Abstract
Metabolomics provides a valuable strategy for describing and annotating the structures, compositions, and functions of mammalian milk. Detailed analyses of the complex components of milk have revealed an unexpected diversity of glycans consisting of oligosaccharides, glycoproteins, and glycolipids, all of which help shape the intestinal environment and in particular the intestinal microbiome of breastfed babies. Using complete and partial ensembles of glycan mixtures, the holistic principles of metabolomics analytics were leveraged for microbial screening studies. The complex glycans of human milk proved to be highly selective in their ability to support the growth of only a very rare group of enteric bacteria. These studies led to the conclusion that a signature achievement of breast milk is the development of a unique milk-oriented intestinal microbiota that results from a functional overlap of stereospecific glycan biosynthesis in maternal mammary epithelia with equally stereospecific glycosidase enzymes encoded within the genome of the commensal bacteria. Clinical evidence in support of that hypothesis has now been generated by the simultaneous administration and quantitation of the entire repertoire of glycans in the milk going in and the feces coming out of human infants. These platforms of systems biology combining separation technologies coupled to highly accurate and sensitive mass spectrometry with exhaustive library development and computational tools provide a model for success in understanding biological processes. Metabolomics is now extending that understanding of the infant microbiota and its phenotype to the role of complex glycans in the microbiota of all ages. The relentless selective pressure on the process of lactation within the mammary epithelial cell over millennia of evolution has been to nourish, protect, and support the survival of the mother infant pair. The principles that have emerged to nourish infants provide a guiding model for diet and health of all humans. The tools of metabolomics are proving successful in revealing the mechanisms behind milk’s “genius.”
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
The mother and breastfed infant dyad provides a model to understand diet in its larger context. The scientific opportunities afforded by this model are transformative. Mechanistic insights to the targets of dietary inputs can be revealed by studying milk genomics, chemical composition, biological properties, and its diversity across mammals and temporally across lactation. The clinical comparison of exclusive breastfeeding against various formulas provides a powerful framework to study structurally defined diets and monitor the consequences of those structures on health and disease. In this respect, studies on milk and the lactating mammary gland provide unique insights into the mechanisms by which diet can act in protection and prevention. The mammary epithelial cell is a bioreactor for bioengineering complex structures and activities that act upon virtually all of the infant’s processes: immunity, growth and development, metabolism, physiology, neurological development, and microbiota colonization and maturation. The components of milk execute on this biological blueprint using integrative and pleiotropic mechanisms that are difficult if not impossible to identify using the reductionist strategies of traditional biological chemistry. The comprehensive nature of the omic sciences and in particular metabolomics is changing the way milk is studied. Milk is the functional output of mammary metabolism, and it’s a biofluid representing maternal genetics, health status, and environments.
A striking example of the principles and technologies of metabolomics applied to breast milk is in the area of glycomics; glycomics has revealed the diversity and abundance of glycans notably the free human milk oligosaccharides (HMOs) that are relatively unique to lactation and the glycosylated proteins, peptides, and lipids. Interestingly, glycans reach the large intestine and can ultimately be excreted and measured in the stool in healthy infants. This is a paradox if milk is considered a source of digestible nutrients for the infant. The resolution of this paradox is found in the fact that in most breastfed infants, these glycans disappear from stool, coincident with the appearance of a group of bacteria capable of digesting and utilizing them as growth substrates. The value of this relationship between maternal milk and intestinal bacteria to shape infant postnatal development is still being revealed, ranging from protection from pathogenic bacteria, viruses, and toxins, to promoting neurological and immune systems and enhancing barrier function of intestinal epithelia. The highly selective digestibility of milk glycans act to shape the intestinal microbiome orchestrating its transition from the sterile uterus through the chaotic introduction of environmental bacteria at birth to a stable milk-oriented microbiome (MOM). This convergence of an entire metabolite class, glycans with the selective metabolism of bacteria and their interaction with the human host, is an opportunity to define metabolism as a dietary variable and study the structure-function relationships between diet, metabolism, and intestinal bacteria development. These studies provide broader principles for nourishing complex microbiota throughout life. At the core, comprehensive and accurate measurement of the structures and composition of milk’s glycan metabolome is required.
Analytical chemistry has only recently brought the tools needed to measure glycobiology, the free oligosaccharides and glycans bound to proteins, peptides, and lipids in milk. Instrumentation is not sufficient; mass spectrometry must be coupled to separation technologies, enzyme biotechnologies, and bioinformatics tools to assemble all of the information into computationally accessible libraries. These technological advancements have led to the discovery that glycans are a central component of all mammalian milks, are variable across lactation and among women, and provide a wide diversity of structures to diverse functions [1–6].
