Abstract
A rapid compression machine is a type of ideal internal combustion reactor which is well suited for gas phase kinetics studies and physical processes of combustion. These studies consist in recording the evolution of the reactivity and of the composition of the reacting mixtures as a function of temperature, pressure, residence time and the composition of the compressed mixture. Pressure measurement is used to analyse the evolution of the combustion process, but more recent studies couple pressure measurement with optical diagnostics in order to assess the homogeneity of the combustion process. Speciation based on rapid sampling of the intermediates formed, during the oxidation of the fuel, is typically carried out by gas chromatography.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Computational Fluid Dynamic
- Particle Image Velocimetry
- Combustion Chamber
- Large Eddy Simulation
- Combustion Process
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Overview of the Rapid Compression Machine
The study of the combustion process needs to be performed with experimental devices which can reproduce thermodynamic conditions closed to that of real combustors (internal combustion engines, gas turbines, etc...). Rapid compression machines (RCM), firstly used in 1906, are able to reproduce these thermodynamic conditions (pressure, temperature) by compressing an air/fuel mixture up to the studied conditions—temperatures of 600–1,100 K, pressures of 1–70 bar. Furthermore, the issues encountered in real engines (blow-by, temperature and mixture heterogeneities, varying volume, metallic swarf, etc.) can be avoided. They also present the advantage of allowing an easy access to the combustion chamber for optical measurements or sampling of the mixture. Therefore, RCMs are more and more being used to study the combustion process mainly auto-ignition phenomenon. RCM was firstly used in order to determine the auto-ignition temperature and is now used for extensive study of the combustion properties of fuels.
The compression process has to be as short as possible in order to assume an adiabatic compression process. Commonly rapid compression machines (RCM) are able to achieve this within a few dozen of milliseconds (10–70 ms). After the compression process, the volume of the combustion chamber has to be kept constant in order to maintain constant thermodynamic conditions and allow the study of the combustion process. Usually, the combustion process can be studied between 2 and 500 ms after the end of the compression stroke.
The rapid compression machine can be used to study the auto-ignition phenomenon and the development of combustion. Most studies consist in recording the evolution of pressure as a function of time and determine the ignition delay time. The evolution of the ignition delay time is then studied as a function of equivalence ratio, fuel mixture composition, and the thermodynamic conditions reached at the end of the compression process: End of compression temperature and pressure. To the author’s knowledge, there are an increasing number of RCM (from 5 at the end of the 1980s to more than 20 in 2011) in operation all over the world.
This Chapter will present the different technologies currently used, the validation and the simulation of RCMs and the diagnostics which can be used to study the combustion process.
2 Design of the Rapid Compression Machine
The critical points while designing a rapid compression machine are the quickness of the compression process, thanks to the driver mechanism, and the ability of the machine, thanks to the braking mechanism, to keep constant the thermodynamics conditions at the end of compression process (pressure, temperature and volume) for the study of the combustion process.
The design of the machine must also take into account the thermodynamic conditions which have to be achieved at the end of the compression process in terms of temperature and pressure range. These conditions can be easily defined thanks to the analysis parameter evolution during the compression which can be assumed as adiabatic. Therefore, the end of the compression pressure, p c, and temperature, T c, will only depend on the initial pressure, p i, and temperature, T i, respectively, the geometry of the machine (volumetric compression ratio ε) and the thermodynamic properties of the mixture composition (ratio of heat capacities, C P/C V = γ) see Eqs. (7.1) and (7.2):
A typical pressure curve recorded during RCM experiments (Fig. 7.1 left) includes the compression phase (from A to B) and the constant volume phase (from B to E) during which the auto-ignition phenomenon occurs (from B to D). The pressure decrease which can be noticed during the constant volume phase is due to a cooling effect because of the heat losses. As the temperature decreases under constant volume and mass conditions, this results in a corresponding pressure decrease. The ignition delay time is measured between the end of the compression process (B) and the onset of the combustion (C). Then, the development of the combustion inside the combustion chamber occurs until the pressure reaches the maximum pressure (D). This pressure curve is typical for a single-stage ignition process. During a two-stage ignition process (Fig. 7.1 right), a first heat release, also called “cool flame” (see Chap. 2), is recorded (first stage) and is followed by the main heat release (second stage).
