Abstract
Surveys ofJatropha curcasL. are being pursued worldwide, but scientific compilations on Jatropha farming are still rare. Since 2008, significant efforts have been made to understand the biological parameters of this species, ranging from genetic diversity to genome sequencing, so that the basics of Jatropha are better known. Emphasis now needs to be placed on crop improvement through selective breeding and biotechnology. However, this effort only makes sense if a cultural system that optimizes the potential of the species has been previously defined. We report our experience over the years concerning the optimization of the propagation system, fertilization, plant nutrition, spacing, crop mechanization, irrigation and culture management, among other farming practices, for Jatropha cultivation. Our discussion applies to the semi-arid region of Brazil that corresponds to the Caatinga biome.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The prospects associated withJatropha curcasL. (hereafter referred to as Jatropha) as a species for biodiesel production have been extensively reported in the literature; however, to date no scientifically valid recommendation for its farming is available for this crop.
Investigations on propagation techniques, crop fertilization, irrigation, plantation spacing, pruning, growth regulators and mechanical harvesting are on-going, and basic trends are already defined. However, official recommendations from government references are necessary to warrant faithful production systems capable of providing acceptable returns on investments to farmers and sustainable oil production.
TheEmpresa de Pesquisa Agropecuária de Minas Gerais(EPAMIG), which can be translated in English as the Agricultural Research Company of Minas Gerais, was incorporated as a public company in 1974. It is the main institution for the implementation of agricultural research in the state of Minas Gerais (Brazil) and functions to provide solutions for agriculture through technological research and development. It also offers specialized services and technical training compatible with customer needs and, more generally, aims to improve the quality of life.
Among the five regional units that Epamig maintains in the state of Minas Gerais, Minas Epamig North has five farms, four in the North of Minas Gerais and one in a region called Vale do Jequitinhonha. Most of Epamig’s experiments on Jatropha were carried out on one farm (Fazenda Experimental Gorutuba) in North Minas Gerais (Nova Porteirinha). The farm lies at a latitude of 15°03′ South, a longitude of 44°01′ West and at an altitude of 452 m. The predominant soil in this region is a red-yellowlatosolin flat terrain, butneosolis also found. Latosol is a type of soil in an advanced stage of weathering, which is highly evolved as a result of significant changes in its constitutive materials. These soils are deficient in minerals, less resistant to weathering and have low cation exchange capacities. They are generally strongly acidic with low base saturation, well drained and very deep. Neosols include a thin layer of minerals or organic materials and did not experience significant changes due to the low intensity of pedogenic processes. The soils of North of Minas Gerais are typically sandier, with low levels of exchangeable aluminum, phosphorus (P) and organic matter (∼0.5%) and are typically of thedry forestbiome.
Dry forest is characterized by forest vegetation with a predominance of deciduous trees that shed their leaves during the dry season. It is a transition zone betweenCaatingaandCerrado, with characteristics of theAtlantic Forestbiome because of its diversity in deciduous trees.
Caatinga (from Tupi: caa (kill) + tinga (white) = white forest) is the only exclusively Brazilian biome, which means that most of its biological heritage cannot be found anywhere else on the planet earth. Its name is derived from the whitish landscape presented by the vegetation during the dry season, when most plants shed their leaves and the trunks become dry and whitish. The Caatinga occupies an area of approximately 800,000 km², about 10% of the country, comprising contiguous parts of the states of Maranhão, Piaui, Ceara, Rio Grande do Norte, Paraiba, Pernambuco, Alagoas, Sergipe, Bahia (Northeast Brazil) and the northern part of Minas Gerais. The botanical heritage includes 2,000–3,000 native plant species with xerophytic species in the understory associated with calcareous outcrops.
The Cerrado is the second largest Brazilian biome, extending over an area of 2,045,064 km² in eight states (Central Brazil: Minas Gerais, Goiás, Tocantins, Bahia, Maranhao, Mato Grosso, Mato Grosso do Sul, Piauí and the District Federal). The landscape has a high biodiversity, although less so than the Atlantic Forest. The vegetation is similar to the savanna, with grasses, shrubs and sparse trees. The average annual temperature is 25°C, eventually reaching 40°C in the spring. The minimum registered temperature can reach values close to 10°C or less.
Rainfalls are unevenly distributed between the months of November and March and account for an average of 750 mm annually. The drought normally extends from April to late October and is characterized by a sharp drop in the relative humidity. Strong winds occur between May and August. Even if growth is reduced to minimum rates during the dry season, leaf loss is not observed in 1-year-old plants. The phenomenon of leaf loss during the dry season in northern Minas Gerais is observed only from the second year of the plant. In northern Minas Gerais, Jatropha remains leafy from November to June (8 months, Fig.12.1a), sheds its leaves during the resting period (winter) from July to September (3 months, Fig.12.1b) and sprouts again in October (1 month, Fig.12.1c).
Depending on regional climatic conditions, the phenology of Jatropha changes according to the time of the year. In the Southeast region of the country, the rainy season is concentrated from October to March. In the northern region of Minas Gerais, especially where Jatropha is planted on a commercial scale for seed production, the rainy season is unevenly distributed in this period (October to March), with peaks between November and December followed by a dry period in January and February and scattered showers in March.
By contrast, in theZona da Mata(a name for a geographic sub-region with specific socio-economic features) in the state of Alagoas in Northeast Brazil, the rainy season is between April and August (70% of total annual rainfall happens in this period), and the dry season occurs from September to February (Souza et al.2005). Under the conditions of Viçosa, which is included in theZona da Mataof Minas Gerais, Matos (2010) concluded that leaf senescence in Jatropha is due to two factors: a decrease in minimum temperature and an increase in the difference between the maximum and minimum temperatures. Interstingly, leaf senescence was not found to be related to water stress or nitrogen deficiency. The rate of leaf fall increased as the minimum air temperature decreased in the period from January to June.
The leaf fall occurred without any significant relationship to the maximum temperature or soil moisture, which remained near field capacity. The sharp leaf fall in this region occurred when the minimum temperature was below 10°C and the difference between the maximum and minimum temperatures was higher than 20°C.
Corroborating the description of Matos (2010) and Oliveira et al. (2011) from Rio Grande do Sul (southern Brazil), the number of chilling hours directly influences the budding, flowering and fruiting of Jatropha. The climatic differences observed during the experiment were correlated with differences in the phenology of Jatropha over 2 years. In the first year of the experiment, the occurrence of only 160 chilling h guaranteed that fruiting began in November and December, while the incidence of 331 h of cold delayed the onset of fruiting until January of the second year.
