Abstract
Over the past several decades much has been learned about arterial stiffness and central blood pressure (BP) and their relations to left ventricular (LV) remodelling and hypertrophy. In addition, the impact of central hemodynamics on LV performance and the development of clinical heart failure is under active investigation and is of particular importance from a public health and therapeutic standpoint. The ability to examine these topics has been vastly accelerated by the development of reliable, noninvasive technology to permit evaluation of cardiac and vascular structure and function on an epidemiologic scale. The present review will discuss data regarding the interaction of arterial stiffness and central BP with LV structure and function; the impact of arterial stiffness on the development of heart failure, particularly with preserved ejection fraction, will also be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Over the past several decades much has been learned about arterial stiffness and central blood pressure (BP) and their relations to left ventricular (LV) hypertrophy and geometry. Central arterial BP and measures of arterial stiffness derived therefrom are of particular importance because central BP represents the load placed on the LV and large coronary and cerebral arteries that develop stenosis and occlusion, and because central BP is variably lower than brachial BP due to the invasively-documented phenomenon of pulse-pressure amplification [1–3]. In addition, the impact of central hemodynamics on LV performance and the development of clinical heart failure is under active investigation and is of particular importance from a public health and therapeutic standpoint. The ability to examine these topics has been vastly accelerated by the development of reliable, noninvasive technology to permit evaluation of cardiac and vascular structure and function on an epidemiologic scale. The present review will discuss data regarding the interactions of arterial stiffness and central BP with LV structure and function. The impact of arterial stiffness on the development of heart failure, particularly with preserved ejection fraction, will also be discussed.
Relation of Central BP and Arterial Stiffness to LV Remodelling
Methodologic Considerations
LV remodelling may be characterized an increase in LV mass (hypertrophy) and/or abnormal relative wall thickness (concentric geometry). LV mass and relative wall thickness can be determined by a variety of methods, with almost all data derived from transthoracic echocardiography. LV mass can be accurately calculated from linear measurement of LV wall thicknesses and internal diameter at mid cavity using an autopsy-validated formula [4, 5]. In view of the strong dependence of LV mass on body size in normal individuals, it is optimal to adjust absolute LV mass for differences in body size. Although body surface area is most commonly used, adjustment of LV mass for its allometric relation to height (ht2.7) better detects increases in LV mass related to obesity [6].
Relative wall thickness is calculated as posterior wall thickness/chamber radius. It is used to classify LV geometry into one of four patterns (normal, eccentric hypertrophy, concentric hypertrophy, and concentric remodeling [Fig. 24.1]). These patterns reflect differences in underlying hemodynamic abnormalities related to hypertension [7]. Recently, it has been suggested that further subdivision of concentric and eccentric LV hypertrophy into subgroups with or without LV chamber dilatation helps stratify LV function and systemic hemodynamics more precisely [8, 9]. However, whether this classification strengthens relations between arterial stiffness and LV geometry has not yet been evaluated.
Classification of LV geometric patterns based on LV mass index and relative wall thickness (Adapted from Ref. [7])
Normative values for LV mass vary according to gender. Greater LV mass in men cannot be fully accounted for by larger body size and may relate to differences in fat-free mass [10, 11]. Based on refinements in image quality and cumulative analyses in large international populations, 116 g/m2 or 48 g/ht2.7 in men and 96 g/m2 or 44 g/ht2.7 in women are currently recommended as the upper limits of normal for LV mass index [5]. The upper normal limit for relative wall thickness is 0.42 [5].
Although the importance of blood pressure as a stimulus to LV hypertrophy has long been known, measures of brachial BP account for a relatively modest amount of variability in LV mass. Thus alternate indices of ventricular afterload have been developed, validated, and examined. Central BP, i.e., BP in the ascending aorta, differs from brachial BP to a variable extent based on pulse pressure (PP) amplification. Because central BP more closely reflects loading conditions of the LV myocardium and coronary and cerebral vasculature, it better predicts cardiovascular target organ damage and clinical disease than does brachial BP, as discussed below. Similarly, arterial stiffness and selected measures of pulse wave transmission may better represent changes in physical properties of the conduit arteries. However, the extent to which arterial stiffness promotes LV hypertrophy is strongly influenced by the method by which arterial stiffness is estimated, i.e., the extent to which the stiffness parameter varies with changes in distending pressure. This phenomenon will be considered as different measures of arterial stiffness are discussed.
