Abstract
Inflammatory bowel disease (IBD) patients have an increased risk for colorectal cancer (CRC). While not always appreciated, this is true not just for patients with ulcerative colitis (UC), but also patients with Crohn disease (CD), particularly those with Crohn colitis. While our understanding of the clinical and molecular basis for this association has improved since the first case descriptions and series were reported nearly a century ago, our means of prevention and treatment, primarily colonoscopic surveillance and prophylactic surgery, remain modest, though circumstantial evidence supports their use. CRC still accounts for a large proportion of the premature mortality in both UC and CD. This chapter will review the pathogenesis and clinical epidemiology of CRC in IBD, as well as the theoretical and literature-based strategies for CRC prevention. Additionally, the available evidence on the association between Crohn ileitis and small intestinal cancer will be presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Inflammatory Bowel Disease
- Ulcerative Colitis
- Primary Sclerosing Cholangitis
- Ulcerative Colitis Patient
- Ursodeoxycholic Acid
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Inflammatory bowel disease (IBD) patients have an increased risk for colorectal cancer (CRC). While not always appreciated, this is true not just for patients with ulcerative colitis (UC), but also patients with Crohn disease (CD), particularly those with Crohn colitis. While our understanding of the clinical and molecular basis for this association has improved since the first case descriptions and series were reported nearly a century ago, our means of prevention and treatment, primarily colonoscopic surveillance and prophylactic surgery, remain modest, though circumstantial evidence supports their use. CRC still accounts for a large proportion of the premature mortality in both UC and CD.
This chapter will review the pathogenesis and clinical epidemiology of CRC in IBD, as well as the theoretical and literature-based strategies for CRC prevention. Additionally, the available evidence on the association between Crohn ileitis and small intestinal cancer will be presented.
Pathogenesis and Molecular Basis of Cancer in IBD
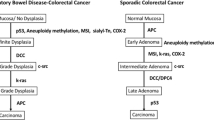
Drawing lessons from the molecular changes that account for colon carcinogenesis in familial adenomatous polyposis and hereditary non-polyposis colorectal cancer, now again called Lynch syndrome, the genetic and molecular basis of colon carcinogenesis have become better understood in recent years. These lessons have been directly applicable to events involved in the development of sporadic colorectal neoplasia, whose pathways mirror those of the familial cancer syndromes. It is currently believed that the vast majority (80–85 %) of sporadic CRCs arise from a pathway that involves chromosomal instability resulting in abnormal segregation of chromosomes, aneuploidy, and altered expression of tumor suppressor genes (primarily APC and p53) and oncogenes (mainly k-ras) (Fig. 49.1). In this pathway, loss of APC function occurs as an initiating or “gatekeeper” event for subsequent molecular alterations that culminate in the development of adenoma. Loss of p53 gene function occurs later in the sequence, typically at the transition of the adenoma to carcinoma. The remaining 15 % of sporadic CRCs arise through a so-called mutator pathway that involves loss of function of DNA base mismatch repair (MMR) genes, mainly hMLH1 and hMSH2. In this pathway, loss of MMR gene function results in a phenotype termed microsatellite instability (MSI). Sporadic CRCs that demonstrate MSI are often diploid (as opposed to the aneuploid state of chromosomal instability pathway-related tumors), tend to occur in the proximal colon, and frequently display rather unique histological features such as a medullary or solid growth pattern, signet ring cell histology, a plethora of tumor infiltrating lymphocytes, and an adjacent inflammatory reaction often referred to as a “Crohn-like reaction.” Another distinguishing feature of MSI-positive sporadic CRCs is the better survival of patients with those tumors compared to the ones without MSI [2].
Timing in Molecular Alterations in Sporadic Colorectal Cancer and Colitis-Associated Colorectal Cancer (From Gastroenterology, 2004) [1]
IBD-associated CRCs share several features in common with sporadic CRC. First, they both arise from a precursor dysplastic lesion. In the case of sporadic CRC, the dysplastic precursor is a discrete, polypoid growth called an adenoma, which typically progresses to cancer by enlarging in size, assuming greater degrees of dysplasia, and often assuming an increasing proportion of villous histology. In chronic colitis, while dysplasia is often polypoid, it may be flat or only slightly raised. Regardless of its growth pattern, colitis-related dysplasia progresses through increasing levels of abnormal development in its path to CRC. Second, stage-based survival of patients with CRC is similar in the two settings. Third, the types of molecular alterations that contribute to the pathogenesis of sporadic CRC are the same ones found in colitis-associated neoplasms [3].
While the similarities between colitis-associated neoplasia and sporadic colorectal neoplasia are notable, they differ in several important ways. First, colitis-associated cancers affect individuals at a much younger age. Second, colitis-associated neoplasia, by definition, arises in the setting of longstanding chronic inflammation, whereas sporadic neoplasms occur in the absence of an inflammatory background. Oxidative stress or other insults may lead to earlier or more frequent genetic changes to the colon, but the precise mechanisms by which chronic inflammation leads to neoplasia remain elusive. Third, dysplasias and even cancers in colitis are often multifocal, suggesting more of a precancerous “field change” of the colitic mucosa compared to the colons of patients with sporadic adenomas and colon cancer; the clinical consequence of this difference accounts for the different surgical approach: colitis-associated neoplasms are usually treated with total proctocolectomy, whereas sporadic adenomas and cancers are treated with polypectomy or segmental resection of affected colon. Fourth, although the two settings of colorectal neoplasia might share several types of molecular changes, the frequency and timing with which these molecular alterations occur is different (Fig. 49.1) [1]. For example, APC mutations are considered to be common and initiating events in sporadic colon carcinogenesis, whereas this molecular alteration is much less frequent and usually occurs late in the colitis-associated dysplasia-carcinoma sequence. In addition, in colitis patients, p53 mutations occur early and have even been detected in mucosa that is non-dysplastic or indefinite for dysplasia [4]. Likewise, MSI has been detected in non-dysplastic mucosa from patients with UC, even those patients with disease of relatively short duration, but not from healthy controls or patients with other types of benign inflammatory colitis [5, 6].
Colorectal Cancer in Ulcerative Colitis: Epidemiology and Clinical Practice
Epidemiology
Crohn and Rosenberg first described rectal cancer complicating UC more than 80 years ago. In their manuscript, they suggested that the malignancy was a complication of the disease [7]. Three years after Crohn and Rosenberg, Bargen, at the Mayo Clinic, reported a series of 17 patients with both chronic colitis and CRC [8]. Other cases and series followed, and the crude frequency calculations from these studies served as evidence supporting a link between UC and CRC. With the application of modern epidemiologic methods, true incidence calculations, cumulative incidence calculations, and standardized incidence rates confirmed the association between UC and CRC. Cumulative incidence rates have largely become the standard by which clinicians and public health experts assess the time-dependent risk of cancer development in colitis. Similarly, standardized incidence rates describe the estimate of the relative risk for developing colon cancer for a segment of a colitis population (such as colitis patients with universal disease) as compared to the general population. While initial series using this more accurate epidemiologic terminology came from large referral centers in which “incident” cases were referred for evaluation and management due to a suspicion for or even the actual presence of CRC, the use of more appropriate terminology was an advance over the previously used crude rates [9–11]. Due to these now obvious referral biases, however, these first “modern” studies overestimated the true risk of CRC in UC. Subsequent studies from population-based data sources used more realistic calculations for determining the incidence of CRC in UC. Without referral and other selection biases, the cancer incidence calculated in these manuscripts was substantially lower than that previously reported [12–16]. These studies, however, may have underestimated the true risk of cancer in longstanding UC, as they included many patients with UC who had undergone previous colectomy in the denominator of the incidence calculations. In a meta-analysis of the risk of CRC in UC in which 116 studies were included, Eaden and colleagues found the overall prevalence of CRC to be 3.7 % and an overall incidence rate of three cases per 1,000 person years duration (95 % confidence interval ranging from two to four cases per 1,000 person years duration). The rate increased with each decade of disease, leading to a calculated incidence of 12 per 1,000 person years in the third decade of colitis [17]. These data corresponded to a cumulative incidence of CRC of 2 % at 10 years, 8 % at 20 years, and 18 % at 30 years disease duration [17]. It is worth noting, however, that referral centers accounted for 64 % of the studies included in Eaden’s study; only 13 population-based reports were located by the Medline search performed as part of the meta-analysis [17]. Based on these and older data, typical estimates of CRC incidence usually range between 0.5 and 1 % per year after 10 years of colitis.