Typical pressure curves measured in the rapid compression machine from NUI Galway (left) and in MIT (right) (Tanaka et al. 2003)
The following section will present the main designs developed in order to achieve these important characteristics. This part will mainly focus on the different strategies used in order to achieve a rapid compression and a constant volume combustion process.
2.1 Review of the Main RCMs Technologies
The first RCM built in 1906 (Falk 1906) was a vertical single piston apparatus whose piston was driven by the fall of a mass. However, there was no braking mechanism. Different technologies are currently used. They differ by the driver mechanism, the stopping mechanism, the number pistons and the size of the combustion chamber. However, three main categories can be defined:
-
Single piston RCM
-
Twin piston RCM
-
Camshaft connection RCM.
2.1.1 Single Piston
The first version of the RCM used in MIT was built in the 1940s. It is a vertical single piston apparatus (Fig. 7.2) fired by the expansion of compressed gas. The piston velocity is controlled by the flow of hydraulic oil. The hydraulic braking mechanism also held the piston at top dead centre (TDC) after the compression process. The stroke of the piston can be changed in order to achieve different end of compression pressure and temperature. This has an impact on the compression time which can vary from 10 to 30 ms.
Cross section view of the MIT rapid compression machine (Lee and Hochgreb 1998; Copyright Elsevier)
The piston has the subject of a particular design in order to limit the temperature heterogeneities inside the combustion chamber. Lee and Hochgreb (1998) carried out a computational fluid dynamics (CFD) study of the aerodynamic and the boundary layer during the compression process. They found that the cold boundary layer is rolled up by the piston during the compression (Fig. 7.3) and results in a vortex which is released at the end of compression. This vortex can be trapped by a specially design crevice behind the piston head.
Sketch of the piston corner vortex (Lee and Hochgreb1998; Copyright Elsevier)
A similar design is used for horizontal machines in Leeds since the late 1970s (Beeley et al. 1979) and in Case Western Research University (CWRU) (Mittal and Sung 2006) since 2006. The machine in CWRU also uses a crevice piston in order to reduce the aerodynamic inside the combustion chamber.
Two RCMs in Japan—one in Tokyo University (Kono et al. 1983) and the other in Keio University (Lim et al. 2004) also use air as a driving force and hydraulic oil in order to control the velocity of the piston and maintain the volume constant after the compression process.
Two fully hydraulically controlled RCMs have also been built. The first one, firstly reported in 2003 (Bysveen and Almas 2003), is used in the Norwegian University of Science and Technology (NTNU); the second one, firstly reported in 2007 (Guibert et al. 2007), was built in University Pierre et Marie Curie (UPMC) in France. This second machine is fully heated thanks to three heating resistance inside the wall of the sleeve and the combustion chamber is wrapped with heating tapes. The aim of such machines is to fully control the piston velocity thanks to a hydraulic cylinder and a servo valve. Thus, the reproducibility of the compression process is guaranteed by the full control of the system.
The last type of single piston RCM was designed by TRW and relocated in the University of Michigan (Donovan et al. 2004). This machine uses a free piston or sabot which is launched by compressed gas. The dimension of this RCM is unusual since the length of the driven section is 2.74 m.
2.1.2 Twin Piston RCM
Two twin piston RCMs are used in the National University of Ireland Galway (NUIG). This machine was firstly designed by Shell-Thornton in 1968, used until the middle of the 1980s and then acquired, re-commissioned and modified by NUIG in the mid 1990s. This machine is composed of two similar systems which include compressed air as a driving force and hydraulic oil in order to control the piston velocity (Fig. 7.4).
This unique design allows reduction of the compression time (below 17 ms) and reducing the aerodynamic thanks to the symmetry of the system (Brett et al. 2001; Würmel et al. 2007a, b). Moreover, a CFD study (Würmel and Simmie 2005) of the combustion chamber was performed. It was shown that there is a stagnation plane in the middle of the combustion chamber. Crevices have also been added to the pistons in order to suppress the vortex created by during the compression phase. This results (Fig. 7.5) in a more homogeneous temperature distribution when crevices are used. A similar RCM was also built in 2005 at Argonne National Laboratory (Gupta et al. 2005).
