Abstract
The current knowledge fits with a scenario where one or more commonly circulating enteroviruses initiate the beta-cell damaging process in early childhood. These viruses may represent certain enterovirus types and strains which have special properties explaining their ability to cause beta-cell damage (e.g., tropism to islet cells). Particular susceptibility for the virus is probably needed and only a small fraction of infected individuals may develop beta-cell damage. In this sense type 1 diabetes has many similarities with polio, the well-known enterovirus disease. Based on experience from polio, both the increasing incidence of type 1 diabetes and the remarkable geographical variation in the incidence rates can be related to varying circulation of diabetogenic enteroviruses in these populations. On the hand, animal experiments have suggested that under certain conditions enteroviruses may also have a protective effect, which seem to be mediated by their ability to activate immunoregulatory mechanisms. Both these aspects (risk vs. protective effect) should be taken into account when possible viral effects on the epidemiology of type 1 diabetes are investigated. Large-scale birth cohort studies, such as the TEDDY study, will play a key role in the identification of these effects and virus–host interactions which determine the outcome of the infection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
To understand possible viral effects on the epidemiological patterns of type 1 diabetes, one needs to combine epidemiological observations with the information on the mechanisms of enterovirus-induced beta-cell damage. Currently, there is no generally accepted single mechanism whereby enteroviruses could cause diabetes, but there are to be two main scenarios, which are relevant to these considerations. The traditional “virus disease” scenario is based on the idea that certain enterovirus destroys pancreatic beta cells, either directly or by immune-mediated mechanisms. This fits well with previous experience from many enterovirus diseases, such as polio. Another scenario is based on the hypothesis that under certain conditions enteroviruses may also have a protective effect: they can induce immunoregulatory responses which can downregulate autoreactive immune responses in analogy with hygiene hypothesis in allergy. This chapter summarizes epidemiological observations in light of these two scenarios.
Epidemic Features of the Beta-Cell Damaging Process
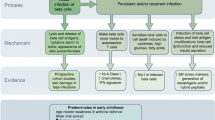
Beta cells are destroyed by an immune-mediated process, which usually progress slowly and may continue for years before clinical type 1 diabetes is diagnosed. The process starts early, and the majority of children who develop type 1 diabetes have turned positive for islet autoantibodies before the age of 3 years, in many cases already before the age of 1 year (Siljander et al. 2009). Therefore, to understand the dynamic relationships between the epidemiology of enterovirus infections type 1 diabetes, the initiation phase which occurs long before the appearance of symptoms of diabetes should be studied.
In fact, several publications support the idea that enterovirus infections play a role in this initiation phase. One of the most interesting observations is the clear seasonal pattern of the onset of this autoimmune process; children turn positive for autoantibodies usually during the late summer and fall, which parallels the seasonality of enterovirus infections (Kimpimaki et al. 2001). This pattern is seen in almost every year. Altogether, these seasonal peaks support the “epidemic” nature of the autoimmune process. Accordingly, assuming that a virus initiates the process, this should happen quite soon after the infection with a relatively constant time-lag. These observations indicate that the search for diabetogenic enteroviruses should focus on viruses occurring at this early age and circulating almost every year. This conclusion is supported by prospective studies showing a “peak” in enterovirus infections a few months before autoantibodies are first detected (Hiltunen et al. 1997; Oikarinen et al. 2011; Salminen et al. 2003) as well as animal experiments showing that enterovirus infection induces islet autoantibodies and diabetes with a few weeks’ time-lag (Chatterjee et al. 1992; Gerling et al. 1991). The diagnosis of clinical type 1 diabetes follows a similar seasonal pattern, even though it may not be as clear as that observed in autoantibody seroconversions (Gamble and Taylor 1969; Moltchanova et al. 2009). Interestingly, the seasonality of type 1 diabetes is the strongest in countries with high incidence of type 1 diabetes and clear seasonality in enterovirus infections (this correlates with the latitude). It is also stronger in boys than in girls which parallels the gender bias in severe enterovirus diseases (Moltchanova et al. 2009).
It is possible that the same infectious agent which initiates the process can also accelerate it and precipitate the symptoms of type 1 diabetes. In fact, enteroviruses have been detected both at the initiation phase (autoantibody seroconversion) and at the diagnosis of clinical type 1 diabetes possibly reflecting this kind of “multiple hit” effect (Yeung et al. 2011).