More recent studies, however, have raised the possibility that prior studies have overestimated the incidence and risk for CRC in this population. More recent publications from Denmark [18], Hungary [19], Canada [20], and Olmsted County, Minnesota (with its relatively small population) [21] have suggested a CRC in UC incidence of between 1 in 500 and 1 in 1,600 per year, far lower than the 1 in 300 rate calculated in Eaden’s meta-analysis [17]. These have corresponded to relative risk calculations ranging from 1.1 to 2.7 times the general population. While some have argued that these more “modern” calculations support a declining incidence over calendar time, as seen by Rutter and colleagues [22], no definitive analysis has been performed to support this hypothesis. To what extent such reductions in incidence (if they exist) are a function of colonoscopic surveillance (see below), chemoprevention with mesalamine-based agents or other medicines (also below), or other factors remain unknown.
Risk Factors
A number of clinical variables have been demonstrated to modify the risk for CRC in UC patients. These variables include duration of UC, anatomic extent of disease, age at UC diagnosis, concomitant primary sclerosing cholangitis (PSC), a family history of CRC, and inflammatory activity. The use of certain medications may lessen the risk of developing CRC, but the impact of these potentially chemopreventive agents is modest. Table 49.1 classifies these different risk modifiers.
Duration of Ulcerative Colitis
A number of investigators have demonstrated that the duration of UC correlates with the risk of cancer [9, 23–25]. Duration of disease, however, can be a rather subjective measurement. Most studies have used the date of UC diagnosis as the point at which the clock starts, but others have argued that the time of symptom onset is a better measure of disease duration. Whichever point is chosen, a number of distortions can be imagined that would impact the findings in any individual study. If date of diagnosis is used as a starting point, then patients with longstanding, subclinical disease would appear to have relatively shorter duration of disease, and such subjects would contribute less to any calculation of the effect of disease duration. Conversely, by using date of first symptoms, subjects who were without colitis might mistakenly contribute years of disease-free time to calculations of duration. This distinction in the definition of disease duration may be particularly problematic for patients with PSC who frequently have clinically quiescent colitis. Without unanimity in definition, there is variability in the estimate of this factor’s effect on subsequent CRC development. In Eaden’s meta-analysis, the effect of duration was made clear as the passage of each successive decade resulted in an increase in incidence. Incidence was calculated to be 2 per 1,000 patient years (95 % CI: 1–4/1,000) at 10 years and 11 per 1,000 (95 % CI: 4–28/1,000) at 30 years; the rate at 20 years was intermediate [17]. As the overall curve for cumulative CRC risk starts to meaningfully exceed that of the general population by 8–10 years, most clinicians will initiate surveillance colonoscopy once this threshold has been reached. Because many of the studies that were entered in to Eaden’s meta-analysis antedated the widespread application of colonoscopic dysplasia surveillance, it remains unclear whether the duration of colitis exerts a seemingly exponential effect, as Eaden found, or a linear effect, which might result if highest risk patients are serially removed from the denominator via colectomy from surveillance-identified dysplasia.
Anatomic Extent of Ulcerative Colitis
The length of involved colon also correlates with cancer risk: the greater the surface area of colitis, the greater the cancer risk. Defining the anatomic extent of UC, as with the duration of disease, can vary from study to study. In initial reports documenting this independent risk factor, anatomic extent was defined by a barium enema at diagnosis. Flexible endoscopy long ago replaced barium radiography for diagnosing colitis and its extent, but there is no consensus as to whether naked eye findings at colonoscopy or microscopic extent determined histologically should be the gold standard for measuring extent. Additionally, definitions of “pancolitis,” “universal colitis,” and “extensive colitis” vary within studies, although they are all typically used to describe disease proximal to the splenic flexure. Another feature that invites confusion into the definition of anatomic extent is the timing of the measurement. As extent can change over time [26], should we take the extent at diagnosis or at some point in follow-up? Like other questions surrounding the issue of extent, this question has been left unresolved, although the majority of studies have used the terms “extent” and “extent at diagnosis” interchangeably. Extent at follow-up has not been well studied as an independent risk factor.
A population-based investigation of a cohort of more than 3,000 patients with UC defined the extent of UC by barium enema exam at diagnosis, and demonstrated an impressive gradient of risk as one moves from proctitis (standardized incidence ratio of 1.7, 95 % confidence interval 0.8–3.2) to left-sided colitis (SIR = 2.8, 95 % CI: 1.6–4.4) to pancolitis (SIR = 14.8, 95 % CI: 11.4–18.9) [13]. Devroede [9], Greenstein [25], Gyde [24], Katzka [27], Mir-Madjlessi [28], and Gilat [14] all reported similar gradients in their studies. This finding was confirmed, though not directly studied, in Eaden’s meta-analysis [17]. In terms of “how” extent should be defined, it is worth noting that a group from University of California San Francisco found CRC in areas proximal to the endoscopically perceived margin of colitis that turned out to have microscopic disease in that region [29]. On this basis, clinicians should consider the most proximal extent of disease microscopically as the proximal extent of disease, and plan any prevention strategy accordingly.
Age of Ulcerative Colitis Onset
Importantly to pediatricians, age of colitis onset, as a variable independent of disease duration, has been implicated in some studies to modify the risk of IBD-related colon cancer. This hypothesis, however, remains in question. Reporting one of the highest published cumulative rates of CRC in colitis, Devroede and colleagues found that at 35 years of follow-up, 43 % of subjects with documented UC prior to age 15 had developed CRC [9]. This study, however, reflected pediatric patients seen at a large referral center; additionally, the number of patients available to analyze after 35 years of follow-up was quite small, with the error surrounding this point estimate correspondingly quite wide. While some investigators have failed to demonstrate a link between age of colitis onset and the subsequent development of CRC [14, 27], others have confirmed the direction if not the magnitude of Devroede’s findings [13, 24]. In the previously mentioned study by Ekbom, for example, the authors found that the relative risk of cancer in colitis decreased with advancing age—younger patients have a higher risk [13]. This overall gradient was confirmed by Eaden, who found that cumulative rates of CRC were greater than the pooled estimates for CRC among adult colitis patients, though this difference did not meet conventional thresholds for statistical significance [17]. Although neither the precise nature nor the precise magnitude of CRC risk for younger patients with UC has been determined, extra caution should be applied to pediatric patients given both the suggestion of an increased risk from the medical literature and the obvious increased lifetime risk given a longer life expectancy.
Primary Sclerosing Cholangitis
PSC is a chronic cholestatic liver disease in which there is progressive inflammatory fibrosis of the biliary tree. It is an infrequent complication of IBD, affecting 2–8 % of patients with UC. However, among patients with PSC, 62–72 % have underlying IBD. Since the intersection of CRC and PSC would be expected to occur in small absolute numbers in patients with UC, it is largely through case–control studies and referral center-based cohort studies that the majority of data have been generated to support an association between PSC and CRC in UC. Although a positive association has not always been noted [30–32], most studies do support such an association, with derived odds ratios from these “positive” studies ranging from 9 to 16 [33–37]. In a population-based study from Sweden, Kornfeld and colleagues found a substantially elevated cumulative incidence of CRC in UC/PSC patients: 33 % at 20 years [38]. As noted above, since colitis activity in PSC is often mild or even subclinical, PSC patients in these studies might well have had a longer duration of disease than that was appreciated, making it difficult to tease out the precise, independent contribution of PSC to the development of CRC.