Central BP and LV Remodelling
It has been firmly established that brachial systolic BP (including ambulatory) is more strongly related to LV mass than is brachial PP [12–14]. Similarly, we demonstrated in the Strong Heart Study a stronger relation of central systolic BP than central PP to LV remodelling (both LV mass index and relative wall thickness) [15]. Importantly, central systolic BP bore a significantly stronger relation with LV remodelling than did brachial systolic BP. Central systolic BP was likewise found to correlate better than brachial systolic BP with LV mass in a large Taiwanese population [16], and central PP was found to relate to LV mass independent of brachial PP in a South African population-based study [17].
Arterial Stiffness and LV Remodelling
A variety of techniques are available for non-invasive assessment of arterial stiffness [18]. The extent to which estimates of arterial stiffness promote LV remodelling is largely a function of their dependence on distending pressure. Thus we have found that elastic modulus, a pressure-dependent measure, is significantly related to LV mass index, whereas the arterial stiffness index (β), a pressure-independent measure, is not [19]. However, the arterial stiffness index was directly related to relative wall thickness and thereby to concentric LV geometry. Although younger and older hypertensive subjects had comparable overall LV mass, relative wall thickness was significantly higher in older hypertensive subjects associated with higher arterial stiffness [19]. As a corollary, among 271 untreated hypertensive subjects, we found elastic modulus to track with systolic blood pressure and therefore to be highest in the group with concentric LV hypertrophy [20].
The pattern of LV concentric remodelling (high relative wall thickness and normal LV mass) in hypertensive individuals is also associated with abnormally high effective arterial elastance (Ea), higher peripheral resistance, and lower LV systolic function. In addition, ventriculo-vascular coupling, calculated as Ea/Ees (where Ees is the ratio of end-systolic pressure to LV end-systolic volume), is increased among hypertensive patients with increased effective arterial elastance, indicative of suboptimal mechanical efficiency [21]. Concentric LV remodelling was also directly related to pulse wave velocity – influenced by the level of arterial distending pressure – among middle-aged and older hypertensive patients studied by Schillaci et al. [22].
Among 1,315 normotensive and untreated hypertensive subjects, Chen et al. found echocardiographic LV mass to be directly related to arterial compliance calculated as LV stroke volume/brachial pulse pressure [12], consistent with the known importance of stroke volume as a stimulus to LV hypertrophy [23] but also possible autocorrelation between mass and stroke volume as both were calculated from similar measurements. In addition, LV mass was directly related to elastic modulus and inversely to arterial elastance, similar to our findings [21]. Importantly, in multivariate analyses in this study, the measures of arterial stiffness examined (elastic modulus, carotid augmentation index, and pulse wave velocity) were only independently related to LV mass when blood pressure was excluded from the analyses, underscoring the pressure-dependence of these parameters.
Relations of Arterial Stiffness and Central BP to LV Systolic and Diastolic Function
Invasive measurement of LV pressure and volume and determination of arterial elastance document an age-associated increase in arterial stiffness which is mirrored by an increase in ventricular stiffness, even in the absence of hypertrophy [24]. Although ventriculo-vascular coupling is maintained on average, there is a much greater sensitivity of systolic BP to changes in LV preload. In a rat model of aortic stiffness (induced elastocalcinosis), prolonged exposure to increased aortic stiffness (characteristic impedance) led to LV hypertrophy, fibrosis reflected as increase in collagen content, and a shift in the myosin heavy chain isoform pattern [25]. This latter phenomenon prolongs systolic ejection to maintain contractile performance but shortens diastole in the setting of increased myocardial stiffness. These important experimental observations have been followed by a number of non-invasive investigations of the chronic impact of arterial stiffness on LV function and its clinical implications.