Temperature distribution inside the combustion chamber at the end of the compression process (top) and during the constant volume phase (bottom) with (right) and without (left) the crevices in the piston head (Würmel and Simmie 2005; Copyright Elsevier)
2.1.3 Camshaft Connection
Two RCMs have been designed with a particular right-angle shape in France. This design solves one of the main problems of RCMs which is to bring the piston to a dead stop at the end of the compression process. The first one, driven thanks to air expansion, was designed in Lille University (Ribaucour et al. 1992) at the end of the 1980s and is composed of two pistons which are perpendicular and are connected by a cam (Fig. 7.6). The second one was designed in the Laboratoire de Combustion et de Détonique in Poitiers (Strozzi et al. 2007). Its driven system is controlled by a hydraulic cylinder.
Sketch of the perpendicular RCM from at Lille University (Carlier et al. 1990)
2.2 Improvements to RCMs
Much effort has gone into improved designs in order to reach steady and homogenous conditions at the end of compression not only to allow a high level of reproducibility but also to create well-defined conditions which can then be successfully simulated. Therefore, research teams often reached the same solutions such as promoting the temperature homogeneity and the control of the velocity of the piston.
Many of the more recent studies have worked with very low vapour pressure (high molecular weight) fuels and this necessitates pre-heating the mixing tank(s), the gas-handling manifold and the RCM itself to high constant initial temperatures in excess of 373 K (see for example Husson et al. 2012). While this is an added complexity, it can reduce the requirement for using high heat capacity ratio, γ, gases to reach high compressed gas temperatures.
2.2.1 Temperature Homogeneity
Lee and Hochgreb (1998) investigated the homogeneity of the temperature inside the combustion chamber, at the end of the combustion chamber and at the combustion process thanks to a CFD study. They noticed that the cold boundary layer along the piston sleeve is rolled up during the compression process and released when the piston reached the TDC. This results in temperature heterogeneities before the combustion process (Fig. 7.5). Therefore, the auto-ignition phenomenon cannot be considered as homogeneous. In order to avoid the issue, they designed a crevice around the piston head in order to trap the cold boundary layer during the compression process. Thus, the temperature is more homogeneous inside the combustion chamber.
In order to improve the impact of the crevice and avoid any disturbance caused by the gases trapped in the crevice during the combustion phase, Mittal and Gupta (2012) computationally studied the impact of using crevice containment thanks to CFD simulations. They implemented a seal between the crevice and the stepped combustion chamber to prevent gas motion from the crevice to the combustion chamber. Results show that there are no negative influences on the flow field and that the roll-up vortex is largely suppressed.
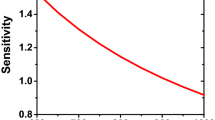
The homogeneity of the temperature also depends on the physical properties of the compressed mixture. The tested mixture is usually composed with fuel, oxygen and a mixture of diluents (carbon dioxide, nitrogen, argon, helium, xenon) in various proportions. Würmel et al. (2007a) studied the impact of different diluent gases on ignition delay times. By using different nature of gases (monatomic and polyatomic), different temperature and pressure range can be achieved. Compared to nitrogen, and due to their higher specific heat capacity ratio, monatomics, such as argon, helium and xenon, will extend upward the temperature and pressure range, whereas polyatomics, such as carbon dioxide will reduce the compressed pressure and temperature. However, monatomics present a lower heat capacity and will result in greater heat losses at the end of the compression process (Fig. 7.7). Thus, during the design, a trade off has to be found between the pressure and temperature range achievable in the RCM and the heat loss issue.
Impact of the diluent on pressure profile (p i = 0.04 MPa, T i = 298 K) (Würmel et al. 2007a; Copyright Elsevier)
2.2.2 Piston Positioning
In the first RCMs, the control of the piston position and speed was only governed by fluid dynamics and mechanics. As the driving force was the expansion of a gas and the control of the velocity of the piston was achieved by hydraulic oil, a careful design of the hydraulic stopping mechanism was needed. After the compression process, both the residual driving pressure and the hydraulic pressure have to ensure that the piston does not move before or during the combustion process. Therefore, the piston displacement profile depends on the opposed pressure applied by the tested mixture on the piston head.