Family History of Colorectal Cancer
Family history of CRC has long been recognized as a risk factor for the development of sporadic CRC. This risk increases according to the number of relatives affected with CRC [39]. In UC, only a few clinical studies have been performed to investigate the independent contribution of a positive family history for CRC. An early study from Lashner’s group at the University of Chicago supported family history of CRC as a potential risk factor for CRC in colitis, although the association did not reach statistical significance [40]. A second report from the Cleveland Clinic documented a lower rate of positive family history of CRC among UC patients with cancer or dysplasia compared to UC controls without colonic neoplasia, though this finding, too, failed to exclude the null hypothesis [41]. Both of these studies, however, were designed to test hypotheses concerning the association between folic acid supplementation and CRC in colitis. Testing for family history as a risk factor was performed as part of a secondary analysis, and these studies did not specify the rigor with which a family history was obtained.
More recently, a handful of studies have suggested an increased risk for CRC in UC when a positive family history of CRC was documented. Nuako and colleagues at the Mayo Clinic were the first to clearly demonstrate this increased risk, calculating an odds ratio of 2.3 (95 % CI: 1.1–5.1) in their case–control study [42]. In a population-based study from Scandinavia, Askling and colleagues found a similar elevated risk of 2.5 (95 % CI: 1.4–4.4) [43], while Eaden (in the UK) found an even greater risk (OR 5.0, 95 % CI: 1.1–22.8) in a multivariable model using case–control derived data [44]. Whatever the absolute magnitude, it appears quite likely that a positive family history confers an increased risk of CRC in UC.
Inflammation
Curiously, although inflammation has been assumed to be a key factor contributing to higher risk of colonic neoplasia in UC, few studies have examined this issue. One well-conducted retrospective case–control study recently reported that histologic inflammation was indeed associated with an increased risk of neoplastic progression based on a retrospective case–control analysis of patients followed at a specialized center [45]. A retrospective cohort study from Mount Sinai, New York has also demonstrated a link between histologic inflammation on dysplasia and cancer risk, with a twofold risk increase for each unit of inflammation derived from a four-point scale [45, 46].
Pharmacotherapy and Chemoprevention
As with sporadic CRC and interest in cyclooxygenase-inhibiting compounds, investigators, clinicians, and patients are actively seeking medications that might decrease the risk of developing CRC in UC. Retrospective studies have been performed examining a number of potential chemopreventive agents with mixed results. As is often the case in retrospectively performed studies of medication use, the dose and duration of use that defines exposure can be arbitrarily chosen. Nevertheless, a number of studies have been performed looking at different hypothesized chemopreventive medications with exposure defined in a number of different ways.
Sulfasalazine/5-Aminosalicylates
Sulfasalazine and the newer 5-amino-salicylic acid (5-ASA) products have been investigated for their chemopreventive effect, mainly by post hoc secondary analyses, yielding conflicting results. In a study designed to investigate the effect of supplemental folic acid on CRC risk, sulfasalazine use was found to have a positive (i.e. predisposing) effect on the development of CRC (slightly but not significantly higher rates of CRC in the exposed group); sulfasalazine allergic patients, however, were noted to have a substantially lower risk of developing CRC [40]. Subsequently, Pinczowski and Eaden were able to demonstrate a protective effect for sulfasalazine or mesalazine [44, 47], when dose and duration were considered. Tung [48] failed to demonstrate a meaningful protective effect, but this study was limited to high-risk PSC patients. A number of additional studies with a variety of definitions for exposure have now been performed with conflicting results. Some have shown benefit with exposure to mesalamine-based agents [49, 50], while others have been less optimistic [51, 52]. A systematic review reviewed a number of these studies, but its conclusion that mesalamine is chemopreventive with nearly a 50 % reduction in cancer incidence must be taken with some caution owing to the heterogeneity of the included studies as well as the different designs that were used (case–control, retrospective cohort, secondary analyses, population-based, and tertiary centers) [53]. Given the lack of unanimity of these studies, it remains in question whether mesalamine-based medications constitute truly chemopreventive agents. For a related question as to whether mesalamines are chemopreventive among patients undergoing dysplasia surveillance, a center-based cohort study that was able to account for changes in exposure over time found no such effect [54]. Given their utility at preventing flares in patients in remission, however, their use should be advocated in all UC patients.
Folic Acid
Folic acid, which has been demonstrated to have a protective effect in sporadic CRC was twice studied by Lashner, once at the University of Chicago [40] and again at the Cleveland Clinic [41]. In neither study was a significant protective effect noted, although the point estimates of risk (0.38 and 0.45) suggested the possibility of a chemopreventive effect. Given the low cost and the low risk of adverse events at conventional doses of 400 μg per day and 1 mg per day, the administration of folic acid as a chemopreventive drug should be strongly considered for all at risk patients.
Ursodeoxycholic Acid
Ursodeoxycholic acid, an exogenous bile acid used in the treatment of PSC, has also been studied. In UC-PSC patients, an impressive chemopreventive effect has been demonstrated, with a 40 percent difference in neoplasia noted between the ursodeoxycholic acid treated group (32 %) and the untreated group (72 %) [48]. This was additionally demonstrated in a randomized clinical trial of ursodeoxycholic acid in which a 74 % reduction in dysplasia or CRC was noted [55]. Newer data, however, from the same group that studied it in the earlier trial, demonstrated that high-dose ursodeoxycholic acid at 28–30 mg/kg per day actually gave rise to more colorectal neoplasia [56]. As the benefits of ursodeoxycholic acid on PSC are questionable at best, it is uncertain whether low-dose administration should be given as a chemopreventive agent.
Methods to Reduce Risk/Mortality
Until we discover or develop a meaningful chemopreventive agent and effective strategies to identify a minimal risk sub-group, only two acceptable forms of CRC prophylaxis exist: surgery and dysplasia surveillance. In dysplasia surveillance, high-risk patients are identified by the identification of neoplasia (either dysplasia or cancer) at colonoscopy and are subsequently referred to surgery, while cancer and dysplasia-free patients continue with periodic colonoscopy. The presumption is that only the highest risk patients will undergo a colectomy, and lower risk patients will be able to maintain a higher quality of life with their colons intact. A third option, watch and wait, with colonoscopy performed only for symptoms, is available, but due to the available evidence that symptomatic cancers are associated with a worse survival than asymptomatic ones [57, 58] is never used in clinical practice.
Surgery
Without question, the most effective method for minimizing CRC risk in UC patients is to perform a total proctocolectomy. This nearly eliminates the risk of colon or rectal cancer, and, while cancers have been reported in case reports and series in patients who have undergone either hand-sewn or stapled anastomoses, the risk of such an event is quite small. In the pre-endoscopic era, this strategy of cancer prevention was often advocated for patients with longstanding colitis, and should still be considered, particularly for patients with medically refractory or difficult disease. As surgery is not without its potential complications and change in quality-of-life, however, and as the absolute risk of developing a lethal colon cancer may not be sufficiently high to warrant such a radical approach in all colitis patients, surgical prophylaxis in asymptomatic patients with longstanding colitis is now viewed with skepticism by both patients and clinicians. At present, surgical options (for CRC prophylaxis or as primary treatment for colitis-related dysplasia or cancer) include total proctocolectomy with creation of an ileal pouch-anal anastomosis (often referred to as a restorative proctocolectomy) or total proctocolectomy with end-ileostomy. Subtotal colectomy with ileorectal anastomosis is to be avoided, although there are no studies comparing this procedure to either of the other conventional choices. Pouch surgery is generally reserved for younger patients, as it requires sufficient anal sphincter tone. Following pouch surgery, patients may expect to have five or more bowel movements per day due to pouch size and ileal flow. Possible complications include sexual and bladder dysfunction, incontinence, pouchitis (which usually responds to short courses of antibiotics but may become chronic and refractory), fistula formation, stricture formation, anastomotic leakage, and pouch failure. The overall failure rate (the proportion of patients eventually converted to end-ileostomy) is approximately 5 % [59]. It should also be noted that the malignant potential of ileal pouch mucosa in colitis patients remains unknown. Initial reports of pouch dysplasia have been reported, and there have been reports of cancer in the cuff of rectal mucosa to which the pouch is anastomosed [60, 61]. While cancer risk following proctocolectomy with Brooke ileostomy is close to nil, the loss of anorectal function and attendant stoma make this option less appealing to most patients who would otherwise be candidates for pouch surgery. Potential complications of total proctocolectomy with end-ileostomy include sexual and bladder dysfunction, stomal fistula, peristomal hernia, and small bowel obstruction [59].
