Abstract
Estimating the postmortem interval is the “bread and butter” component of what the discipline of forensic entomology delivers to the judicial system in matters concerning homicides, suspicious deaths, and suicides. The following chapter introduces the reader to the different requirements necessary to collect and preserve insect material from a cadaver. Apart from the importance of the chronological interval, insect larvae can also be used to extract drugs, poisons, gunshot residues, and DNA. To be able to analyze these substances, it is not only dependent on the context of where the corpse is located but what specimens are collected and how they are preserved. Currently there are no universal procedures and methods, so the practitioner must consider each case and what information is required and apply those techniques. This chapter summarizes those techniques and the relevant tools to complete these undertakings.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
A death occurs. If it is a result of foul play followed by a time interval prior to discovery, and there has been an association of insects with the corpse, then there is a good chance that an entomologist will be required to help determine when the crime occurred. The discipline is called forensic entomology and in more recent times has become the gold standard for estimating the time since death [1]. Within this chapter, the reader will appreciate that there is much more that insects can offer when they become involved with a crime scene. Apart from their value in determining an accurate estimate of the chronological interval of death, forensic entomologists are able to extract drugs and gunshot residues (GSR) (entomotoxicology) from larvae [2] and make determinations on species identification as well as host substrate using DNA from where the larvae were collected [3]. So what is forensic entomology? The following chapter will describe its history; it will define the actual science and detail its many applications as a tool to help solve crime. In addition, the following chapter should serve as a guide for pathologists and other scientists investigating a corpse as to the location of insect material and how to sample and preserve collected specimens.
Forensic entomology is comparable to many of the other sciences that now have an affiliation with forensics. Forensics is a Greek word meaning “in the forum” and, in its more contemporary guise, it is any science or skill set that is used to solve a crime that typically concludes in a court of law.
Forensic entomology has been a useful tool for crime scene investigators for the best part of a century in the Western world. There have been numerous case histories now where entomology has played a crucial role in helping to work out the details of a crime or unattended death. However, three cautionary details apply to forensic entomology [4]:
-
1.
Insects are animals, and it is not always possible to rely on animals to do what is expected of them, even when conditions seem favorable.
-
2.
All care must be taken in the collection of entomological evidence; this should be gathered only by individuals who have knowledge and experience and/or accreditation as a practitioner in this activity.
-
3.
Entomological evidence that is likely to appear before a court should be analyzed by individuals who have demonstrable experience with the aspects and assumptions of entomology that are applied to medicolegal investigations.
As previously stated, the term “forensic entomology” is generally used to describe the study of insects and other arthropods associated with criminal events [5]. In practice, however, most crime scene examiners are not entomologists, so any non-backboned animal associated with a legal investigation has in the past been sent to a forensic entomologist for analysis. Such animals may include snails, millipedes, spiders and mites, land shrimps, and flatworms, which can be passed on by the entomologist to an expert associated with that animal group. This range of invertebrate fauna is further expanded when a corpse is located in a water body and a variety of crustaceans may then be presented for examination. Unfortunately, very few species of animals, apart from a small number of insects and mites [6], have been studied sufficiently to have any reliable use in forensic entomology [4].
What Is Forensic Entomology?
The field of forensic entomology can be divided as follows [7, 8]:
-
Urban entomology (e.g., civil actions relating to insect- and human-built structures, as may occur with termites and buildings) [9, 10]
-
Stored product entomology (e.g., civil actions related to insect infestations of commodities such as food) [11, 12]
-
Medicolegal entomology (e.g., criminal cases involving the estimate of time since death for decomposing remains of humans or animals) [13]
The last of these categories, medicolegal entomology, includes determining where and when the human death occurred, cases involving possible sudden or suspicious death, and criminal misuse of insects [14].
Uses for entomology in forensic situations seem to be broadening continually [15, 16]. This breadth is evidenced by applications in specific legal cases related to detection of:
-
Injuries after death [23]
-
Movement of vehicles and transport of remains [24]
-
Movement of people through bites or infestations [25]
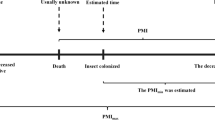
Estimating minimum time since death is still one of the most fundamental questions following a death, and the application of the developmental rates of insects associated with a corpse has become most essential in calculating the postmortem interval (PMI) in legal situations [1]. There are a number of other ways of establishing minimum time elapsed since death [36], including histological, chemical, and bacteriological methods, but such traditional techniques reputedly lose precision if more than a day has elapsed since death [37]. This has now been extended using modern molecular biology techniques, whereby the parameters responsible for changes in DNA yield have been researched as a possible measure [38].
There has always been a need for a broader range of methods that can be applied to determining PMI, and this is where entomology can be of use. Indeed, the case should be argued for pathologists, entomologists, and anthropologists to be considered as part of a continuum, working together for improved information on time since death [1]. Forensic pathology is about the process of autolysis, entomology with the early decay stages, and anthropology with the later stages. As such, the pathologist first calls on the entomologist when insects are present and then the anthropologist to assist with time since death assessments.
Determining minimum time since death is generally based on two considerations:
-
The time it takes for insects attracted by carrion to arrive on a body
-
The temperature-dependent rate at which carrion-eating insect species develop through their life cycles
With respect to time taken for carrion-eating insects to arrive on a body, there are many environmental- and species-specific factors that will determine which insects will utilize a corpse. However, a predicted order of corpse utilization typically occurs. Therefore, the first arrivals will be those insects attracted by and feed only on a fresh corpse [39–41]. In contrast, other types of insects will not be attracted by a corpse until it is in one of the later stages of decay. So, the presence of an insect known to be attracted only by a dry, decayed corpse indicates that the corpse has been dead for some time and has already passed through fresh, bloat, and wet decay stages. Insects involved in feeding on decaying flesh include blowflies, beetles, wasps, and moths, and, by the time the last species arrives, the earliest arrivals are generally no longer present. Within the first hour, blowflies are generally the first insects to arrive at the site of decomposing flesh and are, to date, the only insect group to provide reliable estimates of PMI [42]. There are many species of blowflies, each with its own habits. Therefore, in entomological applications to forensic situations, it is critical that the species is correctly identified. Most forensic entomologists can identify genus and species, but typically the judiciary requests that actual identifications are conducted morphologically and/or genetically by a qualified taxonomist (Dadour 2011, personal communication).
The second consideration concerns the rate of development of insects associated with carrion. This requires knowledge of how climate, topography, vegetation, and other environmental factors will influence how quickly an insect grows from egg to adult (oviparous) (or in some species larvae only are born: ovoviviparous) on a corpse. Temperature (the most important) and rainfall (humidity) are two critical weather factors to consider. It is therefore necessary to find out the recent weather patterns for the area in which a body is found to estimate how overall insect activity in the area and rate of growth of those insects found feeding on a corpse may have been influenced by such factors [43].
History of Forensic Entomology
Sung Tz’u (1235) translated in 1981 [44] was the first known account of entomology being involved in a legal matter. The original details describe an incident involving a Chinese peasant who died of wounds inflicted by a sickle. The investigator assembled the farmers in the village and had them place their sickles on the ground. All were placed in the sun with flies being attracted to one sickle. As a consequence, the owner confessed guilt.
However, forensic entomology stayed unrecognized until the work of several European pioneers whose case studies were published in the last half of the nineteenth century. Dr. Bergeret d’Arbois is credited as being the first Westerner to apply “entomology” to a forensic situation. Bergeret identified some arthropods from a child’s body found in 1850 behind a mantelpiece [14] and as a consequence of their identity and biology suspicion lay with previous rather than current short-term occupants of the house.
Megnin [45] is attributed as establishing entomology as a useful forensic tool when he published a treatise on the fauna of cadavers and their legal applications based on 15 years of work at the Paris morgue. Megnin [46] was the first to suggest that an exposed corpse would undergo a series of predictable changes during decomposition and that these stages would be visited by a succession of specific arthropods. By the end of the nineteenth century, the use of entomology in forensic cases was also well established in North America [47, 48].
Literature during the early part of the twentieth century is depleted on the topic of forensic entomology case work and research. In the UK, the first case utilizing forensic entomology occurred in late 1935 [49]. Police recovered about 70 pieces of butchered human remains belonging to two females from a Scottish ravine that the media of the day dubbed “The Devil’s Beef Tub.” In this case, the forensic pathologists relied heavily on maggots to provide an estimate of the time of death.
The pioneers of regularly using forensic entomological evidence in European courts during the last century were Leclercq and Nuorteva. Publications of their research and involvement in police cases over a number of years were principally responsible for the growing interest by police in Western countries in gathering insect specimens from crime scenes. This resulted in a resurgence of interest in forensic entomology [50–56].
An Overview of Succession
Two types of succession typify the decomposition of a cadaver. The first succession pattern is the actual decomposition of the body, and it is generally categorized as gross morphological changes [1]. This section will not dwell on these changes as overviews of these patterns have been documented extensively [57, 58]. However, from a forensic entomology perspective, probably the most informative sequence is the four stages described by Reed [59] as they are not subjected or altered by climatic change. A fifth and final stage termed “skeletal” was added by Goff [60]. These stages include:
-
1.