Type 1 Diabetes as a Viral Disease
In this scenario enteroviruses are considered as a necessary causal factor for the development of type 1 diabetes. In other words, diabetes is considered as a complication of enterovirus infection. This has been the dominating hypothesis in studies evaluating the association between enteroviruses and diabetes and it has been supported by a number of epidemiological and experimental observations.
One of the key observations is the strong tropism of enteroviruses to human pancreatic islets. Several studies have suggested that human islet cells are highly permissive for a number of different enterovirus types in vitro (Chehadeh et al. 2000; Roivainen et al. 2002; Yin et al. 2002) and that enterovirus proteins and genome are present in the pancreatic islets of both diabetic patients and children who have died of enterovirus infections (Dotta et al. 2007; Foulis et al. 1990; Oikarinen et al. 2008; Richardson et al. 2009; Tauriainen et al. 2011). The virus has been found predominantly in beta cells while the exocrine pancreas has been mostly negative. This tropism correlates also with the expression of one major enterovirus receptor, coxsackie and adenovirus receptor, by the islet cells. Thus, one key mechanism of enterovirus-induced beta-cell damage may be related to the capability of the virus to reach the pancreatic islets. It is possible that such tropism is characteristic to only certain specific enterovirus types and/or strains, analogously with the tropism of the three poliovirus serotypes to motoneurons (polioviruses belong to enteroviruses). In mouse models enteroviruses infect mostly exocrine pancreas but some strains can also infect the islets (Jaidane et al. 2009). In addition, studies carried out with a closely related picornavirus, encephalomyocarditis virus, support this scenario: this virus has two variants (strains), one being highly tropic to insulin-producing beta cells and causing diabetes in infected animals (Jun and Yoon 2001).
If the diabetogenic enterovirus types are common, as indirectly suggested by the epidemiological observations described above, the virus needs to hit a particularly susceptible host to be able to cause diabetes. Susceptibility to these viruses may be linked to the genes which modulate the risk of both type 1 diabetes and immune response to enteroviruses (such as IFIH1, IRF7 network, HLA-DR) as well as other individual factors such as age and gender (young age and male gender increase the risk of complications of enterovirus infections). Again, this scenario resembles closely that previously described in polio (polioviruses used to be very common causing paralytic disease in less than 1% of infected individuals). In addition, the virus may have interactions with other risk factors, such as cow’s milk proteins (Makela et al. 2006).
Lessons from Other Enterovirus Diseases
Type 1 diabetes and the well-known enterovirus disease, polio, share common features which may help to understand the role of enteroviruses in type 1 diabetes (Table 14.1). These similarities include seasonality, time-trends in disease incidence, high incidence in “high hygiene” areas, inflammation in the target organ, and highly selective cell damage. In fact, poliomyelitis was once suggested to be an autoallergic disease, where poliovirus infection induces immune-mediated paralysis in genetically predisposed individuals (Wyatt 1976). One major difference is the long prodromal subclinical phase of disease in type 1 diabetes compared to the rapid appearance of polio paralysis after the infection. This is quite logical since even a limited damage in motoneurons leads immediately to clinical symptoms while up to 90% of beta cells can be destroyed without causing any symptoms leading to the diagnosis of type 1 diabetes.
During the polio era, polioviruses were very common and almost every individual became infected by adulthood and the majority by the age of 5 years. Only less than 1% of the infected individuals developed the paralytic disease while the great majority (about 90%) had subclinical infection. Certain host factors increased the risk of the severe disease including male gender, older age, physical exercise, and muscle damage during infection, but the mechanisms regulating the risk are still largely unknown. Some reports have indicated association with certain HLA genes.
Interestingly, a change in the dynamics of poliovirus circulation turned out to have a major effect on the risk of paralytic disease. The increasing hygiene gradually decreased the transmission of polioviruses during the nineteenth century, which paradoxically led to the first clear epidemics of paralytic disease at the end of the century (Monto 1999). These epidemics started in young infants (the disease was first called infant paralysis) in countries with high standard of living (e.g., Nordic countries and USA) and continued thereafter until the vaccine was developed in the 1950s. These epidemics and the rapid increase in paralytic polio are believed to be caused by a decrease in herd immunity in young children. The basis of this phenomenon was a delay in the age of the first infections; some children were not exposed to polioviruses until later in childhood when they were no more protected by maternal antibodies (Nathanson and Kew 2010). As hygiene, sanitation, and housing improved the proportion of children escaping infection in infancy rose and the number of paralytic diseases rose in parallel.