Using non-invasive echocardiographic parameters and estimated end-systolic pressure, Redfield et al. examined ventricular and vascular stiffening in 2,042 participants in a population-based (Olmstead County, Minnesota) study (Rochester Epidemiology Project) [26]. Similar to the earlier invasive study, both ventricular and vascular stiffening–estimated from Doppler echocardiography and brachial BP as vascular (Ea) and ventricular (Ees) elastances–increased with age. Notably, values were higher in women than in men, and ventricular stiffness increased more steeply in women. These findings were independent of symptom status and provided support for the authors’ hypothesis that parallel arterial and ventricular stiffening might account for age-related heart failure with preserved ejection fraction (HFPEF), especially in women. Of note, the authors also confirmed age-associated concentric remodelling (increase in relative wall thickness), particularly in women.
As further evidence of a potential link between arterial stiffening and diastolic dysfunction as a potential mechanism for HFPEF, aortic and brachial PPs, but not carotid-femoral pulse wave velocity (influenced by mean arterial pressure), were related to the grade of diastolic dysfunction as well as left atrial volume index, a marker of chronic LV diastolic dysfunction, in older patients at risk for development of atrial fibrillation [27]. In an Austrian study of patients with normal LV systolic function undergoing coronary angiography for suspected coronary artery disease, invasively-determined pulse wave velocity was negatively associated with echocardiographic tissue Doppler measures of diastolic relaxation (septal and lateral E′) and directly related to E/E′, an estimate of LV filling pressure (as well as to measured LV end-diastolic pressure) [28]. Of note, pulse wave velocity was directly related to plasma levels of amino terminal pro-brain natriuretic peptide (NT-proBNP, secreted in response to elevated LV filling pressure) and, along with age and female gender, was an independent predictor of the presence of exertional dyspnea.
In a subset (n = 983) of the population-based Northern Manhattan Study in whom non-invasive pulse wave analysis and echocardiography were performed, “global arterial stiffness” was calculated as: central PP/LV stroke volume index [29]. In confirmation of the Austrian patient-based study, arterial stiffness was negatively related to velocity of myocardial relaxation (E′) and directly related to LV filling pressure (E/E′). In multivariable analyses, arterial stiffness was independently related to the presence of diastolic dysfunction.
Regional LV systolic and diastolic strains were determined using cardiac magnetic resonance imaging in 1,100 asymptomatic participants in the Multi-Ethnic Study of Atherosclerosis (MESA Study) who additionally underwent carotid ultrasound study [30]. Carotid artery compliance (calculated using brachial BP) was directly related to both systolic and diastolic regional performance, even following adjustment for blood pressure, supporting a role for arterial stiffening in the development of ventricular dysfunction.
Relation of Arterial Stiffness and Central BP to Clinical Heart Failure
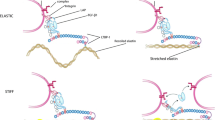
The interaction between arterial and ventricular stiffening and associated consequences as well as the association between arterial stiffness and abnormal LV function described above has led to the consideration of arterial stiffness as a contributor to heart failure with preserved ejection fraction (HFPEF), particularly when it occurs in the absence of significant epicardial coronary artery disease (Fig. 24.2). Thus, arterial stiffening, in the presence or absence of hypertension, leads to compensatory LV stiffening and remodelling to maintain systolic performance at the expense of diastolic relaxation. Increased pulse wave velocity results in augmentation of late-systolic pressure and reduction of early diastolic aortic pressure. The resultant combination of reduced coronary perfusion pressure during diastole, increased metabolically active myocardial mass, and elevated LV filling pressure may promote subendocardial ischemia. Elevation in left atrial pressure may result in atrial fibrillation, pulmonary hypertension and signs and symptoms of HFPEF may ensue. Understanding the pathophysiology and improving treatment of HFPEF is of major public health importance as recent as HFPEF is now the cause of at least 50 % of congestive heart failure, with outcomes similar to patients with heart failure with reduced ejection fraction [31].
The observation in the Framingham Heart Study [32], the East Boston Senior Health Project [33], the Established Populations for Epidemiologic Study of the Elderly [34], and the Systolic Hypertension in the Elderly Program (SHEP) [35] that brachial PP, a surrogate for arterial stiffness, is independently related to incident clinical heart failure supports the importance of conduit artery stiffness as a marker, if not a cause, of heart failure risk. However assessment of LV function was not systematically included in the evaluation of heart failure in these reports.