Fresh stage: begins with death; continues until early stages of bloating.
-
2.
Bloat stage: begins during early stages of bloating; loss of hair begins; ends when bloating ceases.
-
3.
Decay stage: begins when bloating ceases; hair loss is conspicuous; skin is usually broken in one or more places; soil within 30 cm of carcass is pulverized by burrowing activity of insects; ends when most of the carcass remnants are relatively dry.
-
4.
Dry stage: begins when only small amounts of decay tissue remain; the limits of the stage are difficult to define due to lack of pronounced events marking the beginning and end, and diversity in appearance of similar-aged carcasses and fauna living on them; considerable moisture due to rain, dew, or underlying soil and litter may be present; small amounts of semisolid putrefying material is occasionally present on the ground under solid remnants; ends when no carrion fauna remains.
-
5.
Skeletal stage: characterized by skeletal remains and hair and an absence of carrion fauna. During this stage some useful forensic information can be derived from the soil composition under the corpse [6, 60–62].
The second pattern of succession is based upon the behavior and biology of (mostly) immature insects occurring and developing on a cadaver following death. This locality-specific but predictable succession is then correlated with the temperature-dependent developmental data for the immature insects found on the cadaver in order to estimate the PMI [4].
The use of successional data in the estimation of PMI assumes that following death, an orderly and predictable succession of insect species occurs on a cadaver. In a terrestrial environment, insects are generally the first organisms to locate a body following death. The carrion community is comprised of four categories. The first category includes insects classified as necrophages that feed from the body itself and are most useful in estimating the PMI. The second category consists of predators and/or parasites that may feed on other species that have already colonized the body, and as more research is being conducted especially on the parasites, this category is also becoming a useful tool for estimating the PMI [63–65]. The third category is far less useful for estimating the PMI and includes omnivorous species such as beetles, which occur later in the decomposition process. The last category is the adventive or incidental species, which simply visit by chance and generally have little forensic relevance [18]. Insect evidence no matter what category it falls into should only be discounted by a forensic entomologist.
As the body progresses through the stages of decomposition, from fresh dead to bloat, decay, dry, and skeletal stages, the resource changes chemically [66], and as a consequence the odors emitted by the corpse also change [64, 65, 67]. The odors vary in attractiveness to different insects, and as the body decomposes and various resources are depleted, new insect types will colonize, being more suited to the current decompositional stage [68]. These insect taxa reflect the physical and chemical changes in the body and are therefore predictable and useful in estimation of PMI.
Flies (Diptera) and beetles (Coleoptera) are the insects most frequently collected from corpses [69], and although both groups are important, the flies are the focus of most forensic invertebrate research and applications. The blowflies (Diptera: Calliphoridae) are usually the first insects to arrive following death. Female flies will deposit eggs or live larvae around orifices or wound sites on the corpse, and larvae will secrete enzymes and bacteria, facilitating consumption of the soft tissues of the corpse. Larvae will feed through three stages of growth (instars) each punctuated by the molting of their size-restricting cuticle, enabling further growth. At the cessation of feeding, larvae will pupate in soil, clothing, or beneath surrounding objects if inside a dwelling, and following a period of metamorphosis, the adult fly emerges. The empty pupal casings may persist around the corpse for many years and even longer in soil.
The arrival of blowflies, and subsequently their larvae, is coincided or followed quickly by the arrival of the flesh flies (Diptera: Sarcophagidae), other carrion flies (Diptera: Muscidae), and predaceous beetle species such as rove beetles (Coleoptera: Staphylinidae), carrion beetles (Silphidae), clown beetles (Histeridae), skin beetles (Dermestidae), and checkered beetles (Cleridae). A variety of other fly families may be found in association with the body, and hide beetles (Trogidae) and larvae of some of the aforementioned beetle groups may feed on carrion itself, often on remains of hair, skin, and clothing in late decomposition [24].
As mentioned previously, when the body is decomposing in a terrestrial environment, the substrate beneath it is also altered. This initiates a series of changes in vegetation and soil fauna, beginning a succession of arthropods affected by the decomposing corpse above. This is a most important aspect of sampling and collecting for the entomologist or proxy, as the environment directly underneath the corpse may conceal arthropods of forensic importance. Generally, this sampling can only occur after the corpse is removed from the scene and taken to the mortuary (see later section: General Methods for Collecting and Preserving Insect Material).
Bornemissza [61] observed the greatest effect of the decomposing corpse on the soil beneath to occur during the black putrefaction and butyric fermentation stages. Fluid seepage contributes to development of a crust of hair, plant matter, and the uppermost soil layer beneath the body. During fermentation, the decomposition fluids released from the body, along with the waste products excreted by the insects feeding on the body, combine to kill the plants beneath the body and alter the soil fauna, altering the microenvironment [6, 70].
During the decay stage, the soil beneath the carrion may become disturbed to a depth of approximately 3 cm by the action of arthropods, particularly dipteran larvae, burrowing [59]. Decomposition fluids and associated arthropods are reported to affect the soil to a depth of 14 cm, with most effect in the upper soil layers. The area directly beneath the body, the “carrion zone,” serves as a decompositional zone occupied by carrion dwellers, distinct from a surrounding area of approximately 10 cm, which provides an “intermediate zone” of both carrion and regular soil-dwelling invertebrates [61]. Generally, 10–20 cm away from the body, the soil fauna is typical of general litter-dwelling fauna, but perhaps the size of these zones may be dependent on the size of the carrion, as Bornemissza’s work was based on guinea pigs, and human decomposition may produce greater amounts of fluid.
Nomenclature and Insect Life History
The biomass of insects is huge with an estimated (extrapolated) species richness ranging from 3 to 80 million species. Five major insect orders stand out for high species richness: Coleoptera (beetles), Diptera (flies, mosquitoes), Hymenoptera (wasps, ants), Lepidoptera (butterflies and moths), and Hemiptera (true bugs) [71]. The first three orders have adapted directly or indirectly to using carrion as a resource.
The vast majority of the insects lack a common name. A scientific name once applied to a species is recognized throughout the world, although there may be the occasional species being described twice.
Insects owe their success to several adaptations [72]. They mostly produce large numbers of eggs to help compensate for predation or fewer live larvae to escape predation. Wings allow insects to travel far for food or in search of mates and to escape their enemies. A further advantage is the ability of insects to feed on an amazing variety of food materials. Most plant species are utilized by some insect for food and/or shelter. Many insects prey on other insects, a number depend on blood derived from mammals or birds, some feed on the waste products of larger animals or on decaying plants and animals, and there are others that feed only on dried animal remains including hair and feathers. Insects as poikilotherms can reduce their metabolic activity when conditions are unfavorable (i.e., extreme temperatures, water, or food shortages) and can colonize areas of limited food and water supply (i.e., deserts) and as small organisms can adjust to temperature rather quickly to make maximum use of favorable thermal conditions [71].
The external anatomy of most insects includes four wings. However, true flies have only one pair (the forewings, the second pair modified into stabilizers), while beetles use only the hind pair in flight with the forewings serving as a protective covering. Some insects, generally more primitive forms, never developed flight, while some did have wings but due to the niches they now occupy, their wings have been lost.
Another insect attribute is their external skeleton, which is segmented which in turn facilitates movement. However, the tough external skeleton impedes insect growth. The skeleton is constrained as to how much it can grow. As a consequence, insects must molt their exoskeletons. Immediately after molting, they increase in size, but with the hardening of the new skeleton, growth is once again constrained until the next molt. To offset the problem of constantly molting, some insects have developed a soft-bodied larval stage (grub, caterpillar, or maggot) reducing the number of required molts. Amongst the Diptera, the larvae (or maggots) generally undergo 3 molts; the final one forms the pupa and during this period—termed “metamorphosis”—the adult insect forms [73].
Like the Diptera, the more advanced insect groups’ (moths, beetles, flies, wasps, and fleas) development passes through four stages: egg, larva (maggot, grub, caterpillar), pupa (chrysalis, cocoon), and winged adult. Pupation is a period of rest between the feeding and growing larval stage and the final transformation (metamorphosis) into an adult. Those families of flies that have a maggot-like larval stage have simplified the process of pupation whereby the last larval skin hardens and darkens and forms a puparium. In certain blowfly species, the period taken from egg to adult may be as short as 10 days, but as stated previously this time is greatly influenced by climate, especially temperature and humidity [71].
The majority of the other insect groups have no pupal stage, and the young (nymphs) usually resemble the adults except that they lack wings. For example, in grasshoppers, which may molt eight times, the wings grow a little with each molt and appear fully developed only after the last molt. The most primitive insect orders are wingless, and their young closely resemble the adult forms.
In adult flies, the most important external stimuli are those concerned with odors [64, 65], light [71], moisture, and wind direction [74, 75]. Receptors in the antennae detect odor. For blowflies, the most powerful reaction shown by all species is their attraction to the specific odors of decomposition, more especially odors of carrion or decaying animal matter, of the feces of human beings and other animals, and the odors given off under certain conditions by the skin and wool of living sheep. Very importantly, different species of flies react differently to successive stages in the process of decomposition.
