Abstract
Although fuel cells have been known for over 150 years, research on solid oxide fuel cells (SOFCs) based on an oxide ion conducting electrolyte only accelerated in the last 30 years. This chapter, after a brief history of SOFCs, reviews the materials for different cell components (electrolyte and the two electrodes) and cell stacks (seals and interconnects); novel materials and their structures have been investigated and developed to improve electrochemical performance. Different SOFC designs and their relative advantages and disadvantages are then discussed. Finally, various applications of SOFC power systems and the status of their demonstration and commercialization are reviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Solid Oxide Fuel Cell
- Proton Exchange Membrane Fuel Cell
- Lanthanum Chromite
- Solid Oxide Fuel Cell System
- Planar Solid Oxide Fuel Cell
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A solid oxide fuel cell (SOFC) is an energy conversion device that converts chemical energy of a fuel (such as hydrogen or methane) into electricity through a series of electrochemical reactions; no combustion process is involved. As a result, fuel cell efficiencies are not limited by theoretical Carnot efficiencies since the electrical work is directly converted from a substantial fraction of the enthalpy associated with the electrochemical oxidation of the fuel into water and/or carbon dioxide. Hence, fuel cells can deliver higher electrical conversion efficiencies when compared with traditional technologies such as coal-fired power plants and electrical generators based on internal combustion engines. In addition to high efficiency, SOFC, when compared with other fuel cells, has two particular advantages, owing to its high temperature operation; first, SOFC allows the use of a variety of fuels ranging from hydrogen to CO to hydrocarbons, and second, an SOFC produces significant amount of exhaust heat, which can be used in combined heat and power systems (CHP). In addition, quiet, vibration-free operation of fuel cells also eliminates noise usually associated with power generation systems. These fuel cells also produce no or very low levels of SO x and NO x emissions.
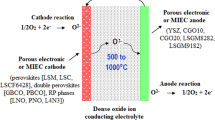
Shown in Fig. 1 is a typical SOFC, which essentially consists of two porous electrodes, separated by a dense, oxide ion conducting electrolyte. Oxygen gas molecules on the cathode side react with incoming electrons coming from the external circuit to form oxygen ions, which migrate through the oxide ion conducting electrolyte to the anode. At the anode, oxide ions react with H2 or other fuels to form H2O (and/or CO2), liberating electrons, which flow from the anode through the external circuit to the cathode to produce electricity. Provided that both fuels and oxygen are supplied constantly, the continuous electrochemical reactions can steadily generate electricity.
Quantitatively speaking, the electromotive force (EMF) of a cell is determined by the chemical potential of oxygen (i.e., oxygen activity) which is expressed by Nernst Equation as:
where Γ is the ionic transference number (ionic conductivity/total conductivity), T is operation temperature, F is Faraday constant, (pO2) a is the oxygen activity on the oxidant side and (pO2) b is the oxygen activity on the fuel side. In the case without external circuit, the EMF corresponds to open circuit voltage (OCV). For instance, an OCV ~1.1 V can be calculated for an SOFC operating at 800 °C when air and room-temperature-humidified H2 are used.
Under cell operating conditions, i.e., when a current passes through it, a fingerprint characteristic of fuel cells is the relationship between voltage and current density. The cell voltage (V) is given by:
where i is the current passing through the cell, R is the electrical resistance of the cell, and η A and η F are the polarization voltage losses associated with the air electrode (cathode) and the fuel electrode (anode), respectively. These polarizations are related to three physical processes: gas diffusion, gas–solid interaction, and ionic migration.
1.1 Brief History
Solid oxide fuel cells have come a long way to become practical power generation devices since the initial discovery of a ceramic material consisting of 85 % ZrO2 and 15 % Y2O3, the so-called “Nernst Mass”, by Walther Nernst in late 1890s that laid the foundation for the electrolyte material for these cells [1, 2]. The first conceptual SOFC is believed to have been demonstrated in 1937 by Bauer and Preis [3]. However, the more focused studies on SOFCs only began after the pioneering 1943 work by Carl Wagner who attributed the electrical conductivity in mixed oxides such as doped ZrO2 to the presence of oxygen vacancies [4]. In 1962, scientists at Westinghouse Electric Corporation published a paper titled simply as “a solid electrolyte fuel cell” [5]. This initial effort became the foundation of Westinghouse’s cathode-supported tubular SOFCs. Based on this cell design, Westinghouse successfully produced and tested several 5–250 kW sized SOFC power systems from the 1980s to the 1990s. ZrO2-based materials remain the most commonly used electrolyte for SOFCs even today.