The Polio Hypothesis
Viskari et al. (2000) launched a new hypothesis to explain the conspicuous increase seen in the incidence of type 1 diabetes in developed countries after World War II as well as the marked geographical variation in the incidence rates. They claimed that these phenomena are connected to the circulation of enteroviruses. They named this hypothesis as the “polio hypothesis” due to the analogy with the epidemiological pattern previously seen in polio. The same idea has also been discussed elsewhere (Zinkernagel 2001).
According to the polio hypothesis, a low frequency of enterovirus infections in the background population increases the risk of type 1 diabetes by making children more susceptible to enterovirus-induced beta-cell damage. In a population with a high prevalence of enterovirus infections and a low risk of type 1 diabetes, the first infections are experienced soon after birth when maternal antibodies protect the child (Fig. 14.1). Infections which occur at the presence of maternal antibodies induce an immune response but remain superficial. This has been called a natural vaccination of the child (Zinkernagel 2001) and is illustrated in Fig. 14.2. The child’s own enterovirus immunity develops gradually as the child experiences serial infections inducing a progressively expanding repertoire of memory T-cells which are known to cross-react between different enterovirus types (Cello et al. 1996; Juhela et al. 1998a). These memory T-cells can boost enterovirus-specific immune responses when the child become infected by the same or different enterovirus type (anamnestic response) speeding up the elimination of the virus and limiting its systemic spread.
Immunological basis of the polio hypothesis. In a population with high prevalence of enterovirus infections and low risk of type 1 diabetes (a), the child experiences the first infections (black arrows) soon after birth when maternal antibodies (dotted curve) still protect the child. The solid curve indicates the development of child’s own enterovirus immunity as a consequence of serial infections and maturation of immune system. In a population with low prevalence of enterovirus infections and high risk of type 1 diabetes (b), the infant lacks maternal antibodies and/or experiences the infections later when maternal antibodies have already disappeared. This creates a susceptibility period in early infancy. If the child becomes infected by a diabetogenic enterovirus (large arrow) during this period, the risk of diabetes is high. In addition, child’s long-term enterovirus immunity develops more slowly and remains at relatively low level due to the lack of booster infections
Biological basis of the polio hypothesis: the role of maternal antibodies in the protection of young infants against the diabetogenic effect of enteroviruses. Panel (a) illustrates a child whose mother has high titers of neutralizing antibodies against the infecting virus serotype. Protective maternal antibodies are transferred to the child through placenta (systemic effect) and via breast-milk (local mucosal effect), both protecting the child against the spread of the virus to the pancreas. Maternal antibodies limit the infection to mucosal surfaces, but the child becomes immunized against the virus. The virus is illustrated with an open circle, the child with a large oval, and the target organ (pancreas) with a gray circle. Panel (b) illustrates a child whose mother does not have antibodies against the infecting serotype and cannot provide protection for the child. The virus replicates effectively in intestinal mucosa and gut-associated immune system, spreading to the pancreas and causing inflammation in the infected tissues. Infection before the age of 1 year is a particular risk factor as child’s own immune system is still immature and the protection depends largely on maternal antibodies
In contrast, in a population with a low prevalence of enteroviruses and a high risk of type 1 diabetes, infants lack maternal antibodies because increasing proportion of the mothers has not experienced the virus which is infecting the child. Immune protection against enteroviruses depends largely on neutralizing antibodies. These antibodies are serotype specific making the infant completely unprotected against those virus types which the mother has never experienced. In addition, the infections occur later when maternal antibodies have already disappeared and breast-feeding has been discontinued. This creates a susceptibility period in early childhood when both passive and acquired immunity are weak and maternal antibodies would be needed to compensate this defect. Thus, the polio hypothesis could be particularly relevant for such enteroviruses which circulate in very young children infecting them during this susceptibility period. Figure 14.3 summarized the changes in the population dynamics of enterovirus infections which led to the past polio epidemics and which may now contribute to the ongoing “epidemic” of type 1 diabetes.