Dysplasia Surveillance
As it results in too many colectomies in patients who would otherwise be unaffected by CRC, prophylactic total proctocolectomy is seldom performed. Even if limited to the high-risk groups of patients with longstanding and extensive UC, with or without PSC or a family history of CRC, a large number of colectomies would be performed in patients who would never develop CRC. What is needed is a tissue marker that better determines the highest risk patients, those with an imminent risk of CRC. While imperfect on many levels, mucosal dysplasia serves as such a marker.
In 1967 Morson and Pang first reported the association between mucosal dysplasia and CRC in patients with UC [62]. In their seminal report they noted that rectal dysplasia, then termed “precancer” and identified by blind rectal biopsy of colitis mucosa, heralded the presence of an invasive adenocarcinoma elsewhere in the colon. If appropriately discriminating, mucosal dysplasia, it was hypothesized, could be used as a diagnostic test to identify the highest risk patients to whom surgery would be offered.
Subsequent studies revealed that, although by no means a perfect test, dysplasia was discriminating enough to be tested in clinical practice. Retrospective studies confirmed Morson and Pang’s findings, noting the presence of dysplasia either adjacent to or remote from cancer in colitis [63–65]. Additionally, cancer foci were discovered in colons resected for the indication of dysplasia [66]. These data, along with the advent of flexible fiberoptic instruments with their ability to deliver multiple mucosal samples to the pathologist’s microscope, led to the development of protocol-based surveillance programs. Unfortunately, no randomized, controlled trials of surveillance were performed. (This may have been a function of difficulty in defining suitable control patients: would patients allow themselves to be randomized to a “no surgery” or “no endoscopy” arm of a surveillance study? Or to a “prophylactic surgery” arm?) Nevertheless, based on the clinical characteristics of dysplasia and the results of numerous surveillance programs, as well as the very limited acceptability of other prevention strategies, namely surgery for all longstanding colitis or waiting for cancer symptoms, periodic colonoscopy with biopsy for dysplasia became an accepted form of cancer prevention in UC. In addition to its widespread use in clinical practice, it has been advocated in the guidelines statements for colon cancer prevention [67] and UC care [68].
Single-armed surveillance programs have demonstrated the feasibility, though not the efficacy, of conventional surveillance [15, 66, 69–79]. When “control” arms were used in these studies, they included patients in whom surveillance at another institution or referral to the institution for malignancy could be considered as “no surveillance.” Nevertheless, the finding that cancers found during surveillance were more often at earlier stages than cancers found in a “watch and wait” strategy contributed to the acceptance of dysplasia surveillance as a form of cancer prevention [57, 58]. Other key features about surveillance programs worth noting include the presence of advanced stage cancers despite inclusion in a surveillance program (some due to patient drop-out and some due to progression while under surveillance); [76, 79, 80] the variable intervals used for surveillance; variable rates of patient drop-out; and the substantially varied rates of dysplasia incidence across studies. For surveillance to be effective, it should reduce CRC mortality in IBD patients. In the absence of prospective controlled studies, a well-designed population-based case–control study sheds light on this issue. Karlen and colleagues compared the exposure to colonoscopy among cases with CRC deaths and alive controls matched for age, gender, disease duration, and disease extent [81]. The point estimate of cancer mortality reduction from either one or only two previous colonoscopic exams was a threefold decrease. Although the odds ratio of 0.3 did not reach statistical significance (95 % CI: 0.1–1.3), this is certainly a clinically impressive result. A recent case–control study from the Mayo Clinic confirmed these findings, and even crossed the threshold of statistical significance with an odds ratio of 0.4 for 1–2 surveillance examination (95 % CI: 0.2–0.7) [82]. While these data were not population-based in their orientation, they nevertheless support the notion that surveillance is likely effective. Additional support comes from decision analysis models [83–85] that demonstrate improved outcomes for a population in surveillance compared to no surveillance. As with all such modeled data, there are many assumptions that lack real-world support, such as a lack of dropout while under surveillance and an orderly progression from no dysplasia to low-grade dysplasia to high-grade dysplasia to CRC [83, 84, 86]. Cost effectiveness analyses have similarly predicted that surveillance was a superior strategy to no surveillance (although prophylactic colectomy, while unacceptable to patients, was the preferred strategy vis-à-vis life-years saved over time).
What then might limit the effectiveness of dysplasia surveillance in UC in practice? One factor may be difficulties in histologic interpretation among pathologists. Indeed this was thought to be so substantial a problem after the initial reports of surveillance studies that in 1983 an international group of experts convened to establish true definitions for the evaluation of dysplasia surveillance specimens: no dysplasia, indefinite for dysplasia (with three subtypes), low-grade dysplasia, high-grade dysplasia, and CRC [87]. Unfortunately, despite these codified definitions, substantial rates of disagreement, even among expert GI pathologists, have been noted [87–90]. Rates of disagreement among community pathologists, not surprisingly, have also been substantial [88]. In these studies, crude rates of agreement have been as low as 40 % and as high as 72 %, with best agreement when no dysplasia was present; kappa values, which can account for chance agreement were fair to good. Clearly, this system needs less subjectivity and overall improvement.
Lack of perfection from practicing pathologists is not the only reason for surveillance not to reach its potential. Gastroenterologists also fall short of ideal practices. One variable that contributes to lack of uniform clinician practices stems from the uncertainty that surrounds the predictive value of dysplasia. While there is near-universal agreement that patients found to have high-grade dysplasia should undergo colectomy due to rates of concurrent adenocarcinoma near 50 % [64], considerable controversy surrounds the management of low-grade dysplasia. Adding to the controversy is the fact that LGD can be flat or polypoid, unifocal, or multifocal, or not repeatedly found on sequential colonoscopic exams. Few studies have directly addressed these variables in patients with LGD.
How to best manage LGD depends in large part on how likely patients with this finding are to either already harbor or progress to more advanced neoplasia (HGD or cancer). More specifically, the essential unanswered question is whether failure to perform a colectomy in patients with LGD results in a poor outcome. In a landmark study from St. Marks Hospital in which the Inflammatory Bowel Disease Morphology Study Group’s 1983 definitions were used [87], the rate of progression to advanced dysplasia from LGD was 54 % at 5 years [91]. In the same year as the St. Mark’s publication, a systematic review of surveillance programs by Bernstein and colleagues noted a 19 % rate of cancer at “immediate colectomy” following the discovery of LGD. These results were confirmed by studies from the Mayo Clinic [92] and Mount Sinai in New York [80], in which the rates of progression for flat LGD were 33 % (95 % CI: 9–56 %) and 53 % (95 % CI), respectively. Furthermore, in the Mount Sinai study, 19 % of patients who underwent colectomy within 6 months of their initial flat LGD finding were found to have CRC in their resection specimens. Of those who progressed, cases of node-positive cancer without intervening HGD were found. Neither the number of biopsies positive for LGD nor any other clinical variable were found to be predictive of subsequent progression, with unifocal flat LGD carrying a 5-year rate of progression of 53 % [80]. Investigators from the University of Washington where an aggressive biopsy protocol is followed [93] and from The Karolinska Institute, Sweden [94], however, discovered less frequent progression and no cancers. Not all investigators have discovered the same high risk for LGD as that noted by St. Mark’s, Mayo Clinic, and Mount Sinai in New York. A group from Karolinska in Sweden noticed a near-total lack of progression following discovery of LGD, but this group’s pathologists did not use the full panoply of IBD Morphology Study Group definitions, as readings of “indefinite” for dysplasia were not allowed. Additionally, a number of patients were included whose discovery of LGD occurred prior to the establishment of the Riddell criteria [95]. Additionally, the Leeds, UK group led by Lim found little progression from LGD, leading him and his co-authors to conclude that continued surveillance with satisfactory biopsy practices was a safe alternative to surgery [96]. Finally, the University of Chicago group found a low actuarial rate of progression among patients with both flat and polypoid low-grade dysplasia [97]. While the variable rates of progression (perhaps secondary to variable biopsy practices, observer variation in the interpretation of dysplasia, or imperfect follow-up) make it difficult to draw absolute conclusions for the management of patients with flat LGD, early colectomy for LGD that is histologically confirmed by two expert pathologists should be strongly considered at the least. For patients who defer or refuse colectomy for LGD, gastroenterologists must make certain that patients return for follow-up examinations and that surveillance is appropriately performed with an adequate number of biopsies taken to exclude dysplasia.