8.2 Metabolomics and Human Milk
The simplifying elegance of the linear encoding of protein structure from DNA, RNA to protein sequence that is so empowering to biology from evolution to function is equally enabling to scientific research. Scientists have been wonderfully successful in annotating DNA-dependent biological processes because of the simplicity of linear sequence. Metabolism does not possess this simplicity. The dizzying complexity of metabolism must now be studied the old fashioned way, by measuring it. Scientists are beginning to assemble the technologies to measure metabolites in the accuracy, sensitivity, and comprehensiveness that reflect actual biology. A broad goal of food research is to build a linear understanding from the genetics of agricultural commodities, through their metabolism and thence composition as foods to the specific actions of those components on the metabolism and ultimately health of individual consumers (Fig. 8.1). Step one is to define the genetic and phenotypic basis of food composition through commodity growth and processing. The next step is to understand the principles by which human metabolism is controlled via these exogenous dietary components. This challenge will be particularly daunting in higher organisms due to the importance of structure to function. In higher organisms metabolites are distributed according to the cells, tissues, organs, and whole bodies. This structural dimension will demand that metabolites are measured as a function of the 3-dimensional structures of their immediate environment, techniques for which are only beginning to emerge. We have taken the approach of using the interaction between milk and microorganisms as a model for dietary metabolomics (Fig. 8.2). The principles emerging from this research provide scientists with examples of the interactions between diet and metabolism that may be instructive for higher animals.
The bidirectional flow of metabolic information through agriculture, food, and health. The genomics of agricultural commodities defines the metabolic machinery of farmed organisms which, once measured by metabolomics, can be guided by selective breeding and explicit genetic engineering. The metabolome compositions of harvested organisms that are the result of their metabolism are in turn alterable post-harvest by a wide variety of processing alternatives. The final compositions of chosen foods measured by food metabolomics define the overall diet compositions of individuals/families. Their diets influence their acute health which is in turn measureable by metabolomics of various body fluids. Departures from desired health trajectories detected by individual and population measurements then feed back into all of the agriculture and food input variables to alter diet composition and guide individuals to improved health
The tripartite evolutionary relationship for mothers, infants, and their microbiota. The importance of the infant microbiota to its survival and genetic success is implied by the substantial investment of lactation in the control of this ecosystem. Understanding how and why lactation controls the infant microbiota provides scientific insights to microbial ecosystems in human intestine and beyond to all complex ecosystems in which food is a selective and discriminating input variable
8.3 Milk Glycomics
Glycans are the biopolymer class that has been largely ignored in spite of their abundance across the phylogenetic tree and through evolution [7]. Despite their importance in health and disease, they are not sequence encoded but rather the products of enzymatic metabolism. As a result of metabolic synthesis, the number of potential structures is massive contributing to the structural diversity seen in milk.
The complexity of a glycan is the result of a number of factors including branching, the number of different sugars, the stereospecific linkages of those sugars all leading to multiple isomers even for a single net mass to which must be added the dimension of conjugation: they are free or bound to proteins, peptides, or lipids again in a heterogeneous but stereospecific manner. Biology has apparently employed the combination of metabolism and structural diversity to leverage glycobiology into a variety of functions and most notably recognition. Perhaps not surprisingly, throughout evolution, the glycans on surfaces of cells are distinct both to “self” and to “foreign” organisms as the molecular basis for individuality. This dimension of diversity that is clearly of value to biological organisms is instead to scientists a nightmare precisely because there is no corresponding genetic template; every glycan must be explicitly analyzed to be identified. Research into the technologies and methodologies to routinely and comprehensively measure the glycans in biological and clinical samples is only now emerging, and as a result, a quantitative, metabolomics approach to glycobiology is becoming possible. Glycomics is defined as the systematic study of the total complement of sugars present in an organism in their free or protein and lipid-bound states [8, 9]. Researchers are now using glycomics to understand diet and health in the context of lactation, milk, and the role of complex glycans in various aspects of the development of the mammalian neonate.
8.3.1 Milk Oligosaccharides
The field of milk glycobiology is not new, and in fact many of the presumed roles of glycans throughout biology have been first discovered by examining milk. Nonetheless, the complexity of glycan structures has been a major hurdle to understanding specific structure – function relationships beyond binding assays. The oligosaccharides of human milk have become of particular interest in large part because they are an abundant (1–2 % w/v) and yet indigestible by the neonate. The biological challenges posed by this apparent paradox propelled a few key laboratories to pursue the analytical challenges of identifying and quantifying them. The revolution that they are bringing to analytical glycomics has been the result of innovations in separation science, enzyme biotechnology, mass spectrometry, automated library development, and computational toolsets.
8.3.2 Separation Science for Oligosaccharides
Liquid chromatography has been applied to glycan separation, yet neither normal nor reverse stationary phases provide sufficient separation power to successfully resolve glycan diversity in structure. Porous graphitized carbon (PGC) has been used as a uniquely selective stationary phase for bulk purification of glycans for many years. Only recently has it been possible to formulate PGC as an HPLC stationary phase for the analysis of native oligosaccharides [10]. This combination of high-efficiency, high reproducibility, and stationary phase selectivity in an HPLC system has provided the first generation of separation platforms capable of the extensive separation of glycan isomers [11]. The future of oligosaccharide and glycan analyses will be extending this selectivity and efficiency to stereospecificity, quantitation, and throughput.
8.3.3 Stereospecificity of Glycan Structures
Glycans are nothing if not a forest of stereospecificity. The total number of possible structures imaginable is astronomical, yet because these glycan polymers are all the results of stereospecific enzymatic reactions, the actual number of structures found in biology is manageably finite and approachable by modern analytics and library systems. Nonetheless, precise oligosaccharide structures cannot be unequivocally identified on chiral separation phases and instead must still be determined the old fashioned way, by cleaving with stereospecific enzymes as an explicit step in the analysis [12]. The use of stereospecific enzymes will likely remain the most efficient means of assigning precise structures to oligosaccharides precisely because once a biological source is accurately described, it is not necessary to perform stereospecific analyses every subsequent analysis. The pragmatic proof of this principle has been demonstrated for the various milk oligosaccharides that have been analyzed for their stereospecificity [13].
