Abstract
Various types of cementless implants with rough or porous-coated surfaces for ongrowth or ingrowth have been used or are currently in use and have shown satisfactory mid- and long-term clinical results [1–4]. In no more than 60–70 % of these surfaces, direct bone apposition has been observed [5–8].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Various types of cementless implants with rough or porous-coated surfaces for ongrowth or ingrowth have been used or are currently in use and have shown satisfactory mid- and long-term clinical results [1–4]. In no more than 60–70 % of these surfaces, direct bone apposition has been observed [5–8].
Trabecular metal (TMT), a three-dimensional structure made of tantalum with interconnecting pores throughout its volume, was developed in an effort to maximize volumetric porosity and improve the microenvironment for bone ingrowth (Fig. 9.1). Unlike most contemporary implants which are made of solid metal, trabecular metal is a space frame with a structure that closely resembles the structure and the mechanical properties of cancellous bone [9]. Tantalum is a relatively soft metal, biologically inert and highly resistant to corrosion and erosion. Medical implants used over the past seven decades like electrodes for pacemakers, femoral stems, and dental implants have proved its safety and biocompatibility. Currently no data supports any possible biological activity of tantalum microparticles and tantalum ions [10].
Structure
Trabecular metal is a composite porous material. Its three-dimensional frame is made of amorphous carbon, and tantalum metal covers this substrate by plasma-spray deposition techniques. Both the pore size and the amount of tantalum deposition can be regulated through the fabrication method, and thus the mechanical properties can be altered [9]. Typically, it is in use for orthopedic applications, its pore size ranging between 400 and 600 μm and with porosity of up to 75–85 % of its entire volume. Porosity, pore size, and elasticity of TMT highly resemble cancellous bone and so does its friction coefficiency which is 40–75 % higher than conventional porous materials [9, 10, 11].
Experimental Data
Animal studies have shown rapid bone ingrowth in TMT implants and no implant-related adverse effects. In vitro experiments demonstrated that pretreated Ta and Ti plates are more resistant to bacteria adhesion [12]. Miyazaki T et al. [13] reported that bone creates a chemical bond with titanium and tantalum plates treated with NaOH in stimulated body fluid. They call this first layer of Ta-Bone tantalite. In an environment that resembles in vivo conditions (stimulated body fluid, SBF), alkali-treated Ti and TMT plates were found to induce apatite formation and direct bonding of the metal/apatite layer to bone. Bobyn et al. [9] have used a canine transcortical model in order to test bone ingrowth and implant stability. Rapid bone ingrowth was evident while 42 % of pores were found to be filled in the 4th week, 63 % in the 16th, and 80 % in the 52nd week. In pullout tests, resistance to shearing was significantly higher compared with porous-coated CoCr surfaces. Bobyn et al. [14] have also implanted 22 total hip arthroplasties with a cup made of TMT in dogs. The interface was examined 6 months postimplantation histologically and by electron microscopy. Bone ingrowth was evident in all specimens ranging from 0.2 to 2 mm in depth while the extent of the bone formation both as a percentage of the implant surface and in depth was found to be comparable with wire-mesh-covered Ti implants. Hacking et al. [15] and more recently Reach et al. [16] studied the ingrowth of fibrous tissue in TMT. They demonstrated that vascularized fibrous tissue rapidly filled the entire volume of the implant. The strength of the tendon-implant bone interface was found to be 99 % of the strength of the normal tendon attachment at 6 weeks and 140 % 12 weeks postimplantation. More interestingly, histology revealed the formation of Sharpey-like fibers within the TMT washers.