It should be noted that a negative exam following LGD can occur for a number of reasons: (1) the previous examination was a false positive due to pathologic interpretation error; (2) the present examination is a false negative due to sampling or interpretation error; or (3) both exams were accurate. Not finding dysplasia on a repeat colonoscopy following one that detected LGD is no reassurance that dysplasia has regressed or will not “recur.” [79] It was estimated that to exclude dysplasia with 95 % confidence, 56 biopsies must be performed, and to exclude 90 % confidence, 33 biopsies should be taken [98]. This number of biopsies is rarely performed even in academic centers [99, 100]. Eaden noted that 57 % of UK gastroenterologists take fewer than ten biopsies in a surveillance exam based on their response to a questionnaire [99]. In a study examining actual gastroenterologists’ practices, Ullman and colleagues found that the mean number of evaluable biopsies in patients with LGD was only 17.5 [80]. Such undersampling represents another limitation for dysplasia surveillance among gastroenterologists. Whether such practices truly limit the effectiveness of surveillance remains unknown.
The appropriate management of polypoid LGD, like that of flat LGD, is equally challenging. Polypoid dysplastic lesions in UC were labeled DALMs (dysplasia-associated lesions or masses), by Blackstone and colleagues in 1981 [70]. In this study, patients had been referred for a suspicion of CRC, and with that obvious selection bias, many lesions were noted to be >2 cm in diameter, and the reported DALMs were noted to harbor a 58% (7 of 12) risk of cancer [70]. Despite the impressive cancer risk of DALMs in the Blackstone report, astute clinicians hypothesized that smaller, adenoma-like lesions might present a lesser risk. Two simultaneously published studies reported on their experience of treating smaller, sessile lesions with endoscopic resection (without surgery). Rubin and colleagues from Mount Sinai, New York followed 48 patients with ulcerative or Crohn colitis in whom dysplastic polyps were detected at colonoscopy [101]. In those patients in whom polyps were endoscopically resected and the remaining colon was dysplasia-free, no patient progressed to CRC after a mean follow-up of 4.1 years [101]. In Engelsgjerd’s study from the Brigham and Women’s Hospital in Boston, none of the 24 colitis patients with adenoma-like polyps treated with polypectomy developed adenocarcinoma after a mean follow-up of 42.4 months [102]. Odze reported on the continued follow-up of the Brigham group 5 years later, and only one case of CRC developed, this in a patient 7.5 years after her initial polypoid lesion had been resected [103]. Similar results were noted in a recent publication by Rutter and colleagues [22]. And both Goldstone [104] and Pekow [97] have documented a more favorable outcome for polypoid LGD as compared to patients with non-targeted LGD findings, likely a function of the success of polypectomy as in sporadic CRC. The need for complete resection of polypoid lesions was underlined in a publication by Vieth, in which 10 of 60 patients in whom residual neoplasia was left behind progressed to CRC [105]. These data seem to indicate the relative safety of endoscopic polypectomy in colitis provided the lesions are small, completely resected, and that the rest of the surveillance run is dysplasia-free. When removing a suspicious polyp in a colitic colon, it is important to separately biopsy the mucosa immediately adjacent to the polyp base because if the polyp resides in a bed of dysplasia, colectomy is warranted.
The colitic colon with numerous inflammatory pseudopolyps presents another challenge to the endoscopist. It is wise to remove any polyp that has unusual features. Molecular studies using global gene expression arrays suggest that DALMs can be distinguished from apparently sporadic adenomas [106] holding promise for managing these difficult lesions. Finding a dysplastic lesion in a sea of inflammatory polyps, however, poses a substantial challenge to the endoscopist. It is not surprising that a recent publication found that the presence of inflammatory pseudopolyps carries a substantial (2.5-fold) risk for subsequent CRC [82].
In addition to a lack of certainty among experts as to how to manage flat, low-grade dysplasia and polypoid dysplasia, other impediments to the success of surveillance exist within the GI community. Poor understanding of dysplasia and surveillance practices exist among trained gastroenterologists [99, 100, 107]. Indeed, only 19 % of the respondents correctly identified dysplasia as neoplastic tissue in Bernstein’s two decade old questionnaire study [107]. Whether gastroenterologists’ understanding has improved since that time remains uncertain.
Patient factors have also limited the effectiveness of colonoscopic surveillance in colitis. Patient drop-out or non-compliance with surveillance programs has been demonstrated to be a substantial source of CRC mortality [58, 74, 76, 79].
Despite the limitations of surveillance based on the difficulties of dysplasia interpretation, poor agreement on dysplasia management, suboptimal surveillance performance, and risks of patient dropout, no other acceptable method for cancer prevention in colitis exists. As such, dysplasia surveillance will remain with us until a superior substitute is found. Current recommendations for how surveillance should be performed have been published in a number of different formats [1, 108, 109]. All of these publications agree that 4-quadrant biopsies, with each quartet of biopsies in a separate jar, should be taken every 10 cm, with suspicious lesions labeled and placed in a separate jar; examinations should be performed every 1–2 years for patients with disease involving one-third or more of their colon after 8 years of disease. Surveillance should begin at diagnosis for all patients with UC and PSC. A recent position statement in Gastroenterology summarizes these findings [110].
Alternatives to Surveillance
Augmentation of white light surveillance has been proposed using chromoendoscopy using the dye stains methylene blue or indigo carmine to better highlight subtle and “flat” lesions. These procedures have demonstrated higher detection rates for dysplasia in head-to-head comparisons [111] with conventional dysplasia surveillance and in back-to-back surveillance in which each patient serves as his/her own control [112, 113]. While an increased yield in the detection of dysplasia is a worthy finding, whether the introduction and application of chromoendoscopic surveillance will alter the outcome for colitis patients remains untested: potential benefits may come in the form of reduced intensity of surveillance for patients with dysplasia-free chromoendoscopic examinations, decreased non-targeted biopsies, decreased cost per examination, and potentially others. Other types of advanced endoscopy have been proposed as well, including narrow band imaging and various forms of spectroscopy. To date, none has demonstrated a benefit to patients. Molecular markers, whether from serum, RNA or stool, may also hold promise for complementing or replacing dysplasia surveillance, but as yet, they have not been incorporated into surveillance protocols.
Colorectal Cancer in Crohn Disease
Like UC, colitis in CD carries a risk for CRC greater than that of the general population. This was not always appreciated, however, as initial reports noted only a small, and sometimes not statistically significant, increase in CRC among patients with CD [114, 115]. A number of factors likely contributed to the dilution of the true effect of CD on colorectal carcinogenesis. First, patients with the disease limited to the small bowel were included in some calculations of incidence. Second, patients who had undergone surgery, particularly colectomy, were often included. And finally, a number of investigators performed their analyses without taking into account the duration of the disease, or more importantly, the extent of colonic involvement for this often segmental disease. Together, these factors resulted in a long-held belief that CD carried a lower risk for colon cancer than UC. Other studies [116–119] and even re-analysis of original data in which only subjects with longstanding and anatomically substantial Crohn colitis were examined [120] demonstrated that Crohn colitis harbored a CRC risk increase similar to that of UC and that both greater duration of colitis and greater length of involved colon increased the risk. As with UC, earlier disease onset resulted in even greater increases in relative risk of CRC, likely as a function decreased risk in the rate of sporadic CRC used in the denominator of these calculations. Population-based studies from separate continents have demonstrated a clear increase in CRC rates not only when limited to cases of Crohn colitis, but even when all patients with CD are considered [20, 121]. A recent meta-analysis by Canavan and colleagues calculated a pooled estimate of CRC relative risk to be 2.5 (95 % CI: 1.3–4.7) for all patients with CD, culled from 12 published manuscripts; for patients with colonic disease (in the four reports where it was available), the pooled RR was 4.5 (95 % CI: 1.3–14.9) [122]. Clearly patients with Crohn have a higher risk than the general population.