General Methods for Collecting and Preserving Insect Material
Numerous accounts of how to collect at a crime scene or a decomposing animal have been documented and published [16, 76, 77]. The following is a précis on much of the current literature and the authors’ experience.
At the Scene
In many cases when insect material is observed at homicides, suspicious deaths, and suicides, then the medical examiner, coroner, pathologist, or trained forensic field officer will call an entomologist. In some cases, although not ideal, any one of these attending persons may do the collections on behalf of an entomologist. At homicides when the entomologist attends, that person is generally integrated into the forensic team dealing with the case. Typically, the entomologist arrives with a kit and dons a disposable and protective type coverall, which includes booties, gloves, and a head covering. At this point the entomologist can, under the direction of forensic officers, assess the evidence and acquire the necessary tools to collect the evidence. The kit should contain tools such as a net and/or sticky paper for the collection of adult fly insect; forceps, spoons, and paintbrushes for collecting immature and some adult insects; collecting jars that are capable of being ventilated for storing insect samples; pens and pencils for writing labels; thermometers (standard but more appropriate infrared) for checking temperatures of large visible masses of fly larvae; and some form of refrigeration—either a cooler containing ice or freezer blocks or a fridge in which to place collected specimens. Prior to delivering specimens to an entomologist, never place insect material directly into a freezer for killing or storage of immature insects.
What to Collect
Although experience is required to sample from a corpse at a scene, it is reasonably straightforward if the person collecting has some basic skills and understanding about what to collect. One major assessment within the environment where the body is situated is if any other decomposing material is closely associated with the corpse. The original observation of the visible insect material on the corpse should include the accessible areas where immature insects are likely to be found. The main areas are the head region, due to the large number of orifices present, and also the hairline; the armpits; between fingers and toes; back of knees or front of elbow if both are bent; where the body and substrate meet; orifices in the groin region; any wound; and the in situ area under the corpse following its removal to the mortuary. It is important to search and sample beneath the corpse as well, so an entomologist or their proxy will be required to collect samples when the corpse is first located and when it is removed. In any one of these locations, a fly larval masses may be present, and the temperature of this mass should be recorded. Studies have shown that these larval masses produce elevated temperatures, which influences the developmental time and consequently affects the PMI [78]. Always place samples into ventilated containers, and do not place live fly and beetle larvae together. Following removal of the corpse, a weather station (generally a miniature Stevenson Screen containing a data logger) should be placed as near as possible to the scene. The weather station should remain in place for 7–10 days.
Preservation
Although a number of different methods are available to preserve collected immature insects, the best procedure following sampling as suggested above is to place all samples into a cooler or refrigerator. All samples can then be conveyed to the entomology laboratory to be processed and preserved. The preferred process in the laboratory is to place the insect material firstly into hot water (approximately 80 °C). Hot water prevents autolysis in the larva by destroying proteins in the gut, which would otherwise darken the specimens during storage, and it reduces the elasticity of the cuticle [79]. Following the hot water treatment, all immatures (eggs, larvae, and pupae) can be placed directly into 70–90 % ethyl alcohol with one exception and that is pupae that must be pierced to allow infusion of the preservative into the pupal case [80]. Sometimes cooling and/or refrigeration is not available, and the preservation processes will need to be conducted at the scene. Processing at the scene can involve a number of steps depending on whether hot water and preservatives are available. In essence, some alcohol will need to be sought, which may include a white spirit such as vodka. If such a liquid is used, this needs to be swapped out into 70–90 % ethyl alcohol at the earliest opportunity. There are many solutions not suited for preserving insect immatures, including methyl alcohol and formalin [79]. During autopsy most preservation issues do not arise as refrigeration is available, and specimens collected can be stored appropriately pending being sent to an entomologist.
However, some other issues can arise at the mortuary, and this is because sampling of the insect evidence on many occasions occurs only during the autopsy. As a consequence, the time period between corpse discovery and the autopsy may range from a few hours to several days, but generally no more than a week. During most of this time, the corpse and the insects feeding on it are stored in a cool room in the mortuary (with temperatures approximating 4 °C). The purpose of refrigeration is to slow down the decomposition of the corpse and the activity and the development of the necrophagous insects (poikilotherms) on or in the corpse. In some cases prior to autopsy, the corpse may be shifted from the cool room to the laboratory to have other procedures (e.g., fingerprinting, inspection of clothing) conducted. These periodic transfers in and out of cooling may affect the development rate of insects reliant on the corpse, which will affect the PMI.
Although there are several studies that have been conducted to determine development of dipterans when subjected to low temperature and its potential effect on the estimation of PMI, no information is available on the effects of moving a corpse in and out of the cool room environment. In addition, all these studies have been focused on larva and pupa, not on the effect of cooling of fly eggs. The results of the effects of cooling on all other life-history stages have demonstrated significant increases in development time [81–87].
Contemporary Research in Forensic Entomology
There is still much to learn about forensically useful insects. Besides their seasonal occurrence in various geographical regions and their rates of development in the many situations of forensic interest, research worldwide continues to explore how this material can be best utilized and increase the credibility of forensic evidence.
Entomotoxicology (Drugs and Gunshot Residue)
It is becoming more commonplace that insects associated with a corpse can be used for toxicological analyses [2, 88]. Forensic toxicology is usually associated with poisons and other illicit substances or their metabolic subproducts found during a death investigation. This combined with the pathology, biology, and pharmacology evidence will typically determine the circumstances of death [2].
Goff and Lord [89] reported that the use of illicit drugs has increased in recent years on a global scale. Many victims having used drugs that cause death may remain undiscovered for different periods of time. Mainstream toxicological technologies require samples of the highest quality and best conditions for analyses. However, such samples become more difficult to extract, especially when the body is highly decomposed. Other factors that may prohibit proper sampling include mummification, a lack of body fluids such as blood or urine, the presence of high levels of alcohol or carbon monoxide, and in some instances due to the religious beliefs of the person that forbid sampling directly from the body [90].
Over the last 35 years, insects have become an alternative source of samples for toxicological analyses. In addition, many toxic substances can modify the development of arthropods [89] and in doing so alter the determination of the postmortem interval. This relatively new science has been coined entomotoxicology, with the first reports showing that adult house flies accumulated metals such as copper, zinc, and iron [91]. Following this, Beyer et al. [17] wrote a technical note based on a highly decomposed corpse discovered after 14 days in a wooded area. The fly larvae collected confirmed that the person had consumed phenobarbital. Later, Nuorteva and Nuorteva [92] extracted mercury from a number of different species of fly larvae feeding on fish tissue. More recently, Roeterdink et al. [21] extracted metals such as antimony, barium, and lead associated with gunshot residues [93]. During the last 25 years, extractions have been made from fly larvae of pesticides and drugs. The following is a compilation of these extractions from fly larvae, which is by no means complete but includes malathion [20, 94], parathion [95], and drugs and narcotics such as alimemazine, bromazepam, clomipramine, levomepromazine, oxazepam, triazolam [96, 97], cocaine [98, 99], amitriptyline, propoxyphene and acetaminophen [100], opiates [101–103], temazepam, trazodone and trimipramine [104], salicylates, paracetamol, amphetamines and barbiturates [105, 106], and derivatives from amphetamines [107]. Furthermore, when a body is highly decomposed, beetle larvae may also be present, but currently limited information is available on drug detection from these larvae [103, 108].
Sampling
The principle to sampling toxicological specimens is that all the apparatus used to collect and store the sample must be clean and preferably autoclaved. Insects, like tissue and fluids, must be collected in vials free of preservatives or any external contamination. Parafilm can be put in a lid of a glass vial to avoid contamination of the sample by rubber seals and metal lids.
Prior to analysis, all samples should be kept refrigerated, and a part of each analyzed specimen should be retained, stored in the refrigerator, and be kept as a reference sample. The toxicological analysis should be conducted as soon as possible to guarantee the integrity of samples.
In the laboratory, live larvae should be sacrificed by freezing prior to analysis. Following death they must be rinsed in distilled water and pupae/puparia rinsed with methanol prior to extraction to avoid contaminants. Prior to toxicological analysis, the substances to be analyzed must be taken into account [109]. It is important to emphasize that all material used for analysis must be stored in clean glass containers to avoid contamination of phthalate present in plastic vials.
Hydrocarbons: A New Tool
A thin epicuticular layer of wax covers the cuticle of all insects. This wax is a compound containing alcohols, hydrocarbons, fatty acids, waxes, acylglycerides, phospholipids, and glycolipids [110]. The purpose of this waxy layer is to prevent desiccation and penetration of microorganisms [71]. In the majority of the insects, the wax layer is dominated by hydrocarbons [111]. Cuticular hydrocarbons are found in all life stages of insects and are biologically stable. Their biosynthesis is genetically based and modulated by factors such as reproductive status [112], developmental stage [113], diet [114], or temperature [115, 116].
Currently, research on cuticular hydrocarbons has identified that the profiles of these compounds found on larvae and pupae change over time [117–119]. If these changes occur as part of the development of necrophagous insects, then these hydrocarbons could be a very useful tool in estimating the age of a larvae or pupae and hence could enhance the accuracy of the PMI.