In order to avoid such issues, hydraulic controlled RCM have been developed. The latest one (Guibert et al. 2007) was developed in the Institut Jean Le Rond d’Alembert (UPMC, Paris). They use a hydraulic piston jack to move the piston. This piston jack is connected to a three-stage servovalve which controls the velocity and the position of the piston during and after the compression process. This design allows a closed-loop control of the piston motion and increase the reproducibility of the machine. This piston displacement profile is defined by the user before the experiment and reproduced accurately by the machine during the test.
3 Validation of the Rapid Compression Machine
As shown previously, the design of a rapid compression machine is difficult and the critical issues to overcome are to ensure the constant volume combustion process and the reproducibility of the machine. This following section deals with the assessment of these two key points of RCM.
3.1 Constant Volume and Pressure Process
The validation of such a device has to go through a few steps. First of all, the main goal of a rapid compression machine is the ability to study the combustion process during a constant volume phase. Therefore, the RCM’s ability to stop the piston dead after the compression process is crucial. In order to validate such a process, the position of the piston has to be measured precisely during both the compression phase and the constant volume phase. The constant volume process is usually checked by compressing perfluoropropane (C3F8). This particular gas has a very advantageous property, namely a specific heat ratio close to 1. Therefore, the temperature only increases slightly during the compression process Eq.( 7.2). This phenomenon leads to a much reduced heat loss during the constant volume phase and the resulting pressure is almost constant (Fig. 7.8).
This type of tests is also used in order to control the airtightness of the combustion chamber after the combustion process. Since the piston stops dead, any pressure drop would be due to leaks from the combustion chamber. Therefore, a constant volume profile validates both the airtightness of the combustion chamber and the constant volume profile. Care must be taken not to exceed the saturated vapour pressure of perfluoropropane during the compression otherwise the test is invalid.
3.2 Reproducibility
The reproducibility of the experiments is always critical and it has to be as high as possible. One of the final results measured in RCM is ignition delay times. Their reproducibility is influenced by many factors so of which are not RCM dependent.
First of all, the reproducibility of the compression process with a non-reactive mixture is necessary preliminary for the reproducibility with reactive mixtures and ignition delay time measurements. The second step is the reproducibility of the initial conditions thanks to a control of the initial pressure and temperature and the composition of the tested mixtures. Usually, the tested mixture is prepared manometrically in stainless steel tanks in order to test the same mixture. When all these necessary conditions are achieved, the reproducibility of the RCM for reactive mixtures can be assessed. Figure 7.9 presents a compilation of 10 experiments for a reactive mixture of iso-octane and air. The 10 pressure profiles present an excellent superposition both during the compression and the combustion processes. Due to the accuracy of the control of initial pressure and temperature, a variation of the ignition delay times within ±5 % of the mean measured values is acceptable (Würmel et al. 2007a).
4 Modelling of the Rapid Compression Machine
Different types of model can be used in order to reproduce numerically the behaviour of a rapid compression machine, from the simplest one, only based on thermodynamics to three-dimensional model based on CFD advanced model using large eddy simulation (LES) and direct numerical simulation (DNS).
4.1 Thermodynamic Model
Thermodynamic models are based on the laws of thermodynamics and the ideal gas law. They are mainly used in order to compare the accuracy of chemical kinetics mechanisms with ignition delay times measured in the RCM thanks to chemical kinetics software.
In order to reproduce the behaviour of the rapid compression machine, the volume profile is needed. For each experiment with a reactive mixture (fuel + oxygen + diluents), an experiment with a non-reactive mixture is performed by replacing the oxygen by nitrogen in the test mixture. Because nitrogen and oxygen have similar thermodynamic properties, the recorded pressure profile presents the same pressure drop as the profile with the reactive mixture due to very similar heat loss properties. The reason for recording a pressure profile with a non-reactive mixture for each experiment is to characterise real heat losses in the kinetic simulations. A volume profile is calculated assuming adiabatic compression and expansion processes and frozen chemistry. This volume profile is used as an input in the model. This adiabatic volume expansion assumption is used in order to take into account the cooling effect due to heat losses. According to such assumption, the loss of energy due to heat losses to the wall is modelled via an expansion of the volume of the combustion chamber. Therefore, this approach takes into account a mean temperature inside the combustion chamber and does not account for potential temperature gradient, due to lower temperature zone close to the wall, higher temperature in the hot core region and mixing between these two regions.