After dormancy, the lush production of leaves and inflorescences begins with the first rains that usually occur in mid-October. Thus, the fruit onset of Jatropha in the northern region of Minas Gerais generally begins in November with a main flowering peak in December-January and a secondary one in March-April. In Alagoas, the period from flowering to fruit maturity is 65 days on average (Santos et al.2010), while it lasts from 43 to 61 days in India (Rao et al.2008). This period gives a window of fruit harvest in northern Minas Gerais that begins in March (3 months after flowering) and can extend to June-July.
Due to its ability to thrive in a semi-arid climate, Jatropha is well adapted to this region, which lacks good agricultural options in terms of biofuels and agriculture in general. In the early 1980s, some researchers from EPAMIG became interested in Jatropha and started planting trials on various experimental farms in the state of Minas Gerais. They collected data that are stored in the company archives. This information is of significant importance for the continuation of current projects on Jatropha, which were resumed in mid-2004 after having been slowed at the end of the 1980s. Issues relevant to ecological conditions for cultivation, physiological aspects, selective breeding, vegetative propagation, seed quality, soil features, fertilization, weed and irrigation management were carefully addressed. Characterization of harmful arthropods (pests), diseases, harvest, post-harvest and use of co-products of oil extraction for animal feeding are also being pursued.
Under the following, we present the important observations on Jatropha farming that we have made over the past several decades.
Propagation
The longevity of profitable production of Jatropha is estimated to be between 20 and 30 years (Dias et al.2007). Because Jatropha is a dioecious plant that is generally out-crossing and entomophilous, large variation of pollen spread between individuals is generally observed and may affect the regularity of seed production. In addition to seed propagation, Jatropha can also be propagated asexually by cuttings, grafting or in vitro culture. The rainy season is the best planting period in the field. Plants originating from seeds flower 9 months after their transplantation in the field, whereas plants reproduced through cuttings generally start to flower after 6 months in the field (Saturnino et al.2005). In general, plants grown from seeds develop a taproot and four lateral roots, and they are economically productive in the fourth year in the field. Plants from cuttings have a less vigorous root system without a taproot, but they exhibit slightly earlier production.
Seed multiplication is recommended because of the better root system (Severino et al.2006). Indeed, multiplication of Jatropha in Brazil has occurred traditionally by collecting seeds from individual plants growing in hedges, gardens and dwelling neighborhoods (Saturnino et al.2005). Currently, specialized nurseries are performing this work of seed multiplication on commercial substrates. Seedlings produced in containers of 120 ml are taller, with larger and more abundant leaves than those produced in 50 ml tubes; thus, 120 ml containers are the best for seedling production (Avelar et al.2005).
Vegetative cloning allows the multiplication of individuals without affecting their genetic structure, which is advantageous to increase the population of elite genotypes (Saturnino et al.2005). Multiplication through cuttings is most widespread (Lima et al.2010; Smiderle and Kroets2008; Vasquez et al.2010). A possible compromise that has yet to be tested is to carry out Jatropha multiplication in vitro by somatic embryogenesis, which should give it a better and uniform rooting system in the field than classical micropropagation by shoot multiplication. Somatic embryogenesis is reported in Jatropha (Cai et al.2011; Jha et al.2007; Nunes et al.2008) and could be a desirable character of a commercial cultivar if the rooting quality is confirmed in the field.
A more pragmatic solution is to produce seedlings from bare roots. This system is among the most cost effective and warrants a better rooting system (Siles et al.1997). According to this technique, seeds are germinated in high density on a ∼50 cm deep sand layer. Seedlings develop a robust pivoting root system and an etiolated aerial part because of the high density. Seedlings are brought to the field in bundles without support, thus reducing costs and improving the planting operations.
The planting of cuttings (clones) is only recommended for replacing plants that are not productive or are attacked by pests or diseases. The stakes for this purpose should be cut from woody branches from plants that have been free of pests and diseases for 1 year. The major limitation for the propagation of cuttings is the large volume of material to be used in commercial fields, as well as the need to know the characteristics of the trees providing the cuttings, emphasizing productivity, health and precocity.
Grafting is an alternative method for propagation of Jatropha. This technique is not used commercially; however, good results have been achieved in experimental fields at EPAMIG by grafting shoots ofJ. curcasonto two wild species, i.e.,Jatropha pohlianaMull. Arg. andJ. gossypiifolia L.Cleft (Fig.12.2). Following this method, a fixation rate of about 90% is to be expected; it is more effective than the simpleEnglishgraft and is the recommended technique for Jatropha (Marques et al.2007). According to Marques et al. (2008), seedlings ofJ. pohlianaobtained from mature seeds without caruncles yield the best rootstocks due to the higher germination rate. Similar results were obtained by graftingJ. curcasonJ. molissimaMull. Arg. (Anjos et al.2007).J. molissimais native from Northeast Brazil and therefore is well adapted to the climate and soil conditions of Caatinga (its area of origin), making it an ideal rootstock for Jatropha.
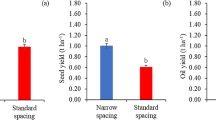
Jatropha’s productivity is influenced by spacing. Optimization of the plant arrangement has been investigated by several research institutions in Brazil. Eight spacing schemes (3 × 1, 3 × 2, 3 × 3, 4 × 1, 4 × 2, 4 × 3, 4 × 4 and 4 × 5 m) were evaluated for Jatropha planting. Silva et al. (2011) recommended spacings of 4 × 4 and 4 × 5 m in the region of Anastasius (Mato Grosso do Sul, Brazil).
The best spacing and planting arrangements for Jatropha are currently under investigation, and these parameters should vary according to the region of cultivation, size of the plant, soil fertility and exploitation system, such as consortium plantation. When intercropped with other cultures, the suggested spacing for Jatropha ranges from 2 × 2 m up to 2 × 8 m (Demartini et al.2009).
Fertilizer Application
Jatropha is preferably cultivated in well-structured and deep soil so that the root system can reach the deeper layers and explore the soil as much as possible, ensuring better absorption of water and nutrients, especially when water is scarce. It can grow in poor sandy soils and clay soils of low fertility. However, productivity of the species is better when it is grown in well-drained, deep, and airy soils with medium to high fertility. At EPAMIG, we have observed that the initial development of Jatropha plants grown in clay soils is better than when grown in sandy soil (Fig.12.3).
Very shallow clay soils with constant humidity, little air and poor drainage should be avoided (Arruda et al.2004). The root growth of Jatropha has been shown to decrease linearly with the compression rate of the top layer ofquartz-sand dystrophic neosol, which means that the plant is sensitive to compacted soil (Abreu et al.2006).