In addition, the extent to which pulse pressure is primarily generated by arterial stiffness vs. LV stroke volume may vary based on ejection fraction. Thus in the Studies of Left Ventricular Dysfunction (SOLVD) trials (LV ejection fraction ≤35 % required for study entry), brachial PP was directly related to both ejection fraction and cardiovascular mortality, resulting in the conclusion that arterial stiffness was the mechanism of risk [36]. Similarly, in 135 patients with chronic heart failure over a wide range of ejection fraction, brachial PP was related to ejection fraction in those with reduced and preserved (≥40 %) ejection fraction, whereas carotid-femoral pulse wave velocity, a direct measure of arterial stiffness, was related to ejection fraction in the low ejection fraction group but not in the preserved ejection fraction group [37].
Subsequent invasive and non-invasive studies in patients with HFPEF have supported the contribution of arterial stiffness to overt heart failure, in addition to evidence of abnormal diastolic function. In a small invasive study of 10 patients with HFPEF, Kawaguchi et al. found increased ventricular and vascular stiffness and an upward shift in the diastolic pressure-volume curve in patients compared to control groups [38]. These findings were confirmed in 244 patients with HFPEF studied at the Mayo Clinic [39]. Both asymptomatic hypertensive patients and HFPEF patients had increased ventricular and arterial stiffness (end-systolic ventricular and arterial elastance calculated using estimated end-systolic pressure and Doppler echocardiography) compared to control subjects, whereas diastolic stiffness (curve-fitting constants derived from Doppler echocardiography) was increased in the HFPEF patients compared to the other two groups.
Two studies from Weber and colleagues have provided important data regarding hemodynamic underpinnings of HFPEF as well as diagnostic utility of non-invasive markers [40, 41]. Two hundred and seventy-one patients referred for cardiac catheterization for suspected coronary artery disease were categorized as having definite diastolic dysfunction (LV end-diastolic pressure >16 mmHg with normal end-diastolic volume and NT-proBNP >125 pg/ml; n = 44), possible diastolic dysfunction (increased LV end-diastolic pressure or elevated NT-proBNP; n = 109), or normal diastolic function [40]. The group with definite diastolic dysfunction had the typical demographic profile of being older and more often female and hypertensive. Patients with diastolic dysfunction had prolonged LV ejection time indexed for heart rate (LVETi) and increased wave reflection (increase in augmentation index and augmented systolic pressure) compared to the other two groups, whereas those with possible diastolic dysfunction had intermediate values (Fig. 24.3). The augmented pressure was directly related to LVETi as well as invasive LV end-diastolic pressure. LVETi was an independent predictor of diastolic dysfunction, as were hypertension, LV mass index, decreased creatinine clearance, and E:E′ ratio from echocardiography. Furthermore, in receiver operating characteristic (ROC) curve analyses, LVETi and E:E′ ratio were comparable in their ability to detect diastolic dysfunction.
Comparisons of LV ejection time index (LVETi), augmentation of central systolic pressure (AP), and augmentation index (Aix) in normal controls, patients with possible diastolic dysfunction, and patients with definite diastolic dysfunction. See text for definitions (Reproduced with permission from Ref. [40])
In a subsequent larger study (n = 359), cardiac catheterization, pulse wave analysis and echocardiography were again performed, and HFPEF was defined as LV EF >50 % with end-diastolic pressure >16 mmHg and NT-proBNP >220 pg/ml [41]. In ROC analyses, the areas under the curve (AUC) for brachial PP, E:E′ ratio, central PP from tonometry and invasively-determined aortic pulse wave velocity were 0.816, 0.823, 0.851, and 0.867, respectively (not significantly different). Importantly, in multivariable models, E:E′ correctly classified 77 % as having HFPEF or not, with significant improvement when a measure of arterial function was added to the model. The addition of central PP to E:E′ was significantly superior to the addition of brachial PP (AUC: 0.901 vs. 0.875; Fig. 24.4).
