Abstract
Dormancy is the temporary failure of a seed to complete germination under favorable conditions. It allows for the dispersal of seeds in space and time. There are several types of dormancy, which include physical, mechanical, or chemical inhibition by the covering layers of the embryo, the inability to germinate because of an undifferentiated or immature embryo, and the repression of germination by metabolic restraints. The breaking of dormancy is governed by environmental cues, including temperature, light, nitrate, and some smoke components. This allows seedling establishment during suitable conditions to maximize survival. The breaking of physiological dormancy and the induction of germination are regulated via hormone signaling pathways and mainly through the GA- (gibberellin) and ABA-(abscisic acid) biosynthetic and catabolic pathways. The ABA–GA balance appears to be a central regulatory feature that integrates multiple interactions among environmental cues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abscisic acid
- Dormancy
- Dormancy classification
- Gibberellin
- Germination
- Hormone signaling
- Light
- Nitrate
- Nitric oxide
- Phytochrome
Whether or not a viable seed germinates and the time at which it does so depend on a number of factors, including those present in the seed’s environment. First, the conditions to support germination must be right. Water must be available, oxygen may have to be present since the seed must respire, and inhibitory chemicals should be absent. Furthermore, the temperature must be suitable and so also, in many cases, must the light quality and quantity. But in many instances all these conditions may be satisfied and nevertheless the seed fails to germinate. The reason is that there exists within the seed (or dispersal unit) itself some block(s) that must be removed or overcome before germination can be completed: such a seed is said to be dormant. To be released from dormancy, a seed must thus experience certain environmental factors for minimal lengths of time, the perception of which induces metabolic and structural changes within the seed that favor germination. Based on the above considerations, seed dormancycan now be defined as a temporary failure of a (viable) seed to complete germination under favorable conditions.
Dormancy is a trait that has been acquired by many species during evolution by selection for the ability to survive in unfavorable environments, such as heat, cold and drought. The origin of dormancy is possibly related to climatic changes that have occurred during the Earth’s history. The number of plant species with seed dormancy tends to increase with the geographical distance from the equator, i.e., as seasonal variation in precipitation and temperature increases. Therefore, a wide range of blocks to germination has evolved as adaptations to the diversity of climates and habitats in which they operate. The relationships between dormancy and germination and the points at which controls exist are shown in Fig. 6.1.
Relationships between seed dormancy and germination. Seeds can be dormant (primary dormancy) or nondormant at the end of seed development depending upon both genotype and maternal environment. Dormancy can be alleviated by various environmental factors. Alternatively, nondormant seeds can be induced into dormancy (secondary dormancy) by some of the same factors. Seeds may cycle seasonally between dormant and nondormant. Nondormant seeds can progress to germination, again influenced by some of the same environmental factors
6.1 Dormancy: Its Biological Role
The function of a seed is to establish a new plant but it can do this only once, because the completion of germination is essentially irreversible. Dormancy provides a strategy for seeds to spread germination in time in order to reduce the risk of premature death in an unfavorable environment. This strategy occurs in three ways:
(1) Seeds are dispersed from the same parent plant with different degrees of dormancy, a phenomenon known as polymorphism, heteromorphy, or heteroblasty. Frequently, the variation in dormancy is reflected in the appearance of the seeds or dispersal units—color, size, and thickness of coat. This may also be a reflection of different levels of maturity of the seeds, for at a given moment they may be at different stages of development on the parent plant and, hence, at different levels of dormancy since this is acquired during development. For example, in many members of the Poaceae grass family caryopses from different spikelets, or from the florets within, have different levels of dormancy. If a developing caryopsis is removed, it will influence the dormancy of those remaining. The green seeds produced by Salsola volkensiihave virtually no dormancy, but the non-green seeds have dormancy. The dispersal unit (fruit) of Xanthiumspp. contains two seeds of which the upper one is deeply dormant whereas the lower one has a very shallow dormancy. In Chenopodium albumfour types of seeds can be found—brown or black ones each with reticulate or smooth coats; of these, the smooth, black seeds have the deepest dormancy. Thus, differences such as these, in both appearance and dormancy, may be displayed by seeds from the same plant or from different plants (e.g., C. album). In the former case, correlative effects are operating, that is plant parts are influencing each other to produce the variation, whereas in the second, both environmental and genetic causes can be traced. When there are polymorphic seeds, germination is spread temporally, with new seedlings emerging at irregular intervals, thus reducing competition and spreading environmental risks, increasing the likelihood that some individuals will survive. Such a temporal distribution clearly can have advantages with regard to the continuation and spread of the species.
(2) Dormancy also results in the distribution of germination in time through the dependence of dormancy breakage on environmental factors which in turn have their own time distribution. For example, seeds are commonly released from dormancy by being chilled, sometimes for several weeks or months at 1–5°C. Since such temperatures occur only during the winter, seeds that rely on this means of dormancy breakage must await the passage of this cold season before they can germinate. The advantage of this strategy is that the young seedling emerges in the spring and establishes itself over the favorable succeeding months; emergence before winter would entail the risk of succumbing to the severe conditions of that season.
(3) Seed dormancy can also lead to a distribution of germination in space—another aspect of its biological importance. Dormant seeds may be dispersed over long distances by wind, water, and animals; these dispersal types are called anemochory, hydrochory, and zoochory, respectively (Sect. 7.1).
6.2 Categories of Dormancy
The completion of germination (radicle protrusion) is the net result of the opposing forces of expansion by the embryo versus the restraints of the surrounding tissues. In the case of embryo dormancythe properties of the embryo are of principal importance. In coat-imposed dormancythe properties of the covering tissues are determinative: these include mechanical, chemical and permeability features, all of which may interfere with or suppress the successful completion of germination by the embryo. Thus, in the case of coat-imposed dormancy, removal of the tissues surrounding the embryo (e.g., endosperm, pericarp, or extrafloral organs) is sufficient for successful completion of germination. In the case of embryo dormancy, removal of the coat does not permit such embryos to germinate normally, and so the block to germination is, in a sense, more profound than in seeds with coat-imposed dormancy. Embryo dormancy is common in woody species, especially in the Rosaceae, but is sometimes found in herbaceous plants such as some grasses (e.g., wild oats). Examples of the two categories of dormancy are given in Table 6.1.
Very often both types of dormancy exist simultaneously or successively. In apple seeds, for example, embryo dormancy predominates, but a contribution is made by the endosperm and testa, and their removal reduces the amount of dormancy-breaking treatment (chilling) that is required (Sect. 6.6.3). Mature sycamore dispersal units (actually fruits, not seeds) possess only coat-imposed dormancy, yet just before the end of their maturation on the plant they have embryo dormancy. And in the grasses Aristada contortaand Bouteloua curtipendula, dormancy of the seed in the newly dispersed units is so deep that removal of the covering hull has no effect, whereas some months later this treatment promotes their germination. The later dormancy is therefore coat imposed.
Different types of seed dormancy also can be distinguished on the basis of the timing of the induction of dormancy rather than the location or mechanism of dormancy. Seeds that are shed from the parent plant in a dormant state display primary dormancy. Seeds in the soil may (gradually) acquire secondary dormancy(Fig. 6.1) if the conditions for germination are unfavorable or if seed germination is inhibited by other means, for example osmotic stress. Secondary dormancy imposed on imbibed light-requiring seeds maintained in the dark is termed skotodormancy, and that imposed by high temperatures is thermodormancyor thermoinhibition.
Seeds of several species display more complex patterns in which the parts of the embryonic axis differ in their depth of dormancy. In the so-called epicotyl dormancy(e.g., in Paeoniaspp. and Liliumspp.), radicle emergence occurs readily but the epicotyl fails to grow. In Trilliumspp. and Caulophyllum thalictroides, the radicle has some dormancy but it is less deep than that of the epicotyl because the two organs differ in the duration of the chilling treatment needed to break dormancy; such cases are said to exhibit double dormancy.
6.3 Mechanisms of Dormancy
Embryo growth potential (mainly associated with the radicle) must increase to allow radicle extension growth and protrusion through the covering layers. The restraint of the covering layers (pericarp, testa, and/or endosperm) must be weakened; in particular, weakening of the micropylar endosperm covering the radicle is of utmost importance (Sect. 4.6.1). The combination of radicle extension growth and weakening of any surrounding tissues are the key processes for dormancy removal and seed germination in most species and share known molecular mechanisms of which several are evolutionarily conserved. Thus, in both embryo and coat-imposed dormancies, the embryo is unable to overcome the constraints imposed on it, in the former case by factors within the embryo itself and in the latter by the enclosing tissues. Whole seed dormancy, therefore, is the result of one or more blocks to germination (in either or both embryo and coat). Removal of these blocks is always governed by environmental cues, including chilling, after-ripening, light, nitrate, and permissive temperatures.
6.3.1 Blocks to Germination Within the Embryo
Among the possible blocks to germination within the embryo are: (1) undifferentiated embryo, (2) immature embryo, (3) chemical inhibitors, and (4) physiological constraints. These are each discussed below.
6.3.1.1 Undifferentiated Embryo
In a number of species, seeds do not follow the general pattern of development. In the genera of some plant families, such as the Orchidaceae and Orobanchaceae, seeds may contain undifferentiated embryos. Orchid seeds have no endosperm and the small embryo is enclosed only by a seed coat that is composed of a thin layer of cell walls (Fig. 6.2a). Orchid seeds are exceptionally small, usually 0.1–0.2 mm wide and 0.2–1 mm long. The embryos may consist of less than 100 cells. They do not contain sufficient food reserves for growth, which necessitates the utilization of exogenous sources of nutrition, e.g., from higher plants (parasitic) or from mycoheterotrophs (fungal associations). Evidently, developmental arrest in these seeds occurs at a very early stage of embryonic histodifferentiation. Thus, these embryos will have to complete their developmental phase before they can start the germination program. Seeds with undifferentiated embryos are not dormant in the strict sense and are therefore not commonly included in current dormancy classifications.
(a) Underdeveloped embryo in seed of the orchid Anacaptis palustris. Note the empty seed at the top, and the small embryo in the lower viable one. From Cafasso et al. (2005). With permission of Oxford Univ. Press. (b) Completion of development of the immature carrot embryo following imbibition of the dry seed dispersed from the mother plant. The initially small embryo (dissected in a) grows into a cavity in the endosperm formed by the dissolution of its cell walls by the hemicellulase, endo-β-mannanase. (a–d) Seeds imbibed for 12, 18, 30, and 40 h, respectively. Completion of germination, noted as emergence of the radicle from the seed, occurs from 2 to 4 days after the start of imbibition, although under field conditions embryo development and germination will be much slower. After Homrichhausen et al. (2003). With permission of Cambridge Univ. Press
6.3.1.2 Immature Embryo
Seeds of other species do complete their morphogenetic phase and have a fully differentiated embryo, but do not appear to enter the maturation phase, i.e., they do not expand and accumulate food reserves. Seeds of this type usually contain relatively large amounts of endosperm tissue, often entirely embedding the small embryo. These embryos have to grow inside the dispersed seed prior to germination, sometimes by cell division, as well as by cell expansion, depending on their extent of development at the time of shedding. This has been studied particularly well in seeds from coffee, celery, carrot (Fig. 6.2b), and Annona crassiflora. In these species the thick cell walls of the surrounding endosperm are enzymically digested in conjunction with growth of the embryo after maturation and dispersal. This growth is facilitated by utilization of the energy-rich carbohydrate degradation products of the endosperm cell walls and, at the same time, the provision of space for expansion of the embryo. Radicle protrusion does not occur until the embryo has attained a predefined length and the micropylar endosperm has been sufficiently degraded.
6.3.1.3 Chemical Inhibitors
Only few cases of dormancy caused by chemical inhibitors have been examined in detail, but in those that have, two factors appear to be involved: (1) the cotyledons and (2) germination inhibitors. Amputation of the cotyledons often allows the embryonic axis of the dormant embryo to germinate and grow. In this way, dormancy is partially or completely broken in hazel and the spindle tree by excising one cotyledon, and in ash by cutting off two. Embryo dormancy in barley can be relieved by removal of the scutellum (which is a modified cotyledon), whereas dormancy of apple embryos is progressively reduced as increasing amounts of cotyledonary tissue are cut off (Fig. 6.3). These results suggest the presence of inhibitory substances in the cotyledons that are transported to the radicle where growth is inhibited. There is good evidence that in apple cotyledons the inhibitor is abscisic acid (ABA). The cotyledons of apple contain bound forms of ABA from which free ABA is gradually released and transported to the radicle, whose extension is thus inhibited. Observations on sunflower embryos suggest that in this species the continuous synthesis of ABA (and not its release from bound forms) is required for the maintenance of embryo dormancy. Embryos lose this dormancy when treated with fluridone, an inhibitor of ABA synthesis; they are then released from inhibition and commence radicle growth. The axes themselves are dormant and when excised they too respond positively to the fluridone treatment. In Arabidopsis, higher ABA contents of seeds clearly correlate with the presence of dormancy. Interestingly, ABA decreases during the first 24 h following imbibition in both dormant and in nondormant seeds, but after that ABA increases only in the dormant seeds (Fig. 6.4a). In seeds of Nicotiana plumbaginifoliaABA synthesis can be inhibited by knocking out the gene that encodes for zeaxanthin epoxidase, an enzyme involved in the synthesis of ABA (seeFig. 6.13a, step: zeaxanthin epoxidation to violaxanthin). The result is a substantial reduction in the lag time for the commencement and completion of germination compared to the wild type (Fig. 6.4b). This also illustrates that dormancy can be evident as delayed germination even when all seeds are eventually able to complete germination.