Such an assumption is very useful and reduces computing time when detailed chemical kinetics mechanisms are used to model the auto-ignition of the fuel and compare measured ignition delay times with chemical kinetics mechanisms predictions. However, since the entire combustion chamber is modelled as a perfectly stirred reactor assuming a uniform heat release, this approach cannot reproduce non-uniform heat release due to temperature heterogeneities caused by the boundary layer.
4.2 Computational Fluid Dynamics
CFD models have been mainly used in order to study the aerodynamics and the temperature homogeneity inside the combustion chamber (Lee and Hochgreb 1998; Würmel and Simmie 2005; Mittal and Sung 2006) and the impact of the temperature on the combustion process (Mittal et al. 2008; Mittal and Gupta 2012; Lodier et al. 2012; Griffiths et al. 2012). Most of the studies used the Reynolds-Averaged Navier-Stokes (RANS) simulation but more advanced simulation such as DNS and LES have been used by Jakirlić et al. (2000) and Lodier et al. (2012).
Most of these studies aimed at reducing the effect of the aerodynamics inside the combustion chamber during and after the compression process (Lee and Hochgreb 1998; Würmel and Simmie 2005; Mittal and Sung 2006; Mittal and Gupta 2012) in order to create a homogeneous temperature field inside the combustion chamber. Therefore, these CFD studies were performed with a non-reactive mixture and aimed at designing appropriate piston crevices in order to trap the cold boundary layer.
The second type of CFD study includes aerodynamics and kinetics modelling thanks to detailed or reduced chemical kinetics mechanisms (Mittal et al. 2008; Mittal and Gupta 2012; Griffiths et al. 2012). They compared the results of zero-dimensional code with CFD codes and evaluated the assumption of uniform heat release throughout the combustion chamber. For the predictions of single stage ignition and in particular the ignition delay times, results show really good agreement between zero-dimensional model and CFD model. However, the auto-ignition phenomenon is not homogeneous and a disagreement appears for the prediction of the ignition delay time of the second-stage of the ignition process for hydrocarbons showing two-stage ignition (Mittal et al. 2010; Griffiths et al. 2012). For such cases, the impact of the cold boundary layer cannot be neglected since the gases which are colder than the core zone react before the core zone and accelerate the reaction which results in a shorter ignition delay time.
However, this type of simulation only models large-scale fluid motion and smaller scale motion needs to be modelled thanks to more detailed such as DNS and LES. This type of simulation includes the calculation of the turbulence inside the combustion chamber and its potential impact on the combustion process cannot be modelled. Jakirlić et al. (2000) studied the aerodynamics inside a rapid compression machine designed to create a swirling flow. The impact of turbulence on combustion inside a rapid compression machine has been studied numerically by Lodier et al. (2012) based on the design from Guibert et al. (2010). They reported different ignition behaviours from homogeneous ignition process to localised spot ignition.
5 Diagnostics and Typical RCM Results
Different diagnostics can be used in RCM in order to study the physics and kinetics prior to and during combustion process. The main diagnostic used in RCM is the measurement of instantaneous pressure inside the combustion chamber thanks to pressure transducer. More complex diagnostics are also used in order to study the development of the combustion process such as rapid sampling coupled with gas chromatography and optical diagnostics with or without on laser-based diagnostics.
5.1 Pressure Profile
Assuming an adiabatic compression process Eq.( 7.3), the pressure profile is used to determine the temperature reached at the end of the compression process, T c, based on the initial pressure, p i, and temperature, T i and the pressure at TDC, p c.
The ignition delay times are also measured thanks to the pressure profile. By varying the initial conditions (pressure, temperature, equivalence ratio, …), it is possible to obtain the evolution of the ignition delay times as a function of temperature and/or equivalence ratio (Fig. 7.10) (Gallagher et al. 2008).