From the mid-1990s to the present, several other cell designs and materials have been explored; in particular, anode-supported planar SOFCs have become quite popular because of performance and cost considerations. Today, SOFCs in many different designs and containing different cell materials are being explored and produced for power generation in small (few watts) to large (several hundred kWs) sizes in residential, commercial, and central power station applications. Although still fairly small in number, most demonstrations of SOFC power systems to date have been made of the 1–5 kW sized residential combined heat and power (CHP) units. The biggest drawback to the large-scale commercialization of SOFC power systems remains their relatively high cost relative to other power generation technologies. Several technical issues have been identified and are being addressed in the development of SOFC products for practical applications. These issues mainly relate to the two drivers to enable commercialization: competitive cost and reliable performance with desired operating characteristics.
The progress in SOFC technology and its commercialization status has been detailed in various scientific publications, books [6, 7] and in proceedings of two international conferences devoted solely to solid oxide fuel cells; first, the biennial international symposia on solid oxide fuel cells starting in 1989 [8−18], and second, the SOFC Forums of the European fuel cell forum [19–27]. These two series of proceedings give an excellent snapshot in time and progress achieved in SOFC technology over the last twenty years.
This chapter briefly reviews the cell and stack materials, cell designs, and present status of SOFC demonstrations, and concludes with expectations for the near future; more detailed description of these subjects is available in the subsequent chapters of this book.
2 Cell and Stack Materials
Materials research for SOFCs is driven by the desire to improve SOFC stability and to reduce various polarization losses. Initially, SOFCs were developed for operation primarily in the 900–1000 °C temperature range to overcome the high ohmic loss from a thick electrolyte. However, reduction of the SOFC operating temperature by 200 °C or more allows the use of a broader set of materials, is less demanding on the seals and the balance-of-plant components, simplifies thermal management, aids in faster start-up and cool down and should result in less degradation of the cell and stack components. Because of these advantages, activity in the development of SOFCs capable of operating in the intermediate temperature (IT) range of 650–800 °C has increased dramatically in the last decade, which necessitates advanced processing and novel materials in order to achieve high electrode kinetics and a low ohmic loss in the IT region. The following sections briefly review the cell (electrolyte and electrodes) and stack (sealant and interconnect) materials as detailed in Ref. [28].
2.1 Electrolyte Materials
In an SOFC, the electrolyte is exposed to both oxidizing (air side) and reducing species (fuel side) at high temperatures; hence, long-term successful SOFC operation requires that the electrolytes have the following: (1) Sufficient ionic conductivity—the electrolyte materials must have an ionic transference number close to unity, i.e., the electronic conductivity in the electrolyte must be sufficiently low in order to provide a high energy conversion efficiency. Also, the oxide ion conductivity must be high to minimize the ohmic loss; (2) Dense structure—in order to produce maximum electrochemical performance, the electrolyte must be gas tight; and (3) Stability—the electrolyte is exposed to both the air and the fuel at elevated temperature and must be chemically stable in these. Also, the thermal expansion coefficients must match at the electrolyte/electrode interfaces.
Typical electrolyte materials for SOFCs are oxides with low valence element substitutions, sometimes named acceptor dopants, which create oxygen vacancies through charge compensation. Yttrium-stabilized ZrO2 (YSZ) is the most common material that satisfies these requirements; acceptor doped CeO2 and perovskite structure oxides have also been investigated as electrolyte materials.
A plot of ionic conductivity as a function of temperature for common SOFC electrolyte materials is shown in Fig. 2. Although YSZ shows the lowest ionic conductivity in Fig. 2, it remains as the only material that has been demonstrated to provide long-term stability under cell operation conditions up to about 80,000 h at 1000 °C. In order for YSZ to be used in the IT regime, a typical approach to minimize ohmic loss is to decrease the thickness of the YSZ electrolyte. Another approach to reduce ohmic loss is to improve the ionic conductivity of a ZrO2-based electrolyte. Sc-doped zirconia shows higher conductivity than yttrium-doped ZrO2; however, the cost and known aging of Sc-doped ZrO2 present challenges in using this material for commercial SOFCs.
Doped CeO2 materials are candidates for the electrolyte for cell operation at temperatures below about 600 °C as discussed by Steele [29] and utilized by Ceres Power Inc. (UK), due to their higher oxide ion conductivity (Ce0.9Gd0.1O1.95: 0.025 Ω−1cm−1 at 600 °C) compared to YSZ (<0.005 Ω−1·cm−1). Gd or Sm doped CeO2 provides the highest ionic conductivity in CeO2-based materials due to similar ionic radii between Gd3+/Sm3+ and Ce4+. The principal challenge with doped CeO2 is the onset of electronic conduction in reducing conditions at temperatures above about 650 °C due to the reduction of Ce4+ to Ce3+ to compensate the formation of oxygen vacancies. Further reduction of Ce4+ results in lattice expansion and often creates microcracking in the electrolyte. Cell temperatures below about 600 °C seem necessary for successful use of doped CeO2 in SOFCs for long-term operation.