Effect of cotyledon removal on embryo dormancy in apple. Portions of cotyledons(s) were removed from isolated, dormant embryos as indicated. The treated embryos were placed on moist cotton wool and their germination recorded. After Thevenot and Côme (1973)
(a) ABA content of Arabidopsis thalianaseeds during imbibition of dormant (●) and after-ripened nondormant (○) seeds and seeds whose dormancy has been broken by cold stratification (□). From Ali-Rachedi et al. (2004). (b) Germination of seeds from Nicotiana plumbaginifolia, comparing wild type (WT), an ABA-deficient mutant (aba2-s1), and two transgenic lines with impaired ABA biosynthesis (Epox-AS0.8 and Epox-AS1.4) as a result of mutations in the gene encoding zeaxanthin epoxidase (Fig. 6.13). From Frey et al. (1999)
It seems likely, therefore, that embryos are held in a state of dormancy by ABA, either generated by the cotyledons, or synthesized within the axis, or possibly both. Dormant embryos of many species contain ABA and detailed studies have confirmed its pivotal role in the regulation of dormancy (Sect. 6.6.1.1).
6.3.1.4 Regulatory and Metabolic Constraints
The term “physiological dormancy” is often used to denote a reversible type of dormancy that is located in the embryo. This type of dormancy is widespread and central to the phenomenon of seasonal dormancy cycling of seeds in the soil, which is crucial to the establishment and survival of plant communities (Sect. 7.3). There is good evidence that physiological dormancy is maintained by active suppression of several germination-related cellular activities. ABA plays an important role in this suppression, whereas GA may counteract its effect. A GA–ABA balance appears to be decisive for the loss or maintenance of dormancy (Sect. 6.6.1).
6.3.2 Blocks to Germination by the Covering Layers
The following are possible effects on germination of the tissues enclosing the embryo: (1) interference with water uptake, (2) interference with gas exchange, (3) prevention of the exit of inhibitors from the embryo, and (4) mechanical restraint. Whereas physiological dormancy is a reversible block to germination, i.e., it can be lost and reinduced as secondary dormancy, coat-imposed dormancy is irreversible. Embryo coverings include seed and fruit tissues that may be dead or alive. Their contribution to the level of dormancy may be of a physical, chemical, or mechanical nature, or combinations of these. Many seeds contain an endosperm that functions as the major source of stored reserves, e.g., cereals and endospermic legumes (Sect. 1.2.2). In others, especially where it is not the main storage tissue, it may contribute considerably to the expression of dormancy and germination. The seeds of fewer species contain a perisperm, a diploid endosperm-like tissue of maternal origin.
6.3.2.1 Interference with Water Uptake
The seed coat, derived from the integuments, and/or surrounding fruit tissues may prevent germination. The term “physical dormancy” is often used to denote absence of germination as the result of seed coat impermeability to water. This is a common effect, especially in seeds of the Leguminosae, but it is also found in at least 15 other families, including the Cannaceae, Convolvulaceae, Chenopodiaceae, and Malvaceae. Many species have seeds with extremely hard coats which, by preventing the entry of water, may delay germination for many years. For example, about 20% of soaked Robinia pseudoacaciaseeds remained ungerminated for 2 years because insufficient water reached the embryo, and some of the seeds may remain in this state for 20 years! It could be argued that these are not really cases of dormancy in the strict sense, because the embryos simply do not have sufficient water, one of the basic requirements for germination; nevertheless, hard-coated seeds, in which water and oxygen entry are the factors limiting germination, are generally considered under the heading of dormancy.
Seed coat impermeability is usually caused by the presence of one or more layers of palisade cells in the testa. These palisade layers are composed of sclereid cells with thick lignified secondary cell walls, as in Melilotus alba(Fig. 6.5). Fruit tissues may also contain sclereid cells. Apart from lignin in the secondary cell walls, a number of other components are water repellent, including cutin, quinones, suberin, waxes, callose, phenolics, and hydrophobic (lipid-like) substances. All of these may contribute to varying extents; sometimes one of them plays a major role in waterproofing of the coat, e.g., the waxy cuticle in some species of Leguminosae. However, the main barrier to water uptake is offered by the osteosclereids, e.g., in coats of the vetch Coronilla varia. Only when this layer is punctured are most seeds capable of imbibing water (Fig. 6.6).
A section of the sclerified seed coat of Melilotus alba. After Hamly (1932)
The effect of puncturing on seed coat permeability to water. The testae of Coronilla variaseeds were punctured to different depths (names of the layers are noted below the graph, increasing in depth from leftto right) with a fine needle and afterward placed on moist filter paper for imbibition. Adapted from McKee et al. (1977)
Some seeds contain mucilage layers that function as a water “gauge.” Too much water will make the layer impermeable to oxygen, e.g., in Blepharis, and too little water will only hydrate the mucilage layer to a certain extent; hence there will be insufficient water reaching the embryo to support its germination.
The testa of many seeds contains specialized structures that regulate the uptake of water. These are generally derived from tissues that close the natural openings in the seed or fruit coat, such as the micropyle, hilum, and chalazal area (Sect. 1.2.3). For example, a chalazal cap is known in members of the Malvaceae and Cistaceae, a strophiole (between hilum and chalaza) in the Papilionoideae and Mimosoideae, and an operculum (derived from the micropyle) in the Musaceae. The development of seed coat impermeability is controlled genetically and also by the relative humidity of the air during the maturation phase of seed development.
6.3.2.2 Interference with Gas Exchange
The initiation of respiration is one of the early events during imbibition; this entails uptake of oxygen and release of carbon dioxide (Sect. 4.4). Any disturbance in gaseous exchange may thus lead to an inhibition of germination or maintenance of dormancy. The tissues surrounding the embryo might limit the capacity for gaseous exchange by the embryo in two ways: entry of oxygen may be impeded or escape of carbon dioxide may be hindered. The possibility that the seed coat imposes dormancy by affecting gaseous exchange was suggested from the fact that in many cases the inhibitory action of the tissues surrounding the fully imbibed embryo is much reduced simply by scratching or puncturing them. Thus, a pinprick through the endosperm, near the radicle, of the lettuce seed or through the pericarp over the embryo of the intact, dormant wheat grain can cause some germination. Numerous cases of such effects are known and it seems unlikely that such moderate “surgical” operations can interfere appreciably with the mechanical resistance of the coat. However, at the same time, it also seems likely that such pinpricks would appreciably increase oxygen diffusion through the covering tissues because also puncturing with a needle at the opposite end from the radicle may induce germination. Stronger evidence that germination of the intact dispersal unit is prevented by insufficient oxygen is that dormancy is frequently overcome by oxygen-enriched atmospheres. These observations suggest that in some species the embryo in the intact, dormant dispersal unit fails to germinate because of the restrictions imposed by the enclosing tissues, including oxygen uptake. Removal, abrasion, or puncturing of these tissues may give the embryo access to oxygen, allowing germination to proceed to completion.
In several species permeability of the seed coat to oxygen is less than that of water. In Sinapis arvensis, for example, it is lower by a factor of about 104and in Xanthium pennsylvanicumby about 102. The reasons for these differences are not clear in all instances, but one possibility is that there is resistance to the entry of oxygen offered by the layer of mucilage around many seeds (S. arvensis, for example). Another way in which the coats act is by consuming oxygen themselves. This is probably due to the enzymatic oxidation of various chemical constituents, an occurrence that is known in the testa of apple and of other seeds, where various phenolic compounds (e.g., phloridzin and chlorogenic acid) are implicated. The dormancy-imposing hull of rice and the glumellae of barley also chemically consume oxygen. As dormancy slowly diminishes during dry storage of the grains, the oxygen-consuming capacity of the hull decreases: this correlation supports the possibility that the hull imposes dormancy because it deprives the embryo of oxygen. Also correlated with the extent of dormancy in different cultivars of rice is the activity in the hull of peroxidases, enzymes that may form part of an oxygen-consuming complex. In barley grains, 40–50% of the total oxygen uptake is accounted for by the activity of the glumellae, which impose dormancy, yet this does not reduce the energy charge (essentially, ATP production) of the embryo. Hence, sufficient oxygen appears to enter it to satisfy the demand in ATP generation. Embryos of several species are satisfied by extremely low partial pressures of oxygen. For example, isolated embryos of birch, Sinapis arvensisand wheat can germinate even in nitrogen atmospheres! Thus, while seed coats can limit oxygen diffusion to the enclosed embryo, they do not seem to impose dormancy simply by restricting the amount of oxygen available for respiration. Oxygen microsensors have been developed that are small enough to be embedded in individual seeds; their use could help resolve the actual oxygen partial pressure inside of dormant seeds.
Nevertheless, high partial pressures of oxygen and facilitated access to air (by pricking or scratching the seed coats) still cause some intact, dormant seeds to germinate. If the oxygen is not needed to support respiration, what is it for? Inhibitors have been invoked, in some cases, to answer this. In Xanthium, for example, growth inhibitors are present in the embryo, which, in the intact seed, are oxidized under high oxygen concentrations but not in air; hence, the effect of exposing intact seeds to elevated oxygen is to inactivate these inhibitors. The inhibitors can also diffuse out of the isolated embryo when it is set on a moist substratum, but they do not cross the seed coat of an intact seed. Thus, there is a twofold effect of the intact coat: primarily, it causes the retention of inhibitor, and secondarily, it can act as a barrier to oxygen, preventing the entry of sufficient oxygen from the surrounding air to support the oxidation of the inhibitor.
6.3.2.3 Prevention of Exit of Inhibitors from the Embryo
A range of chemical compounds derived from seeds or dispersal units, including phenolic acids, tannins, and coumarins, may inhibit germination. Such chemicals have been extracted from all seed and fruit parts. However, the specific role of these compounds in the inhibition of germination is not certain, for in most cases a direct relationship between content and physiological action is lacking. On the other hand, germination may be accelerated by extensive rinsing of seeds containing inhibitory chemicals with water. It is possible that many of these inhibitory substances are primarily present to avoid predation or microbial infections.
A detailed study of the seed coat pigmentation of Arabidopsis has revealed the existence of multiple ways of influencing dormancy and germination, as well as longevity, by modification of seed coat properties. From mutagenesis experiments, a series of mutants were obtained with aberrant seed coat properties, mainly in coloration, which included the so-called transparent testa(tt) mutants. Permeability of the seed coats of these was tested in tetrazolium salt, a compound that turns bright red in living tissue. Wild-type seeds displayed both dormancy and impermeability to the tetrazolium salt. In general, improved germination of the ttmutants was associated with a greater permeability to the tetrazolium compound. Most of the ttmutants displayed a reduced dormancy, perhaps due to a greater porosity of the coats to either an endogenous inhibitor (e.g., leakage of ABA) or to an exogenous stimulant of germination (e.g., uptake of oxygen) or, because of their thinner structure, the testae are less resistant to expansion of the embryo. The enhanced seed coat permeability is likely related to a reduction or absence of proanthocyanidins (condensed tannins) and the concomitant shrinkage of the testa cells.
6.3.2.4 Mechanical Restraint
Tissues surrounding the embryo will almost always impose a certain mechanical restraint to expansion of the embryo. Removal or partial removal of these tissues will often lead to normal embryo growth, indicating that the block to completion of germination is entirely located in the embryo coverings, the endosperm, perisperm, and/or testa. The embryo may overcome the mechanical restraint by the generation of sufficient thrust but, alternatively, the restraint may be weakened by activity of the micropylar endosperm cells, controlled by the embryo, but with no apparent changes in embryonic growth potential. This has been demonstrated for a number of species in which the endosperm is digested by hydrolytic enzymes, e.g., tomato, Datura ferox,tobacco, and coffee (Sect. 4.6.1).
6.4 Embryonic Inadequacy: The Causes
6.4.1 Energy Metabolism of Dormant Seeds
There is a general but incorrect impression that energy metabolism is repressed in the dormant state and, hence, respiration and accumulation of ATP and NAD/NADH are likely being affected. The approach frequently taken is to compare the metabolism of dormant seeds with that of after-ripened, i.e., nondormant, ones. To ensure that the comparison is meaningful, it cannot be made between a dormant seed and one that has germinated, so only metabolism occurring in the early times after imbibition, well before radicle emergence from the nondormant seed, can be considered as relevant. Unfortunately, few studies have taken this into account.
Beginning with respiration, it seems that dormant seeds display a similar respiratory activity to nondormant material. For example, dormant and after-ripened seeds of Xanthiumand wild oat show equivalent oxygen consumptions up to the time of emergence of the radicle from the germinable seed. In seeds of Sisymbrium officinale,oxygen uptake and carbon dioxide evolution are associated neither with the breaking of primary dormancy nor with the breaking or induction of secondary dormancy. Nevertheless, oxygen uptake does decrease with the induction of secondary dormancy when respiration is measured at temperatures above 20°C. The respiratory quotient RQ (O2uptake/CO2evolution) remains constant between 0.55 and 0.7, which is indicative of mostly normal respiratory pathways of oil-containing seeds, without much oxygen consumption by, for example, phenol oxidation in the testa. And hand in hand with the equal oxygen utilization by dormant and nondormant seeds, their ATP contents are comparable. However, there is evidence that it is not the absolute amount of ATP in the seeds that correlates with the dormant vs. nondormant states, but its distribution within the embryo. In tomato, there is no significant difference in ATP content between dormant and nondormant seeds, but when its distribution is visualized within them (Fig. 6.7), the ratio of ATP concentration in the radicle–cotyledons correlates with the dormant state: it is 1.1 in dormant seeds (embryos) and up to 1.7 in the nondormant ones, i.e., there is more present in the radicle of the nondormant seed.