Effect of equivalence ratio on ignition delay time for propane oxidation in “air,” p c ≈ 30 atm. (filled square) φ = 0.5; (filled circle) φ = 1.0; (filled triangle) φ = 2.0. Solid lines HCT simulations, dashed lines RCMBL simulations (code for simulating RCM assuming adiabatic core region and thermal boundary layer) (Gallagher et al. 2008; Copyright Elsevier)
5.2 Optical Diagnostics
The second major area of interest in this field is the optical visualisation of combustion propagation during the combustion process, and is of relevance both to the chemical kineticists and also to fluid dynamicists and engineers. Optical visualisation utilises a wide variety of methods in order to assess different parameters including fuel mixture and temperature gradient and subsequent flame initiation homogeneity, spray visualisation for liquid fuels and the visualisation of the fluid dynamics. Thanks to the constant volume process, RCM can provide an important optical access to the whole combustion event. Different techniques can be used in order to study the initiation and the development of the combustion process.
5.2.1 Direct Visualisation
Simple diagnostics such as direct visualisation of the chemiluminescence and Schlieren imaging can be used to examine the combustion process and analyse its development (Walton et al. 2007; Guibert et al. 2010). In the Schlieren imaging process, a high-intensity point light source is refracted through a gathering lens and the parallel beams of light directed through the combustion chamber. At the exit port, the parallel beams are then re-focused onto the camera CCD array. The interference of light generated by the combustion event is recorded as grey scale images and shows the propagation of the ignition event over time. Since the time from full compression to initiation of ignition is measured in milliseconds, an ultra high speed camera is required. This technique also relies on two diametrically opposed optical access windows. This technique is well established and would provide a relatively simple first run analysis of chamber homogeneity. However, it does not provide a planar image or slice through the access of interest, providing instead a three-dimensional “shadow” of the event. It can be used to show if heterogeneity exists but does not exclusively prove homogeneity (Strozzi et al. 2008).
Guibert et al. (2010) used direct visualisation and noticed a development of the combustion through the combustion chamber (Fig. 7.11). Optical band-pass filter can be added to the camera in order to select particular wavelength corresponding to the emission of selected species (for example, excited OH*: 308 nm).
Direct visualisation of the combustion process of 1-hexene/air mixture (T c = 840 K, p c = 36.6 bar, equivalence ratio: 0.4, Frame rate: 6,250 image/s) (Guibert et al. 2010; Copyright Elsevier)
5.2.2 Laser-Based Diagnostics
Laser-based diagnostics have been used in order to study the thermodynamics conditions (temperature, homogeneity) prior to the combustion event, the aerodynamics and the combustion process.
*Laser Induced Fluorescence (LIF)
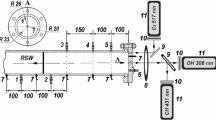
Laser-based diagnostics, such as LIF (for more details about LIF, see Chap. 12), increase the accuracy of the analysis, by exclusively viewing a perpendicular radial plane within the combustion chamber (Clarkson et al. 2001; Strozzi et al. 2009; Tran et al. 2011). This technique requires an optical window perpendicular to the viewing port. Laser light of a specific wavelength is focused to create a planar beam or sheet of light which bisects the volume of interest. The laser light excites specific species on this plane and they re-emit light at a selected wavelength during the relaxation through fluorescence, and it is therefore possible to scrutinise the behaviour of important intermediate species such as OH and CH. Optical filters are then used to isolate the specific wavelength and species concentrations can be recorded using a high-speed camera mentioned. Areas with higher concentrations of these species and/or higher temperature will emit light at a higher intensity, and therefore heterogeneity in terms of light-intensity gradients will indicate areas of thermal gradients. However, only a few studies have been reported in RCM. Figure 7.12 represents the temperature field measured thanks to LIF inside the combustion chamber of a RCM.
Temperature field in the chamber of RCM measured by LIF (Strozzi et al. 2009; Copyright IOP Publishing)
*Particle Image Velocimetry (PIV)
PIV is widely used to characterise the velocity field inside internal combustion engines. However, there are a limited number of applications inside rapid compression machine. This technique requires two perpendicular optical accesses to the combustion chamber which are used in order to illuminate the flow field thanks to a laser sheet and record the Mie scattering images. The flow has to be seeded with particles. Under such high temperature and pressure conditions, solid particles, such as zirconium oxide, are needed. Guibert et al. (2010) and Strozzi et al. (2012) coupled visualisation of the combustion process with PIV measurement (Fig. 7.13 ) of non-reacting mixture and reported different regimes for the development of the combustion process.
