintenance of glucose homeostasis by taurine-fed mice is attributable to the histological changes in the pancreas and the resulting increased insulin production and secretion by the pancreas of these mice when challenged with a glucose load.
Circulating insulin crosses the blood-brain barrier and bind to its cognate membrane-bound receptors on neurons. The IR is widely expressed in the brain but demonstrates denser expression in certain regions (Unger et al. 1991). A higher level of expression is found in the olfactory regions, amygdaloid complex, hippocampus, pyriform cortex and thalamus. Importantly, the IR is also highly expressed in the hypothalamus, specifically in the arcuate, supraoptic and dorsomedial nuclei (Havrankova et al. 1978). There is significant evidence that insulin mRNA is present in certain regions of the brain during development as well as in adult brain (Devaskar et al. 1993). Peripheral insulin enters the brain via a saturable mechanism involving the blood–brain-barrier, and it seems that the rate of entry varies according to the region.
There are numerous studies demonstrating that IR signaling plays a role in both excitatory and inhibitory neurotransmission, functions that are involved in higher brain functions. In addition, short- and long-term memory may affect IR expression levels in the rat hippocampus (Plum et al. 2005). Further support for a role of insulin in neuronal modulation is provided by studies showing that intranasal insulin delivery in mice leads to an increased expression of the potassium ion channel Kv1.3 in the olfactory bulb (Marks et al. 2009). Mice receiving intranasal insulin have improved cognition, as shown by short- and long-term object recognition. These findings suggest that insulin delivered to the CNS increases neuronal activity and improves memory by mechanisms involving changes in Kv1.3 levels. In addition to the role insulin plays in the adult brain, an important function for insulin in the CNS appears to be neuronal survival (Mielke et al. 2006). When rat hippocampal cells in culture are stressed by oxygen or glucose deprivation their survival can be rescued by insulin signaling through IR. Insulin also protects embryonic retinal cells during development from caspase and cathepsin-mediated apoptosis by inhibiting the expression of these pro-apoptotic proteins (Díaz et al. 1999).
The role of insulin receptor in fundamental biological processes (e.g., development, brain function, metabolism, etc.), along with more recent data linking brain insulin function to the etiology of a number of neurodegenerative diseases will, undoubtedly, translate into more clinically-oriented avenues of research in the near future.
5 Conclusion
In summary, the histological changes observed after taurine supplementation on the pancreas are consistent with the hypoglycemic effects of taurine and may have implication in diabetes. Additionally, taurine treatment resulted in a significant upregulation of insulin receptors in the brain. The regional distribution of insulin receptor in the brain coupled with the global upregulation may explain the wide neurobehavioral effects observed after taurine supplementation.
Abbreviations
- Tau:
-
Taurine
- GAD:
-
Glutamic acid decarboxylase
- IR:
-
Insulin receptor
- IGF:
-
Insulin-like growth factor
- WT:
-
Wild type controls
References
Arany E, Strutt B, Romanus P, Remacle C, Reusens B, Hill DJ (2004) Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia 47:1831–1837
Boujendar S, Reusens B, Merezak S, Ahn MT, Arany E, Hill D et al (2002) Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia 45:856–866
Cherif H, Reusens B, Dahri S, Remacle C, Hoet JJ (1996) Stimulatory effects of taurine on insulin secretion by fetal rat islets cultured in vitro. J Endocrinol 151:501–506
Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ (1991) Islet function in offspring of mothers on low protein diet during gestation. Diabetes 40:115–120
Devaskar BS, Singh LR, Carnaghi PA, Rajakumar SJ (1993) Giddings Insulin II gene expression in rat central nervous system. Regul Pept 48:55–63
Díaz B, Pimentel B, de Pablo F, de La Rosa EJ (1999) Apoptotic cell death of proliferating neuroepithelial cells in the embryonic retina is prevented by insulin. Eur J Neurosci 11:1624–1632
El Idrissi A, Trenkner E (1999) Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 19:9459–9468
El Idrissi A, Trenkner E (2004) Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem Res 1:189–197
El Idrissi A, Messing J, Scalia J, Trenkner E (2003) Prevention of epileptic seizures by taurine. Adv Exp Med Biol 526:515–525
El Idrissi A, Boukarrou L, L’Amoreaux WJ (2009) Taurine supplementation and pancreatic remodeling. Adv Exp Med Biol 643:353–358
Foos T, Wu JY (2002) The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 27:21–26
Franconi F, Loizzo A, Ghirlanda G, Seghieri G (2006) Taurine supplementation and diabetes mellitus. Curr Opin Clin Nutr Metab Care 9:32–36
Hansen SH (2001) The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 17:330–346
Havrankova J, Roth J, Brownstein M (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272:827–829
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Lambardini JB (1985) Effects of taurine on calcium ion uptake and protein phosphorylation in rat retinal membrane preparations. J Neurochem 45:268–275
Levinskaya N, Trenkner E, El Idrissi A (2006) Increased GAD-positive neurons in the cortex of taurine-fed mice. Adv Exp Med Biol 583:411–417
Liu Y, Tonna-DeMasi M, Park E, Schuller-Levis G, Quinn MR (1998) Taurine chloramine inhibits production of nitric oxide and prostaglandin E2 in activated C6 glioma cells by suppressing inducible nitric oxide synthase and cyclooxygenase-2 expression. Brain Res Mol Brain Res 59:189–195
Lourenco R, Camilo ME (2002) Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp 17:262–270
Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA (2009) Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci 29:6734–6751
Merezak S, Hardikar AA, Yajnik CS, Remacle C, Reusens B (2001) Intrauterine low protein diet increases fetal beta-cell sensitivity to NO and IL-1 beta: the protective role of taurine. J Endocrinol 171:299–308
Mielke JG, Taghibiglou C, Wang YT (2006) Endogenous insulin signaling protects cultured neurons from oxygen–glucose deprivation-induced cell death. Neuroscience 143:165–173
Militante JD, Lombardini JB (1998) Pharmacological characterization of the effects of taurine on calcium uptake in the rat retina. Amino Acids 15(99):108
Petrik J, Arany E, McDonald TJ, Hill DJ (1998) Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology 139:2994–3004
Plum L, Schubert M, Brüning JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16:59–65
Quinn MR, Harris CL (1995) Taurine allosterically inhibits binding of [35s]-t-butylbicyclophosphorothionate (TBPS) to rat brain synaptic membranes. Neuropharmacology 34:1607–1613
Saransaari P, Oja SS (2000) Taurine and neuronal cell damage. Amino Acids 19:509–526
Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S (1997) Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138:1736–1741
Schaffer S, Takahashi K, Azuma J (2000) Role of osmoregulation in the actions of taurine. Amino Acids 19:527–546
Solis JM, Herranz AS, Erreras O, Lerma J, Martin del Rio R (1988) Does taurine act as an osmoregulatory substance in the rat brain. Neurosci Lett 91:53–58
Unger JW, Livingston JN, Moss AM (1991) Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 36:343–362
Acknowledgements
This work was supported by PSC-CUNY and CSI.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
El Idrissi, A. et al. (2015). Taurine Supplementation Induces Hyperinsulinemia and Neuronal Hyperexcitability. In: Marcinkiewicz, J., Schaffer, S. (eds) Taurine 9. Advances in Experimental Medicine and Biology, vol 803. Springer, Cham. https://doi.org/10.1007/978-3-319-15126-7_32
Download citation
DOI: https://doi.org/10.1007/978-3-319-15126-7_32
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15125-0
Online ISBN: 978-3-319-15126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)