There are two techniques used to collect cuticular hydrocarbons from the cuticle of the insect: via liquid extraction or solid phase micro-extraction (SPME) [110]. It has now been established that many different hydrocarbons exist on the cuticle of insects and that each insect generally has a very distinctive hydrocarbon profile. This profile is fast becoming a valuable tool in identifying species—a research area referred to as chemotaxonomy and now used extensively in plant taxonomy [120, 121].
This latest technique in forensic entomology has the potential to firstly identify the species of immature necrophagous insects. As will be discussed later, DNA has become extremely useful in determining species identification; however, this process is time consuming and not very sensitive when using either spent eggs or pupae. Like DNA, hydrocarbons are species specific and can be compared with a database of profiles of known species; however, species identification can be completed in a few hours [122]. There is also recent evidence that insects could have varying cuticular hydrocarbon profiles, depending on what geographical region where they occur [123, 124]. Once profiles for different regions are established, then hydrocarbon profile could play a role in identifying if a body has been relocated. This would be achieved by comparing the profile of the hydrocarbons on insects associated with the corpse to those of the local insects found in a certain region.
The second area in which cuticular hydrocarbons can be a valuable tool in aiding forensic entomology and alluded to earlier is in establishing the postmortem interval (PMI). It has been demonstrated that a significant change over time of the hydrocarbon profile on the larvae and pupae of the blowfly Chrysomya rufifacies has been observed [117]. Furthermore, Roux et al. [119] provided evidence (with a precision of 1 day) for the importance of hydrocarbons as an alternative method for evaluating the postmortem interval demonstrating changes in hydrocarbon profiles in three calliphorids of forensic interest: Calliphora vomitoria, C. vicina, and Protophormia terraenovae.
The Value of DNA in Forensic Entomology
In forensic entomology case work, there is a growing need to use molecular techniques [125]. This comprises numerous aspects such as identification, host detection, including victim suspect associations, and postmortem interval. The first two aspects have become quite important, whereas research on DNA degradation as a measure for the postmortem interval has not yet become a mainstream technique.
Identification
Research into molecular techniques has become more common as a research tool, providing more equivocal identification of immature stages of flies [126–129]. Globally, an increasing number of publications now emphasize the molecular identification of forensically important blowflies: Canada [130], France [131], USA [132], UK [133], Australia [126, 129], and Harvey et al. [126] for comparison of southern African and Australian species. However, in case work many of these techniques are used only as support for more traditional morphological identifications.
Molecular techniques have many advantages over morphological techniques in that species can potentially be readily identified at all life-history stages. For example, a reliable genetic identification of forensically important flies can be performed from empty puparia and/or their fragments, although DNA degradation can deeply compromise the genetic analysis of older fly puparia [134]. “On-site” identification of first-stage maggots may also become a reality through development of rapid immunodiagnostic assays [135].
To develop suitable diagnostic tests for use in forensic entomology, species-specific molecular markers—regions of DNA used for identification purposes—need to be identified for interspecific distinction. DNA sequencing produces data of high information content and allows both intra- and interspecific comparison. In particular, sequencing of the mitochondrial region encoding the cytochrome oxidase I (COI) gene has proved useful in evolutionary studies, population genetics, and systematics due to the relatively high degree of variation in the region [131]. Generally, mitochondrial DNA (mtDNA) has a higher mutation rate than nuclear DNA and, therefore, an increased chance of generating species-specific markers. In addition, mtDNA may be isolated more easily than nuclear DNA [136]. This is clearly advantageous to forensic studies where specimens may be incomplete or in poor condition.
Ten or so years ago, it would have been thought that DNA analysis would replace any need to rear through fly larvae to adults for identification and possibly would assist in questions of how much time may have been spent in a particular developmental stage. However, in forensic entomology, application of molecular biology techniques and knowledge are still in their infancy. Not only does one need unique stretches of DNA sequence common to all members of the taxon in question (or at least subsets of such a group), but also that such DNA stretches should be distinct from all other taxa [137]. To reach a point of reliable, robust identification from molecular techniques, considerably more geographic sampling and pooling of data from various researchers, is needed.
Host Determination
As stated previously, identification is still the main focus, but DNA analysis of the gut contents of certain arthropods, including maggots, has been used successfully in establishing victim/suspect associations [132, 138], and determining whether the larvae collected from a corpse assumes that these larvae have developed entirely on this resource [138]. Generally, other signs of decomposition, on and around the corpse, will justify this assumption [139]. Situations where host confirmation is necessary include the discovery of a corpse in an area where food scraps are present, or a corpse located in a bushland area where alternate animal carcasses may provide suitable development sites for fly larvae.
Estimating the PMI based on the oldest larvae at a scene may therefore be flawed; as such larvae may have developed on a substrate external to the corpse (see previous discussion of sampling techniques). It may be necessary for the forensic entomologist to confirm the food source on which larvae completed their development. In case work, the PMI may be disputed as a consequence of an alternative feeding resource. As a result, the material present in the digestive system of the larvae needs to be identified.
Recently, molecular biological techniques have been used to identify the sources of blood meals consumed by adult hematophagous insects such as mosquitoes [140] and crab lice [141]. Human DNA has also been isolated from beetles that had fed on human skeletalized remains [142].
Critical in host detection is the quality and quantity of host DNA present in the alimentary canal of the insect. Importantly, the isolation method used to obtain host DNA from the larvae should not result in further degradation of the DNA and should provide a sufficient quantity of DNA, which can be detected and used in subsequent molecular procedures [141].
Sampling
Collecting insects for DNA examination requires the application of different preservation techniques than those required for insects to be used in morphological studies [143]. Appropriate techniques include freeze drying or preserving in 95 % ethanol. While forensic entomologists with appropriate molecular genetic training can undertake the study of insect DNA, it is also likely that the process of identification may be undertaken by a non-entomologist—a molecular technologist using relevant standard operating procedures in the forensic laboratory. Similar to choosing a forensic entomologist for PMI analyses, take the same care when choosing a molecular biologist that he or she has an awareness of the expected analytical standards and nature of legal systems.
Temperature Effects and Maggot Masses
Each fly species has its own unique developmental profile, even those that are genetically close, as demonstrated by Nelson et al. [144] in a study of sibling species of Chrysomya. There are numerous studies on rearing flies associated with cadavers/carcasses at constant temperatures [84–86, 145, 146]. However, these records do not cover all species known to be forensically important. Furthermore, there is an obvious gap in information on development rates of these species at many temperatures, including populations of the same species in different geographic localities; some species have yet to be studied, and thermal limits and optimum temperatures are practically unknown for each of the life stages.
There is a lack of uniformity in investigations that have measured the effect of temperature on larval development. Some have used instar change to measure development, while others have used larval length or weight, and a few studies use a combination of instar and either length or weight [147, 148]. Most of these studies have not recognized that instar change is a reflection of maturation and indicates the “real” age of larvae, whereas length and weight are measures only of somatic growth [149].
Dadour [150] demonstrated the effect of temperature and density on C. dubia. The experiment was set up to measure the effect of density and temperature (fluctuating [30 °C/19 °C] versus constant [24 °C]) on larval development in C. dubia. Densities of 100, 500, 1,000, 2,000, 3,000, 4,000, and 5,000 were selected based on observations of larval masses on carcasses in the field. The parameters measured were larval instar changes and larval length. In general, the effects of temperature and density on the rate of larval instar change were not pronounced. At 20 h of development, temperature had a significant effect on larval instar. The proportion of larvae attaining second instar at the fluctuating temperature regime was greater than at the mean constant temperature, for larvae developing at densities of 100, 1,000, 2,000, 3,000, and 5,000.
Larval density had a significant effect on larval instar between 38 and 42 h of development. At these sampling times, 90 % of the larvae reared at densities of 3,000, 4,000, and 5,000 had reached third instar at both constant and fluctuating temperature regimes. For all other densities at both temperature regimes, less than 20 % of larvae had matured to third instar. Larvae developing at a density of 4,000 and at a constant 24 °C required the least amount of time to reach third instar. At a fluctuating temperature regime, larvae reared at a density of 3,000 had the shortest developmental time to reach third instar.
Overall, there was no significant difference in the rate of larval development of C. dubia at a fluctuating temperature of 30 °C and 19 °C when compared with the mean constant temperature of 24 °C. This suggests that both temperatures of the fluctuating regime lie within the threshold and optimum temperature for the larval development of C. dubia. The only significant difference in proportion of larvae at each larval instar, between constant and fluctuating temperature regimes, was found at 20 h of growth, probably as a result of the initial exposure of larvae to 30 °C at the fluctuating temperature. Exposure to 30 °C in the first 12 h of growth is therefore likely to have initially increased the rate of larval development at the fluctuating temperature regime compared to the mean constant temperature of 24 °C. This initial increase was not sustained as development proceeded.
A significant difference occurred in the length of larvae developing at different temperature regimes and densities. However, there was variation in the length of newly deposited first instar larvae, which implies that there is natural variation in the length of larvae of the same age deposited by adult female C. dubia.