Epidemiological basis of the polio hypothesis: inverse correlation between the circulation of enteroviruses and the risk of severe infection. The incidence of paralytic polio (solid black line) is shown in relation to the circulation of polioviruses in background population (gray dotted line). Similarly, the incidence of type 1 diabetes (dotted black line) is shown in relation to the circulation of diabetogenic enteroviruses in the population (dotted gray line). The threshold of virus circulation which can maintain population immunity and low risk of complications is shown as a horizontal line. In the case of polio, the circulation of the virus dropped below this threshold at the end of nineteenth century and, according to polio hypothesis, in type 1 diabetes 50 years later. The main reason in both cases is an increase in infections which occur in the absence of protective maternal antibodies in young infants
Observations Supporting Polio Hypothesis
The polio hypothesis has been supported by studies comparing the frequency and time-trends of enterovirus infections and type 1 diabetes different populations. Viskari et al. (2000) carried out the first studies in Finland where the incidence of type 1 diabetes is the highest in the world and has increased fivefold during the past 50 years (currently about 60 per 100,000 children). In samples taken from pregnant women during 1983–1995 as a part of the national infectious disease screening program they found a significant decrease in enterovirus antibody levels over time. In a further study they confirmed this finding in larger series in both Finland and Sweden (Viskari et al. 2005). These analyses were done using assays which detect antibodies against several different enterovirus types suggesting that the overall exposure to enteroviruses has decreased. This implies that the proportion of newborns who lack maternal antibodies has increased, making new born children now more susceptible than before. The same group has also compared the prevalence of enterovirus infections in seven countries with either exceptionally high or low/intermediate incidence of type 1 diabetes. They found that enterovirus antibodies were less frequent and at lower levels in countries with high diabetes incidence compared to countries with low diabetes incidence (Viskari et al. 2004, 2005). Finland, in particular, had lower antibody levels compared to other counties. For example, altogether 42% of Finnish pregnant women lacked neutralizing antibodies to coxsackievirus B4, which has been linked to type 1 diabetes in previous studies. The corresponding figure in the neighboring Estonia and Karelian Republic of Russia was only 14%. Such a big difference between these countries has probably biologically relevance and, assuming that this is a diabetogenic virus, could contribute to the high incidence of type 1 diabetes in Finland. Further, Viskari et al. found that while the overall incidence of enterovirus meningitis has decreased during the past decades, the proportion of less than 6 months old cases has increased, supporting also the role of maternal antibodies in the protection of young infants against severe enterovirus infections (Viskari et al. 2000). Same kind of secular increase in severe neonatal enterovirus disease has also been reported in other countries (Shattuck and Chonmaitree 1992). Recently, a rapid increase in severe coxsackievirus B1 infections has documented among young infants in the USA (Wikswo et al. 2009). Altogether, there seems to be an inverse relationship between the frequency of enterovirus infections and type 1 diabetes in the background population, a pattern which is in line with the polio hypothesis.
Even though enterovirus infections seem to have become less frequent during the last decades, they are still common and occur in young children during this susceptibility period. A study carried out in Finland during 1990s indicated that altogether 30% of healthy children had experienced enterovirus infection by the age of 6 months and 60% of children by 12 months (Juhela et al. 1998b). Another study followed newborns during an enterovirus season in New York in 1981, indicating an incidence of 13% during the first month of life (Jenista et al. 1984). Thus, considerable proportion of children becomes infected during this susceptibility period. However, only one study has been carried out to find out how often these infections actually occur in the absence of maternal antibodies. In that study children were followed from birth in Estonia where the incidence of type 1 diabetes is about 17 per 100,000 children. Infections were diagnosed by detecting viral RNA in regularly collected stool samples and the serotype was identified by sequencing the viral genome. Neutralizing antibodies were measured against that particular serotype from cord-blood. The results indicated that 38% of infections, which were experienced during the first months of age, occurred in the absence of maternal antibodies against the causative virus type (Salur et al. 2011).