The establishment of accurate metabolomics today cannot be achieved with online identification systems due to the complexity of the possible glycan structures relative to the separation platforms and mass spectrometry accuracy available. Instead effective methods for the structural identification of HMOs requires the construction of detailed libraries that map structures into analytical platforms taking advantage of the combinations of MS, tandem MS, and exoglycosidase digestion [12]. Neutral [14] and anionic milk oligosaccharides from humans [15] totaling 75 structural isomers have been annotated in this approach (Figs. 8.3, 8.4, and 8.5). Once begun, the library strategy has been extended to milks from other mammals to over 200 complete structures. This basic strategy is appropriate for the vast majority of applications to human milk biology since 50 structures represent 99 % of the total abundances of oligosaccharides in human milks [14–16].
Basic oligosaccharide structures in milk. The structural core of oligosaccharides is illustrated, the key sugar monomers that make up the oligosaccharide compositions and the possible stereospecific glycosidic bonds that are possible. Bifidobacteria longum subspecies infantis contains the genetic capability to synthesize ostensibly all of the enzymes necessary to cleave this array of complex glycans
Structural diversity of complex free glycans in human milk. The cartoon approximations of a subset of oligosaccharide structures in human milk attest to the diversity and yet specificity of this biomolecule class. Milk oligosaccharides as highly structured biomolecules are the result of multiple sugars joined by multiple stereospecific linkages
Metabolomics is of relatively little utility if it only identifies structures and cannot quantify the absolute amounts of metabolites within biological samples. As a subset of the metabolome, glycan quantitation remains a major obstacle to metabolomics of glycobiology. Oligosaccharides lack discriminating chromophores for spectral detectors. As a result, oligosaccharides are often derivatized with absorbing labels including anthranilic acid (AA) or 2-aminobenzamide (AB) for quantitation [17]. To date the varying ionization efficiencies of glycans compromise the use of mass spectrometry of oligosaccharides. The alternative, using isotopically enriched internal standards, while making MS highly accurate [18], requires the synthesis of the entire library of potential structures which is not currently available. Quantitation of metabolites remains the great challenge for the applications of metabolomics to its most relevant applications in health.
The presence of oligosaccharides has been confirmed in very early mammals and marsupials [19]. Thus, indigestible carbohydrate biopolymers provided a selective advantage throughout mammalian lactation. This advantage has apparently continued up to humans. Human milk contains greater concentration and diversity of soluble oligosaccharides than other mammalian milks [20] ranging from on average 7 g/L mature to 23 g/L in colostrum [21, 22]. These soluble oligosaccharides are composed of glucose (Glc), galactose (Gal), N-acetylglucosamine (GlcNAc), fucose (Fuc), and sialic acid (NeuAc) monosaccharides. The basic biochemistry of oligosaccharide synthesis in the mammary gland is initiated by a lactose core of Gal and Glc catalyzed by β-galactotransferase in the presence of α-lactalbumin. The vast majority of HMO structures are based on this lactose core [23]. Lactose is then decorated by β1–3 linkage to lacto-N-biose (GlcNAc linked to Gal by β1–3 linkage) or by β1–6 linkage to N-acetyllactosamine (GlcNAc linked to Gal by β1–4 linkage). This growing chain structure can be further elongated with lacto-N-biose and N-acetyllactosamine by β1–3 and β1–6 linkages; Fuc connected with α1–2, α1–3, or α1–4 linkages and/or NeuAc residues attached by α2–3 or α2–6 linkages at the terminal positions (Fig. 8.3). The terminal sugars are particularly diagnostic of different mammalian milks, 60–80 % of HMOs are fucosylated, and 10–15 % of HMOs are sialylated in human milk [24].
8.4 Annotating the Functions of Human Milk Glycans
The structures of milk oligosaccharides have been selected for an unusual biological value: not to be consumed by infants. This selective pressure on lactation has been particularly intense since milk is the sole source of nourishment for mammalian infants. The genetics, synthesis, and structures of oligosaccharides in milk are unequivocally discoverable. However, the functions of oligosaccharides that were the basis for their emergence and persistence through evolution are not as easily discovered. The process of understanding their actions must first identify their actions, and then each of these actions must then be tested mechanistically as an actual valuable function in vivo. The most extensive approach to evaluating the actions and potential functions of oligosaccharides in human milk has been to establish the detailed support of the growth of specific strains of bacteria notably bifidobacteria [25, 26]. While the mechanisms and extent of microbial diversity in breastfed infants are still being actively documented, the basic observation that bifidobacterial species dominate the microbiota of breastfed infants around the world compared with formula-fed infants has been well established [27]. How an intestinal microbial ecosystem maintains a dominant and consistent bacterial population in the face of repeated and diverse inoculations with environmental microorganisms has been largely speculative until recently. Research has revealed the remarkable interaction between the stereospecific linkages defining the structures of milk oligosaccharides and the genetic repertoire of stereospecific glycosidases and solute-binding proteins that provide these bacteria a distinct competitive growth advantage within the intestine of the breastfed infant.