The variation in larval length between treatments indicated that larvae did not have to reach a certain size before instar change took place. Therefore, the time of instar change was a response to the time of exposure to the environmental conditions and not to larval length. The small amount of variation in larval length within treatments indicates that length is a good measure of larval age only when larval density and environmental temperature are known. The greatest variation in larval length was observed around the time of instar change, when larvae had shed the cuticle of the previous instar. This had also been observed in the larval development of C. vicina and C. vomitoria [151]. Therefore, the age of larvae of the same instar can be determined using larval length. This is particularly useful for determining the age of third instar larvae where an increase in larval size by six to seven times is observed [152].
Further evidence of the effect of temperatures comes from studies in Western Australia inside vehicles. In one set of experiments, Voss et al. [153] showed higher temperatures, rates of decomposition, and insect succession between exposed pig carcasses on the soil surface and those enclosed within a vehicle following carbon monoxide poisoning. Another study, this time of temperatures in the cabin of parked vehicles [154], showed the importance of vehicle color, amount of glass, and if the window was open 2.5 cm or 5 cm on the internal temperature of the vehicle and also produced a model for temperatures in vehicles.
Morris [155] showed the degree to which temperatures where maggots are developing in pigs, goats, rabbits, and sheep can differ from ambient temperatures recorded at the site of the decomposing carcasses. Field experiments with pigs showed that when the pig was not infested by insects at all, the temperature within the pig carcass quickly reflects ambient. Alternatively, when a sheep and a pig were infested with maggots, the temperatures within the abdomen of both animals rise above ambient.
The most distinctive feature of blowfly larval feeding aggregations is heat production, that is, the capacity to generate heat within the aggregations, which can exceed ambient temperatures by 30 °C or more [31, 156, 157]. Although larval mass heat effects are accepted, the relationship between larval mass effect and larval development time remains difficult to assess [13, 158].
From these results, one could presume that the temperatures are almost impossible to estimate in forensic situations where we do not have access to the past history of the corpse or the local weather patterns. However, Morris [155] observed (and recorded on time-lapse film) in field experiments the way in which the maggots move their feeding site in response to temperature stimuli. This behavior was backed up by results of laboratory experiments on responses to temperatures [31]. In particular, maggots move away from extreme temperatures in the carcass or food medium and toward the optimum for their development. Many forensic entomologists use ambient temperatures as their base for estimating a time since death based on insect fauna. The observations and experiments by Morris suggest that in many situations, such as corpses found in summer with high numbers of maggots present (maggot mass), it would be more appropriate to use the optimum temperature for estimates of the temperatures driving development for the maggot species involved, at least during the feeding stage of the third instar, unless evidence exists of mass mortalities of maggots.
Other researchers have come close to suggesting the importance of maggot behavior in controlling the temperature at which the maggot mass is developing. Dallwitz [159] stated that, for life stages capable of movement, the extent of development occurring at high measured temperatures may be of slight importance, since the insect may frequently be able to select a microenvironment where temperatures are nearer to optimal.
Deonier [156] postulated that the heat generated by blowfly larvae in carcasses enables the species to survive periods when weather conditions are unfavorable to adult activity. Waterhouse [160] took up these thoughts and observed that many larvae are killed by high temperatures generated in a carcass by the maggot activity, and the temperatures and mortality vary directly with the degree of overcrowding. Waterhouse [160] also found that when temperatures near 52 °C were reached, larvae, both fully grown and immature, left the carcass. Many perished and the primary species were the first to leave. Morris [31] also found this when carcass temperatures were 22 °C above ambient, L. cuprina, C. augur, and Ch. rufifacies all spilled out of the carcass. Morris [31] suggested that the demise of numbers of maggots is useful in decreasing the overall temperature of the maggot mass and may be part of a behavioral adaptive advantage of some primary flies.
Waterhouse’s study [160] also found that when a carcass becomes a seething mass of Chrysomya spp. larvae, immature primary larvae would leave it in large numbers. It coincided with the time the carcass temperatures rose to their peak. Waterhouse was not able to establish whether the Chrysomya larvae caused the primary flies to depart because of the high temperatures generated by the Chrysomya larvae.
Fuller [161] may have been observing the same temperature phenomenon when carrying out experiments on interspecific competition. She, however, concluded that Lucilia larvae were “definitely repelled by those of Chrysomya [sic].” Even when they were well established and there was an abundance of food, Lucilia larvae became disturbed and some left the carcass when Chrysomya larvae were added.
Influences of clothing on the insect colonization of corpses have had little attention until recently in 2009 and in 2011. Both studies were preliminary in that only small numbers of replicates were used; two seasons (autumn and summer) were used in the Kelly at al. [162] study, and one season (autumn) over 2 years was used in the Voss et al. [163] study. Interestingly the comparisons made between the clothed pigs dressed in T-shirts and shorts in both studies, one in Africa and the other in Australia, largely agree with each other in that there was no evidence of a delay in Calliphoridae arrival or oviposition. However, Lucilia sericata in the Australian study oviposited 24 h earlier on clothed carcasses compared to unclothed carcasses, and this trend was consistent, within years, between all replicates [163]. No such delays were evident in the African study [162], which introduces a potential for error in the estimation of time since death when PMI is based on the arrival and developmental time of this primary colonizing species. Furthermore, within years, the duration of the wet decay stage was approximately 6 days longer for clothed than unclothed carcasses. In both studies maggot masses were present on all carcasses during wet decay, and internal carcass temperatures were consistently above ambient temperature. It seems clothing potentially protects larvae from environmental conditions and facilitates greater movement across the carcass surface contributing to the greater visibility and distribution of maggot masses.
Conclusion
As Jean Fabre [164] so eloquently quoted about the humble maggot:
“At the surface of the soil, exposed to the air, the hideous invasion is possible; aye, it is the invariable rule. For the melting down and remoulding of matter, man is no better, corpse for corpse, than the lowest of the brutes. Then the Fly exercises her rights and deals with us as she does with any ordinary animal refuse. Nature treats us with magnificent indifference in her great regenerating factory: placed in her crucibles, animals and men, beggars and kings are one and all alike. There you have true equality, the only equality in this world of ours: equality in the presence of the maggot.”
This chapter by no means completes all the information that can be extracted from insect material. However, it highlights the “bread and butter” aspect of forensic entomology, which is to provide first and foremost a postmortem interval. Now we know that the decomposition process is much more than this and that the insect material present on a corpse can unlock other secrets to do with the way someone died.
References
Marks MK, Love J, Dadour IR. Chapter 14: Taphonomy and time: estimating the postmortem interval. In: Wolfe Steadman D, editor. Hard evidence – case studies in forensic anthropology. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2009. p. 165–78.
de Carvalho LML. Chapter 9: Toxicology and forensic entomology. In: Amendt J, Campobasso CP, Goff ML, Grassberger M, editors. Current concepts in forensic entomology. Dordrecht: Springer; 2010. p. 163–78.
Carvahlo F, Dadour IR, Groth DM, Harvey ML. Isolation and detection of ingested DNA from the immature stages of Calliphora dubia (Diptera: Calliphoridae): a forensically important blowfly. For Sci Med Path. 2005;1:261–5.
Morris B, Dadour IR. Chapter 91a: Insects and their uses in legal cases. In: Freckleton I, Selby H, editors. Expert evidence. Sydney: The Law Book Company Limited; 2010. p. 8-5291–8-5381.
Nuorteva P. Sarcosaprophagous insects as forensic indicators. In: Tedeschi CG, Eckert WG, Tedeschi LG, editors. Forensic medicine – a study in trauma and environmental hazards. Philadelphia: Saunders; 1977. p. 1072–95.
Goff ML. Gamasis mites as potential indicators of post-mortem interval. In: Channabasavanna GP, Viraktamath CA, editors. Progress in acarology, vol. 1. New Delhi: Oxford & IBH Publishing; 1989. p. 443–50.
Lord WD, Stevenson JR. American registered professional entomologists. Washington DC: Chesapeake Chapter. 1986. p. 42.
Magni P, Guercini S, Leighton A, Dadour I. Forensic entomologists: an evaluation of their status. J Insect Sci. 2013 (in press).
Potter MF. Chapter 3: Termites. In: Mallis A, editor. Handbook of pest control. 10th ed. Cleveland, Ohio: Mallis Handbook and Technical Training Company; 2011. p. 292–441.
Tucker J. Chapter 24: Implementing structural pest management. In: Mallis A, editor. Handbook of pest control. 10th ed. Cleveland, Ohio: Mallis Handbook and Technical Training Company; 2011. p. 1497–515.
Munro J. Pests of stored products. The Rentokil library. Colchester: Benham and Co.; 1966. p. 234.
Weier J. Chapter 13: Stored product pest. In: Mallis A, editor. Handbook of pest control. 10th ed. Mallis Handbook Company; 2011. p. 883–966.
Greenberg B, Kunich JC. Entomology and the law. Flies as forensic indicators. Cambridge: Cambridge University Press; 2002. p. 306.
Leclercq M. Entomological parasitology. The relations between entomology and the medical sciences. In: Leclercq M, editor. Entomology and legal medicine. Oxford: Pergamon Press; 1969. p. 128–42.