The perovskite structure (La,Sr)(Mg,Ga)O3 (LSMG) and other similar materials have also been developed as oxide ion conductors. The use of LSMG is attractive because it has reasonable oxide ion conductivity and is compatible with a variety of cathodes, in particular the highly active ones; hence excellent electrochemical performance has been reported with LSMG electrolytes. The challenges for LSMG are the uncertain costs of Ga sources and its chemical and mechanical stability.
2.2 Cathode Materials
The cathode material is crucial because electrochemical reduction of O2 on the cathode requires a series of elementary reactions and involves the transfer of multiple electrons. The cathode must have: high electronic conductivity, chemical and dimensional stability in environments encountered during cell fabrication and cell operation, a thermal expansion match with other cell components, compatibility and minimum reactivity with the electrolyte and the interconnection with which it comes into contact, and a high catalytic activity for oxygen molecule dissociation and oxygen reduction. Finally, the cathode must have a stable, porous microstructure so that gaseous oxygen can readily diffuse through the cathode to the cathode/electrolyte interface. These stringent electrochemical and mechanical requirements greatly restrict the number of suitable candidate materials.
In addition to noble metals, conducting perovskite family oxides are the preferred cathode materials. Lanthanum manganite (LaMnO3), which, when substituted with low valence elements, such as Ca or Sr, has a superior p-type electronic conduction due to the formation of large amount of Mn4+. Moreover, doped LaMnO3 possesses adequate electrocatalytic activity for oxygen reduction, a reasonable thermal expansion match to YSZ, and stability in the SOFC cathode operating environment. Under the cathode operation conditions, doped LaMnO3 has oxygen excess, or precisely speaking, has cation vacancies, and the oxide ion conductivity is relatively low in Sr doped LaMnO3 (LSM), of the order of 10−7 S/cm at 800 °C.
For SOFCs operating in the IT regime, alternative cathode materials have been developed, since LSM is not the ideal candidate, owing (at least in part) to its low ionic conductivity and slow surface oxygen exchange kinetics. The desirable cathode for IT SOFCs is a mixed ionic and electronic conductor (MIEC). In order to design the MIEC with a single chemical composition, numerous perovskite compositions, typically, containing La on the A site, and transition metals such as Co, Fe, and/or Ni on the B site, for instance (La,Sr)(Co,Fe)O3—the so-called LSCF, have received attention. In general, compared with LSM, these materials offer higher oxide ion diffusion rates and exhibit faster oxygen reduction kinetics. Promising results have been reported using these materials; though in many cases the improved cathodic performance is found to decrease during cell lifetime as a result of chemical or microstructural instability. The decreased electrochemical performance of the cathode often is related to the interdiffusion of LSCF/LSF toward YSZ. A thin interlayer, mostly of a CeO2-based material, is generally used to reduce interdiffusion between the cathode and YSZ.
Shao and Haile [30] have reported on a new cathode material, Ba0.5Sr0.5Co0.8Fe0.2O3 (BSCF), which at ~600 °C possesses fast bulk diffusion coefficients. With BSCF as the cathode, they obtained an excellent cell electrochemical performance of ~1 W/cm2 at 600 °C. They attributed the improved cathode performance to both fast surface exchange and bulk diffusion coefficients. Although thermal and chemical stability of BSCF remains a challenge, the material itself is of interest for use in intermediate temperature SOFCs.
In addition to chemical composition, electrode microstructure has a profound effect on the cathode polarization.
2.3 Anode Materials
The anode must be an excellent catalyst for the oxidation of the fuel, and must be: stable in the reducing environment, electronically conducting, and have sufficient porosity to allow gas species in and out of the electrolyte/anode interface where the fuel oxidation reaction takes place. Other requirements include matching of the thermal expansion coefficient with that of the electrolyte and the interconnect material; chemical stability with the electrolyte and the interconnect; and applicability for use with various fuels and impurities (such as sulfur). In addition, cost effectiveness is always a factor in the commercialization of SOFCs. During the early stages of SOFC development, the anode materials were precious metals (such as Pt and Au) and transition metals (such as Fe and Ni). These candidates, however, cannot meet the stringent aforementioned requirements for the anode in SOFCs. For instance, Ni is an excellent catalyst; however, it possesses a high thermal expansion coefficient (13.4 × 10−6/°C), and exhibits a coarsening of microstructure due to metal aggregation through grain growth. A composite of Ni-YSZ, was found capable of preventing sintering of the nickel particles, decreasing the effective thermal expansion coefficient of Ni, and providing better adhesion of the anode with the electrolyte. Currently, Ni-YSZ cermet is the most commonly used anode material.