ATP distribution in cryosections of (a) ungerminated 24-h-imbibed nondormant and (b) 5-day-imbibed dormant tomato seeds. Low (blue) to high (orange) ATP concentrations are evident in the seed, with the amount of ATP being greatest in the radicle of the nondormant embryo. Images on the leftshow positioning of embryo within the seed. Scale of color from high (red) to low (blue–purple)ATP concentrations is shown to the leftin (a). From Spoelstra et al. (2002). With permission of Cambridge Univ. Press
Secondary dormant seeds held in an imbibed state for prolonged periods exhibit a progressive decline in respiration, perhaps to limit the use of their reserves which eventually will be required during and following germination. When lettuce seeds are released from secondary dormancy there is an increase in respiration during germination, but to a considerably lower extent compared to seeds germinating following the breaking of primary dormancy.
Several other aspects of energy metabolism have received attention with respect to their possible participation in dormancy. Quantitative and qualitative differences have been recorded in some hexose and triose phosphates between dormant and nondormant seeds. Higher concentrations of fructose-2,6-bisphosphate are achieved in nondormant than in dormant grains of oats during the first few hours of imbibition. This is a consequence of the higher amounts of phosphoenolpyruvate and glycerol-3-phosphate in dormant grains, two compounds that inhibit phosphofructokinase, the enzyme phosphorylating fructose-6-phosphate to fructose-2,6-bisphosphate. One important action of fructose-2,6-bisphosphate is to regulate gluconeogenesis through its inhibitory effect on fructose-1,6-bisphosphatase, but the possible significance of this in respect of dormancy is obscure. Changes in the concentration of this hexose phosphate have been reported only in one other species (rice) so it is not known if the phenomenon is widespread.
6.4.2 Genetic Aspects of Dormancy
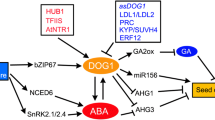
Although environmental factors are important (Sect. 6.5), the entry into dormancy is also under genetic control; heritable variation of this trait has been demonstrated in many species. A wide range of dormancy is encountered in natural accessions of wild oat, for example, from types that have a short-lived dormancy to those whose dormancy is very prolonged. A vast range of accessions with various levels of dormancy is also known in Arabidopsis, which are associated with their geographical distribution. Hybridization studies with dormant and nondormant pure lines have revealed that seed dormancy may be controlled by both the maternal and paternal parent genotypes. Crossing of accessions with a large parental disparity in dormancy and the subsequent derivation of recombinant inbred lines (RIL) have provided the tools to study the genetic aspects of the natural variation in dormancy. One gene discovered in such studies is DELAY OF GERMINATION 1(DOG1), which appears to correlate very closely with the onset and depth of dormancy in Arabidopsis. DOG1encodes a protein of currently unknown function, but which is likely a transcription factor. Similarly, a genetic analysis of a RIL population of lettuce, combining lines with low (cv. UC96US23) and high (cv. Salinas) susceptibility to thermodormancy, revealed that the LsNCED4gene is largely responsible for the observed variation. This gene encodes the enzyme 9-cis-epoxycarotenoid dioxygenase, which is involved in ABA synthesis (Sect. 6.6.1.1). The sensitivity of LsNCED4expression to elevated temperature, resulting in higher ABA and lower germination, may thus determine the upper temperature limit for lettuce seed germination (Fig. 6.8). Similarly, Arabidopsis seeds germinate well at 22°C but become dormant at high temperatures (e.g., 34°C) due to an increase in NCED9expression and ABA synthesis. In contrast, seeds of the nced9mutant, which lacks this gene, are resistant to thermoinhibition.
(a) Germination time courses of Lactuca sativacv. Salinas (Sal, circles) and L. serriolacv. UC96US23 (UC, triangles) seeds at 20°C (open symbols) and 35°C (closed symbols) in the light. (b) Relative expression (mRNA content) of LsNCED4in seeds of cv. Salinas and UC96US23 seeds imbibed at 20 and 35°C in the light. (c) ABA contents of cvs. Salinas and UC96US23 seeds during imbibition and germination at 20°C and 35°C in the light. ABA values are plotted on a logarithmic scale. Error bars indicate ± SE (n = 3). After Argyris et al. (2008). Copyright American Society of Plant Biologists
In this context, it is important to note that dormancy can depend on an interaction between coat and embryo, and therefore three genetically distinct components are involved: (1) the diploid embryo with maternal and paternal genes, (2) the diploid testa, pericarp, and hull of maternal constitution only, and (3) the triploid endosperm bearing two sets of maternal genes. It may well be, then, that the genetics of coat-imposed dormancy are rather complex.
6.5 The Environment in Dormancy Inception
In addition to genetic factors, the environment has a profound influence on the acquisition of dormancy during seed development. The effect of environmental factors largely explains why dormancy varies with provenance. Species commonly studied by seed physiology researchers, such as Arabidopsis, are notorious in this respect, and one cannot assume that a particular batch will have any dormancy at all: this depends on where it was grown and what environmental factors were operative at the time of its development and maturation. What are these factors that can have such a profound effect on the development of dormancy? Some of the major ones are discussed below.
(1) Soil. This edaphic influence (i.e., that of soil characteristics rather than of the climate) can play a role in the establishment of dormancy. A clear example is the uptake and distribution of nitrate, an important regulator of dormancy and germination (Sect. 7.2.4). The nitrate content of mature seeds depends on the amount of this anion taken up by the mother plant and allocated to the seeds. This, in turn, depends, among other things, on soil moisture and nitrate content, the nitrate reductase activity in the roots of the mother plant, and activity of a seed-specific nitrate transporter, such as the Arabidopsis thalianaNITRATE TRANSPORTER2.1 (AtNRT2.1). The amount of nitrate accumulated in dry seeds is directly related to the amount of nitrate in the soil during their development and determines the extent of their germination (Fig. 6.9a, b). There is very little information about the possible influence of other nutrients on dormancy acquisition, such as phosphate, cations such as sodium and potassium, or micronutrients such as iron, zinc, and copper.
(a) Effect of potassium nitrate fertilization of Chenopodium albumand Sisymbrium officinaleplants on the nitrate content of produced seeds. The plants were cultivated in plots in an open field. (b) Nitrate content and germination of Sisymbrium officinaleseeds. Seeds were taken from 20 lots, derived from plants grown in liquid culture or in the field, treated with red light and germinated at 24°C. Seed nitrate content is plotted on a log scale and germination percentage on a probit scale. From Hilhorst and Karssen (2000). Courtesy of CAB International
(2) Temperature. Much more is known about the effects of temperature on the acquisition of dormancy during seed development. In general, its occurrence is promoted by relatively low temperatures, as in Rosaspp., in grasses such as wild oats, and the cereals, wheat, and barley. In line with this, Arabidopsis seeds developing under cool or warm temperatures display high and low dormancy, respectively (Fig. 6.10a). A global gene expression (transcriptomic) analysis of the dry seeds has identified a low-temperature-regulated set of genes, including DOG1, GA2ox6, and NCED4(Fig. 6.10b), that are strongly associated with dormancy. GA2ox6encodes the enzyme GA-2-oxidase, which is a component of the inactivation pathway of gibberellins, whereas DOG1 and NCED4 are involved in the acquisition of dormancy and ABA biosynthesis, respectively (Sect. 6.6.1.1). In contrast, the lower level of dormancy of seeds matured at higher temperature is associated with elevated expression of CYP707A2encoding cytochrome P450 707A2, which is a principal component of ABA catabolism (Fig. 6.10b). It is clear that, at least in Arabidopsis, the control of dormancy by the maternal environment is mediated by a GA–ABA balance (Sect. 6.6.1.4). However, there are species in which seed dormancy is induced by elevated temperatures. Syringa vulgaris, for example, can be made to produce dormant seeds by holding the mother plant at relatively high temperatures (18–24°C) during the last week of seed maturation, a treatment that appears to make the endosperm (the tissue imposing dormancy) tougher.
(a) Germination of freshly harvested wild-type Arabidopsis Columbia-0 seeds matured at (▲) 10°C, (○) 15°C, and (●) 20°C in response to moist chilling. (b) Gene expression changes in Arabidopsis seeds developed at 10 or 20°C. Low seed maturation temperatures induced GA2ox6, DOG1, and NCED4expression, whereas warm seed maturation temperatures induce high CYP707A2expression, the transcripts of which were present in mature dry seeds. From Kendall et al. (2011). Copyright American Society of Plant Biologists
(3) Light. Light quantity, its daily distribution, and spectral quality can all have a profound influence on the development of dormancy. Photoperiodic effects on the inception of seed dormancy are well known in several species, and Chenopodiumspp. provide good examples. C. album(Fig. 6.11) has deeply dormant seeds when the fecund plants are held under long days, but nondormant seeds under short days. Not only is the dormancy pattern affected by day length but also the structure of the seeds, for those maturing in long days are smaller and thicker coated than those in short days. Dormancy induced by photoperiod is not always associated with coat thickness, however, since a short-term dormancy of seeds with thin coats is brought about by long photoperiods given for just a few days after the end of flowering. An effect of photoperiodic conditions on coat structure is also seen in seeds of several other species. Seeds of Arabidopsis matured under short-day conditions are in general less dormant than those ripened under long days. However, there is a strong dependency on accession type and temperature for the expression of dormancy. An interaction of flowering time and dormancy appears to be pivotal for germination timing and, hence, dispersal and survival of the species (Sect. 7.4.2). Light, quite apart from photoperiod, has an important role in dormancy induction in several species. Seeds of Arabidopsis maturing in white fluorescent light lose their dormancy with time after harvesting, whereas those which have experienced incandescent light during maturation remain deeply dormant for at least several months (Fig. 6.12); similar results have been reported for lettuce. This is because white fluorescent light is relatively rich in the red wavelengths whereas incandescent light has a relatively high component of far-red light. Under the former illumination conditions, more of the active Pfr form of phytochrome (Sect. 6.6.5) accumulates in seeds than under the latter type of light. Seeds with a high Pfr content can often germinate in darkness because they have already exceeded the required threshold of Pfr for germination, while those with a low Pfr concentration remain dormant. Treatment with far-red light alone, of course, has the same effect: cucumber seeds are made dormant when fruits are irradiated with far-red light. It appears that this kind of phenomenon also occurs in nature, where the source of far-red light is light filtered through green tissues ( Sect.7.2.3.1). Chlorophyll absorbs red light (peak ca. 660 nm) but not wavelengths longer than about 710 nm; hence the transmitted light, being rich in the far-red component, serves to lower the amount of Pfr.
Germination of Chenopodium albumseeds that developed under different photoperiods. Plants with developing seeds were held under short days or long days. After harvesting and being maintained in the dry state, germination of the seeds was tested at intervals of several months. Seeds developing and maturing under short days (●) have a high germination percentage whereas those from long-day-treated plants (■) have a dormancy that lasts for at least 3.5 months. All germination tests were carried out in darkness. Adapted from Karssen (1970)
Light quality during seed maturation and its influence on Arabidopsis thalianadormancy. Seed-bearing plants were kept in white incandescent or white fluorescent light during seed maturation. Germination was subsequently tested in darkness. Seeds maturing in white fluorescent light (●) have less dormancy than those maturing in incandescent light, which are deeply dormant for more than 100 days after harvest (■). Adapted from Hayes and Klein (1974)
6.6 The Release from Dormancy
Because seeds can germinate only once, those of many species have evolved sophisticated means to sense the environment and determine the right moment to proceed towards the completion germination. Flowering time and dormancy are the principal cues that determine germination timing. Thus, in many instances there are seasonal components involved in the ultimate “decision” of a seed to germinate, which will be discussed in Chap. 7. Here the different factors by which dormancy is terminated are detailed while making a distinction between their long-term and short-term efficacies. For example, dry after-ripening and moist chilling are often associated with seasonal length (i.e., the temporal environment), whereas light and chemicals may be active instantaneously and appear to be indicators of the prevailing (or spatial) environment.
For simplicity, the different factors are discussed separately but it must be appreciated that in nature a seed is not subject to the influence of just one factor but to several simultaneously. For example, the release of Arabidopsisseeds from primary dormancy requires the removal of several “blocks” to germination, including after-ripening, chilling, and exposure to nitrate and light, often in a certain order. Thus, the termination of dormancy in the field is not likely to be the prerogative of just one factor in the seed’s environment but will be influenced by several, and in some circumstances seeds of the same species might have their dormancy ended by different cues.
6.6.1 Perception, Signaling, and Role of Hormones with Respect to Dormancy and Germination
Before the diverse environmental factors affecting release from dormancy are discussed, this section focuses on the function and quantitative changes in the expression of genes associated with the metabolism of the major seed hormones which suppress or promote germination. Hormones play a vital role in dormancy maintenance or release in response to the environment, so consideration is given here to their synthesis and catabolism, and to the mechanisms by which cells perceive their presence and respond appropriately. Although there are other dormancy-breaking chemicals, GA and ABA in particular have a profound influence on the dormancy and germination of seeds. Whether or not the latter is completed is effected during Phase II of germination (Fig. 4.1) by hormone biosynthetic and catabolic enzymes, whose abundance is controlled primarily at the level of transcription. Also important is perception of the hormones by receptors and subsequent signal transduction pathways to influence gene expression. Therefore, identifying hormone metabolism genes and analyzing their expression patterns are critical to understanding the control of dormancy and germination. Posttranslational modifications of signaling proteins by phosphorylation or dephosphorylation, and ubiquitination, which affects their stability, play critical roles. Cold, light, and other effectors, such as smoke and soil nitrate, affect dormancy and germination, at least in part, through hormone metabolism and signal transduction. Cross talk between environmental signals and hormonal regulation provides a complex integrated network through which seeds can adjust their germination potential to match ecological opportunities.