Retrieval Studies
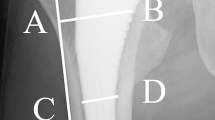
Although bone ingrowth in TMT has been experimentally tested and proved clinically by radiological and outcome studies, data from retrieval studies are rare. D’Angelo et al. [17] performed a histological evaluation of bone-implant interface in a human specimen removed from a patient. Their study was based on polarized light microscopy, and they reported 90 % of pore filling by bone. According to our own unpublished data from a retrieved stable implant, ingrowth of bone was complete in the first two rows of cells (2–3 mm) while vascularized fibrous tissue was evident beyond the second row, and in areas of the surface that there was not bone formation. During ingrowth, bone follows the topology of the scaffold by attaching to the tantalum struts and then fills the empty space of the cells. In other words, bone attaches first to the cell boundary and then grows further to the interior of the cell. By applying EDS elemental analysis, we could compare the composition of new bone material within the cells of the trabecular metal with that of the bone attached to the surface of the cup. The bone material grown inside the first row of cells had almost identical composition with the attached bone verified by similar Ca:P ratio, indicating complete densification into hydroxyapatite. However, the composition of the bone-like material in the second row of cells had a different composition (Fig. 9.2).
Clinical Studies
TMT has been used up today in a variety of implants and clinical experience with some of them exceeds one decade. Historically the first implant released for commercial use was an acetabular component. This cup incorporated, besides a TMT metal shell, a number of unique design characteristics. The TMT cup is elliptic in shape, designed for an interference fit at the rim of the prepared acetabular cavity and compact with the compression-molded polyethylene liner (sterilized with gamma radiation) solidly seated in the porous tantalum cell. The Monoblock TMT cup has a modulus of elasticity almost identical with subchondral bone. As the cup is elliptical, peripheral fit may prevent the complete sitting of the cup in the prepared acetabular cavity. As a consequence, gaps at the interface of the dome have been reported to be relatively frequent (13–32 %) [18–21]. Gruen et al. [21] reported that 84 % of these gaps were filled within 5 years. In our series [18], big polar gaps did not fill entirely, but they did not compromise the stability of the cup.
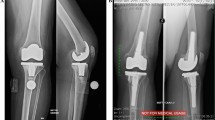
Several authors have reported satisfactory midterm results with this construct [18–25]. We have reported [18, 20] excellent midterm results with this particular implant demonstrating that all cups were radiographically stable with a follow-up ranging between 3 and 9 years with an overall survivorship of 98.75 %. Furthermore, in serial radiographs, thickening of the trabeculae and increased bone density at the periphery of the cup as well as at the dome were observed. This was attributed to the load transfer pattern and the elasticity of the cup. Recent studies proved that instead of stress shielding that occurs behind Ti and CoCr cementless cups, there is increased bone density and remodelling of the subchondral bone with TMT cups [26, 27] (Fig. 9.3).
Trabecular metal implants have been used for acetabular revision surgery also. An acetabular component made of TMT with multiple screw holes is available. The cup can be fixed in a fashion that allows maximum contact of metal with viable bone, and the liner can be cemented in the desired anteversion and inclination for joint stability. Augments are available for the filling of rim and wall deficiencies. They are secured with screws and filled with bone graft, and the cup is then placed and secured. It has been proposed that a thin cement layer be placed between the two implants to prevent the production of microparticles [28, 29]. The augments support the cup in a similar fashion with structural allografts. The theoretical advantage is that augments allow bone ingrowth, and they are not subject to resorption and fatigue fractures as do the structural grafts. Few studies with optimal results have been published with revision TMT cups and the use of augments. Although bone ingrowth and healing of the pelvic discontinuity is evident in serial radiographs, the long-term behavior of this implant is as yet unknown [30–33].
A cementless tibia tray made of porous tantalum with a compression-molded polyethylene is also available. Clinical studies with relatively short follow-up time demonstrate encouraging results [34, 35]. A recent radiostereometric comparison of TMT versus a titanium tibia demonstrated a higher rate of posterior tilt and migration of the TMT tray but no loosening or revision [36]. Recent studies also suggest that stress shielding does not occur in the metaphyseal area of the tibia [37, 38].
A variety of new applications and materials are currently under development, and titanium-made porous materials resembling TMT are already being marketed. Trabecular bone-like materials represent a novel approach for cementless metallic implants (Fig. 9.4). Experimental data and retrievals support the fact that bone ingrowth is both rapid and to a better extent than traditional surfaces. Short- and midterm clinical results of tantalum trabecular metal implants support this hypothesis. Yet, the long-term clinical performance and the significance of the unique properties of the material described above require further investigation and experience. Surgeons should use TMT and other porous materials with caution based on site and implant-specific studies, keeping in mind that TMT is still a very promising but costly alternative for hip and knee replacement surgery.