In addition, Jatropha should not be planted in soils where the electrical conductivity (EC) is elevated or where the irrigation water has a high salt content (Vale et al.2006). We observed a reduction of plantlet height from 19.7 to 13.3 cm using water with EC values of 0.06 dS. m−1and 4.2 dS. m−1, respectively.
Jatropha adapts to low-fertility soils; however, application of nitrogen (N), phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) provides significant increases in production. A favorable level of soil fertility is necessary for the plants to express their yield potential. According to Silva et al. (2009), the macronutrients that most affect (by more than 85%) the production of total dry matter are Ca, Mg and K. The series of the relative importance of macronutrients is Ca > Mg > K > N > P > S. P and S are less important macronutrients because their omissions result in the smallest reductions of dry matter production of the macronutrients. In the greenhouse, Kurihara et al. (2006) observed a highly significant response towards P input, especially in soils with low available P. In research conducted at EPAMIG in Northern Minas Gerais, Moura Neto et al. (2007) found that the relationship (Fig.12.4) of (1) plantlet height (Fig.12.4a), (2) stem diameter (Fig.12.4b), (3) number of leaves (Fig.12.4c), and (4) weight of roots (Fig.12.4d), stems (Fig.12.4e) and leaves (Fig.12.4f) to P input follows a quadratic function (ax2 + bx + c = 0). Compared to the control without P, these parameters increased under P fertilization by 59% (height), 31% (diameter), 205% (leaf number), 59% (root weight), 87% (stem weight) and 223% (leaf weight). These authors also concluded that P is extremely important for the early development of Jatropha plants, as observed from the effects of increasing doses of P on seedling development (Fig.12.5).
Plant height (a), stem diameter (b), number of leaves per plant (c), root weight (d), stem weight (e) and leaf weight (f) of Jatropha as a function of P input (Moura Neto et al.2007). The t-test was significant at probability levels of 1% and 5%
In sandy soil under controlled conditions, a maximum seed production of 1,538 kg. ha−1. year−1was obtained with 21-month-old plants fertilized with an application of 240 and 400 kg. ha−1. year−1of N and P2O5, respectively (Silva et al.2007a). Maximum seed production (2,137 kg. ha−1. year−1) after 33 months, estimated with the equation Ŷ = 1,390 +2.24 * N + 2.11 * P2O5– 0.0053* (P2O5)2(R2 = 0.70), was obtained with the application of 240 and 192 kg. ha−1. year−1of N and P2O5, respectively (Fig.12.6). The seed yield increased with age and stabilized between the fifth and sixth year, but increasing doses of N and P2O5did not result in significant effects on the oil content of the seeds (Table12.1).
As described above, N occupies the fourth place in the ranking of macronutrient importance in Jatropha (Silva et al.2009), in contrast with castor beans for which N is the most limiting nutrient (Lavres et al.2005). N is the nutrient that most promotes plant vegetative development in the presence of water. Inter-cropping with Fabaceae legume species is a way to partially supply the N that is needed for Jatropha development. According to Saturnino et al. (2005), Jatropha is highly productive when grown in areas that receive large amounts of organic manure.
The mass of dry matter produced by Jatropha grown onyellow-red latosolwith medium texture supplemented with four doses of N and five doses of K was found to increase with N following a quadratic function, but to decrease with K (Silva et al.2007b), suggesting that Jatropha does not require a large amount of K for its initial development. Thus, the native content of K in soils (87 mg dm−3) is sufficient for the plant demand, as was confirmed by the fact that K application during the first 5 months do not significantly affect vegetative development (Oliveira et al.2007). K is expected to be essential at the stages of seed formation and maturation, as K is present in large amounts in mature seeds (CETEC1983). The absence of K at this stage could be a bottleneck for seed production.
Omission of Fe, Cu, Zn, Mn and B led to reductions of the total dry matter by 84%, 69%, 43%, 31% and 17%, respectively. The importance of micronutrients follows the series Fe > Cu > Mn ∼ Zn > B. In Jatropha, the largest micronutrient requirement is for Fe, similar to the requirement of castor beans (Lange et al.2005).
The diagnosis of the nutritional status of plants is an important tool for the proper use of fertilizers; its main objective is to identify nutrients that limit plant growth, plant development and crop yield.
There is a well-defined relationship between nutrient content in tissues, plant growth and crop production (Martinez et al.1999). This relationship is characterized by a curve that can be divided into five regions. The first and second are called regions ofdisability. In these regions, an increase in nutrient supply is followed by an increase in their content in plant tissues, which results in an increased plant growth and yield. In the third region, called the region ofadequacy, an increase in the nutrient supply and in the nutrient content in plant tissues is not accompanied by a significant increase in plant growth and yield. In the fourth region, called theluxury absorptionregion, the increase in the supply of nutrients and their content in tissues is not accompanied by any increase in plant growth or yield, which means that the addition of nutrients does not result in any benefit for plant growth or yield, i.e., it is wasted. The fifth region is called thetoxicregion and is characterized by a decline in the plant growth or yield with an increase in the nutrient supply and tissue content.
Studies on Jatropha nutrition are still at an infancy. With the goal of identifying the leaf indicators of nutritional status in Jatropha, we found a significant correlation of N and P in the limbo of the fifth leaf with the doses of N and P applied to the soil; by contrast, only a small correlation was observed between these variables in the leaf petiole. For this correlation to be achieved, the fifth leaf must be (1) counted only among the fully formed leaves, (2) from a branch in the median part of the plant and (3) with an inflorescence (Fig.12.7). The nutrient levels found in the limbo of the fifth leaf of a highly productive plant are presented in Table12.2.
Roots exhibit poor development when the soil acidity is below pH 4.5. Limestone should be added at a depth of 20–30 cm approximately 2 months before planting to reduce the acidity of the soil. Correction of the free aluminum content by liming has a positive effect on the development of Jatropha. In a sample ofred clayey latosol, the addition of 55% limestone to correct for acidity was needed to obtain maximum production (12 g) of the dry mass of Jatropha seedlings. Insandy neosol, the maximum dry mass production (24 g) of seedlings was reached by adding 60% limestone (Kurihara et al.2006). In typicalortic sandy neosol, 55% limestone provided further development of seedlings grown in a greenhouse (Tanure2006). Several greenhouse surveys have shown that the Jatropha plants develop better when Ca is applied. It is known that Ca stimulates the development of pivoting roots, ensuring better water absorption and formation of secondary roots. These factors are critical for better plant use of soil fertility and for adaptation to stress, particularly under water deficit conditions. Calcium is one of the most important nutrients for root growth because it stimulates water and nutrient uptake by roots. Upon application of combinations of Ca and Mg doses in Jatropha seedlings grown in soil samples with low fertility, Pacheco et al. (2006) found a larger stimulating effect of Ca compared to Mg on seedling development, which indicates that Ca is much more important than Mg to support early seedling growth (Fig.12.8).