6.6.1.1 Regulation by ABA
According to the hormone balance theory, the relative actions of ABA (inhibitory) and GA (promotive) are the primary determinants of seed dormancy and germination. The relative activities of ABA and GA are in turn a result of the amounts of each present and the sensitivity of the target cells or tissues to them, dependent on their respective perception and signaling pathways. The amounts of these hormones in seeds are regulated by their rates of synthesis and deactivation. ABA is produced via the carotenoid pathway, and β-carotene is an important upstream substrate in its synthesis (Fig. 6.13a). This is converted to other forms of C40 carotenoids, such as zeaxanthin and violaxanthin, which are eventually processed into xanthoxin, a C15 precursor of ABA, via oxidative cleavage. While active ABA is produced from ABA aldehyde by ABA aldehyde oxidase (AAO), the rate-limiting (most critical) step in ABA synthesis is the conversion of 9′-cis-neoxanthin and 9-cis-violaxanthin to xanthoxin by 9-cis-epoxycarotenoid dioxygenase (NCED) (Fig. 6.13a). Therefore, the rate of ABA production, which is associated with the induction and maintenance of seed dormancy, is considered to be regulated primarily by NCED.
Hormones involved in the regulation of germination and dormancy. (a) Abscisic acid (ABA) is derived from geranyl-geranyl diphosphate (GGDP) through the carotenoid pathway. The conversion of 9′-cis-neoxanthin and 9-cis-violaxanthin to xanthoxin is the rate-limiting (most critical) reaction, which is catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED). The conversion of abscisic aldehyde to active ABA is catalyzed by ABA aldehyde oxidase (AAO). ABA is then deactivated by CYP707A2, an 8′-hydroxylase, or by conjugation with glucose to form ABA glucose ester (ABA-GE). ABA biosynthesis can be blocked by fluridone, an herbicide that inhibits phytoene desaturase (PDS), an enzyme that functions in the upstream, carotenoid biosynthesis pathway. (b) Gibberellin (GA) biosynthesis is initiated by the conversion of GGDP to ent-kaurene by coparyl diphosphate synthase (CPS) and ent-kaurene synthase (KS); kaurene is then converted to ent-kaurenoic acid by kaurene oxidase (KO). The rate-limiting reaction in the GA biosynthesis pathway is the last step of the conversion of an inactive to an active form, for example, GA9to GA4in Arabidopsis seeds and GA20to GA1in lettuce seeds, both of which are catalyzed by GA-3-oxidase (GA3ox). GA-2-oxidase (GA2ox) catalyzes the deactivation of active GAs to the inactive forms GA34and GA8. Paclobutrazol (PAC) inhibits KO, and hence GA biosynthesis. (c) Ethylene is derived from S-adenosylmethionine (SAM), which is converted to 1-aminocyclopropane carboxylate (ACC) by ACC synthase (ACS). ACC, in turn, is converted to ethylene (and CO2and cyanide) by ACC oxidase (ACO). Methionine is regenerated for reuse in the ethylene pathway via the Yang cycle and SAM synthetase. ACS can be inhibited by aminoethoxyvinyl glycine (AVG), and ACO can be inhibited by anaerobiosis. The structures of ABA, GA and ethylene are shown in Fig. 2.7. Only representative intermediates in the pathways are shown. A single arrow does not necessarily represent a single step in the pathway. (┤) indicates the sites of action of inhibitors typically used in seed biology experiments. Modified from Yamaguchi et al. (2007)
The NCEDgene was first identified during characterization of the maize mutant viviparous 14(vp14) (Sect. 2.4.2) and is now known to be present in many other species. In addition, multiple NCEDfamily members are present within a species, providing opportunities for temporal and tissue-specific expression. For example, because ABA is involved in both seed germination and in responses to water stress, those functions often involve different NCEDgene family members. Even in the seed, NCED6is expressed specifically in the endosperm while NCED9is expressed in the endosperm/testa and in the peripheral cells of the embryos of developing Arabidopsis seeds, suggesting the involvement of both of these regions in the induction and maintenance of seed dormancy. When NCED6gene expression is experimentally induced in imbibed nondormant seeds, germination is suppressed. This indicates that the carotenoid and ABA biosynthesis pathways are operating in nondormant seeds, and that the substrates for NCED are being synthesized even in seeds that are capable of completing germination. This ready availability of substrate for ABA synthesis is probably important in allowing seeds to respond quickly to produce the inhibitor when there are environmental changes, and also in the induction of secondary dormancy under adverse conditions (e.g., by high temperatures; Sect. 6.4.2). Thus, NCED can be regarded as a “rate-limiting” enzyme for ABA synthesis in seeds and is important in the control of their germination.
Continuous ABA synthesis is apparently necessary for the suppression of germination because carotenoid biosynthesis inhibitors such as fluridone and norflurazon promote the germination of dormant seeds. The carotenoid biosynthesis pathway is upstream of that for ABA synthesis (Fig. 6.13a); therefore, blockage of the former hinders the latter and as a consequence alleviates seed dormancy.
The ABA content of seeds is controlled not only by its synthesis but also by its deactivation. One of the many cytochrome P450s, CYP707A2, is an ABA-8′-hydroxylase and deactivates ABA, reducing its content in seeds and releasing seed dormancy. CYP707A2is expressed during early germination of nondormant seeds of Arabidopsis, for example, while those of the cyp707a2mutant that are defective in ABA deactivation exhibit hyperdormancy. Thus CYP707A2 is a key regulatory enzyme for ABA deactivation and dormancy release, although ABA can also be deactivated through its conjugation to sugar (e.g., ABA-glucose esters) by ABA glucosyltransferase.
Developing and imbibed mature seeds respond promptly to changes in ABA concentration because they contain the components for hormone perception and response. The hormone is initially perceived by binding to the ABA receptor PYRABACTIN RESISTANCE1 (PYR1), followed by a sequence of downstream events involving multiple proteins and their modification (Fig. 6.14). In the absence of ABA, protein phosphatase 2C (PP2C) binds to and represses Sucrose non-fermenting-Related Protein Kinase 2 (SnRK2), a positive regulator of ABA responses. The ABA-receptor protein changes its conformation upon binding to the hormone and then binds PP2C, releasing SnRK2 from its inhibition (Fig. 6.14). SnRK2 then phosphorylates downstream target proteins, such as ABRE BINDING FACTOR (ABF), which then induce ABA responses, including inhibition of seed germination and dormancy. Seeds of mutants lacking the ABA receptor (e.g., pyr1), PP2C (e.g., abi1, abi2), SnRK2 (snrk2.2/snrk2.3double mutants), or ABF (e.g., abi5) exhibit ABA-insensitive germination phenotypes, i.e., they complete germination even in the presence of the inhibitor.
ABA perception and signaling in the control of seed germination. In the absence of ABA, protein phosphatase 2C (PP2C), a negative regulator of ABA responses, binds to and suppresses protein kinase (SnRK2: Sucrose Non-fermenting-Related Protein Kinase 2), a positive regulator of ABA responses. Upon ABA perception, ABA receptors such as PYRABACTIN RESISTANCE 1 (PYR1, also called RCAR: regulatory components of ABA receptor) change their conformation, forming a pocket to which PP2C binds. In this way, PP2C function is suppressed, which releases and activates SnRK2. The downstream factors such as ABRE BINDING FACTOR (ABF) are then phosphorylated (P) and activated by SnRK2, which triggers ABA responses including germination suppression and dormancy. Based on Park et al. (2009)
These basic mechanisms of ABA metabolism are conserved among species, and are common to seeds of monocots and dicots. In barley, the expression of HvNCED(Hordeum vulgareNCED) maintains a high ABA content in grains and imposes dormancy. In contrast, the expression of HvABA8′OH-1(encoding the ABA-deactivating ABA-8′-hydroxylase), an ortholog of the Arabidopsis CYP707A2gene, is associated with dormancy release. This gene is expressed mainly in the coleorhiza, which surrounds the radicle tip, and is probably associated with weakening of this covering tissue in the absence of ABA (Sect. 4.6.1).
6.6.1.2 Regulation by GA
GA is involved in many aspects of plant development in addition to germination, including vegetative growth, flowering, and pollen production. Typical symptoms of GA-deficient mutants are dwarfism and reduced or completely suppressed germination. There are many forms of GA molecules in plants, the majority of which are inactive and are intermediates in the GA biosynthetic pathway. Here, only key steps in the pathway that are critical to an understanding of seed dormancy and germination are considered.
The GA biosynthetic pathway shares a common precursor, geranyl-geranyl diphosphate (GGDP), with that for ABA (Fig. 6.13b). GGDP is converted to ent-kaurene by ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS). The ga1mutant in Arabidopsis is defective in CPS and synthesis of GA is inhibited; the seeds are absolutely dependent on an exogenous supply of the hormone for germination. Notably, the ga1embryo is able to grow when excised from its surrounding structures, indicating that the lack of CPS does not affect seed viability, but rather the ability of the embryo to overcome coat-imposed dormancy (Sect. 4.6.1). The tomato gib-1mutant has a similar defect and its seeds also require exogenous GA to complete germination. However, the germinated seeds produce dwarfed seedlings. GA-deficient mutants of other species, such as rice, still are able to complete germination without the addition of GA. The reason for this is unknown, but other factors such as the properties of the covering tissues, or ABA metabolism and signaling, might play a role.
ent-Kaurene is converted to ent-kaurenoic acid by ent-kaurene oxidase (KO) (Fig. 6.13b). Plant growth retardants, such as paclobutrazol (PAC), uniconazole-P, tetcyclacis, and ancymidol, inhibit KO and when applied to seeds cause a decline in GA content and prevent germination. However, caution is required in the interpretation of data from experiments using these inhibitors because they not only influence a specific enzyme involved in GA synthesis but can affect others, such as those involved in ABA deactivation.
ent-Kaurenoic acid is converted to several inactive forms of GA before GA12is converted to GA9by GA-20-oxidase (GA20ox). The last step in the pathway (GA9to GA4in Arabidopsis; GA20to GA1in lettuce) is the rate-limiting one, which is catalyzed by GA-3-oxidase (GA3ox) (Fig. 6.13b). There are two major genes for GA3ox in Arabidopsis seeds; ga3ox1or ga3ox2single mutant seeds can still complete germination while those of the ga3ox1 ga3ox2double mutant cannot. This indicates that these two genes play overlapping (redundant) roles in the rate-limiting step of GA synthesis, with one substituting for the other when it is mutated. When both are mutated, however, GA synthesis is prevented. Genes encoding GA3oxare expressed in the embryonic axis and are most likely associated with the generation of embryo growth potential (Sect. 4.6.1).
It is noteworthy that in Arabidopsis two key genes for enzymes of the GA biosynthesis pathway (AtGA3ox1and AtGA3ox2) are expressed specifically in cells in the hypocotyl transition zone immediately behind the radicle that will expand during germination (Figs. 4.19, 6.15), which is suggestive of an intimate association between GA synthesis and cell expansion.
Expression of the GA biosynthetic genes (AtGA3ox1and AtGA3ox2) in the radicle/hypocotyl region of Arabidopsis embryonic axes. The photographs are of longitudinal sections of the embryos, with the dark signals indicating gene expression. The localization of mRNAs was detected by hybridization with antisense RNA probes and visualized with a colorimetric enzyme reaction. No signal was detected in Control sections (hybridized with a sense GA3ox1probe), whereas strong signals were detected in the cortical cells of the hypocotyl using the antisense probes for both genes. cot, cotyledon; CT, cortical tissue; hy, hypocotyl; PV, provasculature; r, radicle; SA, shoot apical meristem. Scale bar = 100 μm. Modified from Yamaguchi et al. (2007). Courtesy of Wiley-Blackwell
There are more than 130 different structures of GA molecules in plants, the majority of which are precursors and/or inactive forms, including GA9. Only a small number, such as GA4and GA1, are active, and vary among species. For example, GA4and GA1are the active endogenous GAs in Arabidopsis and lettuce, respectively, although many seeds respond positively to application of the commercially available GA3.
When active GA is produced excessively or is not required, it is converted into an inactive form (GA4to GA34or GA1to GA8; Fig. 6.13b). The major enzyme catalyzing these reactions is GA-2-oxidase (GA2ox), which reduces the GA content in seeds and negatively affects germination. GA deactivation by GA2ox plays an important role in the regulation of seed germination by light (Sect. 6.6.5). GA can also be deactivated by GA methyltransferase, although this enzyme functions mainly during seed development.
Identification of GA metabolism genes and analysis of their expression have contributed to an understanding of the mechanisms underlying seed responses to environmental signals. For example, high temperatures inhibit germination of Arabidopsis seeds not only through the promotion of NCED expression and ABA synthesis but also through the suppression of expression of GA20oxand GA3ox,and hence of GA synthesis.
As with ABA signaling, the GA signal transduction pathway involves multiple protein components and their modification. GA-inducible genes that are most likely required for germination completion are suppressed in the absence of this hormone by RGA-LIKE2 (RGL2), a seed-germination-repressor protein. RGL2 and its homologs also are called “DELLA” proteins due to a characteristic amino acid sequence that they contain. GA binds to its receptor GA-INSENSITIVE DWARF (GID1), which then interacts with RGL2 (Fig. 6.16). RGL2 is then recognized by SLEEPY1 (SLY1), an E3 ubiquitin ligase that is a component of the SCF (Skp, Cullin, and F-box) complex, which attaches ubiquitin peptides to RGL2. The ubiquitinated RGL2 is recognized by the 26S proteasome complex and is degraded or inactivated. This removal of repression (i.e., de-repression) is central to dormancy release and germination induction. Similar posttranslational modifications play an important role also in the perception and transduction of light signals during dormancy release (Sect. 6.6.5).