In a Ni/YSZ anode, nickel has the dual role of being a catalyst for hydrogen oxidation as well as being an electrical current collector. In addition to being an excellent catalyst for the oxidation of hydrogen, nickel is also highly active for the steam reforming of methane. This catalytic property is exploited in the so-called internal reforming SOFCs that can operate on fuels composed of mixtures of methane and water. However, Ni can catalyze the formation of carbon from hydrocarbons under reducing conditions. Unless adequate amount of steam is present along with the hydrocarbon to remove this carbon from the nickel surface, the anode activity may suffer. As a result, even when using methane as the fuel, relatively high steam-to-carbon ratio is needed to suppress this deleterious carbon deposition reaction. Unfortunately, due to the high catalytic activity of nickel for hydrocarbon cracking, this approach does not work for higher hydrocarbons. Therefore, a pre-reformer is necessary when using higher hydrocarbon-containing fuels.
Incorporating oxide-based catalysts is an approach that has been tried to overcome the limitations of the nickel-based anodes to directly utilize hydrocarbons, and has met with some success. A composite of Cu/CeO2 has been investigated as the SOFC anode for the direct utilization of hydrocarbon fuels. Unlike Ni, Cu is not an active catalyst for carbon formation. The addition of CeO2 provides active catalytic sites. However, sintering of copper at cell operating temperatures has limited its use in practical SOFCs. In terms of sulfur tolerance, ceramic oxides based on ceria or strontium titanate, have yielded some promising results, but the benefits obtained are counterbalanced by other limitations (such as high electrical resistivity and the difficulty of integrating such materials with existing cell and stack fabrication processes and materials). Research is progressing on further development of such oxide-based anodes.
2.4 Sealing Materials
In planar SOFC stacks, seals are critical and must prevent (or at least minimize sufficiently for acceptable performance) leakage of both fuel and oxidant gases from the stack to the outside environment, as well as mixing of the fuel and oxidant gases within the stack. Successful development of sealing materials and the concepts for planar SOFCs are probably the most important issues for the long-term performance stability and lifetime of planar SOFC stacks and hence their commercialization at competitive costs. The primary challenge in developing seals resides in a variety of strict requirements that effective seals need to meet. First, an effective seal must be thermochemically stable under the operational conditions of the stack, they must be electrically insulating, and must have a thermal expansion match to the fuel cell components. Additionally, the seal should be compatible with other components, and should be formed at a low enough temperature to avoid damaging the cell components and also should not migrate or flow from the designated sealing region during sealing or cell operation. It is also necessary to develop a sealing system that can withstand thermal cycling between the cell operational temperature and room temperature. In order to satisfy these requirements, a number of different sealing approaches have been developed, which have met with some success, including rigid, bonded seals (e.g., glass-ceramics and brazes), compliant seals (e.g., viscous glasses), and compressive seals (e.g., mica-based composites); multiple sealants may be used in any given stack design between different components.
Rigid seals typically rely on a glass that softens and “glues together” the adjacent stack components during stack fabrication (at a temperature above the operating temperature), but then become rigid and immobile when cooled to the operating temperature. A wide variety of glasses and glass-ceramics have been developed, including alkali silicate, alkaline earth silicate, borosilicate, aluminoborosilicate, and others. Glass-based seals represent a relatively straightforward means of sealing an SOFC stack (at least initially), but the brittle nature of glasses (below the glass transition temperature) and glass–ceramics makes these seals vulnerable to cracking (resulting from stresses related to thermal expansion mismatches with the adjacent components). As a consequence, the coefficient of thermal expansion (CTE) must be similar to that of the other components. While glass compositions can be tailored to optimize their physical properties, the selection of glasses offering appropriate thermal expansion behavior is relatively narrow, and the selection is further limited by the need for the glass to have appropriate wetting behavior and viscosity at the sealing temperature. The best results to date have been obtained using compositions based on silica. Alkali silicate glasses tend to be very reactive toward other SOFC components, though alkaline-earth aluminosilicate glasses have yielded promising results. One of the primary advantages of the latter (particularly those containing BaO) is the ability to tailor their CTE to match that of other SOFC stack components.