Breast-feeding is known to protect children against many virus infections including enterovirus infections (Jenista et al. 1984; Sadeharju et al. 2007). In fact, maternal antibodies in breast-milk may provide even stronger protection against enteroviruses than transplacentally acquired antibodies in circulation (Sadeharju et al. 2007). This is logical since the transmission of enteroviruses happens via mucosal route and ingested breast-milk antibodies can neutralize the virus before it can infect the host. Thus, one can argue that breast-feeding should also protect against enterovirus-induced diabetes. Breast-feeding may indeed have a protective effect against type 1 diabetes, even though conflicting observations also exist (Knip and Akerblom 2005).
In summary, epidemiological observations support polio hypothesis and more studies are indicated to test it further. It would be important to find out if diabetogenic enteroviruses belong to certain serotypes and to study if these observations hold true for these particular virus types. Large-scale prospective studies in different populations will play a key role in this effort.
Type 1 Diabetes and the “Hygiene Hypothesis”
In this scenario type 1 diabetes is considered as an autoimmune disease which develops spontaneously or is initiated by other factors than enteroviruses. The idea of a spontaneous autoimmune process as an underlying mechanism is largely based on studies in NOD mice, the most widely used animal model for type 1 diabetes. These mice develop an autoimmune process which starts “spontaneously” and damages beta cells leading to type 1 diabetes-like disease. Several microbes can prevent or delay this process, suggesting that under certain conditions microbes can have a protective effect resembling that previously suggested in allergies (hygiene hypothesis). In fact, recent studies have widened the scope of the hygiene hypothesis from allergies to type 1 diabetes and other immune-mediated diseases, and the concept that microbes can be important regulators of immune system is under vigorous investigation (Bach 2005). This idea has also been supported by a recent observation showing that of type 1 diabetes and IgE-mediated allergic sensitization co-occur in such subjects who are seronegative for hepatitis A virus (Seiskari et al. 2010). Thus, living in very hygienic conditions may lead to a defect in immune regulations and predispose to immune-mediated diseases.
Among other microbes enteroviruses prevent the development of diabetes in NOD mice (Drescher et al. 2004). This effect seems to be mediated by the induction of immunoregulatory mechanisms such as regulatory T-cells which can suppress the autoimmune phenotype in these animals (Filippi et al. 2009; Tracy and Drescher 2007). The timing of the infection is critical, since this protection can be seen only when the mice are infected before the autoimmune process has started. In older mice, which are already affected by an inflammation process in the pancreas, the virus can even accelerate the process, particularly when given in high doses (Serreze et al. 2000). Thus far, two enterovirus serotypes have been studied in NOD mouse model (coxsackieviruses B3 and B4) and it is not known if other serotypes could have a similar protective effect.
These observations have raised the question if certain enteroviruses could also have a protective effect in man. However, the number of human studies addressing this question is very limited. One study showed that neutralizing antibodies against coxsackieviruses B3 and B4, the same serotypes which have had a protective effect in NOD mice, are decreased in type 1 diabetes patients compared to controls (Palmer et al. 1982). Theoretically, this difference could reflect a protective effect. Coxsackievirus B3 can also selectively inhibit major histocompatibility complex class I presentation pathway which reduces cytotoxic T-cell responses to infected cells (Cornell et al. 2007). Interestingly, recent studies have suggested that enterovirus infections may protect from IgE-mediated allergic sensitization (Seiskari et al. 2007) also supporting possible immunoregulatory effects. Such an effect could be mediated, e.g., by induction of immunoregulatory cytokines such as IL-10 by the virus (bystander suppression mechanism). However, further studies are still needed to find out if enteroviruses have immunoregulatory effects which play a role in the pathogenesis of human type 1 diabetes.