Similarities Between CRC in Crohn and UC and Rationale Behind Recommendation for Surveillance
In addition to the greater rate and earlier appearance of CRC in Crohn colitis when compared to the general population, investigators have noted other important similarities between Crohn-related CRC and UC-related CRC [123]. These include:
-
A higher proportion of mucinous and signet-ring histology
-
A greater proportion of synchronous lesions compared to sporadic CRC
-
Similar survival rates once detected (also true of sporadic CRC)
-
Presence of tumor in areas of macroscopic disease (although this point remains in question for CD)
-
Presence of dysplasia adjacent to and distant from tumor suggesting a field effect
This latter feature has led a number of experts to recommend a strategy of serial surveillance colonoscopy for patients with longstanding, extensive Crohn colitis as is performed and recommended for UC patients. To date, only one single-practice-based retrospective Crohn surveillance program has been reported in the literature [124]. In this study, Friedman and colleagues demonstrated both the feasibility and practicality of surveillance in Crohn patients with colitis affecting at least one-third of their colon for a minimum of 8 years. The authors detected dysplasia or cancer in 16 % of their 259 patients over a 16-year period, in which 663 examinations were performed; there were no cancer deaths [124]. As this is the only available study describing a surveillance program in CD, and there is no available control arm (i.e., no surveillance) against which to compare mortality rates, the efficacy of surveillance in CD is not yet established. Nevertheless, it has been recommended that all patients with extensive Crohn colitis (greater than one-third of colon involved) undergo periodic surveillance or be recommended prophylactic surgery after 8 years of disease, as is done with extensive UC. Guidelines have suggested that practices used in surveillance should be similar to those demonstrated to be able to rule out dysplasia in UC [1]. The effects of agents thought to be chemopreventive in UC are untested in CD.
Crohn Disease and Adenocarcinoma of the Small Intestine
As with colonic adenocarcinoma in Crohn colitis, an increased risk of small intestine adenocarcinoma has been demonstrated in patients with small bowel CD. Unlike CRC, the second most common lethal malignancy in the US, adenocarcinoma of the small intestine is uncommon. Even when evaluated in population-based reports, the absolute numbers are quite small, with the largest such series having only five patients with small bowel adenocarcinoma [20]. A summary of these studies is presented in Table 49.2. Since the absolute rates for these cancers is so small, and the best means of prevention is uncertain if a pre-clinical, precancerous finding were detected, it would be impractical to perform screening and surveillance in all patients with small bowel CD. When there is a change in clinical symptoms or a change in barium exams, however, the possibility of a small bowel malignancy should be entertained, particularly in a patient with longstanding disease.
Other Malignancies
Following case reports and series of extraintestinal malignancies, investigators questioned whether certain neoplasms might be related to either the presence or treatment of IBD. Greenstein and colleagues performed one of the first studies in which relative risks were calculated [126]. Using patients hospitalized for IBD at a tertiary care hospital, the authors determined that there was an increased incidence of leukemias, lymphomas, and squamous cell cancers when compared to published population-based controls [126]. Given the source of their sampling, a likelihood of selection and detection biases must be considered. Other referral-based studies examining this issue have demonstrated increased incidence of leukemias [127, 128] as well as bile duct, [28] and endometrial cancers [28]. Despite the large number of Crohn and UC patients, low absolute numbers of extraintestinal malignancies with broad confidence intervals have resulted in claims of “significance,” when one less case would have resulted in “no significance.” Ultimately, population-based analyses of cancer incidence in IBD have replaced the center-specific studies with their inherent biases. One such population-based study came from Ekbom and colleagues who determined that in a cohort from Uppsala, Sweden there was no increase in the incidence of leukemias, lymphomas, bile duct cancers, or uterine cancers [121]. However, an increase was noted in connective tissue cancers and squamous cell cancers of the skin, as well as brain cancers among patients with extensive UC [121]. It is worth noting, however, that no adjustments were made for the multiple comparisons in Ekbom’s studies. Other population-based studies have also failed to detect an increased number of extraintestinal malignancies. These include another Swedish study in which Crohn patients from Stockholm county were analyzed [125]—only a slight increase in bladder cancer was found and no increase in leukemias, lymphomas, bile duct cancers, or endometrial cancers was demonstrated—and one from North America [20]. In this latter population-based study from Manitoba, Canada that included over 6,000 IBD subjects, Bernstein and colleagues found an increase in liver and biliary tumors in both Crohn and UC (with only five such cases) and a small increase in lymphomas only among men with CD. As increased rates of lymphoma and other hematologic malignancies have been raised as possible adverse effects of either azathioprine or 6-mercaptopurine use in other conditions [129–131] and rates of these malignancies have been calculated in series from large referral practices and centers [132, 133], it is notable that Bernstein and colleagues demonstrated no relation to an increased risk of hematologic malignancies and purine analog use, the first population-based dataset to do so [20]. More recently, rare lymphomas (hepatosplenic T-cell lymphomas), particularly in younger patients, have been noted with anti-TNF therapy particularly in combination with purine analog immunomodulators [134]. This topic is reviewed elsewhere in this volume.
Summary
CRC remains a major threat to patients with longstanding UC and Crohn colitis. Due to patients’ and physicians’ desires to avoid unnecessary surgery, prophylactic colectomies are rarely performed in these patients. Instead, caregivers and IBD patients tend to elect a program of dysplasia surveillance in an effort to simultaneously minimize cancer mortality and unnecessary colectomies. Although only circumstantial evidence supports the use of such a strategy as a means of reducing CRC-related mortality, dysplasia surveillance will remain the standard of care until better tests are available. Extra caution should be given to pediatric patients whose relative risk and lifetime risk of cancer are increased. Small intestinal cancer occurs at an increased rate in patients with Crohn enteritis, but the absolute risk remains low. Extraintestinal malignancies are uncommon in IBD but lymphomas, biliary tract cancers, and squamous cell cancers of the skin may occur at an increased rate in IBD patients. The mechanisms for all of these processes remain elusive, but it is hoped that advances in molecular medicine will help to unravel these issues in the future.
References
Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–48.
Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17.
Itzkowitz SH. Inflammatory bowel disease and cancer. Gastroenterol Clin North Am. 1997;26:129–39.
Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–78.
Brentnall TA, Crispin DA, Bronner MP, Cherian SP, Hueffed M, Rabinovitch PS, et al. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996;56:1237–40.
Suzuki H, Harpaz N, Tarmin L, Yin J, Jiang HY, Bell JD, et al. Microsatellite instability in ulcerative colitis-associated colorectal dysplasias and cancers. Cancer Res. 1994;54:4841–4.
Crohn BB, Rosenberg H. The sigmoidoscopic picture of chronic ulcerative colitis. Am J Med Sci. 1925;170:220–8.
Bargen TA. Chronic ulcerative colitis associated with malignant disease. Arch Surg. 1928;17:862–8.
Devroede GJ, Taylor WF, Sauer WG, Jackman RJ, Stickler GB. Cancer risk and life expectancy of children with ulcerative colitis. N Engl J Med. 1971;285:17–21.
Bargen JA, Gage RP. Carcinoma and ulcerative colitis: prognosis. Gastroenterology. 1960;39:385–92.
Slaney G, Brooke BN. Cancer in ulcerative colitis. Lancet. 1959;2:694–8.
Rozen P, et al. Low incidence of significant dysplasia in a successful endoscopic surveillance program of patients with ulcerative colitis. Gastroenterology. 1995;108:1361–70.
Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33.
Gilat T, Fireman Z, Grossman A, Hacohen D, Kadish U, Ron E, et al. Colorectal cancer in patients with ulcerative colitis. A population study in central Israel. Gastroenterology. 1988;94:870–7.