Response of Jatropha to increasing Ca doses corresponding to 0.08 and 1.6 times the lime required to neutralize the pH (Source: Pacheco et al.2006)
However, the omission of Mg resulted in a decrease in dry matter production statistically equal to that of Ca, showing the importance of Mg as a liming factor of Jatropha development and explaining the benefit of using lime with a higher magnesium content (Silva et al.2009).
According to the results above, it is possible to suggest preliminary fertilizing recommendations for Jatropha cultivation (Tables12.3,12.4and12.5) in the semi-arid region of Montes Claros (North of Minas Gerais, Brazil).
Post-planting fertilization is performed in accordance with the projection of the plant canopy. Soil sample analysis must be carried out in the region prior to fertilizer application to calculate the correct dose. Soil analyses must be carried out once a year to assess the evolution of soil fertility. In addition to soil analyses, nutrient analyses of the fifth leaves are also recommended to check which nutrient(s) may be a limiting factor for optimized fruit production. Leaf analysis is an important tool for determining if a fertilization program is in agreement with production simulations.
Irrigation
In an eco-physiology study of water and gas exchange, Lima Filho et al. (2007) measured the water potential, gas exchange, stomatal conductance, photosynthesis and transpiration of leaves exposed to sun in 18-month-old plants spaced 2.0 × 2.0 m in irrigated and non-irrigated plots, following a completely randomized design. The water potentials of the irrigated and non-irrigated plants were −0.57 and −0.95 MPa, respectively, at 5:00 am (before sunrise) and −1.4 and −1.7 MPa after sunrise when evapotranspiration is increased. Photosynthesis was much more impaired than evapotranspiration within the levels of water potential and stomatal conductance observed. The parameters of leaf temperature (Tf), photosynthesis, water pressure, stomatal conductance (gs) and transpiration (E) measured at 8:00 am, 10:00 am, 12:00 pm, 2:00 pm and 6:00 pm showed that Jatropha presented the typical behavior of a woody plant under warm climates and a rainy season. The values of gs were higher in the early morning (between 1.0 and 1.5 mol m−2s−1) and then fell to values approximately 0.7–0.9 mol m−2s−1between 12:00 am and 2:00 pm depending on the temperature of the largest leaf. The stomata were closed between 12:00 pm and 4:00 pm; however, photosynthesis reached its highest values during this time interval (between 8 and 9 mmol m−2s−1), certainly as a result of the higher air evaporative demand (Araújo et al.2007).
Although the cultivation of Jatropha has been described as drought tolerant to water shortages, we observed a positive effect of an artificial water supply to the crop in periods when it is subjected to water stress. This positive contribution of water to Jatropha development and production has also been observed in regions where rainfalls are higher and evenly distributed. However, successful irrigation of this species requires a rational system together with other necessary agricultural inputs.
Irrigation of Jatropha can be performed using several methods and systems; there is not a system more suitable than the others, but rather advantages and drawbacks for each of the system. Thus, in situ experiments can be used to learn about the most appropriate irrigation system. Appropriate irrigation methods for Jatropha are (1) localized irrigation (micro-sprinkling systems and drip), (2) overhead irrigation (central pivot and conventional sprinkling with restrictions) and (3) surface irrigation.
In the localized irrigation methods, water is applied directly to each plant above the root system. In overhead irrigation, water is applied above the plants, resembling natural rain. By contrast, surface irrigation refers to irrigation methods where water is moved from the soil surface to the plants. Linear and central pivot are considered as automated overhead irrigation systems. In linear irrigation, an automated sprinkler moves in a straight line. In central pivot irrigation, automated sprinklers moves in a circle around a central point or pivot.
According to Costa et al. (2008), the root system is an important parameter to be considered for crop irrigation. In addition to providing plant support, it is the main organ responsible for the absorption of water and soil nutrients. As described above, Jatropha plantations derived from seeds exhibit larger vegetative development and fruit production than plantations derived from cuttings, which has been attributed to better root development from effective use of irrigation water. On average, Jatropha cuttings develop five roots, a central and four peripheral roots, indicating good soil use when the appropriate growth conditions are provided.
In farming under irrigation, plant spacing is normally managed according to the plant characteristics. Physical restriction may also occur, depending on the irrigation system used. In experimental areas of irrigated Jatropha, the most widely used spacings have been 4 × 2 and 5 × 2 m when mechanical harvesting was applied. The spacing is related to the tree size and irrigation system. For example, “central pivot” or “linear” systems can be difficult to apply or require pruning. According to Saturnino et al. (2005), Jatropha sheds leaves during the dry season or the cold period. The plant remains dormant until the beginning of next rainy season, and the end of dormancy is marked by new sprouts developing at the tips of branches. However, the periodic leaf loss typical of non-irrigated plantations was not observed under irrigation in Northern Minas Gerais. These results indicate the adaptation potential of Jatropha and an opportunity for further irrigation management strategies. For example, an irrigation pause during certain periods could enable nutrient accumulation, among other benefits.
In northern Minas Gerais, it appears that Jatropha is able to produce and develop under non-irrigated conditions, with only water from the poorly distributed precipitation (rainfall concentrated between the months of November and February) of approximately 1,200 mm. However, preliminary results show positive effects of irrigation including (1) better plant development, (2) increased precocity of production, (3) maximized harvest period, and (4) increased yield. Although tolerant to periods of water shortage, Jatropha needs a proper and constant water supply to achieve its yield potential.
Under irrigation, Jatropha’s production starts earlier and is greater. Drummond et al. (1984) reported that in an experimental Jatropha area in Janaúba (northern Minas Gerais), 18-month-old plants under surface irrigation produced 2,500 kg of seeds per hectare at an oil rate of 38% of seed weight. In another region, the seed yield conducted under non-irrigatedyellow-red latosolat Felixlândia Cerrado (Central region of Minas Gerais) only reached 500 kg ha−1. Thus, although adapted to dry regions and having a thick stem able to store enough water to survive in dry regimes, Jatropha is far less productive under non-irrigated conditions. Jatropha is productive in warm climates with more abundant and regular rainfalls, such as Zona da Mata (Minas Gerais). In irrigated and fertile areas, Jatropha can start producing soon in the second planting year, reaching 2 t ha−1in the third year (unpublished data).