Dadour IR, Harvey ML. The use of insects and associated arthropods in legal cases: a historical and practical perspective. In: Oxenham M, editor. Forensic approaches to death, disaster and abuse. Bowen Hills: Australian Academic Press; 2008.
Byrd JH, Castner JL. Entomological evidence. The utility of arthropods in legal investigations. Boca Raton: CRC Press; 2010. p. 681.
Beyer JC, Enos WF, Stajic M. Drug identification through analysis of maggots. J Forensic Sci. 1980;25:411–2.
Catts EP, Goff ML. Forensic entomology in criminal investigations. Ann Rev Entomol. 1992;37:253–72.
Crosby TK, Watt JC, Kistemaker AC, Nelson PE. Entomological identification of the origin of imported cannabis. Forensic Sci Soc. 1986;26:35–44.
Gunatilake K, Goff ML. Detection of organophosphate poisoning in a putrefying body by analysing arthropod larvae. J Forensic Sci. 1989;34:714–6.
Roeterdink EM, Dadour IR, Watling RJ. Extraction of gunshot residues from the larvae of the forensically important blowfly Calliphora dubia (Macquart) (Diptera: Calliphoridae). Int J Legal Med. 2004;118:63–70.
Goff ML, Lord WB. Entomotoxicology: insects as toxicological indicators and the impact of drugs and toxins on insect development. In: Byrd JH, Castner JL, editors. Entomological evidence. The utility of arthropods in legal investigations. Boca Raton: CRC Press; 2010. p. 427–36.
Haglund WD, Reay DT, Swindler DR. Canid scavenging/disarticulation sequence of human remains in the Pacific Northwest. J Forensic Sci. 1989;34:587–606.
Smith KGV. A manual of forensic entomology. London: Trustees of the British Museum of Natural History; 1986. p. 205.
Prichard JD, Kossoris PD, Leibovitch RA, Robinson LD, Lovell WF. Implications of Trombiculid mite bites; report of a case and submission of evidence in a murder trial. J Forensic Sci. 1986;31:301–6.
Goff ML, Brown WA, Hewadikaram KA, Omari AI. Effect of heroin in decomposing tissues on the development rate of Boettcherisca peregrina (Diptera: Sarcophagidae) and implications to the estimations of postmortem intervals using arthropod development patterns. J Forensic Sci. 1991;36:537–42.
Benecke M, Lessig R. Child neglect and forensic entomology. Forensic Sci Int. 2001;120:155–9.
Benecke M. Forensic entomology special issue. Forensic Sci Int. 2001;120:1–160.
Benecke M. Cases of neglect involving entomological evidence. In: Byrd JH, Castner JL, editors Entomological evidence. The utility of arthropods in legal investigations. Boca Raton: CRC Press; 2010. pp. 627–49.
Berenbaum MR. Bugs in the system: insects and their impact on human affairs. Reading: Addison-Wesley; 1995.
Morris B. Physiology and taxonomy of blowflies. MAgSc Thesis, University of Adelaide; 1993.
Anderson GS. Wildlife forensic entomology: determining time of death in two illegally killed black bear cubs. J Forensic Sci. 1999;44:856–9.
Watson EJ, Carlton CE. Spring succession of necrophilous insects on wildlife carcasses in Louisiana. J Med Entomol. 2003;4:338–47.
Watson EJ, Carlton CE. Insect succession and decomposition of wildlife carcasses during fall and winter in Louisiana. J Med Entomol. 2005;42:193–203.
Merck MD. Veterinary forensics: animal cruelty investigations. Ames: Blackwell Publishing; 2007.
Easton AM, Smith KG. The entomology of the cadaver. Med Sci Law. 1970;10:208–15.
Snyder Sachs J. Corpse: nature, forensics, and the struggle to pinpoint time of death. London: Arrow Books; 2001. 270 pp.
Larkin B, Iaschi S, Tay G, Dadour IR. Using accumulated degree-days to estimate post mortem interval (PMI) from the DNA yield of porcine skeletal muscle. For Sci Med Path. 2010;6:83–92.
Morris B. First reported case of human aural Myiasis caused by the flesh fly Parasarcophaga crassipalpis (Diptera: Sarcophagidae). J Parasit. 1987;73:1068–9.
Morris B, Weinstein P. A case of aural myiasis in Australia. Med J Aust. 1986;145:634–5.
Zumpt F. Myiasis in man and animals in the old world. London: Butterworths; 1965. p. 267.
Cook DF, Dadour IR. Larviposition in the ovoviviparous blowfly Calliphora dubia. Med Vet Entomol. 2011;25:53–7.
Amendt J, Campobasso CP, Goff ML, Grassberger M. Current concepts in forensic entomology. Dordrecht/London: Springer; 2010. p. 376.
McKnight BE. The washing away of wrongs: forensic medicine in thirteenth-century China. Ann Arbor: University of Michigan; 1981. p. 181.
Megnin JP. La Faune des Tombeaux. Compte Rendu Hebdomadaire des Séances de l’Academie des Sciences. 1887;105:948–51.
Megnin JP. La faune des cadavres; application de l’entomologie a la medecine legale. In: Léauté H, editor. Encyclopedie Scientifique des Aides-Memoires. Paris: Masson et Gauthiers-Villars; 1894. p. 214.
Johnston W, Villeneuve G. On the medico-legal applications of entomology. Montreal Med J. 1897;26:81–90.
Motter MG. A contribution to the study of the fauna of the grave. A study of one hundred and fifty disinterments, with some additional experimental observations. J NY Entomol Soc. 1898;6:201–31.
Glaister J, Brash JC. Medico-legal aspects of the Ruxton case. Baltimore: William Wood & Company; 1937. p. 144–70, 245–59.
Nuorteva P, Isokoski M, Laiho K. Studies on the possibilities of using blowflies (Dipt.) as medicolegal indicators in Finland. Ann Entomol Fenn. 1967;33:217–25.
Nuorteva P, Schumann H, Isokoski M, Laiho K. Studies on the possibility of using blowflies (Dipt, Calliphoridae) as medicolegal indicators in Finland. 2. Four cases where species identification was performed from larvae. Ann Entomol Fenn. 1974;40:70–4.
Nuorteva P. Age determination of a blood stain in a decomposing shirt by entomological means. Forensic Sci. 1974;3:89–94.
Leclercq M. Entomologie et Medecine Legale. Etude des Insectes et Acariens Necrophages pour determiner la date de la mort. Spectrum Int. 1974;17:1–7.
Leclercq M. Entomologie et Medecine Legale, Datation de la Mort, Coll Med Legale Toxicol Med No 108. Paris: Masson; 1978. p. 100.
Goff ML. Forensic entomology. In: Resh VH, Cardé R, editors. Encyclopedia of insects. 2nd ed. London: Academic Press; 2009. p. 381–5.
Haskell NH, Williams RE, Catts EP. Entomology and death: a procedural guide. 2nd ed. Clemson, SC: East Park Printing; 2008. p. 216.
Mann RW, Bass WM, Meadows L. Time since death and decomposition of the human body-variables and observations in case and experimental field studies. J Forensic Sci. 1990;35:103–11.
Bass WM. Time interval since death. A difficult decision. In: Rathbun TA, Buikstra JE, editors. Human identification: case studies in forensic anthropology. Springfield: Charles C. Thomas; 1984. p. 136–47.
Reed HB. A Study of dog carcass communities in Tennessee, with special reference to the insects. Am Mid Nature. 1958;59:213–45.
Goff ML. Estimation of postmortem interval using arthropod development and successional patterns. Forensic Sci Rev. 1993;5:81–94.
Bornemissza GF. An analysis of arthropod succession in carrion and the effect of its decomposition on the soil fauna. Aust J Zool. 1957;5:1–12.
Goff ML. Comparison of insect species associated with decomposing remains recovered inside dwellings and outdoors on the Island of Oahu. J Forensic Sci. 1991;36:748–53.
Goff ML. A fly for the prosecution. How insect evidence helps solve crimes. Cambridge: Harvard University Press; 2000. p. 225.
Voss S, Spafford H, Dadour I. Temperature-dependant development of Nasonia vitripennis on five forensically important carrion fly species. Entomol Exp Appl. 2010;135:37–47.
Voss S, Spafford H, Dadour I. Temperature-dependant development of the parasitoid Tachinaephagus zealandicus on five forensically important carrion fly species. Med Vet Entomol. 2010;24:189–98.
Forbes SL, Dadour IR. The soil environment and forensic entomology. In: Byrd JH, Castner JL, editors. Entomological evidence. The utility of arthropods in legal investigations. 2nd ed. Boca Raton: CRC Press; 2010. p. 407–26.
Anderson GS. Minimum and maximum developmental rates of some forensically important Calliphoridae (Diptera). J Forensic Sci. 2000;45:824–32.
Vass AA, Barshick SA, Sega G, Caton J, Skeen JT, Love JC, Synstelien JA. Decomposition chemistry of human remains: a new methodology for determining post-mortem interval. J Forensic Sci. 2002;47:542–53.