Velocity magnitude fields and flow streamlines within the reference inert mixture at the compression end (Guibert et al. 2010; Copyright Elsevier)
5.3 Rapid Sampling
In order to investigate the important intermediate species during the combustion process, a direct measurement of species concentration is needed. Rapid sampling inside the combustion can provide such information. Minetti et al. (1994) designed a rapid sampling apparatus in order to collect the reaction products. On triggering, the sampling apparatus connect the combustion chamber with an expansion chamber initially separated by a diaphragm. Due to the rapid adiabatic expansion, the sample undergoes a high rate of cooling which quenches the chemical reactions. The quenched sample is then analysed qualitatively and quantitatively. With successive experiments and sampling at different times, concentration profiles as a function of time can be obtained for initial, intermediate and final products, Fig. 7.14.
Species concentration-time profiles expressed as the percentage of the carbon atoms initially present in PCH (%C) during the two-stage ignition of a propylcyclohexane/O2/inert mixture. φ = 0.5, p c = 0.7 MPa, T c = 669 ± 9 K. The cool flame (light grey) (see Chap. 2) and ignition (dark grey) are shown as grey zones. (Crochet et al. 2010; Copyright Elsevier)
While periodic sampling and subsequent analyses of a reacting mixture provide useful information on stable species, the alternative approach of continuous sampling has not yet been attempted to my knowledge. Given that the timescales in RCM experiments are much slower than those encountered in shock tubes (see Chap. 6), coupling of a RCM to a time-of-flight mass spectrometer should be eminently possible. The additional refinement of tunable photo-ionisation, currently achieved via synchrotron radiation, would be desirable.
6 Conclusion
Quite apart from the technological improvements which have been mentioned above as being desirable, the single most important advance would be to couple CFD solutions with a detailed chemical kinetic mechanism. Only in this way can gas motions, heat losses, etc. and realistic chemistries be properly allowed for as opposed to the ad hoc present-day approaches.
References
Beeley P, Gray P, Griffiths JF (1979) Shock sensitivity of liquid propellants: the exothermic decomposition of isopropyl nitrate under rapid compression. Proc Combust Inst 17:1415–1423
Brett L, MacNamara J, Musch P et al (2001) Simulation of methane autoignition in a rapid compression machine with creviced pistons. Combust Flame 124:326–329
Bysveen M, Almas T (2003) Fuel quality evaluation by changing the compression ratio in a hydraulic dynamic combustion rig. SAE Technical Paper 2003-01-3081
Carlier M, Corre C, Minetti R et al (1990) Autoignition of butane: a burner and a rapid compression machine study. Proc Combust Inst 23:1753–1758
Clarkson J, Griffiths JF, MacNamara JP et al (2001) Temperature fields during the development of combustion in a rapid compression machine. Combust Flame 125:1162–1175
Crochet M, Minetti R, Ribaucour M et al (2010) A detailed experimental study of n-propylcyclohexane autoignition in lean conditions. Combust Flame 157:2078–2085
Donovan MT, He X, Zigler BT et al (2004) Demonstration of a free-piston rapid compression facility for the study of high temperature combustion phenomena. Combust Flame 137:351–365
Falk KG (1906) The ignition temperatures of hydrogen-oxygen mixtures. J Am Chem Soc 28(11):1517–1534
Gallagher SM, Curran HJ, Metcalfe WK et al (2008) A rapid compression machine study of the oxidation of propane in the negative temperature coefficient regime. Combust Flame 153:316–333
Griffiths JF, Piazzesi R, Sazhina EM et al (2012) CFD modeling of cyclohexane auto-ignition in RCM. Fuel 96:192–203
Guibert P, Kéromnès A, Legros G (2007) Development of a turbulence controlled rapid compression machine for HCCI combustion. SAE Technical Paper 2007-01-1869
Guibert P, Kéromnès A, Legros G (2010) An experimental investigation of the turbulence effect on the combustion propagation in a rapid compression machine. Flow Turbul Combust 84(1):79–95
Gupta S, Bihari B, Sekar R et al (2005) Ignition characteristics of methane-air mixtures at elevated temperatures and pressures. SAE Technical Paper 2005-01-2189