In NNE Minas Agro Florestal Ltda. (Janaúba), flowering initiation took place 7 months after crop planting underdrip irrigationwith a volume of 15 l per plant per week, given in three irrigation events over the course of the week.
MSEA (2008) and Reyadh (1999) reported that 5,000 ha of Jatropha were planted with 3 × 3 m spacing (1,260 seedlings per hectare) in sandy soils of the desert in the Luxor region (Egypt) under irrigation with effluent water from sewage treatment (EC 1.04 and pH 7.47). The plants did not receive any organic or mineral fertilization other than the nutrients contained naturally in the irrigation water from the effluents of sewage treatment. The production began 18 months after seedling transplantation and reached an average yield of 3–4 kg per plant 2 years after planting. The oldest and largest plants produced between 12 and 18 kg per plant. These reports not only indicated the feasibility of fertirrigation on Jatropha plantations, but also the possibility of using waste water for a productive activity that neither harms human health nor pollutes the environment.
In an area on the experimental farm of EPAMIG in Jaíba (northern Minas Gerais), nitrogen and potassium were successfully applied to a Jatropha plantation using localized irrigation systems (drip and micro-sprinkling).
To determine the yield potential of Jatropha in semiarid conditions, Drummond et al. (2007) compared Jatropha’s productivity under dry and irrigated conditions in an experimental field (9°09′ S, 40°22′ W at an altitude of 365.5 m) of Embrapa (ENT-Petrolina, Petrolina, Pernambuco, Brazil). In this region, the average annual rainfall ranges from 400 to 500 mm and is concentrated in February to April. The average temperature is 26.4°C, the average evaporation is 7.4 mm d−1, the average day length is 7.3 h d−1and the annual average relative humidity is 61.8%. Nine rows of 23 plants spaced 2.0 × 2.0 m with surface irrigation were planted at the beginning of the rainy season. The area was divided into two parts of four rows, separated by a central row. The plants of all rows were grown under non-irrigated conditions until 4 months after planting. After this period, four rows were irrigated each week. Nine months after planting, 63 plants from both of the four dry rows and the four irrigated rows were assessed individually for (1) plant height, (2) stem diameter, (3) branch number at one meter and (4) numbers and weights of fruits and seeds. The results obtained for these parameters showed that Jatropha’s performance 4 months after planting was far superior when complemented with irrigation compared to the control plants grown with rain precipitation. The average seed productivity under irrigation was 871 kg ha−1, i.e., which is 3.5 times larger than the control (246 kg ha−1).
In a comparison of vegetative development under dry and irrigated conditions in the region of Vale do Jequitinhonha, plants were spaced 2 × 2 m, and one irrigated row was separated from the other by a non-irrigated row. Irrigation was performed by dripping. Ninety-six plants, including 48 from dry rows and 48 from irrigated rows, were evaluated after 5 months for plant height and stem diameter. As expected, plants under irrigation exhibited better development in terms of both plant height and stem diameter (Evaristo and Moreira2008).
An average production of 63.72 and 83.02 g of seeds under non-irrigated and irrigated conditions, respectively, was reported in 9-month-old plants by Coletti et al. (2008). By contrast, Drumond et al. (2008) found that 12-month-old Jatropha planted in a scheme of three rows containing 21 plants spaced 2 × 2 m produced an average of 50 (330 kg ha−1) and 210 (1,156 kg ha−1) fruits per plant under dry and irrigated (drip) conditions, respectively. More recently, Drumond et al. (2010) reported average seed yields ranging between 2,853 and 3,542 kg ha−1in 12-month-old genotypes under irrigation. However, investigations of Jatropha’s productivity are not generally based on field realities. Experiments based on small sample sizes may give unreliable results because of statistical inconstancies. Other important parameters were not considered by these investigators, such as the genotype interaction of this undomesticated species with the edapho-climatic conditions of the environment. However, irrigation and fertirrigation are technologies that have great potential for the cultivation of Jatropha. To be successful, they must be adapted to farming techniques that still need to be optimized for this crop.
Frigo et al. (2008) analyzed the expenses of different energy sources (renewable and non-renewable) of a Jatropha agro-ecosystem under drip irrigation to evaluate its long-term sustainability based on energy balance and use of non-renewable resources. Data from primary (collected in the field through oral reports) and secondary sources (data from bibliographies of the area), as well as manual or mechanical operations such as land cleanup, pruning, rowing, mechanical, digging, seedlings plantations, insecticide application, fungicide manual application, manual weeding, irrigation and harvesting, were used to calculate an energy balance of 2,141.66 MJ ha−1. Thus, for each kg of Jatropha fruit produced (i.e., a gross energy of 12.80 MJ), 4.62 MJ are from non-renewable energy sources, which, in the case of this study, corresponds to fossil fuel sources (fuel, grease and lubricants). Because the energy efficiency is 2.77 for every kg of fruit produced (12.80 MJ), an additional 35.56 MJ of non-renewable energy sources is needed. Finally, the culture efficiency of irrigated Jatropha is 0.36%, meaning that for every 12.80 MJ produced (kg of fruits), 35.67 MJ of fossil fuels are needed as energy input. Thus, due to the heavy use of non-renewable energy in the irrigation process, Jatropha under irrigation is an untenable agro-ecosystem in the long run. Jatropha can be used to convert solar energy into oil with lower energy input than the energy effectively released in the oil after its extraction. However, this study only looked at the first year of cultivation, which is insufficient to draw conclusions about the energy ratio over the 20 years of a Jatropha perennial plantation. Nonetheless, this study may serve as a reference for future analyses needed to identify in which agro-system Jatropha may become sustainable as a member of the energy matrix. There is an imperative need for economic investigations on the financial viability of irrigation of Jatropha before making any recommendation on its use, especially when referring to small- and medium-sized producers who have few resources to invest in such a productive process.
Fruit Harvesting
The fruits of Jatropha are considered mature when they reach a yellow coloration and are generally harvested by hand. The fruits at this stage of development are at the peak of oil accumulation in seeds and get detached easily from stalks. Fructification occurs in bunches, but maturation is not uniform, and flowering occurs continuously as long as there is heat and moisture. Thus, continuous harvesting is needed throughout the maturation period.
An alternative faster and easier method is to shake plants at half their height to allow fruits to fall, which can then be easily collected on a canvas extended on the soil (Saturnino et al.2005). The drawback of this method is that some fruits do not tear off and others are pulled out when falling on the ground, which also may lead to contamination and the need to collect the fallen fruit.
Farmers and the scientific community believe that the implementation of a mechanical harvesting system for Jatropha would be a critical step to improve crop feasibility. Today, equipment used in other crops, such as coffee, is being tested for adaption to Jatropha.