Lord WD. Case histories of the use of insects in investigations. In: Catts EP, Haskell NH, editors. Entomology and death: a procedural guide. Clemson: Joyces Print Shop; 1990. p. 9–37.
Anderson GS, VanLaerhoven SL. Initial studies on insect succession on carrion in southwestern British Columbia. J Forensic Sci. 1996;41:617–25.
Gullan PJ, Cranston PS. The insects: an outline of entomology. 3rd ed. Malden: Blackwell Publishing; 2005. p. 505.
Chapman RF. The insects: structure and function. 4th ed. Cambridge: Cambridge University Press; 1998. p. 770.
Imms AD, Richards OW, Davies RG. Imms’ general textbook of entomology: structure, physiology, and development. London: Chapman and Hall; 1977. p. 1354.
Vogt WG, Woodburn TL. The influence of temperature and moisture on the survival and duration of the egg stage of the Australian sheep blowfly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Bull Entomol Res. 1980;70:665–71.
Vogt WG, Woodburn TL, Morton R, Ellem BA. The analysis and standardisation of trap catches of Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Bull Entomol Res. 1985;73:609–17.
Lord WD, Burger JF. Collection and preservation of forensically important entomological materials. J Forensic Sci. 1983;28:936–44.
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, Hall MJR. Best practice in forensic entomology – standards and guidelines. Int J Legal Med. 2007;121:90–104.
Slone DH, Gruner SV. Thermoregulation in larval aggregations of carrion-feeding blow flies (Diptera: Calliphoridae). J Med Entomol. 2007;44:516–23.
Tantawi TI, Greenberg B. The effect of killing and preservative solutions on estimates of maggot age in forensic cases. J Forensic Sci. 1993;38:702–7.
Davies K, Harvey ML. Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J Forensic Sci. 2012. doi:10.1111/j.1556-4029.2012.02196.x.
Myskowiak JB, Doums C. Effects of refrigeration on the biometry and development of Protophormia terraenovae (Robineau–Desvoidy) (Diptera: Calliphoridae) and its consequences in estimating post-mortem interval in forensic investigations. Forensic Sci Int. 2002;125:254–61.
Johl HK, Anderson GS. Effects of refrigeration on development of the blowfly Calliphora vicina (Diptera: Calliphoridae) and their relationship t time of death. J Entomol Soc BC. 1996;93:93–8.
Davies L, Ratcliffe GG. Development rates in some pre-adult stages in blowflies with reference to low temperatures. Med Vet Entomol. 1994;8:245–54.
Byrd JH, Butler JF. Effects of temperature on Cochliomyia macellaria (Diptera: Calliphoridae) development. J Med Entomol. 1996;33:901–5.
Byrd JH, Butler JF. Effects of temperature on Chrysomya rufifacies (Diptera: Calliphoridae) development. J Med Entomol. 1997;34:353–8.
Byrd JH, Butler JF. Effects of temperature on Sarcophaga haemorrhoidalis (Diptera: Sarcophagidae) development. J Med Entomol. 1998;35:694–8.
Huntington TE, Higley LG, Baxindale FP. Maggot development during morgue storage and its effect on estimating the post-mortem interval. J Forensic Sci. 2007;52:453–8.
Introna Jr F, Campobasso CP, Goff ML. Entomotoxicology. Forensic Sci Int. 2001;120:42–7.
Goff ML, Lord WD. Entomotoxicology: a new area for forensic investigation. Am J Forensic Med Pathol. 1994;15:51–7.
Pounder DJ. Forensic entomo-toxicology. J Forensic Sci Soc. 1991;31:469–72.
Sohal RS, Lamb RE. Storage excretion of metalic cations in the adult housefly Musca domestica. J Insect Physiol. 1979;25:119–24.
Nuorteva P, Nuorteva SL. The fate of mercury in sarcosaprophagous flies and in insects eating them. Ambio. 1982;11:34–7.
Romolo FS, Margot P. Identification of gunshot residue: a critical review. Forensic Sci Int. 2001;119:195–211.
Liu X, Shi Y, Wang H, Zang R. Determination of malathion levels and its effect on the development of Chrysomya megacephala (Fabricius) in south China. Forensic Sci Int. 2009;192:14–8.
Wolff M, Builes A, Zapata G, Morales G, Benecke M. Detection of parathion (0,0-diethyl 0-(4-nitrophenyl) phosphorothioate) by HPLC in insects of forensic importance in Medellin, Colombia. Aggrawal’s Internet. J Forensic Med Toxicol. 2004;5:6–11.
Kintz P, Tracqui A, Mangin P. Toxicology and fly larvae on a putrefied cadaver. J Forensic Sci Soc. 1990;30:243–6.
Kintz P, Tracqui A, Ludes B, Waller J, Boukhabza A, Mangin P, Lugnier AA, Chaumont AJ. Fly larvae and their relevance in forensic toxicology. Am J Forensic Med Pathol. 1990;11:63–5.
Manhoff DT, Hood I, Caputo F, Perry J, Rosen S, Mirchandani HG. Cocaine in decomposed human remains. J Forensic Sci. 1991;36:1732–5.
Nolte KB, Pinder RD, Lord WD. Insect larvae used to detect cocaine poisoning in a decomposed body. J Forensic Sci. 1992;4:179–85.
Wilson Z, Hubbard S, Pounder DJ. Drug analysis in fly larvae. Am J Forensic Med Pathol. 1993;14:118–20.
Introna Jr F, Lo Dico C, Caplan YH, Smialek JE. Opiate analysis of cadaveric blow fly larvae as an indicator of narcotic intoxication. J Forensic Sci. 1990;35:118–22.
Kintz P, Tracqui A, Mangin P. Analysis of opiate in fly larvae sampled on putrefied cadaver. J Forensic Sci Soc. 1994;34:95–7.
Bourel B, Fleurisse L, Hedouin Y, Cailliez JC, Creusy C, Goff ML, Gosset D. Immunohistochemical contribution to the study of morphine metabolism in Calliphoridae larvae and implications in forensic entomotoxicology. J Forensic Sci. 2001;46:596–9.
Sadler DW, Fuke C, Court F, Pounder DJ. Drug accumulation and elimination in Calliphora vicina larvae. Forensic Sci Int. 1995;71:191–7.
Sadler DW, Patl MR, Robertson L, Brown G, Fuke E, Pounder DJ. Barbiturates and analgesics in Calliphora vicina larvae. J Forensic Sci. 1997;42:481–8.
O’Brien C, Turner B. Impact of paracetamol on Calliphora vicina larval development. Int J Legal Med. 2004;118:188–9.
Goff ML, Miller ML, Paulsson JD, Lord WD, Richards E, Omori AI. Effects of 3, 4-methylenedioxymethamphetamine in decomposing tissues on the development of Parasarcophaga ruficornis (Diptera: Sarcophagidae) and detection of the drug in postmortem blood, liver tissue, larvae and puparia. J Forensic Sci. 1997;42:276–80.
Miller ML, Lord WD, Goff ML, Donnelly B, McDonough ET, Alexis JC. Isolation of amitriptyline and nortriptyline from fly puparia (Phoridae) and beetle exuvia (Dermestidae) associated with mummified human remains. J Forensic Sci. 1994;39:1305–13.
Gagliano-Candela R, Aventaggiato L. The detection of toxic substances in entomological specimens. Int J Legal Med. 2001;114:197–203.
Drijfhout FP. Cuticular hydrocarbons: a new tool in forensic entomology? In: Amendt J, Campobasso CP, Goff ML, Grassberger M, editors. Current concepts in forensic entomology. Dordrecht: Springer; 2010. p. 179–203.
Lockey KH. Lipids of the insect cuticle: origin composition and function. Comp Biochem Physiol. 1988;89B:595–645.
Monnin T. Chemical recognition of reproductive status in social insects. Ann Zool Fenn. 2006;43:515–30.
Martin C, Salvy M, Provost E, Bagneres AG, Roux M, Crauser D, Clement JL, Le Conte Y. Variations in chemical mimicry by the ectoparasitic mite Varma jacobsoni according to the developmental stage of the host honey bee Apis mellifera. Ins Biochem Mol Biol. 2001;31:365–79.
Buczkowski G, Kumar R, Suib SL, Silverman J. Diet- related modification of cuticular hydrocarbon profiles of the Argentine ant Linepithema humile diminishes intercolony aggression. J Chem Ecol. 2005;31:829–43.
Savarit F, Ferveur J-F. Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster. J Exp Biol. 2002;205:3241–9.
Rouault J-D, Marican C, Wicker-Thomas C, Jallon J-M. Relations between cuticular hydro carbons (HC) polymorphism resistance against desiccation and breeding temperature; a model for HC evolution in D. melanogaster and D. stimulans. Genetica. 2004;120:195–212.
Zhu GH, Ye GY, Hu C, Xu XH, Li K. Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: potential for determining larval age. Med Vet Entomol. 2006;20:438–44.
Zhu GH, Xu XH, Yu XJ, Zhang Y, Wang JR. Puparial case hydrocarbons of Chrysomya megacephala as an indicator of the postmortem interval. Forensic Sci Int. 2007;169:1–5.