Leidenius M, Kellokumpu I, Husa A, Riihela M, Sipponen P. Dysplasia and carcinoma in longstanding ulcerative colitis: an endoscopic and histological surveillance programme. Gut. 1991;32:1521–5.
Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–51.
Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35.
Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088–95.
Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, et al. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205–11.
Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62.
Jess T, Loftus Jr EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039–46.
Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–8.
Edwards F, Truelove S. The course and prognosis of ulcerative colitis. III and IV. Gut. 1964;5:1.
Gyde SN, Prior P, Allan RN, Stevens A, Jewell DP, Truelove SC, et al. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29:206–17.
Greenstein AJ, Sachar DB, Smith H, Pucillo A, Papatestas AE, Kreel I, et al. Cancer in universal and left-sided ulcerative colitis: factors determining risk. Gastroenterology. 1979;77:290–4.
Langholz E, Munkholm P, Davidsen M, Nielsen OH, Binder V. Changes in extent of ulcerative colitis: a study on the course and prognostic factors. Scand J Gastroenterol. 1996;31:260–6.
Katzka I, Brody RS, Morris E, Katz S. Assessment of colorectal cancer risk in patients with ulcerative colitis: experience from a private practice. Gastroenterology. 1983;85:22–9.
Mir-Madjlessi SH, Farmer RG, Easley KA, Beck GJ. Colorectal and extracolonic malignancy in ulcerative colitis. Cancer. 1986;58:1569–74.
Mathy C, Schneider K, Chen YY, Varma M, Terdiman JP, Mahadevan U. Gross versus microscopic pancolitis and the occurrence of neoplasia in ulcerative colitis. Inflamm Bowel Dis. 2003;9:351–5.
Gurbuz AK, Giardiello FM, Bayless TM. Colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Dis Colon Rectum. 1995;38:37–41.
Loftus Jr EV, Sandborn WJ, Tremaine WJ, Mahoney DW, Zinsmeister AR, Offord KP, et al. Risk of colorectal neoplasia in patients with primary sclerosing cholangitis [see comments]. Gastroenterology. 1996;110:432–40.
Nuako KW, Ahlquist DA, Sandborn WJ, Mahoney DW, Siems DM, Zinsmeister AR. Primary sclerosing cholangitis and colorectal carcinoma in patients with chronic ulcerative colitis: a case–control study. Cancer. 1998;82:822–6.
Brentnall TA, Haggitt RC, Rabinovitch PS, Kimmey MB, Bronner MP, Levine DS, et al. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis [see comments]. Gastroenterology. 1996;110:331–8.
Broome U, Lindberg G, Lofberg R. Primary sclerosing cholangitis in ulcerative colitis—a risk factor for the development of dysplasia and DNA aneuploidy? Gastroenterology. 1992;102:1877–80.
Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential [see comments]. Hepatology. 1995;22:1404–8.
D’Haens GR, Lashner BA, Hanauer SB. Pericholangitis and sclerosing cholangitis are risk factors for dysplasia and cancer in ulcerative colitis. Am J Gastroenterol. 1993;88:1174–8.
Marchesa P, Lashner BA, Lavery IC, Milsom J, Hull TL, Strong SA, et al. The risk of cancer and dysplasia among ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1997;92:1285–8.
Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study [see comments]. Gut. 1997;41:522–5.
Burt RW. Familial risk and colorectal cancer. Gastroenterol Clin North Am. 1996;25:793–803.
Lashner BA, Heidenreich PA, Su GL, Kane SV, Hanauer SB. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case–control study [see comments]. Gastroenterology. 1989;97:255–9.
Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology. 1997;112:29–32.
Nuako KW, Ahlquist DA, Mahoney DW, Schaid DJ, Siems DM, Lindor NM. Familial predisposition for colorectal cancer in chronic ulcerative colitis: a case–control study. Gastroenterology. 1998;115:1079–83.
Askling J, Dickman PW, Karlen P, Brostrom O, Lapidus A, Lofberg R, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356–62.
Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case–control study. Aliment Pharmacol Ther. 2000;14:145–53.
Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9.
Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105.
Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO. Risk factors for colorectal cancer in patients with ulcerative colitis: a case–control study. Gastroenterology. 1994;107:117–20.
Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV, et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89–95.
van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–8.
Rubin DT, LoSavio A, Yadron N, Huo D, Hanauer SB. Aminosalicylate therapy in the prevention of dysplasia and colorectal cancer in ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:1346–50.
Bernstein CN, Blanchard JF, Metge C, Yogendran M. Does the use of 5-aminosalicylates in inflammatory bowel disease prevent the development of colorectal cancer? Am J Gastroenterol. 2003;98:2784–8.
Terdiman JP, Steinbuch M, Blumentals WA, Ullman TA, Rubin DT. 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:367–71.
Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–53.
Ullman T, Croog V, Harpaz N, et al. Progression to colorectal neoplasia in ulcerative colitis: effect of mesalamine. Clin Gastroenterol Hepatol. 2008;6:1225–30.
Pardi DS, Loftus Jr EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–93.
Eaton JE, Silveira MG, Pardi DS, Sinakos E, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106(9):1638–45.
Choi PM, Nugent FW, Schoetz Jr DJ, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–24.
Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis [see comments]. Gastroenterology. 1994;107:934–44.
Becker JM. Surgical therapy for ulcerative colitis and Crohn disease. Gastroenterol Clin North Am. 1999;28:371–90. viii–ix.
Stern H, Walfisch S, Mullen B, McLeod R, Cohen Z. Cancer in an ileoanal reservoir: a new late complication? Gut. 1990;31:473–5.
Thompson-Fawcett MW, Marcus V, Redston M, Cohen Z, McLeod RS. Risk of dysplasia in long-term ileal pouches and pouches with chronic pouchitis. Gastroenterology. 2001;121:275–81.
Morson BC, Pang LS. Rectal biopsy as an aid to cancer control in ulcerative colitis. Gut. 1967;8:423–34.
Cook MG, Goligher JC. Carcinoma and epithelial dysplasia complicating ulcerative colitis. Gastroenterology. 1975;68:1127–36.
Ransohoff DF, Riddell RH, Levin B. Ulcerative colitis and colonic cancer. Problems in assessing the diagnostic usefulness of mucosal dysplasia. Dis Colon Rectum. 1985;28:383–8.
Taylor BA, Pemberton JH, Carpenter HA, Levin KE, Schroeder KW, Welling DR, et al. Dysplasia in chronic ulcerative colitis: implications for colonoscopic surveillance. Dis Colon Rectum. 1992;35:950–6.
Dickinson RJ, Dixon MF, Axon AT. Colonoscopy and the detection of dysplasia in patients with longstanding ulcerative colitis. Lancet. 1980;2:620–2.
Levin B, Lennard-Jones J, Riddell RH, Sachar D, Winawer SJ. Surveillance of patients with chronic ulcerative colitis. WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1991;69:121–6.
Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:204–11.
Brostrom O, Lofberg R, Ost A, Reichard H. Cancer surveillance of patients with longstanding ulcerative colitis: a clinical, endoscopical, and histological study. Gut. 1986;27:1408–13.
Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366–74.
Lashner BA, Silverstein MD, Hanauer SB. Hazard rates for dysplasia and cancer in ulcerative colitis. Results from a surveillance program. Dig Dis Sci. 1989;34:1536–41.
Lashner BA, Kane SV, Hanauer SB. Colon cancer surveillance in chronic ulcerative colitis: historical cohort study. Am J Gastroenterol. 1990;85:1083–7.
Lennard-Jones JE, Morson BC, Ritchie JK, Shove DC, Williams CB. Cancer in colitis: assessment of the individual risk by clinical and histological criteria. Gastroenterology. 1977;73:1280–9.
Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31:800–6.
Lofberg R, Brostrom O, Karlen P, Tribukait B, Ost A. Colonoscopic surveillance in long-standing total ulcerative colitis—a 15-year follow-up study. Gastroenterology. 1990;99:1021–31.