GA perception and signaling for seed germination control. RGA-LIKE2 (RGL2) suppresses expression of GA-inducible genes and germination in the absence of GA. GA binds to the GA receptor GA-INSENSITIVE DWARF (GID1), which triggers its interaction with RGL2. This then triggers ubiquitination (Ub) of RGL2 by SLEEPY1 (SLY1), an E3 ubiquitin ligase component of the SCF (Skp, Cullin, and F-box) complex. Ubiquitinated RGL2 is recognized by the 26S proteasome and is degraded or inactivated. In this way, the repression of GA-inducible genes and of seed germination by RGL2 is removed. Based on Seo et al. (2009)
6.6.1.3 Regulation by Ethylene and Brassinosteroids
While ABA and GA are the primary inhibitory and promotive hormones in regulating seed dormancy and germination, other hormones and compounds also play roles in these processes, often through their interactions with the ABA/GA biosynthetic and regulatory pathways. Ethylene (ETH), in particular, often exhibits a promotive effect on germination. Ethylene (C2H4) is a gaseous hormone that is synthesized from S-adenosylmethionine (SAM) in only two enzymatic steps. The enzyme ACC synthase (ACS) converts SAM to the unusual cyclic amino acid 1-aminocyclopropane carboxylate (ACC), which is then converted to ETH in the presence of oxygen by ACC oxidase (ACO) (Fig. 6.13c). Expression of genes encoding ACS and ACO and release of ETH often increase during germination in parallel with expression of GA20ox and GA3ox. Provision of ETH (or ACC) can improve the germination of some seeds, particularly under stressful conditions, while mutations in ACO have been shown to reduce germination capacity.
As for ABA and GA, the signal transduction pathway for ethylene also involves specific receptors that remove downstream inhibitors and thus de-repress germination. In the absence of ETH, the ETH receptor proteins (ETR or ERS) interact with a kinase protein (CONSTITUTIVE TRIPLE RESPONSE 1, CTR1) that represses ETHYLENE INSENSITIVE 2 (EIN2), a positive regulator of downstream signaling processes (Fig. 6.17). When ETH binds to the receptor proteins, this repression of EIN2 by CTR1 is removed, triggering a signaling cascade that results in production of ETHYLENE RESPONSE FACTOR proteins (ERFs) that upregulate transcription of genes associated with germination. A number of genes expressed late during germination, just prior to radicle emergence, including for pathogenesis- and wounding-related proteins such β-1,3-glucanase and chitinase, are regulated by ERFs. Mutations in the ETH signaling pathway were isolated in screens to restore ABA sensitivity in ABA-insensitive mutants, suggesting that ETH acts by reducing the sensitivity of germination to inhibition by ABA.
Ethylene perception and signaling pathway for seed germination control. ETH receptor protein (ETR1, also called an ERS) dimers interact with the CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1) protein that represses the positive regulator EIN2 (left). When ETH binds to the receptor protein (right), conformational changes result in the release and deactivation of CTR1, freeing EIN2 from repression. This results, through a number of intermediate kinases and proteins, in production of ETHYLENE RESPONSE FACTOR proteins (ERFs) that upregulate transcription of genes associated with germination
Brassinosteroids (BRs) are another group of plant hormones that can promote germination (Fig. 2.7). BRs are involved in various aspects of plant growth and development, including flowering time, plant architecture, seed yield, and stress tolerance. BR-insensitive and BR-deficient mutants are more sensitive to inhibition of germination by ABA, and BR treatment rescues germination of seeds having defects in GA biosynthetic enzymes, as can mutations in ABA biosynthetic enzymes. Thus, like ETH, BRs apparently promote germination at least in part by reducing the sensitivity of seeds to inhibition by ABA.
6.6.1.4 ABA–GA Balance and Hormonal Cross Talk in the Regulation of Dormancy
The inhibitory and promotive effects of ABA and GA on seed dormancy and germination have been known for several decades. Central to our current understanding of how they interact is the fact that there is mutual regulation or “cross talk” between these two hormones, i.e., ABA regulates GA metabolism and signal transduction while GA reciprocally affects ABA metabolism and signal transduction.
The expression of GA3oxand GA20ox, GA synthesis genes, is elevated in seeds of the Arabidopsis ABA-deficient mutant aba2-2, suggesting that GA synthesis is normally suppressed by ABA in the wild type. Furthermore, the expression of GA2ox, which results in GA deactivation, is reduced in these mutant seeds, which suggests that GA deactivation is promoted by ABA in wild-type seeds (Fig. 6.18). These results indicate that there is double regulation of GA metabolism by ABA, i.e., suppression of its synthesis and promotion of its deactivation, both of which result in less GA in the seeds and lower completion of germination.
Interactions and cross talk between GA, ABA, ETH, and BR metabolism, perception, signaling, and response pathways. Examples of regulated proteins are shown to indicate the respective pathways for hormone production (GA3ox, NCED, ACO) or deactivation (GA2ox, CYP [CYP707A]). Receptors for GA (GID1), ABA (PYR1), and ETH (ETR) interact with the respective hormones and lead to de-repression of downstream signaling pathways involving the indicated intermediates. These pathways result in either the removal (in the case of DELLAs [e.g., RGL2]) or activation (ABFs [e.g., ABI5] or ERFs [e.g., ERF1]) of transcriptional regulators of germination-related genes (e.g., EXP, expansins; XET, xyloglucan endotransglycosylases; MAN, endo-β-mannanase; β-GLU, β-1,3-glucanase; CHI, chitinase). Blue arrowsindicate promotion of the indicated interaction and red barsindicate repression. Dashed linesindicate where the interaction is known, but the specific mechanisms involved are not
On the other hand, GA can also influence ABA metabolism and signal transduction (Fig. 6.18). RGL2, a key DELLA component of the GA signal transduction pathway and a germination repressor, stimulates ABA biosynthesis and the activity of ABI5, one of the ABFs in the ABA signaling pathway (Fig. 6.14). Since RGL2 is subjected to degradation or inactivation upon GA perception (Fig. 6.16), the hormone causes a decrease in RGL2 activity and hence also in ABA synthesis and signaling, both of which have a positive effect on germination. The multiple layers of regulation involved in the ABA–GA balance in seeds seem to function in such a way that each signal is amplified rapidly (i.e., GA triggers an increase in GA content and response by eliminating ABA production and signal transduction, or ABA increases its synthesis and signal transduction by causing a decline in GA). This rapid amplification of hormonal signals may be important for seeds to respond to environmental cues promptly and thus to commence or suspend germination. Altering the ABA–GA balance in seeds is also an important part of light signal transduction for germination control (Sect. 6.6.5).
Ethylene and brassinosteroids also influence the ABA signaling pathway. Both hormones act by as yet unclear mechanisms to reduce the ability of ABA to inhibit germination, apparently by acting downstream of ABA synthesis in its signal transduction pathway (Fig. 6.18). In turn, ABA negatively regulates the transcription of ACOgenes, reducing ethylene synthesis. Although not confirmed specifically in seeds, BR can also elevate ETH biosynthesis, suggesting that BR may also act indirectly through enhancing ETH action.
The picture that emerges is one of extensive cross talk and interaction among the primary hormones regulating germination. Rather than thinking of these as independent pathways, they more likely constitute a network of reciprocally interacting regulatory factors that are constantly adjusting the relative strengths of the different inputs to the downstream transcriptional regulators. These master integrators (e.g., DELLAs, ABFs, ERFs) can then shift transcriptional patterns between dormancy or germination modes in response to internal or external cues (Sects. 4.5.1, 7.3.2).
6.6.2 After-Ripening
Seeds of many species require variable periods of dry after-ripening or moist chilling (“cold stratification”) to relieve their dormancy, varying from as little as a few weeks (e.g., barley) to as long as 60 months (e.g., Rumex crispus). Under natural conditions dry after-ripening may occur in winter annuals in which dormancy is broken by high summer temperatures in order to make the seeds germinable in the fall, whereas moist chilling is effective in many summer annuals to break dormancy during the cold winter months. However, this distinction is not absolute. For example, dormancy in an Arabidopsis accession from the Cape Verde Islands (Cvi), a winter annual, can also be broken by a short period of cold stratification. The rate of after-ripening and decrease of dormancy can vary, depending on environmental conditions during seed maturation, seed storage and germination conditions.
After-ripening occurs in dry dormant seeds. Seeds are considered “dry” when they have less than ∼20% water content (dry weight basis; <∼−20 MPa) (Sect. 8.4.1). In this range (hydration level II), seeds contain both strongly (<∼4%) and weakly bound (4–20%) water but there is no free water available for enzymatic catalysis and other biochemical events (Figs. 2.29, 8.9). The efficacy of after-ripening depends on the environmental conditions—moisture, temperature, and oxygen. Since it generally occurs in seeds below a certain water content, it may be prevented at higher seed water contents. Indeed, at intermediate moisture contents (>20–40%, or >−20 MPa to −8 MPa) not only might after-ripening fail but the seed may also lose viability (Sect. 8.2.2), and at higher water contents dormancy is maintained or secondary dormancy may be induced (Sect. 7.3). On the other hand, if seeds become too dry (e.g., <4% water content or <∼−300 MPa), after-ripening is delayed or prevented (Sect. 7.2.1.3).
Besides moisture, the rate of after-ripening depends on the temperature. Hydrotime- and hydrothermal-time models can be used to study its progress and predict its duration (Sect. 7.2.1.2). This is particularly useful to monitor and accelerate the loss of dormancy in agriculturally important species, such as barley for malting. Also, these models provide information about how physiological and environmental factors may interact to control the termination of dormancy. After-ripening is delayed when oxygen tensions are low and accelerated when they are high; however, this has been determined experimentally using concentrations of oxygen from near zero to 100%, a range that dry seeds in nature will never encounter.
Our understanding of what occurs in dry seeds during after-ripening is very limited (Sect. 8.5). The obvious constraint that restricts the study of dry organisms is that any biochemical or molecular technique that requires the use of aqueous solutions can initiate a rapid response of preformed enzymes, leading to an alteration in their activity and/or contents of substrates and products. Also it is not always clear how dry a seed is, and this may be variable between experiments. Therefore, the interpretation of results from different laboratories, often using different seeds, seed lots and experimental approaches, should be approached with caution. Nevertheless, there are some convincing indications of changes that occur in dry seeds that may be associated with the release of dormancy.
Seeds of several species display a decrease in ABA content during after-ripening. A small decrease of approximately 10% occurs during 6 months of after-ripening of deeply dormant Arabidopsis Cvi seeds. Nevertheless, dormancy is completely broken and this suggests that the sensitivity of the seed to ABA also plays a role. Seeds of Nicotiana plumbaginifoliaafter-ripen in approximately 10 months (Fig. 6.19a), during which their ABA content may drop to ∼40% of the initial amount, with a concomitant reduction in the lag time of germination and a tenfold decrease in sensitivity to ABA upon subsequent imbibition (Fig. 6.19b). As ABA is a potent inhibitor of seed germination, these changes in its content and sensitivity are related to the increased germination potential of after-ripened seeds. When after-ripening results in an increase in germination of Sisymbrium officinaleseeds on water, they also exhibit a greater sensitivity to GA (Fig. 6.20), allowing for a more rapid completion of germination, perhaps due to an increase in germination potential following this treatment.
(a) Effect of the duration of dry storage on the time course of germination of seeds of Nicotiana plumbaginifolia. Mature seeds were after-ripened for 14 days, 2, 4, 6, 10, and 16 months, reducing the lag time of germination with time of treatment. (b) Effect of ABA on the germination of 14-day (WT-D) and 12-month AR (WT-ND) seeds of N. plumbaginifolia,showing that long-time after-ripened seeds are less sensitive to the inhibitor. From Grappin et al. (2000)
The sensitivity to exogenous GA4+7increases with after-ripening of Sisymbrium officinaleseeds. Germination was tested in water (■, □) and 100 μM of GA4+7(●, ○) of non-after-ripened (closed symbols) or 6-month-after-ripened (open symbols) seeds. From data in Iglesias-Fernández and Matilla (2009)
The molecular mechanisms that decrease the dormancy status during after-ripening are not well understood but they apparently involve various chemical changes in the dry seed. The presence of stored (residual) mRNAs in mature dry seeds is seemingly universal in flowering plant species. Their function is debatable (Sect. 4.5.1) but they do participate in protein synthesis early during germination. As after-ripening proceeds in seeds of several species, including tobacco, barley, and Arabidopsis, there are changes in the transcript pool concomitant with their release from dormancy, as well as in the spectrum of proteins identified on 2D gels (e.g., Fig. 4.17). Some transcriptomic studies have identified a few mRNAs that appear to increase during after-ripening, but in general, a decrease in abundance seems to be the most frequent occurrence. In Arabidopsis, the mRNAs of 30 dormancy-associated genes, including DOG1(Sect. 6.4.2), decrease in seeds during after-ripening. In dry seeds of Arabidopsis,also several proteins appear to accumulate during after-ripening, but the mobility of existing proteins on 2D gels could have been altered by chemical modifications (e.g., glycosylation or oxidation). While the majority of transcripts and proteins do not change in amount during after-ripening, those that do may be related to loss of dormancy. There are plausible alternative reasons for the variations in the mRNA and protein pools during after-ripening, other than due to changes in transcription and translation; these are elucidated in Sect. 8.5.
6.6.3 Low Temperatures (Chilling)
A high proportion of species—probably the majority of nontropical ones—can be released from dormancy when, in the hydrated condition, they experience relatively low temperatures, generally in the range 1–10°C, but in some cases as high as 15°C. The importance of this kind of control in nature is obvious: dormancy of the hydrated seed is slowly broken over the winter (in temperate regions). This is presumably a means of preventing germination during frequently occurring short spells of elevated temperatures until after the winter has passed (Sect. 7.3). Chilling of seeds to break dormancy is a long-standing practice in horticulture and forestry and is generally referred to as “stratification,” because the seeds are sometimes arranged in layers (i.e., stratified) in moist substrata.