References
Böhm P, Bösche R. Survival analysis of the Harris-Galante I acetabular cup. J Bone Joint Surg Br. 1998;80B:396–403.
Huo MH. What’s new in hip arthroplasty. J Bone Joint Surg Am. 2002;84A:1894–905.
Latimer HA, Lachiewicz PF. Porous-coated acetabular components with screw fixation: five to ten-year results. J Bone Joint Surg Am. 1996;78A:975–81.
Udomkiat P, Dorr LD, Wan Z. Cementless hemispheric porous-coated sockets implanted with press-fit technique without screws: average ten-year follow-up. J Bone Joint Surg Am. 2002;84A:1195–2000.
Engh CA, Zettl-Schaffer KF, Kukita Y, Sweet D, Jasty M, Bragdon C. Histological and radiographic assessment of well functioning porous-coated acetabular components. A human postmortem retrieval study. J Bone Joint Surg Am. 1993;75A:814–24.
Bobyn JD, Tanzer M, Miller JE. Chapter 9. Fundamental principles of biologic fixation. In: Morrey BF, editor. Reconstructive surgery of the joints. New York: Churchill Livingstone; 1991. p. 75–94.
Bragdon CR, Jasty M, Lowenstein JD, Burke DW. The histology of bone ingrowth at the implant/bone interface under known amount of micromotion. Trans Orthop Res Soc. 1993;18:468–73.
Pidhorz LE, Urban RM, Jacobs JJ, Sumner DR, Galante JO. A quantitative study of bone and soft tissues in cementless porous-coated acetabular components retrieved at autopsy. J Arthroplasty. 1993;8:213–25.
Bobyn JD, Stackpool GJ, Hacking A, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81B:907–14.
Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006;27:4671–81.
Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin Orthop. 1980;150:263–70.
Schildhauer TA, Robie B, Muhr G, Köller M. Bacterial adherence to tantalum versus commonly used orthopedic metallic implant materials. J Orthop Trauma. 2006;20:476–84.
Miyazaki T, Kim HM, Kokubo T, Ohtsuki C, Kato H, Nakamura T. Mechanism of bonelike apatite formation on bioactive tantalum metal in a simulated body fluid. Biomaterials. 2002;23:827–32.
Bobyn JD, Toh KK, Hacking A, Eng M, Tanzer M, Krygier JJ. Tissue response to porous tantalum acetabular cups. A canine model. J Arthroplasty. 1999;14:347–53.
Hacking SA, Bobyn JD, Toh K, Tanzer M, Krygier JJ. Fibrous tissue ingrowth and attachment to porous tantalum. J Biomed Mater Res. 2000;52:631–8.
Reach Jr JS, Dickey ID, Zobitz ME, Adams JE, Scully SP, Lewallen DG. Direct tendon attachment and healing to porous tantalum: an experimental animal study. J Bone Joint Surg Am. 2007;89A:1000–9.
D’Angelo F, Murena L, Campagnolo M, Zatti G, Cherubino P. Analysis of bone ingrowth on a tantalum cup. IJO. 2008;42:275–8.
Malizos KN, Bargiotas K, Papatheodorou L, Hantes M, Karachalios T. Survivorship of monoblock trabecular metal cups in primary THA: midterm results. Clin Orthop. 2008;466:159–66.
Macheras GA, Papagelopoulos PJ, Kateros K, Kostakos AT, Baltas D, Karachalios TS. Radiological evaluation of the metal-bone interface of a porous tantalum monoblock acetabular component. J Bone Joint Surg Br. 2006;88B:304–9.
Xenakis T, Machairas G, Bargiotas K, Stafylas K, Malizos KN. A multi-center study of the use of a porous Tantalum Monoblock Acetabular component. Five years clinical and radiographic results. Int Orthop. 2009;33:911–6.