Roux O, Gers C, Legal L. Ontogenetic study of three Calliphoridae of forensic importance through cuticular hydrocarbon analysis. Med Vet Entomol. 2008;22:309–17.
Urech R, Brown GW, Moore CJ, Green PE. Cuticular hydrocarbons of buffalo fly Haematobia exigua and chemotaxonomic differentiation from horn fly H-Irritans. J Chem Ecol. 2005;31:2451–61.
Page M, Nelson LJ, Blomquist GJ, Seybold SJ. Cuticular hydrocarbons as chemotaxonomic characters of pine engraver beetles (Ips spp.) in the grandicollis subgeneric group. J Chem Ecol. 1997;23:1053–99.
Ye GY, Li K, Zhu JY, Zhu GH, Hu C. Cuticular hydrocarbon composition in pupal exuviae for taxonomic differentiation of six necrophagous flies. J Med Entomol. 2007;44:450–6.
Rouault J, Capy P, Jallon JM. Variations of male cuticular hydroca rbons with geoclimatic variables: an adaptative mechanism in Drosophila melanogaster? Genetica. 2001;110:117–30.
Ugelvig LV, Drijfhout FP, Kronauer DJC, Boomsma JJ, Pedersen JS, Cremer S. The introduction history of invasive garden ants in Europe: integrating genetic chemical and behavioural approaches. BMC Biol. 2008. doi:10.1186/1741-7007-6-11.
Wells JD, Stevens JR. Molecular methods for forensic entomology. In: Byrd JH, Castner JL, editors. Entomological evidence. The utility of arthropods in legal investigations. Boca Raton: CRC Press; 2010. p. 437–52.
Harvey ML, Mansell MW, Villet MH, Dadour IR. Phylogeny of some forensically important Calliphoridae (Diptera) in Australia and southern Africa. Med Vet Entomol. 2003;17:1–7.
Wallman JF, Adams M. Molecular systematics of Australian carrion-breeding blowflies of the Genus Calliphora (Diptera: Calliphoridae). Aust J Zool. 1997;45:337.
Wallman JF, Adams M. The forensic application of allozyme electrophoresis to the identification of blowfly larvae (Diptera: Calliphoridae) in southern Australia. J Forensic Sci. 2001;46:681.
Wallman JF, Donnellan SC. The utility of mitochondrial DNA sequences for the identification of forensically important blowflies (Diptera: Calliphoridae) in southeastern Australia. Forensic Sci Int. 2001;120:60–7.
Sperling FAH, Anderson GS, Hickey DA. A DNA-based approach to the identification of insect specimens used for post-mortem interval estimation. J Forensic Sci. 1994;39:418.
Malgorn Y, Coquoz R. DNA typing for identification of some species of calliphoridae. An interest in forensic entomology. Forensic Sci Int. 1999;102:111–9.
Benecke M, Wells J. Molecular techniques for forensically important insects. In: Byrd JH, Castner JL, editors. Entomological evidence: the utility of arthropods in legal investigations. Boca Raton: CRC Press; 2000. p. 341–52.
Stevens J, Wall R. Evolution of ectoparasitism in the Genus Lucilia (Diptera: Calliphoridae). Int J Parasitol. 1997;27:51.
Mazzanti M, Alessandrini F, Tagliabracci A, Wells JD, Campobasso CP. DNA degradation and genetic analysis of empty puparia: genetic identification limits in forensic entomology. Forensic Sci Int. 2010;195:99–102.
McDonagh L, Thornton C, Wallman JF, Stevens JR. Development of an antigen-based rapid diagnostic test for the identification of blowfly (Calliphoridae) species of forensic significance. Forensic Sci Int. 2009;3:162–5.
Harrison RG. Animal mtDNA as a genetic marker in population and evolutionary biology. Trends Ecol Evol. 1989;4:6–11.
Harvey ML, Gaudieri S, Villet MH, Dadour IR. A global study of forensically significant Calliphorids: implications for identification. Forensic Sci Int. 2008;177:66–76.
Campobasso CP, Linville JG, Wells JD, Introna F. Forensic genetic analysis of insect gut contents. Am J Forensic Med Pathol. 2005;26:161–5.
Wells JD, Introna Jr F, Di Vella G, Campobasso CP, Hayes J, Sperling FA. Human and insect mitochondrial DNA analysis from maggots. J Forensic Sci. 2001;46:685–7.
Kreife J, Kempfer S. Isolation and characterization of human DNA from mosquitoes (Culicidae). Int J Legal Med. 1999;112:380–2.
Lord WD, DiZinno JA, Wilson MR, Budowle B, Taplin D, Meinking TL. Isolation, amplification, and sequencing of human mitochondrial DNA obtained from human crab louse, Phthirus Pubis (L.), blood meals. J Forensic Sci. 1998;43:1097–100.
DiZinno JA, Lord WD, Collins-Morton MB, Wilson MR, Goff ML. Mitochondrial DNA sequencing of beetle larvae (Nitidulidae: Omosita) recovered from human bone. J Forensic Sci. 2002;47:1337–9.
Benecke M. Asservierung von Insekten, Spinnen und Krebsmaterial fur die Forensisch Kriminalistische Untersuchung (collecting insects, spiders and crustaceans for criminal forensic study). Arch Kriminol. 1997;199:167–76.
Nelson LA, Dowton M, Wallman JF. Thermal attributes of Chrysomya species. Entomol Exp Appl. 2009;133:260–75.
Dadour IR, Cook DF, Wirth N. Rate of development of Hydrotaea rostrata under summer and winter (cyclic and constant) temperature regimes. Med Vet Entomol. 2001;15:177–82.
O’Flynn MA. The succession and rate of development of blowflies in carrion in Southern Queensland and the application of these data to Forensic Entomology. J Aust Entomol Soc. 1983;22:137–48.
von Zuben CJ, Bassanezi RC, von Zuben FJ. Theoretical approaches to forensic entomology: II. Mathematical model of larval development. J Appl Entomol. 1998;275:122.
Wells JD, Kurahashi H. Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) development rate, variation and the implications for forensic entomology. Jap J San Zool. 1994;45:303.
Thompson RCA, Lymbery AJ. Echinococcus and hydatid disease. Wallingford: CAB International; 1995. p. 477.
Dadour IR, Cook DF, Fissioli JN, Bailey WJ. Forensic entomology: application, education and research in Western Australia. Forensic Sci Int. 2001;120:48–52.
Ratcliffe GG. Comparative studies on the developmental rates of the larvae of certain blowflies (“Diptera: Calliphoridae”) at constant and alternating temperatures. MSc Thesis, University of Durham; 1984.
Erzinclioglu YZ. Areas of research in forensic entomology. Med Sci Law. 1986;26:273–8.
Voss SC, Forbes SL, Dadour IR. Decomposition and insect succession on cadavers inside a vehicle environment. Forensic Sci Med Pathol. 2008;4:22–32.
Dadour IR, Almanjahie I, Fowkes ND, Keady G, Vijayan K. Temperature variations in a parked vehicle. Forensic Sci Int. 2011;207:205–11.
Morris B. Towards an entomological timing of death. In: Proceedings of the 8th Australian forensic science symposium, Perth; 1983. p. 347–54.
Deonier CC. Carcass temperatures and their relation to winter blowfly populations and activity in the southwest. J Econ Entomol. 1940;33:166–70.
Charabidze D, Bourel B, Gosset D. Larval-mass effect: characterisation of heat emission by necrophageous blowflies (Diptera: Calliphoridae) larval aggregates. Forensic Sci Int. 2011;211:61–6.
VanLaerhoven SL. Blind validation of postmortem interval estimates using developmental rates of blow flies. Forensic Sci Int. 2008;180:76–80.
Dallwitz R. The influence of constant and fluctuating temperatures on the development rate and survival of the Australian sheep blowfly Lucilia cuprina. Appl Exp Entomol. 1984;36:89–95.
Waterhouse DF. The relative importance of live sheep and of carrion as breeding grounds for the Australian sheep blowfly Lucilia cuprina. Council Sci Ind Res Bull. 1947;1:217.
Fuller ME. The insect inhabitants of carrion: a study in animal ecology. Bull Council Sci Ind Res. 1934;82:1–62.
Kelly JA, v.d. Linde TC, Anderson GS. The influence of clothing and wrapping on carcass decomposition and arthropod succession during the warmer seasons in Central South Africa. J Forensic Sci. 2009;54:1105–12.
Voss S, Cook DF, Dadour IR. Decomposition and insect succession of clothed and unclothed carcasses in Western Australia. Forensic Sci Int. 2011;211:67–75.
Fabre JH. Souvenirs entomologiques. 6th Ser. Paris: Librairie Ch. Delagrave; 1890. p. 113–29. Chapter 7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Dadour, I.R., Morris, B. (2014). Forensic Entomology: A Synopsis, Guide, and Update. In: Rutty, G. (eds) Essentials of Autopsy Practice. Springer, London. https://doi.org/10.1007/978-1-4471-5270-5_6
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5270-5_6
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5269-9
Online ISBN: 978-1-4471-5270-5
eBook Packages: MedicineMedicine (R0)