8.4.1 Screening Bacteria for Growth on Oligosaccharides
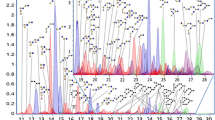
In an ongoing search for biological activities of these molecules to justify their abundance and diversity in milk, a prevailing hypothesis was that they are substrates for bacterial growth. However, no studies had yet documented that fact nor whether growth was selective among bacteria. Initial growth experiments in fact failed to demonstrate significant growth of bacteria when human milk oligosaccharides were the sole source of carbon in an otherwise supportive medium [25]. A series of subsequent experiments revealed that among gut-related bacteria tested (including Lactobacillus, Clostridium, Eubacterium E. coli, Veillonella, Enterococcus isolates), only Bifidobacterium and Bacteriodes species grew to high cell densities [28]. Growth on HMO was found in a select group of B. bifidum and B. longum subsp. infantis strains. In these same isolated growth conditions, even isolates of B. longum subsp. longum and B. breve showed poor growth, and other strains of B. adolescentis and B. animales were ostensibly unable to grow on HMO [29] (Fig. 8.6).
Growth curves and human milk oligosaccharide consumption by Bifidobacteria longum subspecies infantis. The growth curves of isolated bacteria (indicated) on media containing only isolated milk oligosaccharides as carbon source of B. longum, B. longum subspecies infantis, and B. breve are shown, illustrating the conspicuously greater growth of B. infantis. The most abundant oligosaccharides in human milk are histogrammed in the figure on the right before and after consumption by B. infantis illustrating the complete consumption of the majority of oligosaccharides by this bacterium [25]
The complex mechanisms by which milk oligosaccharides guide bacterial growth within the ecosystem of the infant intestine have been elaborated in a series of microbial studies. Among the bifidobacteria that are able to consume HMO, different strategies are present to use HMO as a substrate. In isolated growth studies of HMO consumption, B. longum subsp. infantis ATCC15697 most efficiently consumed oligosaccharides seven sugars (DP) or below [25]. Oligosaccharides below ten sugars are the majority of species human milk [10]. Other bifidobacteria including B. longum subsp. longum DJO10A and B. breve ATCC15700 that grew slowly on pooled HMO were found to be consuming mostly a single, nonfucosylated/nonsialylated species, LNnT. LNnT is present in breast milk yet a small portion of the overall HMOs. B. breve did grow in culture on all the monomer constituents of HMO and thus if present within the gastrointestinal tract could grow on liberated monosaccharides.
The bacteria that were found to be capable of growing on HMO were analyzed for the presence of metabolic activities towards complex oligosaccharides including the key sialidase and fucosidase activities required to deconstruct complex glycan structures. Among the strains examined, fucosidase activity was present in B. longum subsp. infantis and was only detected upon growth on HMO [25].
Distinct strategies for catalytic activity on complex biopolymer destruction are known among intestinal bacteria. The majority of intestinal bacteria secrete extracellular glycosidase enzymes that liberate free sugars that are then subsequently taken up by bacteria and metabolized. Select bifidobacteria use lacto-N-biosidase activity to break down oligosaccharides [30]. LNB is transported into B. bifidum via an ABC transporter and an associated LNB-specific solute-binding lipoprotein whereby it is further processed and fed into the central metabolic pathway [31].
The discovery that B. longum subsp. infantis ATCC15697 was uniquely capable of growing on human milk oligosaccharides led to an immediate project to sequence its genome. No prior experience prepared the investigators for the elegance of the genetic repertoire of this organism’s sequence. B. longum subsp. infantis ATCC15697 has become the blueprint for understanding the genetic basis of glycan-specific growth and phenotype [32]. This specific strain possesses clusters of genes associated with its unique phenotype distributed in the genome into four loci. The most informative, HMO cluster 1 (Fig. 8.7), contains all the necessary glycosidases (sialidase, fucosidase, galactosidase, and hexosaminidase) and transporters necessary for importing and metabolizing HMO. Sequencing more isolates for HMO-related genomic architecture among B. longum subsp. infantis isolates provides a detailed genetic map of the mechanisms behind the vigorous growth of this clade on HMO.
Gene cluster 4 from the complete genome of B. infantis illustrating the location of the genes encoding glycosidases and oligosaccharide transporters [32]. The putative glycosidic linkages on which the glycosidic enzymes react are shown below left and the model of the cell membrane-bound solute-binding protein complex is shown below right
The bacterial model of metabolomics illustrates the complexity of structure within metabolic pathways. Within the large HMO cluster (Fig. 8.7) are genes encoding an interesting group of extracellular solute-binding proteins (SBP; pfam 01547) demonstrated to bind oligosaccharides. These proteins provide two functions for the bacteria in their ecological niche of the breastfed infant intestine. These solute-binding proteins would tether the bacteria to glycans on the luminal side of the infant intestine and provide a net coverage of microbial binding sites thus blocking potential pathogens from the infant. Of more direct value to the bacterium, these solute-binding proteins would internalize free oligosaccharides directly infusing substrate into its endogenous metabolism. This substrate sequestering mechanism provides the B. longum subsp. infantis a unique foraging advantage in the overall microbial community. These solute-binding proteins also appear to be of singular advantage to the mammalian infant gut. A subset of these genes shows a pronounced evolutionary divergence from other SBP family 1 proteins in bifidobacteria [32]. The emergence of these genes is consistent with their functions as a mechanistic basis of symbiosis with humans through their interaction with milk oligosaccharides. The B. longum subsp. infantis genome has been shown to contain 21 family 1 SBP, more than most bifidobacteria.