Gruen TA, Poggie RA, Lewallen DG, Hanssen AD, Lewis RJ, O’Keefe TJ, et al. Radiographic evaluation of a monoblock acetabular component: a multicenter study with 2 to 5 year results. J Arthroplasty. 2005;20:369–78.
Komarasamy B, Vadivelu R, Bruce A, Karshaw C, Davison J. Clinical and radiological outcome following total hip arthroplasty with an uncemented trabecular metal monoblock acetabular cup. Acta Orthop Belg. 2006;72:320–5.
Mulier M, Rys B, Moke L. Hedrocel trabecular metal monoblock acetabular cups: mid-term results. Acta Orthop Belg. 2006;72:326–31.
Macheras GA, Kateros K, Koutsostathis SD, Tsakotos G, Galanakos S, Papadakis SA. The Trabecular Metal Monoblock acetabular component in patients with high congenital hip dislocation: a prospective study. J Bone Joint Surg Br. 2010;92:624–8.
Simon JP, Bellemans J. Clinical and radiological evaluation of modular trabecular metal acetabular cups. Short-term results in 64 hips. Acta Orthop Belg. 2009;75:623–30.
Wright JM, Pellicci PM, Salvati EA, Ghelman B, Roberts MM, Koh JL. Bone density adjacent to press-fit acetabular components: a prospective analysis with quantitative computed tomography. J Bone Joint Surg Am. 2001;83A:529–36.
Meneghini RM, Ford KS, McCollough CH, Hanssen AD, Lewallen DG. Bone remodeling around porous metal cementless acetabular components. J Arthroplasty. 2010;25:741–7.
Paprosky W, Perona P, Lawrence J. Acetabular defect classification and surgical reconstruction in revision arthroplasty: a 6-year follow-up evaluation. J Arthroplasty. 1994;9:33–44.
Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21(S2):87–90.
Gross AE, Goodman SB. Rebuilding the skeleton: the intraoperative use of trabecular metal in revision total hip arthroplasty. J Arthroplasty. 2005;20 S2:91–3.
Weeden SH, Schmidt RH. The use of tantalum porous metal implants for Paprosky 3A and 3B defects. J Arthroplasty. 2007;22(6 S2):151–5.
Malkani AL, Price MR, Crawford 3rd CH, Baker DL. Acetabular component revision using a porous tantalum biomaterial: a case series. J Arthroplasty. 2009;24:1068–73.
Lachiewicz PF, Soileau ES. Tantalum components in difficult acetabular revisions. Clin Orthop. 2010;468:454–8.
Lingaraj K, Teo YH, Bergman N. The management of severe acetabular bone defects in revision hip arthroplasty using modular porous metal components. J Bone Joint Surg Br. 2009;91B:1555–60.
O’Keefe TJ, Winter S, Lewallen DG, Robertson DD, Poggie RA. Clinical and radiographic evaluation of a monoblock tibial component. J Arthroplasty. 2009;25:785–92.
Henricson A, Linder L, Nilsson KG. A trabecular metal tibial component in total knee replacement in patients younger than 60 years: a two-year radiostereophotogrammetric analysis. J Bone Joint Surg Br. 2008;90:1585–93.
Harrison AK, Gioe TJ, Simonelli C, Tatman PJ, Schoeller MC. Do porous tantalum implants help preserve bone?: evaluation of tibial bone density surrounding tantalum tibial implants in TKA. Clin Orthop. 2010;468:2739–45.
Minoda Y, Kobayashi A, Iwaki H, Ikebuchi M, Inori F, Takaoka K. Comparison of bone mineral density between porous tantalum and cemented tibial total knee arthroplasty components. J Bone Joint Surg Am. 2010;92:700–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Bargiotas, K.A. (2014). Trabecular Metal: Bone Interface in Total Joint Arthroplasty. In: Karachalios, T. (eds) Bone-Implant Interface in Orthopedic Surgery. Springer, London. https://doi.org/10.1007/978-1-4471-5409-9_9
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5409-9_9
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5408-2
Online ISBN: 978-1-4471-5409-9
eBook Packages: MedicineMedicine (R0)