A semi-mechanized system with lateral vibrating fingers is being tested at the Federal University of Viçosa (Minas Gerais). This type of equipment is widely used in the cultivation of coffee. The performance of the prototype has been considered to be excellent when tested on Jatropha (Dias et al.2007). Ideally, a harvester should cause minimal plant damage and fruit loss. However, tests conducted in the field in Janaúba (Minas Gerais) using this equipment caused extensive damage to branches and plants.
A self-propelled harvester designed for coffee was also tested on Jatropha by Biojan Ltda in Janaúba. This harvester uses a system to drag and shake branches (Fig.12.9). Fruits are collected by mugs as they fall, led by elevators and dispensed into a cart. According to Biojan, the results are promising because the fruit harvest yield was very good. An evaluation performed shortly after harvesting revealed the occurrence of damage to the trunks, branches, loss of leaves and eventually partial loss of inflorescences. In addition, many immature fruits were collected together with the mature ones. However, despite all the above constraints, the plant recovery was quickly verified, even if the equipment was used throughout the year.
In order for a mechanical harvesting system to be viable, a series of technological advances must be achieved. For example, pruning is a viable alternative to reduce the crown size for mechanical harvesting (Silva et al.2012). Pruning at 80 cm in the primary branch with thinning below 60 cm and promotion of three secondary branches gave the best results for mechanical fruit harvesting. Dwarf cultivars or those with different architectures should be developed in conjunction with the harvester to allow for better circulation of the equipment over plantation lines. Such dwarf cultivars should present similar flower numbers despite their small size, good/prolific flowering and fructification in discrete periods of the year and greater fruit uniformity within the same cluster. Knowledge of plant physiology for inducing synchronous flowering and maturation under irrigation control, pruning and/or plant regulators are needed. Pruning is a viable alternative to reduce the canopy size for adaptation to mechanical harvesting; however, this method requires human power, which inevitably increases costs. Biophysical analyses of branch, fruit and stem resistance should be carried out to measure the average force needed to collect mature fruits without breaking new inflorescences, productive branches and damaging immature fruits.
Conclusions
Jatropha is an oilseed crop with good potential for biodiesel production; it is hardy, tolerant to drought, widespread through tropical to sub-tropical climates and some temperate regions. However, under inadequate conditions of light, air temperature, relative humidity, rainfall, soil fertility and moisture, this species does not reach the expected productivity. Production costs should be considered based on the specific environment where Jatropha is grown. Damages caused by biotic and abiotic factors also need to be assessed.
Research on the fertilization of Jatropha is still at an early stage in Brazil. To exploit this crop on a large scale, additional information is needed to warrant sustainability of its commercial cultivation.
The information available on the physiology of Jatropha is very meagre. The limited information on the vegetative and reproductive growth in various environments and cropping systems came from observations made in the early stages of plant life without reference to the physiological mechanisms that explain these processes. Investigations on gas exchange and water balance are lacking. Moreover, there is no information on hormonal relationships, nitrogen metabolism, assimilate partitioning, and root physiology, among other things. Molecular biology is in the early stages of applications for understanding the mechanisms of regulation of gene expression in response to environmental stresses.
Irrigation is a technology that holds great potential for cultivation of Jatropha. However, to be successful, it must be adapted to other cultural treatments, performed using good-quality equipment, and maintained periodically to ensure proper performance in the long run. An economic survey is needed to verify the financial viability of irrigation in the cultivation of Jatropha to understand the conditions under which it is recommended.
Because flowering and fruit development is asynchronous in Jatropha, fruit harvesting is one of the main challenges to be overcome for the viability of the culture as an industrial crop. In the present stage of crop development, the process simply needs to be sufficiently efficient to be economically viable, i.e., there must be a positive balance between fruit crop and crop costs; the higher the harvesting frequency, the lower the fruit loss, but the higher the crop cost. On the other hand, if the harvest occurs only once, losses can be so high that they may challenge Jatropha’s sustainability. Postharvest steps leading to biodiesel production will be subject to both losses in quantity and quality of oil, which can only be prevented by using several agronomic and industrial technologies. High-quality seeds are needed for storage and industrial processing to ensure good yield and quality of biodiesel.
References
Abreu HA, Guerra GM, Mesquita DN, Pereira VC, de Assis RL, Silva OA et al (2006) Crescimento aéreo e radicular de pinhão manso sob diferentes níveis de compactação do solo. In: Proceedings of the Congresso da Rede Brasileira de Tecnologia do Biodiesel, vol 1. MCT/ABIPTI, Brasília, pp 144–149, Portuguese
Anjos JB, Drumond MA, Morgado LB (2007) Enxertia de pinhão-bravo com pinhão manso. In: Proceedings of the Congresso Internacional de Bioenergia e Biocombustíveis, Energia de Resultados [CD-ROM]. Teresina, (Embrapa Meio Norte, Documentos 143). 11–15 June 2007. Portuguese