The results of genomic analyses of bifidobacteria illustrate that HMO-related clusters are shared among all B. longum subsp. infantis isolates that have been examined to date, yet they are notably absent in other sequenced bifidobacteria, such as B. longum subsp. longum DJO10A [33] and B. adolescentis ATCC15703 (GenBank AP009256), which grow weakly or not all (respectively) on HMO [29]. The elegance of microbial genetics is illustrated by the HMO-related gene set shared between ATCC15697 and DJO10A. This seven-gene operon is responsible for LNB metabolism further evidence of evolution selecting for metabolic substrate utilization [34]. Given that DJO10A is able to weakly grow on HMO and glycoprofiling indicated a small consumption of LNnT, it is tempting to speculate that this operon is linked to consumption of that particular HMO moiety.
While it is very hard to generalize the mechanisms of HMO catabolism across bifidobacteria because of strain heterogeneity and taxonomic confusion [35] within the genera, several important trends have emerged. The most common infant-borne bifidobacteria, B. bifidum, B. longum subsp. infantis, B. longum subsp. longum, and B. breve, possess different modes for consumption of HMO (Fig. 8.6). B. longum subsp. infantis likely imports the lower molecular weight oligosaccharides via an army of dedicated ABC transporters. Once inside the cell, these oligosaccharides are catabolized by a complement of glycosidases prior to entry of the monosaccharides into central metabolic pathways. In contrast, B. bifidum exports fucosidases and lacto-N-biosidase to remove LNB from the HMO structure (leaving the free fucose and sialic acid behind) [48], internalize the free LNB and catabolize it intracellularly. Both B. breve and B. longum subsp. longum are able to consume free LNnT from an HMO pool, whereas B. breve can also grow on the various monomer constituents of HMO [48]. These different strategies suggest a possible mechanism for niche partitioning among the different bifidobacterial species within the developing infant gastrointestinal tract microbiota. Taken together this data provides a mechanism of action for glycan structures.
8.4.2 Prebiotics for Infant Gut Bifidobacteria
Breastfed infants that are colonized by protective strains of bifidobacteria benefit from the microbial activities within their developing intestine [36], which supports a valuable function in vivo for the emergence and persistence of glycans. Henry Tissier observed by microscopic analysis and culture techniques that the feces of breastfed infants were unique in containing a bacterial isolate he termed “Bacillus bifidus communis” [37]. For the 100 years since that initial identification, methodological techniques have wrestled to accurately type much less understand the specific bacteria within breastfed infants largely due to technical problems [38–40]. The challenges have become understandable in retrospect. Initial, culture-based studies failed to isolate significant proportions of bifidobacteria from infants, but these culture techniques failed to appreciate the oxygen sensitivity of infant bifidobacteria and were omitted. The major breakthroughs in DNA-based culture-independent methods should have identified bifidobacteria, yet unfortunately the 16s rDNA primers that are the basis of detection in these methods were not designed to effectively amplify bifidobacteria. Finally, both 16s rDNA surveys and metagenomic techniques that ostensibly sequence all DNA and again should have unequivocably identified bifidobacteria failed to appreciate the physical integrity of the double cell wall of bifidobacteria and the need to selectively handle the disruption of these barriers to DNA release for sequencing. These technical difficulties are now being resolved, and accurate measures of infant fecal microbiota are now available. With these techniques in place, studies are demonstrating very high proportions of specific strains of bifidobacteria in breastfed infants prior to transition to an adult microbiota [41]. The analyses of the breastfed intestinal track have revealed Bifidobacterium longum and B. breve with B. bifidum and B. pseudocatenulatum and B. catenulatum also present [42].
The basic concept that milk itself was influencing the microbial population was proposed by Gorgy and colleagues [43] on the basis of observations that B. bifidum (then termed Lactobacillus bifidus) grew on human milk fractions. The concept however implied that there was a single component responsible, the so-called Bifidus factor. Various studies since have demonstrated that human milk does indeed contain indigestible matter that since humans cannot break them down into digestible monomers would invariably reach the intestine [44–46]. The selectivity of growth promotion by bifidobacterial species growing on human milk oligosaccharides was first demonstrated in vitro by Ward et al. [47, 48]. A series of detailed studies have extended this initial observation demonstrating that only certain bifidobacterial species consume the majority of the stereospecific oligosaccharides of human milk [1, 25, 42]. Within specific strains, growth on oligosaccharides differed leading to the conclusion that B. infantis and select B. breve preferentially consume fucosylated and sialylated HMOs. These results indicate that bifidobacterial strains that grow well on specific glycan structures possess genetic adaptations for select growth on human milk in the infant intestine [32, 49].