Receiver operating characteristic curves comparing the ability to identify patients with HFPEF. The combination of central PP (aoPP) to E:E′ resulted in the highest area under the curve (0.901). bPP brachial pulse pressure (Reproduced with permission from Ref. [41])
Vascular stiffness and impaired ventriculo-arterial coupling are exaggerated with exercise in patients with HFPEF. Among 23 patients with HFPEF compared to 15 normal controls, exercise resulted in significant increases in elastic modulus, pulse wave velocity, and arterial elastance (calculated using central pressures derived from carotid applanation tonometry) and a lesser increase in stroke volume measured by echocardiography [42]. Of note, for a given end-diastolic volume, E/E′ ratio, an estimate of LV filling pressure was higher at rest (20 vs. 11, p < 0.001) and remained elevated with exercise in the HFPEF group.
These results are complemented by a study of 15 patients with HFPEF and 15 matched control subjects who underwent rest and exercise echocardiography and radial applanation tonometry [43]. Exercise in HFPEF patients resulted in an increase in E:E′ which was independently associated with an increase in central pressure augmentation but not with change in brachial systolic or pulse pressure. In contrast, exercise in control subjects resulted in decreases in E:E′, augmentation index and augmented pressure.
In a small study of 10 patients with HFPEF who underwent cardiac magnetic resonance imaging at rest, pulsatile changes in aortic area and distensibility (calculated using brachial blood pressures) were lower than in age-matched controls [44]. Subsequent exercise showed diminished peak exercise oxygen consumption in the patient group that was associated with lower distensibility of the thoracic aorta.
In summary, there is strong support for the importance of increased arterial stiffness and central blood pressure in the development of LV remodelling, abnormal LV function (particularly diastolic relaxation), and clinical manifestations of heart failure, most notably in the presence of apparently preserved systolic function. The inability thus far to identify pharmacologic approaches of clear benefit in patients with HFPEF supports the need to better treat hypertension in the general population, to better identify patients in whom arterial stiffness is a leading pathophysiologic mechanism for diastolic dysfunction and for clinical heart failure, and to identify therapies that are more effective in ameliorating arterial stiffening than current heart failure regimens that have been proven to benefit heart failure patients with systolic dysfunction.
Abbreviations
- AUC:
-
Area under the curve
- BP:
-
Blood pressure
- HFPEF:
-
Heart failure with preserved ejection fraction
- LV:
-
Left ventricular
- LVETi:
-
Left ventricular ejection time index
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptides
- PP:
-
Pulse pressure
- ROC:
-
Receiver operating characteristic
References
Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–7.
Westerhof BE, Guelen I, Stok WJ, Lasance HAJ, Ascoop CAPL, Wesseling KH, Westerhof N, Bos WJW, Stergiopulos N, Spaan JAE. Individualization of transfer function in estimation of central aortic pressure from the peripheral pulse is not required in patients at rest. J Appl Physiol. 2008;105:1858–63.
Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH. Validation of a generalized transfer function to noninvasively derived central blood pressure during exercise. Hypertension. 2006;47:1203–8.
Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and of the impact of overweight. J Am Coll Cardiol. 1992;20:1251–60.
Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodelling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–8.
Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas Heart Study. Circ Cardiovasc Imaging. 2010;3:164–71.
Bang CN, Gerdts E, Aurigemma GP, Boman K, Dahlof B, Roman MJ, Kober L, Wachtell K, Devereux RB. Systolic left ventricular function according to left ventricular concentricity and dilatation in hypertensive patients: the Losartan Intervention For Endpoint reduction in hypertension study. J Hypertens. 2013;31:2060–8.
Bella JN, Devereux RB, Roman MJ, et al. Relations of left ventricular mass to fat-free and adipose body mass: the Strong Heart Study. Circulation. 1998;98:2538–44.
Kuch B, Hense H-W, Gneiting B, et al. Body composition and prevalence of left ventricular hypertrophy. Circulation. 2000;102:405–10.
Chen C-H, Ting C-T, Lin S-J, His T-L, Ho S-J, Chou P, et al. Which arterial and cardiac parameters best predict left ventricular mass? Circulation. 1998;98:422–8.
Flack JM, Gardin JM, Yunis C, Liu K, for the CARDIA Research Group. Static and pulsatile blood pressure correlates of left ventricular structure and function in black and white young adults: the CARDIA Study. Am Heart J. 1999;138:856–64.
Verdecchia P, Schillaci G, Borgioni C, Gaatobigio R, Ambrosio G, Porcellati C. Prevalent influence of systolic over pulse pressure on left ventricular mass in essential hypertension. Eur Heart J. 2002;23:658–65.
Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens. 2010;28:384–8.
Wang K-L, Cheng H-M, Chuang S-Y, Spurgeon HA, Ting C-T, Lakatta EG, et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–7.
Norton GR, Majane OHI, Maseko MJ, Libhaber C, Redelinghuys M, Kruger D, Veller M, Sareli P, Woodiwiss AJ. Brachial blood pressure-independent relations between radial late systolic shoulder-derived aortic pressures and target organ changes. Hypertension. 2012;59:885–92.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, on behalf of the European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
Roman MJ, Ganau A, Saba PS, et al. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–94.
Roman MJ, Pickering TG, Schwartz JE, et al. Relation of arterial structure and function to left ventricular geometric patterns in hypertensive adults. J Am Coll Cardiol. 1996;28:751–6.
Saba PS, Ganau A, Devereux RB, et al. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens. 1999;17:1007–15.
Schillaci G, Mannarino MR, Pucci G, Pirro M, Helou J, Savarese G, Vaudo G, Mannarino E. Age-specific relationship of aortic pulse wave velocity with left ventricular geometry and function in hypertension. Hypertension. 2007;49:317–21.
Ganau A, Devereux RB, Pickering TG, Roman MJ, Schnall PL, Santucci S, Spitzer MC, Laragh JH. Relation of left ventricular hemodynamic load and contractile performance to left ventricular mass in hypertension. Circulation. 1990;81:25–36.
Chen C-H, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–7.
Lartaud-Idjouadiene I, Lompré A-M, Kieffer P, Colas T, Atkinson J. Cardiac consequences of prolonged exposure to an isolated increase in aortic stiffness. Hypertension. 1999;34:63–9.
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62.
Abhayaratna WP, Barnes ME, O’Rourke MF, Gersh BJ, Seward JB, Miyasaka Y, Bailey KR, Tsang TSM. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients ≥65 years of age. Am J Cardiol. 2006;98:1387–92.
Weber T, O’Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–202.
Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–8.
Fernandes VRS, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O’Leary DH, Bluemke DA, Lima JAC. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201.
Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis and treatment. Eur Heart J. 2011;32:670–9.
Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–6.
Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–9.
Vaccarino V, Holfrod TR, Krumholz HM. Pulse pressure and risk of myocardial infarction and heart failure in the elderly. J Am Coll Cardiol. 2000;36:130–8.
Kostis JB, Lawrence-Nelson J, Ranjan R, Wilson AC, Kostis WJ, Lacy CR. Association of increased pulse pressure with the development of heart failure in SHEP. Am J Hypertens. 2001;14:798–803.
Domanski MJ, Mitchell GF, Normal JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–8.
Tartière J-M, Logaert D, Safar M, Cohen-Solal A. Interaction between pulse wave velocity, augmentation index, pulse pressure and left ventricular function in chronic heart failure. J Hum Hypertens. 2006;20:213–9.
Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–20.
Lam CSP, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–90.
Weber T, Auer J, O’Rourke MF, Punzengruber C, Kvas E, Eber B. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart. 2006;92:1616–22.
Weber T, Wassertheurer S, O’Rourke MF, Haiden A, Zweiker R, Rammer M, Hametner B, Eber B. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–83.
Tartière-Kesri L, Tartière J-M, Logeart D, Beauvais F, Cohen SA. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59:455–61.
Holland DJ, Sacre JW, Leano RL, Marwick TH, Sharman JE. Contribution of abnormal central blood pressure to left ventricular filling pressure during exercise in patients with heart failure and preserved ejection fraction. J Hypertens. 2011;29:1422–30.
Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Roman, M.J., Devereux, R.B. (2014). Arterial Stiffness, Central Blood Pressure and Cardiac Remodelling: From Cardiac Hypertrophy to Heart Failure. In: Safar, M., O'Rourke, M., Frohlich, E. (eds) Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases. Springer, London. https://doi.org/10.1007/978-1-4471-5198-2_24
Download citation
DOI: https://doi.org/10.1007/978-1-4471-5198-2_24
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-5197-5
Online ISBN: 978-1-4471-5198-2
eBook Packages: MedicineMedicine (R0)