Araújo ECE, Prado CHBA, Veloso MEC, Costa SEM, Freire CL, Novaes P (2007) Curso diário de parâmetros do estado hídrico do pinhão manso (Jatropha curcasL.) no período chuvoso do município de Teresina, Piauí. In: Proceedings of the Congresso Internacional de Bioenergia e Biocombustíveis, Energia de Resultados [CD-ROM]. Teresina, (Embrapa Meio Norte, Documentos 143). 11–15 June 2007. Portuguese
Arruda FP, Beltrão NEM, Andrade AP, Pereira WE, Severino LS (2004) Cultivo de pinhão manso (Jatropha curcasL.) como alternativa para o semi-árido nordestino. Revista Brasileira de Oleaginosas e Fibrosas 8(1):789–799, Portuguese
Avelar RC, Deperon Júnior MA, Dourado DC, Quintiliano AA, Danfa S, Fraga AC (2005) Produção de mudas de pinhão manso (Jatropha curcasL.) em tubetes. In: Proceedings of the 2nd Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel [CD-ROM], vol 1. MG, Varginha. Biodiesel: combustível ecológico. UFLA, Lavras, pp 298–301, Portuguese
Cai L, Fu L, Ji L (2011) Regeneration ofJatropha curcasthrough efficient somatic embryogenesis and suspension culture. GM Crops Food 2(2):110–117. doi:10.4161/gmcr.2.2.16126
CETEC (1983) Produção de combustíveis líquidos a partir de óleos vegetais: relatório final, vol 2. CETEC, Belo Horizonte, Portuguese
Coletti AJ, Dallacort R, Martins JA, Dalchiavon FC, Silva KD (2008) Produtividade inicial da cultura do pinhão manso em condições irrigadas e de sequeiro, na região de Tangará da Serra-MT. In: Proceedings of the 4th Congresso Interno De Iniciação Científica Da Universidade do Mato Grosso, vol 1. Universidade do Mato Grosso, Cáceres, pp 1–4, Portuguese
Costa EL, Coelho EF, Simão FR, Coelho Filho MA, Oliveira PM (2008) Irrigação da Bananeira. Informe Agropecuário Belo Horizonte 29(245):38–46, Portuguese
Demartini D, Muller MD, Nascimento Junior ER, Fernandes EN (2009) Correlação entre características agronômicas de pinhão manso (Jatropha curcasL.) em sistema de consórcio com pastagens. In: Proceedings of the 6th Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel. Montes Claros. Biodiesel, inovação tecnológica: Revista de resumos. UFLA, Lavras, 6 p. Portuguese
Dias LAS, Leme LP, Laviola BG, Palline A, Pereira OL, Dias DCFS et al (2007) Cultivo de pinhão manso (Jatropha curcasL.) para produção de óleo combustível. MG, Viçosa, 40 p. Portuguese
Drummond AO, Purcino AAC, Cunha LHS, Veloso JM (1984) Cultura do pinhão manso. EPAMIG, Belo Horizonte. Não paginado. (EPAMIG. Pesquisando, 131). Portuguese
Drumond MA, Anjos JB, Morgado LB, Paiva LE (2008) Comportamento do pinhão manso no semi-árido brasileiro, resultado do 1º ano. In: Proceedings of the Simpósio Brasileiro de Agroenergia, 2008, Botucatu. Agroenergia e desenvolvimento sustentável. Faculdade de Ciências Agronômicas, UNESP. Available from:http://www.infoteca.cnptia.embrapa.br/bitstream/CPATSA-2009-09/39519/1/OPB1998.pdf. Accessed 30 Jan 2010. Portuguese
Drumond MA, Anjos JB, Paiva LE, Morgado LB, Reis EM (2007) Produção de pinhão manso no semi-árido brasileiro. In: Proceedings of the Congresso Internacional de Bioenergia e Biocombustíveis, Energia de Resultados [CD-ROM]. Teresina, (Embrapa Meio Norte, Documentos 143). 11–15 June 2007. Portuguese
Drumond MA, Santos CAF, Oliveira VR, Martins JC, Anjos JB, Evangelista MRV (2010) Desempenho agronômico de genótipos de pinhão manso no Semiárido pernambucano. Ciência Rural. 2010. Santa Maria. 40(1):44–47. Available from:http://www.scielo.br/scielo.php?script=sci_pdf&pid=S0103-84782010000100008&lng=pt&nrm=iso&tlng=pt. Accessed 30 Mar 2010. Portuguese
Evaristo AB, Moreira TMB (2008) Desenvolvimento de plantas de pinhão manso em regime irrigado e sequeiro na região do Médio Vale do Jequitinhonha. In: Proceedings of the Congresso Brasileiro de Agroenergia & Simpósio Internacional de Biocombustível [CD-ROM]. MG, Uberlândia, 4 p. Portuguese
Frigo MS, Bueno OC, Esperancini MST, Frigo EP, Klar AE (2008) Análise energética do primeiro ano de cultivo do pinhão manso em sistema irrigado por gotejamento. Irriga 13(2):261–271, Portuguese
Jha TB, Mukherjee P, Datta MM (2007) Somatic embryogenesis inJatropha curcasLinn., an important biofuel plant. Plant Biotechnol Rep 1:135–140
Kurihara CH, Roscoe R, Silva WM, Maeda S, Gordin CL, Santos G (2006) Crescimento inicial de pinhão manso sob efeito de calagem e adubação, em solos do Mato Grosso do Sul. In: Proceedings of the 25th Reunião Brasileira de Fertilidade do Solo e Nutrição de Plantas [CD-ROM]. Sociedade Brasileira de Ciência do Solo, Bonito-MT. Portuguese
Lange A, Martines AM, Silva MAC, Sorreano MCM, Cabral CP, Malavolta E (2005) Efeito de deficiência de micronutrientes no estado nutricional da mamoneira cultivar Íris. Pesq Agropec Bras 40:61–67, Portuguese
Lavres J Jr, Boaretto RM, Silva MLS, Correia D, Cabral CP, Malavolta E (2005) Deficiências de macronutrientes no estado nutricional da mamoneira cultivar Íris. Pesq Agropec Bras 40:145–151, Portuguese
Lima Filho JMP, Silva FFS, Lopes AP, Anjos JB, Drumond MA (2007) Comportamento ecofisiológico do pinhão manso (Jatropha curcasL.) sob condições semi-áridas. In: Proceedings of the Congresso Internacional de Bioenergia e Biocombustíveis, Energia de Resultados [CD-ROM]. Teresina, (Embrapa Meio Norte, Documentos 143). 11–15 June 2007. Portuguese
Lima RLS, Severino LS, Pereira WE, Lucena AMA, Gheyi HR, Arriel NHC (2010) Comprimento das estacas e parte do ramo para formação de mudas de pinhão-manso. R Bras Eng Agríc Ambiental 14(11):1234–1239, Portuguese
Marques DS, Dias MSC, Saturnino HM, Vitorno Júnior D, Barbosa AP, Barbosa JG (2008) Enxertia de pinhão-manso (Jatropha curcasL.) emJatropha pohlianana região Norte de Minas Gerais. Proceedings of the 5th Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel [CD-ROM]. MG/UFLA, Varginha/Lavras. Portuguese
Marques DS, Saturnino HM, Faria MAV, Santos PG, Morais DLB (2007) Enxertia de pinhão manso (Jatropha curcasL.) sobre espécies nativas deJatrophado Norte de Minas Gerais. In: Proceedings of the 4th Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel. MG/UFLA, Varginha, pp 981–985, Portuguese