Chilling is effective in seeds with embryo, coat-imposed, primary and secondary dormancy. In apple seeds, for example, it is possible to show how the different components of dormancy are differentially broken by chilling. Chilling for 50 days suffices to remove embryo dormancy; the presence of the endosperm increases the required time to about 60 days, whereas the whole seed needs even longer (Fig. 6.21). Evidently, this is a reflection of the gradual increase in germination potential of the embryo which becomes great enough to overcome, first, the resistance of the endosperm alone and eventually of both enclosing tissues. In general, woody species of the temperate regions require fairly extensive treatment times, sometimes as much as 180 days (Crataegus mollis), but usually 60–90 days are satisfactory. On the other hand, dormancy in some herbaceous species may be broken by just a few days of low temperature (e.g., 7 and 14 days, respectively, in Poa annuaand Delphinium ambiguum) and by just 12 h in wheat!
Termination of apple seed dormancy by chilling. Imbibed seeds were kept at 5°C and periodically removed for testing. The percentage germination of the following was determined for (a) isolated embryos, (b) seeds with testa removed but endosperm intact, and (c) intact, whole seeds. Note that embryo dormancy is terminated completely by 40–50 days of chilling, endosperm-imposed dormancy by 60 days of chilling, and whole-seed dormancy partially by more than 80 days of chilling. After Visser (1956)
Recorded optimum temperatures for chilling are generally in the region of 5°C, but this figure may be misleading, as exemplified by Rumex obtusifolius(Fig. 6.22). The seeds are released from dormancy almost as effectively by 1.5, 10, and 15°C within an initial 2-week treatment period. When the treatment time is prolonged, secondary dormancy sets in, predominantly at 10 and 15°C. Thus, in this case 1.5°C is the most suitable temperature for dormancy relief (Fig. 6.22).
Temperature–time relationships for the termination of dormancy of Rumex obtusifoliusseeds. Imbibed seeds were held in the light at three temperatures for up to 12 weeks. They were then transferred to 25°C for 4 weeks, after which the percentage of germinated seeds was determined. After Totterdell and Roberts (1979)
Early studies showed that chilling increases the seed’s sensitivity to environmental factors, such as light and nitrate, and also to applied GAs. Cold treatment of crab apple, hazel, and Arabidopsis seeds results in a higher abundance of bioactive GAs than in untreated samples. Cold appears to induce genes that encode enzymes of the GA-biosynthetic pathway. GA3ox1,encoding the enzyme that converts inactive GA9to active GA4(Fig. 6.13b), is specifically upregulated by cold in Arabidopsis seeds. Interestingly, this gene is also upregulated by red light (Sect. 6.6.5.4).
There are also reports of a decrease in ABA content as a result of combined alternating temperatures and chilling as well as due to cold stratification, as in Taxus maireiseeds (Table 6.2), but more often ABA content does not significantly decrease during the chilling treatment but only afterwards, when the nondormant seeds display a rapid decline in ABA content, which does not occur in the non-chilled seeds. The decrease in ABA content is not caused only by its simple leakage from the seed but also by ABA catabolism. Furthermore, although dormancy release of yellow cedar seeds by a combined warm and cold treatment results in a decrease of ABA content of the embryo by ∼50%, this alone is not sufficient for dormancy release; a concurrent decrease in sensitivity to ABA is also required. In beech tree seeds, expression of an ABA-inducible protein phosphatase type-2C (PP2C) gene increases during the first weeks of cold stratification. Overexpression of the beechnut PP2Cgene in Arabidopsis results in less dormant seeds with a markedly reduced ABA sensitivity. This supports the role of PP2C protein as a negative regulator of ABA signaling (Fig. 6.14) and suggests that phosphorylation/dephosphorylation is involved in seed dormancy release during chilling.
6.6.4 Other Effects of Temperature on Dormancy
In the field, dormant seeds are commonly subjected to fluctuating temperatures, for example, low night temperatures and high daytime temperatures. Such temperature fluctuations, or temperature alternations, are frequently effective in dormancy breakage, in cases such as Bidens tripartitus,tobacco, and Rumexspp., which all have coat-imposed dormancy. While alternating temperatures break dormancy of Rumex obtusifoliusseeds, the seeds remain dormant at constant temperatures, as is shown by the valley running across the diagonal of Fig. 6.23. Dormancy is broken when different temperatures are combined, with the maximum effect at the greatest temperature differentials (i.e., amplitudes). So to be effective, the temperature alternation must have a certain minimum amplitude, and in some species this need be only a few degrees. In addition, the temperatures of the pair must be above and below certain values, the duration of exposure to each is important, and the number of cycles of fluctuating temperatures can be decisive. This illustrates some of the exacting environmental requirements that seeds can sense in relation to the breakage or maintenance of dormancy.
Breaking dormancy in Rumex obtusifoliusby alternating temperatures. Imbibed seeds were held in darkness for 28 days at different temperature combinations: 16 h at one temperature followed by 8 h at another. Germinated seeds were counted after 28 days. Note that high germination percentages (i.e., termination of dormancy) occur in the following temperature and time combinations: (a) 16 h at 25–35°C, 8 h at 1.5–15°C; (b) 8 h at 25–35°C, 16 h at 1.5–20°C. After Totterdell and Roberts (1980)
In a few species, relatively high temperatures can break, or assist in breaking, dormancy. Seeds of Hyacinthoides non-scriptarequire several weeks at 26–31°C followed by a germination phase at 11°C. Several species that are chilling sensitive need a period at relatively high temperature before the cold. Fraxinusspp., for example, require a few weeks at about 20°C prior to chilling at 1–7°C. Softening of the seed coat of woody species might occur at the higher temperature. In contrast, seeds of Annona crassifloracannot be germinated in the lab under any favorable temperature regime. In the soil seed bank they require several months of below-average temperatures (<15°C) to break physiological dormancy and allow the embryo to grow to maturity when the field temperature rises and the rainy season commences. Numerous examples of the various temperature regimes that break dormancy can be found in the literature, a reflection of how species and their seeds are adapted to a range of climatic conditions.
6.6.5 Light
Light is an extremely important factor for releasing seeds from dormancy. Almost all light-requiring seeds have coat-imposed dormancy. Seeds of many species are affected by exposure to white light for just a few minutes or seconds (e.g., lettuce) or even milliseconds, whereas others require intermittent illumination for sometimes prolonged periods of time (e.g., Kalanchoë blossfeldiana). Photoperiodic effects also exist, so that some species require exposure to long days and others to short days (Table 6.3). The light requirement frequently depends on the temperature. Grand Rapids lettuce seeds, for example, generally are dormant in darkness only above about 23°C, so below this value they germinate without illumination. Seeds of some species of Betula, on the other hand, are dormant in darkness at lower temperatures (e.g., 15°C) but not at 25–30°C. Sensitivity to light in many species is enhanced by chilling and various temperature alternations and temperature shifts also interact with light.
6.6.5.1 Phytochrome: Action Spectra
In nature, white light (i.e., sunlight) breaks dormancy, although wavelengths in the orange/red region of the spectrum are the most effective. In 1954, a detailed action spectrum for the breaking of dormancy in the Grand Rapids cultivar of lettuce, obtained by Borthwick, Hendricks, and their colleagues, revealed that the major promotive activity is at 660 nm (Fig. 6.24a). Prior to this, inhibitory parts of the spectrum were also known, with an especially potent waveband in the far-red, i.e., wavelengths longer than about 700 nm; the action spectrum shows that 730 nm is the wavelength of maximum activity (Fig. 6.24b). At about the same time as the action spectrum was discovered, Borthwick, Hendricks, Parker, E. H. Toole and V.K. Toole showed that red (R) and far-red (FR) light are mutually antagonistic. This was done by exposing lettuce seeds to a sequence of red and far-red irradiations: only when the last exposure in the sequence was to red light was dormancy terminated (Table 6.4). This established the fact of photoreversibility; i.e., the two wavelengths 660 nm and 730 nm are able to reverse each other’s effect. To act, light must be absorbed by molecules of a pigment, and the one participating in the breaking of dormancy, as well as in other photoresponses, came to be called phytochrome. This pigment, therefore, exists in two forms (Fig. 6.24c). One is present in unirradiated, dormant seeds; its absorption spectrum shows that it absorbs red light (peak at 660 nm) and is therefore designated as Pr (Fig. 6.24d). This form of phytochrome obviously cannot break dormancy (if it could, the seeds would not be dormant!), but when activated by 660-nm light, it is changed into an active, dormancy-breaking form. But this active form absorbs FR light (730 nm) (Fig. 6.24d); hence it is designated as Pfr, and is then reverted to Pr at this wavelength.
(a, b) Action spectra showing the wavelengths of light that affect germination and dormancy of lettuce seeds, cv. Grand Rapids. In (a) seeds were imbibed in darkness for 16 h before irradiation with the shown wavelengths of light and then returned to darkness and the percentage of seeds germinated later counted. The energy of each wavelength required to achieve 50% germinated seeds (i.e., break dormancy of 50% of the seeds) was determined. In (b) the energies of different wavelengths required to reverse the effects of red light and to maintain dormancy are shown. (c) Pictorial model of a phytochrome dimer molecule and the interconversion of the inactive Pr and the active Pfr forms by red and far-red light. The lower part of the molecule has kinase activity. (d) Absorption spectra for the two forms of phytochrome (Pr and Pfr). The shaded area shows the wavelengths that both forms of the pigment absorb. a, b, and dbased on Borthwick et al. (1954)
Ignoring for the moment the dark-reversion path, it is easy to see how the reversibility of dormancy breakage works: if Pfr is left in the seed at the end of the radiation sequence, dormancy is terminated, but if Pr is left, dormancy is retained. Note that photoreversion only stops the breaking of dormancy if Pfr has been given insufficient time in which to act. If dosage with far-red light is delayed for a few hours, Pfr can then operate, and even if photoreversion occurs later, dormancy has been terminated and a point-of-no-return (i.e., the “escape time”) has been passed.
The energies needed to carry out these photoconversions are relatively small. A saturating dose of R light in lettuce seeds is about 10 J m−2, an amount given by about 0.2 s of direct, summer sunlight. Up to saturation value, the effect is directly proportional to the total amount of energy, irrespective of how the energy is delivered, i.e., by a lower fluence rate (irradiance) for a longer time, or by a higher fluence rate (irradiance) for a shorter time. As long as the products, fluence rate x time, are equal, the same effect (e.g., percentage of seeds breaking dormancy) is achieved. This is to say that the phytochrome system shows reciprocity. It is intriguing to note that this reciprocity is analogous to the concept of thermal time, where temperature and time requirements are inversely related (Sect. 7.2.2.2), just as photon fluence and time of exposure are for phytochrome action. In a more complex response type, e.g., where repeated doses of R light are required, reciprocity is shown for each exposure, and not for the total amount of light involved. The dosage of FR light needed for photoreversion is higher; in lettuce, for example, approximately 600 J m−2of light at 730 nm causes about 50% reversion of the effect of saturating R light. In the laboratory, higher doses of FR are usually secured by higher fluence rates and/or longer irradiation times.
The scheme for pigment photoconversion (Fig. 6.24c) also contains a dark-reversion component. This was discovered when lettuce seeds that were transferred to a relatively high temperature in the dark for a few hours immediately after exposure to R light failed to germinate; they then required a second dose of R light. Thus, the high temperature caused a slow loss of active phytochrome by a reversion of Pfr to Pr, so-called dark reversion. Loss of Pfr might also occur by its destruction; Pfr is labile and has a half-life of about 1.25 h.
Discussion so far has centered on the photoconversions of phytochrome by R and FR light. In nature, seeds are exposed to mixtures of wavelengths such as those which exist in sunlight. Conversions of phytochrome can occur at any wavelength or mixtures thereof, in both directions, to generate a certain proportion of Pfr, as there is some absorption by both forms across the visible spectrum (Fig. 6.24d). It is the proportion of Pfr established under a given condition that determines dormancy breakage, as explained in the next section.
6.6.5.2 Phytochrome: Photoequilibria
The properties of phytochrome so far discussed were all derived simply from the experimental work on the physiology of light action in seed dormancy. Complete confirmation of these points came when phytochrome was isolated from plants, not from seeds but from dark-grown (i.e., non-green) tissues such as oat or rye coleoptiles. Phytochrome is a blue chromoprotein, the chromophore being an open-chain tetrapyrrole not unlike the phycocyanins of the blue–green algae. In solution, it shows photoreversibility with R and FR light, and the absorption spectra of the two forms can be determined (Fig. 6.24d). Peak absorption of Pr is at 660 nm and of Pfr is at 730 nm, just as predicted from the experiments with lettuce seeds! An important point to note is the overlap in absorbances of Pr and Pfr. Because both forms absorb over the spectrum from about 300 nm to about 730 nm (Fig. 6.24d), irradiation with monochromatic light in this range sets up an equilibrium mixture of Pr and Pfr, the photoequilibrium or photostationary state of Pfr/Ptotal or φ(phi). At 660 nm, for example, Pr is photoconverted to Pfr, but Pfr also absorbs at this wavelength and hence some Pfr molecules are phototransformed back to Pr. A mixture of approximately 80% Pfr and 20% Pr is thus established (Pfr/Ptot, φ = ∼0.8). Even at 730 nm, where Pfr absorbs most strongly, there is also some absorption by Pr; here the φvalue is 0.02, i.e., 2% Pfr. Inspection of the absorption spectrum shows that no waveband region can produce 100% Pfr; on the other hand, almost 100% Pr can be achieved by irradiation at 740–800 nm. Consequently, irradiation with mixed wavelengths also establishes a certain φvalue. In midday sunlight, φis about 0.55, in white incandescent light it is about 0.45, and in white fluorescent light about 0.65. The effectiveness of phytochrome in terminating dormancy is determined by the φvalue generated in the seeds, the required value depending on the species (Table 6.5). Lettuce is barely satisfied by the φvalue brought about by sunlight, and Wittrockia superbacan be stimulated to germinate even by broad-band far-red light (φ > 0.02).