References
Bach JF (2005) Infections and autoimmune diseases. J Autoimmun 25(Suppl):74–80
Cello J, Strannegard O, Svennerholm B (1996) A study of the cellular immune response to enteroviruses in humans: identification of cross-reactive T cell epitopes on the structural proteins of enteroviruses. J Gen Virol 77(Pt 9):2097–2108
Chatterjee NK, Hou J, Dockstader P, Charbonneau T (1992) Coxsackievirus B4 infection alters thymic, splenic, and peripheral lymphocyte repertoire preceding onset of hyperglycemia in mice. J Med Virol 38:124–131
Chehadeh W, Kerr-Conte J, Pattou F, Alm G, Lefebvre J, Wattre P, Hober D (2000) Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. J Virol 74:10153–10164
Cornell CT, Kiosses WB, Harkins S, Whitton JL (2007) Coxsackievirus B3 proteins directionally complement each other to downregulate surface major histocompatibility complex class I. J Virol 81:6785–6797
Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P (2007) Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 104:5115–5120
Drescher KM, Kono K, Bopegamage S, Carson SD, Tracy S (2004) Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329:381–394
Filippi CM, Estes EA, Oldham JE, von Herrath MG (2009) Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest 119:1515–1523
Foulis AK, Farquharson MA, Cameron SO, McGill M, Schonke H, Kandolf R (1990) A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia 33:290–298
Gamble DR, Taylor KW (1969) Seasonal incidence of diabetes mellitus. Br Med J 3:631–633
Gerling I, Chatterjee NK, Nejman C (1991) Coxsackievirus B4-induced development of antibodies to 64,000-Mr islet autoantigen and hyperglycemia in mice. Autoimmunity 10:49–56
Hiltunen M, Hyoty H, Knip M, Ilonen J, Reijonen H, Vahasalo P, Roivainen M, Lonnrot M, Leinikki P, Hovi T, Akerblom HK (1997) Islet cell antibody seroconversion in children is temporally associated with enterovirus infections. Childhood Diabetes in Finland (DiMe) Study Group. J Infect Dis 175:554–560
Jaidane H, Sane F, Gharbi J, Aouni M, Romond MB, Hober D (2009) Coxsackievirus B4 and type 1 diabetes pathogenesis: contribution of animal models. Diabetes Metab Res Rev 25:591–603
Jenista JA, Powell KR, Menegus MA (1984) Epidemiology of neonatal enterovirus infection. J Pediatr 104:685–690
Juhela S, Hyoty H, Lonnrot M, Roivainen M, Simell O, Ilonen J (1998a) Enterovirus infections and enterovirus specific T-cell responses in infancy. J Med Virol 54:226–232
Juhela S, Hyöty H, Lonnrot M, Roivainen M, Simell O, Ilonen J (1998b) Enterovirus infections and enterovirus specific T-cell responses in infancy. J Med Virol 54:226–232
Jun HS, Yoon JW (2001) The role of viruses in type I diabetes: two distinct cellular and molecular pathogenic mechanisms of virus-induced diabetes in animals. Diabetologia 44:271–285
Kimpimaki T, Kupila A, Hamalainen AM, Kukko M, Kulmala P, Savola K, Simell T, Keskinen P, Ilonen J, Simell O, Knip M (2001) The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab 86:4782–4788
Knip M, Akerblom HK (2005) Early nutrition and later diabetes risk. Adv Exp Med Biol 569:142–150
Makela M, Vaarala O, Hermann R, Salminen K, Vahlberg T, Veijola R, Hyoty H, Knip M, Simell O, Ilonen J (2006) Enteral virus infections in early childhood and an enhanced type 1 diabetes-associated antibody response to dietary insulin. J Autoimmun 27:54–61
Moltchanova EV, Schreier N, Lammi N, Karvonen M (2009) Seasonal variation of diagnosis of type 1 diabetes mellitus in children worldwide. Diabet Med 26:673–678
Monto AS (1999) Francis field trial of inactivated poliomyelitis vaccine: background and lessons for today. Epidemiol Rev 21:7–23
Nathanson N, Kew OM (2010) From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol 172:1213–1229
Oikarinen M, Tauriainen S, Honkanen T, Vuori K, Karhunen P, Vasama-Nolvi C, Oikarinen S, Verbeke C, Blair GE, Rantala I, Ilonen J, Simell O, Knip M, Hyoty H (2008) Analysis of pancreas tissue in a child positive for islet cell antibodies. Diabetologia 51:1796–1802
Oikarinen S, Martiskainen M, Tauriainen S, Huhtala H, Ilonen J, Veijola R, Simell O, Knip M, Hyoty H (2011) Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 60:276–279
Palmer JP, Cooney MK, Ward RH, Hansen JA, Brodsky JB, Ray CG, Crossley JR, Asplin CM, Williams RH (1982) Reduced Coxsackie antibody titres in type 1 (insulin-dependent) diabetic patients presenting during an outbreak of Coxsackie B3 and B4 infection. Diabetologia 22:426–429