Husson B, Bounaceur R, Tanaka K et al (2012) Experimental and modeling study of the oxidation of n-butylbenzene. Combust Flame 159:1399–1416
Jakirlić S, Volkert J, Pascal H et al (2000) DNS, experimental and modelling study of axially compressed in-cylinder swirling flow. Int J Heat Fluid Flow 21:627–639
Kono M, Shiga S, Kumagai S et al (1983) Thermodynamic and experimental determinations of knock intensity by using a spark-ignited rapid compression machine. Combust Flame 54:33–47
Lee D, Hochgreb S (1998) Rapid compression machines: heat transfer and suppression of corner vortex. Combust Flame 114:531–545
Lim OT, Sendoh N, Iida N (2004) Experimental study on HCCI combustion characteristics of n-heptane and iso-octane fuel/air mixture by the use of a rapid compression machine. SAE Technical Paper 2004-01-1968
Lodier G, Merlin C, Domingo P et al (2012) Self-ignition scenarios after rapid compression of a turbulent mixture weakly-stratified in temperature. Combust Flame 159:3358–3371
Minetti R, Ribaucour M, Carlier M et al (1994) Experimental and modelling study of oxidation and Autoignition of butane at high pressure. Combust Flame 96:201–211
Mittal G, Gupta S (2012) Computational assessment of an approach for implementing crevice containment in rapid compression machines. Fuel 102:536–544
Mittal G, Sung CJ (2006) Aerodynamics inside a rapid compression machine. Combust Flame 145:160–180
Mittal G, Raju MP, Sung C (2008) Computational fluid dynamics modeling of hydrogen ignition in a rapid compression machine. Combust Flame 155:417–428
Mittal G, Raju MP, Sung C (2010) CFD modeling of two-stage ignition in a rapid compression machine: Assessment of zero-dimensional approach. Combust Flame 157:1316–1324
Ribaucour M, Minetti R, Carlier M et al (1992) Autoinflammation à haute pression. Conception, réalisation et test d’une machine à compression rapide. J Chim Phys 89:2127–2152
Strozzi C, Sotton J, Bellenoue M et al (2007) Self-ignition of a lean methane-air mixture at high pressure in a Rapid Compression Machine. In: Proceedings of the European Combustion Meeting, Chania, Greece, vol 3
Strozzi C, Sotton J, Mura A et al (2008) Experimental and numerical study of the influence of temperature heterogeneities on self-ignition process of methane-air mixtures in a rapid compression machine. Combust Sci Technol 180:1829–1857
Strozzi C, Sotton J, Mura A et al (2009) Characterization of a two-dimensional temperature field within a rapid compression machine using a toluene planar laser-induced fluorescence imaging technique. Meas Sci Technol 20:125403
Strozzi C, Mura A, Sotton J et al (2012) Experimental analysis of propagation regimes during the autoignition of a fully premixed methane–air mixture in the presence of temperature inhomogeneities. Combust Flame 159:3323–3341
Tanaka S, Ayala F, Keck JC (2003) A reduced chemical kinetic model for HCCI combustion of primary reference fuels in a rapid compression machine. Combust Flame 133:467–481
Tran K, Morin C, Guibert P (2011) Anisole laser induced fluorescence (LIF) for imaging local heterogeneities in temperature in a rapid combustion machine. In: Proceedings of the European Combustion Meeting, Cardiff, Wales, vol 4
Walton SM, He X, Zigler BT et al (2007) An experimental investigation of iso-octane ignition phenomena. Combust Flame 150:246–262
Würmel J, Simmie JM (2005) CFD studies of a twin-piston rapid compression machine. Combust Flame 141:417–430
Würmel J, Silke EJ, Curran HJ et al (2007a) The effect of diluent gases on ignition delay times in the shock tube and in the rapid compression machine. Combust Flame 151:289–302
Würmel J, Simmie JM, Curran HJ (2007b) Studying the chemistry of HCCI in rapid compression machines Int J Vehicle Design 44:84–106
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Kéromnès, A. (2013). Rapid Compression Machines. In: Battin-Leclerc, F., Simmie, J., Blurock, E. (eds) Cleaner Combustion. Green Energy and Technology. Springer, London. https://doi.org/10.1007/978-1-4471-5307-8_7
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5307-8_7
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5306-1
Online ISBN: 978-1-4471-5307-8
eBook Packages: EnergyEnergy (R0)