Martinez HEP, Carvalho JG, Souza RB. Diagnose foliar (1999) In: Comissão De Fertilidade Do Solo Do Estado De Minas Gerais. Recomendações para o uso de corretivos e fertilizantes em Minas Gerais: 5aaproximação. Viçosa, pp 143–168, Portuguese
Matos FS (2010) Caracterização fisiológica da senescência foliar em populações deJatropha curcasL. Dissertation, Universidade Federal de Viçosa/MG, Viçosa. Portuguese
Moura Neto A, Silva JTA, Silva IP, Costa EL (2007) Efeito da aplicação de diferentes doses de fósforo no pinhão manso (Jatropha curcasL.). In: Proceedings of the 31th Congresso Brasileiro de Ciência do Solo [CD-ROM]. SBCS, Gramado. Portuguese
MSEA – Ministry of State for Environmental Affairs (2008). Environmental affairs agency. The national programme for safe use of treated sewage water for afforestation: planting jatropha in Egypt. Available from:http://www.eeaa.gov.eg/english/main/greencorner.asp. Accessed 11 set. 2008
Nunes CF, Pasqual M, Santos DN, Custódio TN, Araújo AG (2008) Diferentes suplementos no cultivo in vitro de embriões de pinhão-manso. Pesq agropec bras 43(1):9–14, Portuguese
Oliveira EL, Faria MA, Morais AR, Fraga AC, Castro Neto P (2007) Efeito da adubação potássica no crescimento inicial do pinhão manso irrigado por gotejamento. In: Proceedings of the 4th Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel. MG/UFLA, Varginha/Lavras, p 149. Portuguese
Oliveira RJP, Silva DAS, Lemoes LS, Fonseca ER, Eicholz ED (2011) Estudo da fenologia de pinhão-manso nas safras 2008/09 e 2010/11 em Pelotas-RS. In: Proceedings of the 2nd Congresso Brasileiro de Pesquisas de Pinhão-Manso [Internet]. Brasília, 2011. Available from:http://www.alice.cnptia.embrapa.br/bitstream/doc/920775/1/ESTUDODAFENOLOGIADEPINHAOMANSONASSAFRAS200809E201011EMPELOTASRS.pdf
Pacheco DD, Saturnino HM, Mendes LD, Soares FR, Paula TOM, Prates FBS et al (2006) Produção de massa vegetal e composição mineral de plantas de pinhão manso. In: Proceedings of the 3rd Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel [CD-ROM]. MG/UFLA, Varginha. Portuguese
Rao GR, Korwar GR, Shanker AK, Ramakrishna YS (2008) Genetic associations, variability and diversity in seed characters, growth, reproductive phenology and yield inJatropha curcasL. accessions. Trees Struct Func 22:697–709
Reyadh M (1999) The cultivation ofJatropha curcasin Egypt. In: INTERNATIONAL EXPERT MEETING, 1997, Cairo. Proceedings. Medicinal, culinary and aromatic plants in the Near East. FAO, Cairo, 1999. Available from:http://www.fao.org/documents/show_cdr.asp?url_file=/docrep/x5402e/5402e00.htm. Accessed 30 July 2004
Santos CM, Endres L, Wanderly Filho HCL, Rolim EV, Ferreira VM (2010) Fenologia e crescimento do pinhão-manso cultivado na Zona da Mata do estado de Alagoas, Brasil. Scientia Agraria 11(3):201–209, Portuguese
Saturnino HM, Pacheco DD, Kakida J, Tominaga N, Gonçalves NP (2005) Produção de oleaginosas para o biodiesel. Informe Agropecuário Belo Horizonte-MG 26(229):44–74, Portuguese
Severino LS, Lima RLS, Beltrão NEM (2006) Produção de mudas de pinhão manso. Embrapa Algodão, Campina Grande, (Folder). Portuguese
Siles MC, Montoya A, Vásquez W, Foidl N (1997) PlantingJatropha curcasusing bare roots. In: Proceedings of the SYMPOSIUM “JATROPHA 97”. 1997. Managua, Nicaragua. Biofuels and industrial products fromJatropha curcas. University of Technology, Graz, 1997. Available from:http://www.jatropha.de/conferences/abstracts-Jatropha97.htm. Accessed 5 ago. 2004
Silva CJ, Silva YK, Staut LA, Schiavo JA (2011) Produção de pinhão manso em diferentes espaçamentos em Anastácio, MS. In: Proceedings of the 2nd Congresso Brasileiro de Pesquisas de Pinhão Manso [CD-ROM]. II CBPPM, Brasília. Portuguese
Silva EB, Tanure LPP, Santos SR, Resende PS Jr (2009) Sintomas visuais de deficiências nutricionais em pinhão-manso. Pesq Agropec Bras 44(4):392–397
Silva IP, Silva JTA, Moura Neto A, Costa EL (2007a) Resposta do pinhão manso (Jatropha curcasL.) a adubação com N e K. In: Proceedings of the 31st Congresso Brasileiro de Ciência do Solo [CD-ROM]. SBCS, Gramado. Portuguese
Silva JTA, Costa EL, Silva IP, Moura Neto A (2007b) Adubação do pinhão manso(Jatropha curcasL.) com nitrogênio e fósforo. In: Proceedingsof the 4th Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel. MG/UFLA, Varginha, p 178. Portuguese
Silva VA, Morais DLB, Kakida J, Ferreira EA, Silva VF (2012) Concentração do ciclo de produção de pinhão-manso por meio de podas de formação ou de produção. Pesq Agropec Bras 47(1):134–137, Portuguese
Smiderle OJ, Kroetz VJ (2008) Produção de mudas de Pinhão-manso propagadas por estaquia. Embrapa Meio Norte. [Comunicado Técnico 22]. Portuguese
Souza JL, Nicácio RM, Moura MAL (2005) Global solar radiation measurements in Maceió Brazil. Renew Energ 30(8):1203–1220
Tanure LPP (2006) Avaliação do crescimento de pinhão manso (Jatropha curcasL.) em diferentes níveis de saturação por bases. MG/UFVJM, Diamantina, p 21. (Monograph). Portuguese
Vale LS, Severino LS, Beltrão NEM (2006) Efeito da salinidade da água sobre o pinhão manso. In: Proceedings of the 1st Congresso da Rede Brasileira de Tecnologia de Biodiesel. MCT/ABIPTI, Brasília, 1(1):87–90. Portuguese
Vasquez GH, Lazarini E, Ribeiro TC, Gradela AS, Silva TF, Viana RL (2010) Produção de mudas de pinhão-manso via estaquia. Rev Bras Ol Fibros 14(3):97–105, Portuguese
Acknowledgements
The authors thank the Research Support Foundation of Minas Gerais and Petrobras for financial support between 2007 and 2012 as well as Dr. Nicolas Carels for help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this chapter
Cite this chapter
de Resende, J.C.F., da Silva, J.T.A., Simão, F.R., de Azevedo Pimentel, R.M., de Lourdes Batista Morais, D. (2012). Phytotechnical Aspects of Jatropha Farming in Brazil. In: Carels, N., Sujatha, M., Bahadur, B. (eds) Jatropha, Challenges for a New Energy Crop. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4806-8_12
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4806-8_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4805-1
Online ISBN: 978-1-4614-4806-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)