The variation in φvalues required for the breaking of dormancy makes clear that different species have different Pfr thresholds. This has significant ecological implications in the fact that light-requiring seeds can use phytochrome to detect different light qualities when the proportions of R and FR light change. This happens to some extent according to the time of day, but more importantly it occurs when light is transmitted through green leaves, whose chlorophyll absorbs R light but allows FR light to pass. Thus, a seed under a leaf canopy is in light rich in FR, which sets up a φvalue too low to satisfy most (but not all) light-requiring seeds. In a sense, phytochrome is the device used by the seed to detect where it is, especially in relation to burial in the soil or to neighboring plants. Phytochrome is also involved in the photoinhibition of germination, when light of rather high fluence rates given for relatively long periods of time prevents even nondormant seeds from germinating. This is returned to with respect to the ecophysiology of dormancy in Sect. 7.2.3.
6.6.5.3 Phytochrome: Multiple Forms
The formation of Pfr alone does not break dormancy. Rather, Pfr engages signaling pathways that ultimately lead to the termination of dormancy and the completion of germination by the seed. These signaling pathways appear to be rather complex (Sect. 6.6.1) and show extensive cross interactions with other environmental factors. First of all there are different kinds of phytochromes. Two that have received the most attention are phytochrome A and phytochrome B, which are encoded by different genes, PHYAand PHYBrespectively. PHYA protein accumulates in dark-grown seedlings while PHYB, a light-stable form, is found in non-etiolated, green plants; it is now clear that the two types have different roles in photomorphogenesis. In addition to phytochromes A and B, three more phytochrome genes, PHYC, PHYD, and PHYE,are present in Arabidopsis which encode phytochromes C through E. A powerful experimental approach to classifying the roles of the discrete phytochromes has been the generation of mutations in the apoproteins of each of the phytochromes and by subsequently crossing individuals with single mutations to create plants containing multiple mutations with progressively fewer functional phytochromes. The results of these analyses show that there are substantial redundancies among the functions for each type of phytochrome. For example, mutants lacking PHYE only, both PHYA and PHYB, or PHYA, PHYB and PHYD still respond in a very similar way to white, R and FR light as the wild type Ler(accession Landsberg erecta), but those that lack PHYA, PHYB, and PHYE do not respond to light anymore (Fig. 6.25). Thus, there is redundancy of function, but absence of PHYA, B and E together leaves the seeds blind to light. In addition, the different phytochromes respond differently to temperature, implying that the breaking or maintenance of dormancy at a given temperature may depend on one or more specific phytochromes. More studies are required to establish the exact roles of the phytochromes in the control of dormancy and regulation in different environments. As mentioned above, the existence of several types of phytochromes and their overlapping functions considerably increases the complexity of phytochrome signaling in concert with the other factors that affect dormancy, e.g., temperature.
Induction of germination by light pulses of wild-type (Lerecotype: Landsberg erecta) and single (phyE), double (phyA phyB) and triple phytochrome loss-of-function mutant (phyAand Bwith phyDor E) seeds of Arabidopsis thaliana. Imbibed seeds were incubated for 24 h at 4°C in the dark, followed by continuous white light (W) or hourly light pulses of the indicated light quality at 25°C for 3 days, or continuously in the dark (D). R, red; FR, far-red; R/FR, R pulse followed by FR pulse. From Hennig et al. (2002). Copyright American Society of Plant Biologists
6.6.5.4 Phytochrome: Downstream Signaling
From physiological experiments it has long been known that the action of light on seeds, through phytochrome, may lead to the synthesis of GAs that then engage a signaling pathway to effect the completion of germination. Now, gene expression analysis has shown that phytochromes (Pfr) increase the amount of bioactive GAs in seeds by activating the transcription of GA-3-oxidasegenes (GA3ox1and GA3ox2), which are also positively regulated by cold (Sect. 6.6.3), and repressing the transcription of a GA-2-oxidase gene (GA2ox2) that is involved in the degradation of active GAs (Fig. 6.13b).
The regulation of ABA metabolism genes by light is mediated through phytochrome-interacting factors (PIFs). A protein called PHYTOCHROME-INTERACTING FACTOR3-LIKE5 (PIL5), a light-labile basic helix-loop-helix transcription factor, inhibits seed germination by repressing the above-mentioned GA3ox1and GA3ox2genes and upregulating GA2ox2(Fig. 6.26). Furthermore, PIL5 causes an increase in ABA by activating genes whose expression are required for its synthesis, and repressing a gene required for ABA catabolism. When PIL5is knocked out in Arabidopsis, pil5mutant seeds become resistant to FR inhibition while seeds overexpressing PIL5are incapable of responding to R and do not germinate in response to the light stimulus. Therefore, PIL5 is probably a critical component of phytochrome responses and a negative regulator of seed germination.
Proposed molecular events leading to light-, nitrate-, nitric oxide-, or karrikin-induced seed germination in Arabidopsis thaliana. In the dark or presence of Pr, PIL5 activates the expression of DELLA genes (RGL2, GAI, RGA) by binding directly to their promoters through G-box elements. PIL5 also represses genes of the GA biosynthetic and ABA deactivation pathways as well, promoting expression of those responsible for GA deactivation and ABA biosynthesis. This results in decreased GA and increased ABA. The decrease in GA stabilizes DELLA proteins, leading to their increase and the suppression of GA responses and of subsequent germination. These events are highlighted in purple. Upon light irradiation, activated phytochromes (Pfr) induce PIL5 degradation, leading to decreased DELLA proteins, less ABA biosynthesis and increased GA biosynthesis. DELLA proteins also decrease due to the increased bioactive GA. Nitrate and nitric oxide also reduce ABA content through enhancement of CYP707A2expression, while also promoting GA3oxexpression. Karrikins, components of smoke, promote expression of genes encoding GA biosynthetic enzymes. As a result of the increased GA synthesis and action, and the decrease in ABA synthesis, the seeds are able to complete germination. These events are highlighted in green. The reciprocal regulatory circuit between GA and ABA biosynthesis and degradation is shown by the lighter purpleand green lines. Modified from Oh et al. (2007) and Martin et al. (2010)
Finally, PIL5 also increases the expression of DELLAgenes (e.g., GA-INSENSITIVE[GAI] and REPRESSOR OF GA1-3[RGA]) that results in the repression of GA action. Light perceived by phytochromes represses this inhibition due to a reduction in the amount of PIL5 protein, which in turn results in a decrease in the transcription of DELLAdirectly, in addition to the indirect effects of reduced PIL5 on ABA and GA metabolism, resulting in the breakage of dormancy (Fig. 6.26). Thus, PIL5 modulates both ABA and GA metabolism through the stabilization of DELLA (e.g., RGL2, Fig. 6.16) and plays a critical role at the intersection of light and hormone signaling.
6.6.6 Dormancy Release of Seeds with Impermeable Coats
Many seeds with impermeable coats contain specialized structures that regulate the uptake of water (Sects. 1.2.3, 6.3.2.1). They are generally derived from tissues that close the natural openings in the seed or fruit coat, such as the micropyle, hilum, and chalazal area. It appears that the breaking of dormancy of seeds with impermeable coats proceeds mostly through these specialized structures. Under natural conditions physical dormancy is often released by exposure to extremes in temperature and temperature fluctuations. Detailed studies of species such as Stylosanthes humilis, S. hamata, and Heliocarpus donnell-smithiisuggest that (extreme) temperature fluctuations rather than absolute temperature are decisive for the unplugging of seed openings. The required day–night temperature amplitudes may range from 15 to 40°C. It is likely that the perception of large temperature fluctuations represents a gap-detecting mechanism. The best known condition of exposure of seeds to extreme heat is fire, which is a natural component of many ecosystems. The regeneration of many plant species after a fire is essentially due to the breaking of dormancy of seeds in the soil. Evidently, seeds cannot withstand fire temperatures of over 600°C but a steep temperature gradient in the soil top layer results in more moderate temperatures a few centimeters beneath. Many seeds lose physical dormancy when exposed to temperatures between 50 and 100°C for a limited time. A spectacular response is seen in seeds such as Albizzia lophantha,in which the strophiolar plug is audibly ejected from the seed as high temperature is reached, leaving a strophiolar crater through which water can enter. Such effects of high temperature are thought to be important in pyric species whose seedlings emerge as a consequence of forest fires. Burnt vegetation leaves gaps and open spaces which creates greater day–night temperature fluctuations. Apart from unplugging of the seed coat openings, exposure to dry heat also causes cracks, often starting from the opening. It is likely that this type of damage not only increases the permeability of the seed coat but also decreases its mechanical restraint.
However, depending on the moisture content of the seed and the relative humidity of the air, imbibition of water may also occur across the whole seed coat. In many species, seed coat impermeability increases as seed moisture content decreases. Seed coats become fully impermeable in a range of moisture contents from 2 to ∼20%. It has been suggested frequently that microbial action can mediate in the opening of impermeable seed coats. However, there is no conclusive evidence to support this suggestion. Similarly, there are few data available to support the possibility of dormancy relief by passage through an animal’s digestive tract, although it has been documented for the passage of seeds of wild tomatoes through Galapagos tortoises! Although studies have shown that such passage through an animal enhances germination, it is not known how this is accomplished. Within the animal, dormancy may be broken by either mechanical scarification or by acid in the digestive tract. Also, passage through the animal may indirectly break the dormancy, because its waste material is usually dropped on the surface, thereby exposing seeds to higher (fluctuating) temperatures, as well as elevated temperatures as a result of fermentation of the fecal material and perhaps some of its chemical constituents.
6.6.7 Breaking of Dormancy by Chemicals
A selected list of chemicals that can break dormancy is given in Table 6.6. Only a few of these are likely to be encountered by seeds in their natural environment, but they are nevertheless of great interest because they may help provide an understanding of the mechanism of dormancy breakage. The possible action of many of these substances has led to hypotheses concerning the mechanisms and regulation of dormancy breakage. Only a few, if any, of these hypotheses have stood the test of time. A short account of two of them follows:
(1) The membrane hypothesis of dormancy breakage. This hypothesis was built on the observation that anesthetics, such as ethyl ether, chloroform, acetone, ethanol, propanol, and other alcohols, can break dormancy of some seeds, e.g., Panicumspp., Digitariaspp., grasses, and also lettuce. Anesthetics affect cells by entering membranes, thus altering the relationships among membrane components by decreasing their packing density. Consequently, these components, including membrane-associated proteins such as receptors, may become more accessible to substances that bind to them. It was thought that these receptors could bind such dormancy-breaking factors as phytochrome and nitrate. Furthermore, membranes are highly sensitive to changes in temperature, an important factor in the regulation of dormancy, and it is possible that anesthetics mimic these. The germination of red rice grains can only be stimulated by those alcohols that are metabolized by the enzyme alcohol dehydrogenase. Thus, besides exerting possible anesthetic effects, alcohols may affect germination and dormancy through (unknown) metabolic modifications.
(2) The pentose phosphate pathway hypothesis. The hypothesis that the pentose phosphate pathway (PPP) could play a unique role in dormancy breakage arose largely from studies of the effect of certain chemicals on dormancy. Dormancy of seeds of several species, including lettuce, rice, and barley, is broken by application of inhibitors of respiration, including those that inhibit terminal oxidation and the tricaboxylic acid cycle in the mitochondria (e.g., cyanide and malonate) and some that inhibit glycolysis (e.g., fluoride). Electron acceptors such as nitrate, nitrite, and methylene blue can also break dormancy, as can high oxygen concentrations. Explanations for the effects of these substances could include that respiratory inhibitors block the consumption of oxygen by conventional respiration and thus it becomes available for other processes, or alternatively, that oxygen could be made more available by elevating its external concentration. The PPP has been suggested to be the important “other” process and it may require the oxygen for the oxidation of reduced NADP (NADPH + H+), which this pathway generates. Oxidation of NADPH can also be brought about directly by the electron acceptors methylene blue, nitrate, and nitrite, in which case oxygen is not needed. Although the special significance and function of the PPP has never been explained, the hypothesis has been thoroughly investigated. The results, however, are mixed and it has been suggested that the techniques that were used can give misleading results. Thus, evidence for the participation of the PPP in dormancy breakage is, at best, equivocal.
However, some chemicals do play an important natural role in the breaking of dormancy and their involvement has been investigated in several species. These chemicals are nitrate, nitroxides (NO x ) and active smoke components, the butenolides.
6.6.7.1 Breaking of Dormancy by Nitrate
There is little doubt that nitrate is an indicator of soil fertility for seeds in the soil seed bank; this and its other ecological roles are discussed in Sect. 7.2.4. Nitrate has long been known to break the dormancy of seeds of a variety of species, often in combination with light. Some, such as Sisymbrium officinale, show an absolute requirement for both of these factors; only the combination is successful in the breaking of dormancy. The mechanism of action of nitrate has often been associated with the pentose phosphate pathway (previous Section), but there is no conclusive evidence for this. Moreover, in S. officinaleseeds nitrate remains effective even when nitrate reductase activity is completely inhibited. Therefore, the nitrate signal for the breaking of dormancy may be nitrate itself in its unreduced form.
In the deeply dormant Cvi accession of Arabidopsis, nitrate can substitute for the long period (7–12 months) of dry storage or several days of cold stratification required to break dormancy. There is good evidence that nitrate signaling is targeted at the ABA and GA signaling pathways. Nitrate, either from endogenous pools or when applied, decreases ABA content in seeds of Arabidopsis (Fig. 6.27a, b). Moreover, it stimulates the expression of the CYP707A2(Fig. 6.27c) gene that encodes for the cytochrome P450 protein that catalyzes the degradation of ABA (Fig. 6.13a). Interestingly, transcript profiling of imbibed seeds treated with or without nitrate revealed that the latter results in an mRNA profile very similar to that of stratified or after-ripened seeds, which corroborates the observation that nitrate can substitute for these. This role of nitrate in the breakage of dormancy supports the model displayed in Fig. 6.26.