The interaction between human milk oligosaccharides and bifidobacteria provides a unique opportunity to map the continuum of metabolites from a food, through the genetics of their disassembly by a “consumer” through the metabolic pathways that utilize them for growth. The genes in bifidobacteria that specifically bind and catabolize HMOs for energy have been identified, expressed, and verified for enzymatic activity [25, 28, 50–53]. The process of annotating these genes has demonstrated that different bifidobacterial species grow on HMO by distinct catalytic mechanisms. B. infantis possess a 43-kb gene cluster (termed HMO cluster I), encode for glycosyl hydrolases, and transport systems using a unique and highly efficient pathway to internalize and metabolize milk oligosaccharides [54, 55]. In contrast, B. bifidum is equipped with genes encoding a different set of catalytic activities toward HMO consumption. This strain exports fucosidases and a lacto-N-biosidase to hydrolyze lacto-N-biose from HMO structures which is in turn transported into the bacterium and metabolized [56].
The process of annotating the detailed mechanisms of the metabolism of human milk oligosaccharides by bifidobacteria has revealed consequences of that metabolism that were unanticipated. This group of metabolites causes a fundamental shift in the phenotype of the bacterium itself. Milk oligosaccharides trigger a specific HMO phenotype to B. infantis. In effect the bacterium shifts to a phenotypic state that is linked to its competitive success in establishing itself within the microbial ecosystem. The phenotype is also associated with interactions between the bacterium and the infant host. Chichlowski et al. [57] reported that the HMO-specific phenotype of B. infantis ATCC15697 on HMOs increases binding to intestinal epithelial cells in vitro. These studies suggest that the specific phenotype of bifidobacterial populations grown on human milk oligosaccharides as metabolites provides mechanisms to the organism supporting greater growth, microbiota persistence interactions with the host epithelium. This model of a metabolically distinct bacterial population induced by its “food” source is supported by in vivo administration of B. infantis to premature infants fed either formula or breast milk. The human breast milk-fed infants, when supplemented with B. infantis, had increases in fecal bifidobacteria and decreases in γ-Proteobacteria compared with the formula-fed group [58]. The ability of these specific bacteria to deconstruct HMOs that is encoded in their genome suggests the co-evolution of human lactation and specific commensal organisms. Thus, mothers are shaping the protective milk-oriented microbiota (MOM) of their infants through breast milk (Fig. 8.8) [53, 59]. This is one example of how milk glycans are being annotated.
Fecal analyses of a single infant at several time points through the first 3 months of life. Above are shown the oligosaccharides from human milk in the infant feces, normalized to week 1 in which all oligosaccharides from milk appeared unmodified in the feces. Successive weeks later time points illustrate the oligosaccharide abundances in the feces relative to week 1. Below is shown the bacteria profile of the same fecal samples through the first 3 months of life. (modified from de Leoz [60] unpublished)
8.5 A Vision for Metabolomics in the Future
The science of nutrition is faced with a daunting challenge: improving human health. The enabling principles of reductionist chemistry that were so effective in identifying essential nutrients are failing to address the more complex problems of non-communicative but diet-dependent diseases that are epidemic around the world. Single molecules delivered to everyone in the population will not solve these problems. Nutrition as a field must lead the world into more integrative, biology-driven strategies that are not only quantitatively precise and mechanistically complete but mapped to actual foods and deployable as individual solutions. The first proofs of principle of such strategies are emerging from the, admittedly more narrowly defined, nourishment of breastfed infants. The toolsets of systems biology including genomics, metabolomics, proteomics, and glycomics have shown their power to interrogate the complexity of milk and reveal how it accomplishes an astonishingly successful biological feat, the colonization and development of the infant microbiota. Evolution clearly identified this to be an important target for mammalian health. Human mothers are nourishing the bacteria within their infants almost as enthusiastically as their infants. Yet, the strategy of nourishing the infant microbiota is a lesson for all of nutrition research. Rather than a single, simple molecule, the mammary gland produces an entire metabolome that includes: a spectrum of complex oligosaccharides and glycans that evade digestion by the infant and continue through to the infant’s lower intestine. The complexity of glycans provides an intense selectivity that rewards only those bacteria genetically capable digesting the glycans and accessing their sugars. The combination of glycan complexity as available substrate and genetic capability as enzymatic specificity is a model for nutrition’s microbiota research going forward. The knowledge assembled to date has begun the process of mapping the detailed mechanistic understanding of the functions of different microbial ecosystems in the infant. Key questions remain: How does a particular microbiota protect infants from pathogens and what are its weaknesses? How does a particular microbiota educate immunity in the face of the bewildering array of both pathogenic insults and completely benign passersby, and what are the causes of its failures? How does the microbiota prevent the massive activation of immunity and the anticipated increase in inflammation that would be expected from dropping a naïve, ostensibly sterile infant in the “real world,” and can we apply these same principles to adults? How does a particular influence whole body metabolism and ensure appropriate food intake and suitable direction of fuels to peripheral tissues, and could these same mechanisms take visceral fat out of adults and put back “baby fat”? The successes of the first generation of metabolomics research tools applied to understand the interactions between mammary-produced oligosaccharides, and the infant microbiota are a glimpse of what this new field of biology can achieve.
References
Smilowitz JT, O’Sullivan A, Barile D, German JB, Lönnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr. 2013;143(11):1709–18.
Molinari CE, Casadio YS, Hartmann BT, Arthur PG, Hartmann PE. Longitudinal analysis of protein glycosylation and β-casein phosphorylation in term and preterm human milk during the first 2 months of lactation. Br J Nutr. 2012;1(1):1–11.