Lynch DA, Lobo AJ, Sobala GM, Dixon MF, Axon AT. Failure of colonoscopic surveillance in ulcerative colitis [see comments]. Gut. 1993;34:1075–80.
Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis [see comments]. Gastroenterology. 1991;100:1241–8.
Rutegard J, Ahsgren L, Stenling R, Janunger KG. Ulcerative colitis. Cancer surveillance in an unselected population. Scand J Gastroenterol. 1988;23:139–45.
Woolrich AJ, DaSilva MD, Korelitz BI. Surveillance in the routine management of ulcerative colitis: the predictive value of low-grade dysplasia [see comments]. Gastroenterology. 1992;103:431–8.
Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–9.
Karlen P, Kornfeld D, Brostrom O, Lofberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711–4.
Velayos FS, Loftus Jr EV, Jess T, Harmsen WS, Bida J, Zinsmeister AR, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: a case–control study. Gastroenterology. 2006;130:1941–9.
Gage TP. Managing the cancer risk in chronic ulcerative colitis. A decision-analytic approach. J Clin Gastroenterol. 1986;8:50–7.
Delco F, Sonnenberg A. A decision analysis of surveillance for colorectal cancer in ulcerative colitis. Gut. 2000;46:500–6.
Inadomi JM. Cost-effectiveness of colorectal cancer surveillance in ulcerative colitis. Scand J Gastroenterol Suppl. 2003;237:17–21.
Provenzale D, Kowdley KV, Arora S, Wong JB. Prophylactic colectomy or surveillance for chronic ulcerative colitis? A decision analysis [see comments]. Gastroenterology. 1995;109:1188–96.
Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–68.
Eaden J, Abrams K, McKay H, Denley H, Mayberry J. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol. 2001;194:152–7.
Dixon MF, Brown LJ, Gilmour HM, Price AB, Smeeton NC, Talbot IC, et al. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology. 1988;13:385–97.
Melville DM, Jass JR, Morson BC, Pollock DJ, Richman PI, Shepherd NA, et al. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum Pathol. 1989;20:1008–14.
Connell WR, Talbot IC, Harpaz N, Britto N, Wilkinson KH, Kamm MA, et al. Clinicopathological characteristics of colorectal carcinoma complicating ulcerative colitis. Gut. 1994;35:1419–23.
Ullman TA, Loftus Jr EV, Kakar S, Burgart LJ, Sandborn WJ, Tremaine WJ. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol. 2002;97:922–7.
Brentnall T, Bronner M, Rubin C, Rabinovitch P, Kimmey M, Kowdley K, et al. Natural history and management of low-grade dysplasia in ulcerative colitis. Gastroenterology. 1999;116:A382.
Befrits R, Ljung T, Jaramillo E, Rubio C. Low grade dysplasia in flat colonic mucosa in patients with extensive longstanding inflammatory bowel disease—a follow-up study. Gastroenterology. 1999;116:A376.
Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum. 2002;45:615–20.
Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127–32.
Pekow JR, Hetzel JT, Rothe JA, Hanauer SB, Turner JR, Hart J, et al. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16(8):1352–6.
Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, et al. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611–20.
Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc. 2000;51:123–8.
Ullman T, White J, Harpaz N, Itzkowitz S. Assessment of biopsy practices in colonoscopic surveillance in ulcerative colitis. Gastroenterology. 2001;120:A-446.
Rubin PH, et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295–300.
Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis [see comments]. Gastroenterology. 1999;117:1288–94. discussion 1488–91.
Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004;2:534–41.
Goldstone R, Itzkowitz S, Harpaz N, Ullman T. Progression of low-grade dysplasia in ulcerative colitis: effect of colonic location. Gastrointest Endosc. 2011;74(5):1087–93.
Vieth M, Behrens H, Stolte M. Sporadic adenoma in ulcerative colitis: endoscopic resection is an adequate treatment. Gut. 2006;55:1151–5.
Selaru FM, Xu Y, Yin J, Zou T, Liu TC, Mori Y, et al. Artificial neural networks distinguish among subtypes of neoplastic colorectal lesions. Gastroenterology. 2002;122:606–13.
Bernstein CN, Weinstein WM, Levine DS, Shanahan F. Physicians’ perceptions of dysplasia and approaches to surveillance colonoscopy in ulcerative colitis [see comments]. Am J Gastroenterol. 1995;90:2106–14.
Rubin DT, Turner JR. Surveillance of dysplasia in inflammatory bowel disease: the gastroenterologist-pathologist partnership. Clin Gastroenterol Hepatol. 2006;4:1309–13.
Itzkowitz SH, Present DH. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21.
Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, et al. AGA Institute Medical Position Panel on Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138(2):738–45.
Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–8.
Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–60.
Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103(9):2342–9.
Gollop JH, Phillips SF, Melton 3rd LJ, Zinsmeister AR. Epidemiologic aspects of Crohn disease: a population based study in Olmsted County, Minnesota, 1943–1982. Gut. 1988;29:49–56.
Munkholm P, Langholz E, Davidsen M, Binder V. Intestinal cancer risk and mortality in patients with Crohn disease. Gastroenterology. 1993;105:1716–23.
Weedon DD, Shorter RG, Ilstrup DM, Huizenga KA, Taylor WF. Crohn disease and cancer. N Engl J Med. 1973;289:1099–103.
Greenstein AJ, Sachar DB, Smith H, Janowitz HD, Aufses Jr AH. A comparison of cancer risk in Crohn disease and ulcerative colitis. Cancer. 1981;48:2742–5.
Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–2.
Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn disease with colonic involvement. Lancet. 1990;336:357–9.
Sachar DB. Cancer in Crohn disease: dispelling the myths. Gut. 1994;35:1507–8.
Ekbom A, Helmick C, Zack M, Adami HO. Extracolonic malignancies in inflammatory bowel disease. Cancer. 1991;67:2015–9.
Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn disease. Aliment Pharmacol Ther. 2006;23:1097–104.
Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950–4.
Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn colitis. Gastroenterology. 2001;120:820–6.
Persson PG, Karlen P, Bernell O, Leijonmarck CE, Brostrom O, Ahlbom A, et al. Crohn disease and cancer: a population-based cohort study. Gastroenterology. 1994;107:1675–9.
Greenstein AJ, Gennuso R, Sachar DB, Heimann T, Smith H, Janowitz HD, et al. Extraintestinal cancers in inflammatory bowel disease. Cancer. 1985;56:2914–21.
Mir Madjlessi SH, Farmer RG, Weick JK. Inflammatory bowel disease and leukemia. A report of seven cases of leukemia in ulcerative colitis and Crohn disease and review of the literature. Dig Dis Sci. 1986;31:1025–31.
Cuttner J. Increased incidence of acute promyelocytic leukemia in patients with ulcerative colitis. Ann Intern Med. 1982;97:864–5.
Wilkinson AH, Smith JL, Hunsicker LG, Tobacman J, Kapelanski DP, Johnson M, et al. Increased frequency of posttransplant lymphomas in patients treated with cyclosporine, azathioprine, and prednisone. Transplantation. 1989;47:293–6.
Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342:1514–6.
Silman AJ, Petrie J, Hazleman B, Evans SJ. Lymphoproliferative cancer and other malignancy in patients with rheumatoid arthritis treated with azathioprine: a 20 year follow up study. Ann Rheum Dis. 1988;47:988–92.
Loftus Jr EV, Tremaine WJ, Habermann TM, Harmsen WS, Zinsmeister AR, Sandborn WJ. Risk of lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2000;95:2308–12.
Korelitz BI, Mirsky FJ, Fleisher MR, Warman JI, Wisch N, Gleim GW. Malignant neoplasms subsequent to treatment of inflammatory bowel disease with 6-mercaptopurine. Am J Gastroenterol. 1999;94:3248–53.
Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ullman, T.A. (2013). Malignant Tumors Arising in Inflammatory Bowel Disease. In: Mamula, P., Markowitz, J., Baldassano, R. (eds) Pediatric Inflammatory Bowel Disease. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5061-0_49
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5061-0_49
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5060-3
Online ISBN: 978-1-4614-5061-0
eBook Packages: MedicineMedicine (R0)