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG (2009) The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52:1143–1151
Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T (2002) Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 45:693–702
Sadeharju K, Knip M, Virtanen SM, Savilahti E, Tauriainen S, Koskela P, Akerblom HK, Hyoty H (2007) Maternal antibodies in breast milk protect the child from enterovirus infections. Pediatrics 119:941–946
Salminen K, Sadeharju K, Lonnrot M, Vahasalo P, Kupila A, Korhonen S, Ilonen J, Simell O, Knip M, Hyoty H (2003) Enterovirus infections are associated with the induction of beta-cell autoimmunity in a prospective birth cohort study. J Med Virol 69:91–98
Salur L, Oikarinen S, Tauriainen S, Mandel M, Hyöty H, Uibo R (2011) Enterovirus infections in young infants – are children still protected by maternal antibodies? Hum Vaccin 7(9):966–971
Seiskari T, Kondrashova A, Viskari H, Kaila M, Haapala AM, Aittoniemi J, Virta M, Hurme M, Uibo R, Knip M, Hyoty H (2007) Allergic sensitization and microbial load – a comparison between Finland and Russian Karelia. Clin Exp Immunol 148:47–52
Seiskari T, Viskari H, Kondrashova A, Haapala AM, Ilonen J, Knip M, Hyoty H (2010) Co-occurrence of allergic sensitization and type 1 diabetes. Ann Med 42:352–359
Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA (2000) Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 49:708–711
Shattuck KE, Chonmaitree T (1992) The changing spectrum of neonatal meningitis over a fifteen-year period. Clin Pediatr (Phila) 31:130–136
Siljander HT, Simell S, Hekkala A, Lahde J, Simell T, Vahasalo P, Veijola R, Ilonen J, Simell O, Knip M (2009) Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes 58:2835–2842
Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H (2011) Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol 33:45–55
Tracy S, Drescher KM (2007) Coxsackievirus infections and NOD mice: relevant models of protection from, and induction of, type 1 diabetes. Ann N Y Acad Sci 1103:143–151
Viskari HR, Koskela P, Lonnrot M, Luonuansuu S, Reunanen A, Baer M, Hyoty H (2000) Can enterovirus infections explain the increasing incidence of type 1 diabetes? Diabetes Care 23:414–416
Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Knip M, Hyoty H (2004) Relationship between the incidence of type 1 diabetes and enterovirus infections in different European populations: results from the EPIVIR project. J Med Virol 72:610–617
Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Kaplan B, Laron Z, Koskela P, Vesikari T, Huhtala H, Knip M, Hyoty H (2005) Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia 48:1280–1287
Wikswo ME, Khetsuriani N, Fowlkes AL, Zheng X, Penaranda S, Verma N, Shulman ST, Sircar K, Robinson CC, Schmidt T, Schnurr D, Oberste MS (2009) Increased activity of Coxsackievirus B1 strains associated with severe disease among young infants in the United States, 2007–2008. Clin Infect Dis 49:e44–e51
Wyatt HV (1976) Is poliomyelitis an auto-allergic disease triggered by virus? Med Hypotheses 2:262–268
Yeung WC, Rawlinson WD, Craig ME (2011) Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. Br Med J 342:d35
Yin H, Berg AK, Westman J, Hellerstrom C, Frisk G (2002) Complete nucleotide sequence of a Coxsackievirus B-4 strain capable of establishing persistent infection in human pancreatic islet cells: effects on insulin release, proinsulin synthesis, and cell morphology. J Med Virol 68:544–557
Zinkernagel RM (2001) Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med 345:1331–1335
Acknowledgments
I wish to thank Hanna Viskari, M.D., Ph.D., Maria Lönnrot, M.D., Ph.D., Anita Kondrashova, M.D., Ph.D., Sisko Tauriainen, Ph.D., Sami Oikarinen, M.Sc. and all other collaborators contributing to the findings summarized in this chapter. The studies summarized in this chapter have been financially supported by the Juvenile Diabetes Research Foundation, European Commission, Sohlberg’s Foundation, and the Academy of Finland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Hyöty, H. (2013). Enterovirus Immunity and the “Hygiene Hypothesis”. In: Taylor, K., Hyöty, H., Toniolo, A., Zuckerman, A. (eds) Diabetes and Viruses. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4051-2_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4051-2_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4050-5
Online ISBN: 978-1-4614-4051-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)