Froehlich J, Dodds E, Barboza M, McJimpsey E, Seipert R, Francis J, et al. Glycoprotein expression in human milk during lactation. J Agric Food Chem. 2010;58(10):6440–8.
Uchiyama S-i, Sekiguchi K, Akaishi M, Anan A, Maeda T, Izumi T. Characterization and chronological changes of preterm human milk gangliosides. Nutrition. 2011;27(10):998–1001.
Liao Y, Alvarado R, Phinney B, Lönnerdal B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J Proteome Res. 2011;10(4):1746–54.
Ruvoën-Clouet N, Le Pendu J, Mas E, Marionneau S, Guillon P, Lombardo D. Bile-salt-stimulated lipase and mucins from milk of ‘secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem J. 2006;393:627–34.
Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14(8):351–60. Epub 2008/07/09.
Aoki-Kinoshita KF. An introduction to bioinformatics for glycomics research. PLoS Comput Biol. 2008;4(5):e1000075.
Reinhold V, Zhang H, Hanneman A, Ashline D. Toward a platform for comprehensive glycan sequencing. Mol Cell Proteomics. 2013;12(4):866–73.
Ninonuevo MR, Perkins PD, Francis J, Lamotte LM, LoCascio RG, Freeman SL, et al. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J Agric Food Chem. 2008;56(2):618–26. Epub 2007/12/20.
De Leoz ML, Wu S, Strum JS, Ninonuevo MR, Gaerlan SC, Mirmiran M, et al. A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal Bioanal Chem. 2013;405(12):4089–105. Epub 2013/03/08.
Lebrilla CB. Is high throughput glycomics possible? Mass Spectrom (Tokyo). 2013;2(Spec Iss):S0016. doi:10.5702/massspectrometry.S0016. Epub 2013 Apr 15.
Sela DA, Garrido D, Lerno L, Wu S, Tan K, Eom HJ, et al. Bifidobacterium longum subsp. Infantis ATCC 15697 α-fucosidases are active on fucosylated human milk oligosaccharides. Appl Environ Microbiol. 2012;78(3):795–803.
Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9(8):4138–51.
Wu SA, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10(2):856–68.
Ninonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B, Lebrilla C. Electrophoresis. 2005;26:3641–9.
Leo F, Asakuma S, Fukuda K, Senda A, Urashima T. Determination of sialyl and neutral oligosaccharide levels in transition and mature milks of Samoan women, using anthranilic derivatization followed by reverse phase high performance liquid chromatography. Biosci Biotechnol Biochem. 2010;74(2):298–303.
Ninonuevo MR, Ward RE, LoCascio RG, German JB, Freeman SL, Barboza M, et al. Methods for the quantitation of human milk oligosaccharides in bacterial fermentation by mass spectrometry. Anal Biochem. 2007;361(1):15–23.
Messer E, Trifonoff W, Stern JG, Collins JH. Bradbury structure of a marsupial-mild trisaccharide. Carbohydr Res. 1980;83:327–34.
Tao N, Wu S, Kim J, An HJ, Hinde K, Power ML, et al. Evolutionary glycomics: characterization of milk oligosaccharides in primates. J Proteome Res. 2011;10(4):1548–57. Epub 2011/01/11.
Coppa GV, Gabrielli O, Pierani P, Catassi C, Carlucci A, Giorgi PL. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics. 1993;91(3):637–41.
Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, et al. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011;128(6):e1520–31.
Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722.
Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54(20):7471–80. Epub 2006/09/28.
LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, et al. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. 2007;55(22):8914–9. Epub 2007/10/06.
Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18(7):298–307. Epub 2010/04/23.
Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast-and formula-fed infants during the first 18 months of life. Microbiology. 2010;156(11):3329–41.
Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10(5):507–14. Epub 2011/11/01.
Locascio RG, Ninonuevo MR, Kronewitter SR, Freeman SL, German JB, Lebrilla CB, et al. A versatile and scalable strategy for glycoprofiling bifidobacterial consumption of human milk oligosaccharides. Microb Biotechnol. 2009;2(3):333–42. Epub 2009/05/01.
Wada J, et al. Bifidobacterium bifidum lacto-N-biosidase, a critical enzyme for the degradation of human milk oligosaccharides with a type 1 structure. Appl Environ Microbiol. 2008;74(13):3996–4004.
Suzuki R, et al. Structural and thermodynamic analyses of solute-binding protein from Bifidobacterium longum specific for core 1 disaccharide and lacto-N-biose I. J Biol Chem. 2008;283(19):13165–73.
Sela D, Chapman J, Adeuya A, Kim J, Chen F, Whitehead T, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci. 2008;105(48):18964.
Lee JH, et al. Comparative genomic analysis of the gut bacterium Bifidobacterium longum reveals loci susceptible to deletion during pure culture growth. BMC Genomics. 2008;9:247.
Mattarelli P, Bonaparte C, Pot B, Biavati B. Proposal to reclassify the three biotypes of Bifidobacterium longum as three subspecies: Bifidobacterium longum subsp. longum subsp. nov., Bifidobacterium longum subsp. infantis comb. nov. and Bifidobacterium longum subsp. suis comb. nov. Int J Syst Evol Microbiol. 2008;58(Pt 4):767–72.
Falk P, Roth KA, Boren T, Westblom TU, Gordon JI, Normark S. Proc Natl Acad Sci U S A. 1993;90:2035.
Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci. 2000;97(22):12222–6.
Tissier H. Recherches sur la flore intestinale des nourrissons: état normal et pathologique: G. Carré et C. Naud; 1900.
Sakata S, Tonooka T, Ishizeki S, Takada M, Sakamoto M, Fukuyama M, et al. Culture‐independent analysis of fecal microbiota in infants, with special reference to Bifidobacterium species. FEMS Microbiol Lett. 2005;243(2):417–23.
Roger LC, McCartney AL. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology. 2010;156(11):3317–28.
Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30(1):61–7.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7.
Turroni F, Bottacini F, Foroni E, Mulder I, Kim JH, Zomer A, et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc Natl Acad Sci U S A. 2010;107(45):19514–9. Epub 2010/10/27.
György P, Norris RF, Rose CS. Bifidus factor. I. A variant of Lactobacillus bifidus requiring a special growth factor. Arch Biochem Biophys. 1954;48(1):193–201.
Coppa G, Pierani P, Zampini L, Bruni S, Carloni I, Gabrielli O. Characterization of oligosaccharides in milk and feces of breast-fed infants by high-performance anion-exchange chromatography. Bioactive components of human milk. Springer; 2001. p. 307–14. ISBN 978-1-4613-5521-2.
Chaturvedi P, Warren CD, Buescher CR, Pickering LK, Newburg DS. Survival of human milk oligosaccharides in the intestine of infants. Bioactive components of human milk. Springer; 2001. p. 315–23. ISBN 978-1-4613-5521-2.
Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71(6):1589–96.
Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl Environ Microbiol. 2006;72(6):4497–9.
Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol Nutr Food Res. 2007;51(11):1398–405.
Ruiz-Moyano S, Totten SM, Garrido DA, Smilowitz JT, German JB, Lebrilla CB, et al. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of bifidobacterium breve. Appl Environ Microbiol. 2013;79(19):6040–9. Epub 2013/07/31.
Garrido D, Nwosu C, Ruiz-Moyano S, Aldredge D, German JB, Lebrilla CB, et al. Endo-beta-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol Cell Proteomics. 2012;11(9):775–85. Epub 2012/06/30.
Garrido D, Ruiz-Moyano S, Jimenez-Espinoza R, Eom HJ, Block DE, Mills DA. Utilization of galactooligosaccharides by Bifidobacterium longum subsp. infantis isolates. Food Microbiol. 2013;33(2):262–70. Epub 2012/12/04.
Garrido D, Ruiz-Moyano S, Mills DA. Release and utilization of N-acetyl-D-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe. 2012;18(4):430–5. Epub 2012/05/15.
Zivkovic AM, Lewis ZT, German JB, Mills DA. Establishment of a milk-oriented microbiota (MOM) in early life: how babies meet their MOMs. Funct Food Rev. 2013;5:3–12.
Garrido D, Dallas DC, Mills DA. Consumption of human milk glycoconjugates by infant-associated bifidobacteria: mechanisms and implications. Microbiology. 2013;159(Pt 4):649–64. Epub 2013/03/06.
Garrido D, Kim JH, German JB, Raybould HE, Mills DA. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS One. 2011;6(3):e17315.
Kitaoka M. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv Nutr: Int Rev J. 2012;3(3):422S–9S.
Chichlowski M, De Lartigue G, German JB, Raybould HE, Mills DA. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastroenterol Nutr. 2012;55(3):321–7. Epub 2012/03/03.
Underwood MA, Kalanetra KM, Bokulich NA, Lewis ZT, Mirmiran M, Tancredi DJ, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatr. 2013;163(6):1585–91.e9. doi:10.1016/j.jpeds.2013.07.017. Epub 2013 Aug 29.
Bode L, Jantscher-Krenn E. Structure-function relationships of human milk oligosaccharides. Adv Nutr: Int Rev J. 2012;3(3):383S–91.
De Leoz ML, Kalanetra K, Bokulich N, Strum J, Underwood M, German J, Mills D, Lebrilla C. Determination of human milk glycomics and gut microbial genomics in infant feces shows correlation between lactation and development of gut microbiota. (2014 unpublished)
Acknowledgements
We acknowledge all of the researchers in the UC Davis Foods for Health Institute and the Milk Bioactives Program for their enthusiasm, imagination, and collective contribution to this subject matter. The work by the Milk Bioactives Program has been supported by the UC Davis Research Investments in the Sciences and Engineering Program; the UC Discovery Grant Program; the California Dairy Research Foundation; the Dairy Research Institute; the Bill & Melinda Gates Foundation; and the National Institutes of Health awards R01HD059127, R01HD065122, R01HD061923, R21AT006180, and R01AT007079. D.A.M. acknowledges support as the Peter J. Shields Endowed Chair in Dairy Food Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
German, J.B., Smilowitz, J.T., Lebrilla, C.B., Mills, D.A., Freeman, S.L. (2015). Metabolomics and Milk: The Development of the Microbiota in Breastfed Infants. In: Kochhar, S., Martin, FP. (eds) Metabonomics and Gut Microbiota in Nutrition and Disease. Molecular and Integrative Toxicology. Springer, London. https://doi.org/10.1007/978-1-4471-6539-2_8
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6539-2_8
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6538-5
Online ISBN: 978-1-4471-6539-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)