ABA contents of nitrate-treated seeds of Arabidopsis thaliana(Col-0). (a) ABA contents of mature seeds that were produced on plants grown on 10 mM (C10) or 50 mM (C50) nitrate and seeds from the nia1nia2nitrate reductase mutant grown on 10 mM nitrate (nia10). In the nia1nia2mutant much less nitrate is converted to nitrite and, hence, a larger nitrate pool is available to the seed. (b) ABA contents of seeds treated with exogenous nitrate. Freshly harvested seeds from plants grown on 10 mM of nitrate were imbibed for 20 h in water or 10 mM nitrate. (c) Differences in CYP707A2transcript content (relative to a gene expression standard) of C10 seeds imbibed for 6 h in water (W, control) or nitrate. a, b and c indicate significant statistical differences between treatments. After Matakiadis et al. (2009). Copyright American Society of Plant Biologists
6.6.7.2 Breaking of Dormancy by Nitric Oxide
Nitric oxide (NO) is a very reactive gaseous free radical that is a ubiquitous and potent signaling molecule in plants and animals. Among many other plant processes, NO is involved in the regulation of dormancy and germination in many species. It is produced in plant cells from nitrite and arginine. Nitrite is the first product of the reduction of nitrate by nitrate reductase, and is then further reduced, both enzymatically and nonenzymatically, to NO. Since NO is a highly reactive gaseous substance that partitions into hydrophobic and hydrophilic environments, it is challenging to use in experiments; instead, chemicals are used that function as NO-donors in vivo. These are, for example, sodium nitroprusside (SNP) and S-nitroso-N-acetylpenicillamine (SNAP). As controls, NO scavengers, such as (carboxy)2-phenyl-4,4,5,5,-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) are utilized. Application of SNP and SNAP to light-requiring lettuce seeds results in the breaking of dormancy, which is effectively counteracted by PTIO, providing evidence that NO can bypass the light-requirement for germination (Table 6.7).
Arabidopsis mutants lacking the AtNOS1gene and which are thus deficient in endogenous NO have increased dormancy and lower seed germination and seedling establishment rates than wild-type seeds due to enhanced ABA inhibitory action. These effects can be reversed by application of NO to the mutant seeds. There is good evidence that NO enhances the degradation of ABA through activation of the CYP707A2gene and the biosynthesis of GAs through enhancing the activity of the GA3oxgenes (Fig. 6.13a, b) and thus contributes to the breaking of dormancy.
6.6.7.3 Breaking of Dormancy by Smoke
Smoke, as well as charcoal extracts, has long been known to break seed dormancy in many species. Smoke is an effective stimulant that breaks dormancy in some 1,200 species of more than 80 genera worldwide. Fire offers an important opportunity for the breaking of dormancy, germination, and subsequent seedling establishment by altering key environmental factors such as light, space, and nutrients. The flush of new growth immediately after the fire event indicates a massive breaking of dormancy of seeds in the soil seed bank. Adding to what was discussed earlier (Sect. 6.6.6), heat is not the only factor required for this type of response since the application of cold smoke is also effective. In other words, this effect is not physical, as in the cracking of hard seed coats, but is of a chemical nature.
One of the active chemicals in smoke is karrikinolide, previously referred to as “butenolide,” (3-methyl-2H-furo[2,3-c]pyran-2-one), a member of the class of karrikins. There are several active karrikins, denoted KAR1 through KAR4. These have been compared with strigolactones that bear some structural resemblance. Strigolactones, including the synthetic compound GR-24, are effective in breaking dormancy of parasitic weed seeds (Sect. 7.2.6). Most of the karrikins are similarly effective in breaking dormancy of Arabidopsis seeds and even can be more effective than GAs (Fig. 6.28)! It must be noted, however, that the effectiveness of karrikin action depends on the seed ecotype. As to their mechanism of action, they induce an increase of expression of the two principal genes for GA-3-oxidase, GA3ox1and GA3ox2(Fig. 6.13b), but do not affect any of the key genes related to ABA synthesis or catabolism (Fig. 6.26). Interestingly, cyanohydrins have been identified also as a group of active chemicals in smoke, stimulating germination. Cyanide, which is slowly released from cyanohydrin, has long been known to break dormancy and stimulate germination by virtue of its capacity to inhibit respiration (Sect. 6.6.7 and Table 6.6).
Karrikins (KAR) break Arabidopsis thalianaseed dormancy. Germination of dormant Lerseeds after 7 days from the start of imbibition on water, 1 nM to 10 μM KAR1, KAR2, KAR3, or KAR4, GR-24 (a synthetic strigolactone), or GA4. After Nelson et al. (2009). Copyright American Society of Plant Biologists
As this and the previous two sections suggest, many dormancy responses induced by environmental factors ultimately affect ABA and/or GA signaling, often via the control of the same genes and proteins involved in ABA and GA synthesis and catabolism (Fig. 6.26).
Useful Literature References
Section 6.1
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Academic, New York (Compendium of dormancy types and ecological relationships)
Section 6.2
Baskin CC, Baskin JM (2004) Seed Sci Res 14:1–16 (Dormancy classification)
Hilhorst HWM (2007) In: Bradford KJ, Nonogaki H (eds) Seed development, dormancy and germination. Blackwell Publishing, Oxford, pp 50–71 (Definitions and hypotheses of dormancy)
Werker E (1997) Seed anatomy. Schweizerbart Scientific Productions, Stuttgart (Superior coverage of seed anatomy)
Section 6.3
Ali-Rachedi S, Bouinot D, Wagner MH, Bonnet M, Sotta B, Grappin P, Jullien M (2004) Planta 219:479–488 (ABA contents in relation with dormancy)
Cafasso D, Widmer A, Cozzolino S (2005) J Hered 96:66–70 (Genetics of dormancy)
Debeaujon I, Koornneef M (2000) Plant Physiol 122:415–424 (Seed coat pigmentation of Arabidopsis and dormancy)
Frey A, Audran C, Marin E, Sotta B, Marion-Poll A (1999) Plant Mol Biol 39:1267–1274 (Genetic modification of ABA content)
Hamly DH (1932) Bot Gaz 93:345–375 (Seed coat structure)
Homrichhausen TM, Hewitt JR, Nonogaki H (2003) Seed Sci Res 13:219–227 (Carrot seeds shed with immature embryos)
Le Page-Degivry M-T, Garello G (1992) Plant Physiol 98:1386–1390 (ABA and embryo dormancy)
Lenoir C, Corbineau F, Côme D (1986) Physiol Plant 68:301–307 (Oxygen uptake by enclosing tissues of barley grains)
McKee GW, Pfeiffer RA, Mohsenin NN (1977) Agron J 69:53–58 (Seed coat impermeability to water)
Thevenot C, Côme D (1973) CR Acad Sci Ser D 277:1873–1876 (Cotyledon and embryo dormancy)
Section 6.4
Argyris J, Dahal P, Hayashi E, Still DW, Bradford KJ (2008) Plant Physiol 148:926–947 (Thermodormancy and ABA)
Bentsink L, Jowett J, Hanhart CJ, Koornneef M (2006) Proc Natl Acad Sci USA 103:17042–17047 (Cloning of the DOG1gene)
Derkx MPM, Smidt WJ, Van der Plas LHW, Karssen CM (1993) Physiol Plant 9:707–718 (Respiration and changes in Sisymbriumdormancy)
Finkelstein R, Reeves W, Ariizumi T, Steber C (2008) Ann Rev Plant Biol 59:387–415 (Review of molecular aspects of dormancy)
Footitt S, Cohn MA (1995) Plant Physiol 107:1365–1370 (Fructose 2,6-bisphosphatase and red rice dormancy)
Gubler F, Millar AA, Jacobsen JV (2005) Curr Opin Plant Biol 8:183–187 (Review of vivipary and pre-harvest sprouting)
Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M (1983) Planta 157:158–165 (ABA-mutants and Arabidopsis dormancy)
Okamoto M, Kuwahara A, et al (2006) Plant Physiol 141:97–107 (ABA in Arabidopsis seed)
Spoelstra P, Joosen RVL, Hilhorst HWM (2002) Seed Sci Res 12:231–238 (ATP localization in dormant and non-dormant tomato seeds)
Section 6.5
Hayes RG, Klein WH (1974) Plant Cell Physiol 15:643–663 (Spectral quality of light and onset of dormancy)
Hilhorst HWM, Karssen CM (2000) In: Fenner M (ed) Seeds. The ecology of regeneration in plant communities, 2nd ed. CAB International, Wallingford, pp 293–310 (Influence of chemical environment on germination)
Karssen CM (1970) Acta Bot Neerl 19:81–94 (Photoperiodic induction of dormancy in Chenopodium)
Kendall SL, Hellwege A, Marriot P, Whalley C, Graham IA, Penfield S (2011) Plant Cell 23:2568–2580 (Effect of temperature during seed maturation on dormancy)
Section 6.6
Bazin J, Langlade N, Vincourt P, Arribat S, Balzergue S, El-Maarouf-Bouteau H, Bailly C (2011) Plant Cell 23:2196–2208 (Targeted oxidation of mRNA during after-ripening)
Beligni MV, Lamattina L (2000) Planta 210:215–221 (Effect of NO on lettuce germination)
Bethke PC, Libourel IGL, Aoyama N, ChungY-Y, Still DW, Jones RL (2007) Plant Physiol 143:1173–1188 (Effect of NO on Arabidopsis dormancy)
Borthwick HA, Hendricks SB, Toole EH, Toole VK (1954) Bot Gaz 115:205–225 (Action spectrum for breaking of dormancy in lettuce)
Bradford KJ (2005) New Phytol 165:338–341 (Threshold models applied to phytochrome action)
Chien C-T, Kuo-Hang L-L, Lin T-P (1998) Ann Bot 81:41–47 (Content and sensitivity to ABA during cold stratification)
Da Silva EAA, de Melo DLB, Davide AC, de Bode N, Abreu GB, Faria JMR, Hilhorst HWM (2007) Ann Bot 99:823–830 (Breaking of morpho-physiological dormancy)
Finch-Savage WE, Cadman CSC, Toorop PE, Lynn JR, Hilhorst HWM (2007) Plant J 51:60–78 (Transcriptomics of dormancy breaking in Arabidopsis)
Flematti GR, Merritt DJ, Piggott MJ, Trengrove RD, Smith SM, Dixon KW, Ghisalberti EL (2011) Nat Commun 2:Article 360 (Cyanohydrins in smoke)
Grappin P, Bouinot D, Sotta B, Miginiac E, Julien M (2000) Planta 210:279–285 (After-ripening of tobacco seeds)
Hennig L, Stoddart WM, Dieterle M, Whitelam GC, Schäfer E (2002) Plant Physiol 128:194–200 (Different forms of phytochrome)
Iglesias-Fernández R, Matilla A (2009) J Exp Bot 60:1645–1661 (After-ripening and sensitivity to GAs)
Martin RC, Pluskota WE, Nonogaki H (2010) In: Pua EC, Davey MR (eds) Plant developmental biology: biotechnological perspectives. Springer, Heidelberg, 383–404 (Interaction of ABA and GA metabolism)
Matakiadis T, Alboresi A, Jikumaru J, Tatematsu K, Pichon O, Renou J-P, Kamiya Y, Nambara E, Truong H-N (2009) Plant Physiol 149:949–960 (Nitrate signaling in dormancy relief)
Matilla AJ, Matilla-Vázquez MA (2008) Plant Sci 175:87–97 (Involvement of ethylene in seed physiology)
Nelson DC, Riseborough J, Flematti GR, Stevens J, Ghisalberti EL, Dixon KW, Smith SM (2009) Plant Physiol 149:863–873 (Active smoke components that break dormancy)
Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee H-S, Sun T, Kamiya Y, Choia G (2007) Plant Cell 19:1192–1208 (Phytochrome downstream signalling)
Park S-Y, Fung P, Nishimura N, et al (2009) Science 324:1068–1071 (ABA perception and signal transduction)
Reyes D, Rodriguez D, Gonzalez-Garcia MP, Lorenzo O, Nicolas G, Garcia-Martinez JL, Nicolas C (2006) Plant Physiol 141:1414–1424 (Role of PP2C in ABA and GA signaling)
Seo M, Nambara E, Choi G, Yamaguchi S (2009) Plant Mol Biol 69:463–472 (GA perception and signal transduction)
Stepanova AN, Alonso JM (2009) Curr Opin Plant Biol 12:548–555 (Ethylene signaling and response)
Totterdell S, Roberts EH (1979) Plant Cell Environ 2:131–137 (Chilling of Rumex)
Totterdell S, Roberts EH (1980) Plant Cell Environ 3:3–12 (Alternating temperatures and germination)
Visser T (1956) Proc K Ned Akad Wet C 59:314–324 (Chilling and apple seed dormancy)
Yamaguchi S, Kamiya Y, Nambara E (2007) In: Bradford KJ, Nonogaki H (eds) Seed development, dormancy and germination. Blackwell Publishing, Oxford, 224–247 (ABA and GA metabolism pathways)
Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S (2004) Plant Cell 16:367–378 (Gene expression in the cold)
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Bewley, J.D., Bradford, K.J., Hilhorst, H.W.M., Nonogaki, H. (2013). Dormancy and the Control of Germination. In: Seeds. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4693-4_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4693-4_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4692-7
Online ISBN: 978-1-4614-4693-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)