In addition to glass seals, brazing has been developed as a possible sealing approach, whereby a filler metal, the liquidus of which is well below that of the materials to be joined, is heated to a point at which it is molten, is allowed to flow and fill the gap between the two joining pieces under capillary action, and then cooled to solidify the joint. Although most commercial brazes do not wet ceramic surfaces, the addition of a reactive metal, such as Ti or Zr, results in the reduction of the ceramic phase at the joining interface to give an intermediate layer that is in chemical equilibrium with both the ceramic and braze-metal phases. Once this reduced phase forms, wetting of the ceramic surface by the filler metal is improved greatly. While active metal brazing solves the wetting issue, preferential oxidation of the active element in the braze at SOFC operating temperatures can lead to rapid deterioration of the joint. The desire to accomplish sealing in an inexpensive oxidizing environment greatly complicates the search for an appropriate joining material. Use of a reducing environment at joining temperatures of the order of 800 °C or greater adds cost and can damage cathode materials, resulting in a severe loss in cell performance. This processing restriction reduces the list of candidate joining materials to noble metal-based brazes.
Compressive seals, which typically utilize materials such as sheet-structure silicates to act as a gasket between components, can help to improve the stack’s tolerance to thermal expansion mismatch between various stack components. A compliant high-temperature material is placed between the two sealing surfaces and compressed, using a load frame external to the stack, to accomplish sealing. Because the seal conforms to both sealing surfaces and is under constant compression during use, it forms a dynamic structure that reduces the need for CTE matching. It is also possible for the sealing surfaces to slide past one another without disrupting the hermeticity of the seal. The primary challenge associated with this technology is the need for a compliant high-temperature sealing material that functions adequately as a reliable compressive seal. A number of materials have been considered for compressive seals, including mica, nickel, and copper. Each has been found to have limitations when used in a simple gasket configuration, ranging from oxidation in the case of the metals to relatively high leak rates in the case of the mica. The route to enhanced performance probably lies with new hybrid and composite seal approaches, in which mica is combined with other materials.
2.5 Interconnect Materials
An interconnect provides the electrical connection between cells and ensures air and fuel separation within the cell stack. Typical requirements of an interconnect material include: high electronic conductivity with low ionic conductivity; chemical stability in both fuel and air; a thermal expansion match to other cell components; high mechanical strength; high thermal conductivity and chemical stability with regard to other cell components. In addition, when considering a stack design, one must take into account ease of fabrication. To satisfy these requirements, doped lanthanum chromite has been used as the interconnect for cells intended for operation at about 1000 °C. Lanthanum chromite is a p-type conductor; its conductivity is due to small polaron hopping from room temperature to 1400 °C at oxygen pressures as low as 10−18 atm. The conductivity is enhanced as lower valence ions (e.g., Ca, Mg, Sr, etc.) are substituted on either the La3+ or the Cr3+ sites. In cells intended for operation at lower temperatures (<800 °C), it is possible to use oxidation-resistant metallic materials for the interconnect. Compared to lanthanum chromite ceramic interconnects, metallic alloys offer advantages such as improved manufacturability, significantly lower raw material and fabrication costs, and higher electrical and thermal conductivity. But to be useful for the interconnect application, the metallic alloys must satisfy additional requirements, particularly resistance to surface oxidation and corrosion in a dual atmosphere (exposure to oxidizing atmosphere on one side and reducing atmosphere on the other side). An oxide protective layer, formed in situ during oxidation or ex situ by a coating technique, can provide resistance to surface oxidation and corrosion. As a result, the oxide must have the following characteristics: slow growth kinetics, sufficient electrical conductivity, strong adhesion to the underlying alloy, even in severe thermal cycling conditions, and good chemical compatibility with the electrodes, with no significant diffusion or evaporation of components that can influence electrode performance or oxide integrity.
Ferritic stainless steels are the most promising materials as the interconnect, owing to the fact that some alloys in this family offer a protective and conductive Cr-based oxide scale, appropriate thermal expansion behavior, ease of manufacturing, and low cost. Several new ferritic stainless steels, such as Crofer22 APU, have been developed specifically for the SOFC interconnect application. Although these alloys demonstrate improved performance over traditional compositions, several critical issues remain. Among these are the formation of chromium containing species at electrolyte/cathode interface and subsequent poisoning of cathodes; corrosion and spalling under interconnect exposure conditions, and compatibility with the adjacent components such as seals and electrical contact layers. As an alternative approach to developing new alloys, surface modification can result in enhanced performance. For example, electrically conductive perovskites and spinels can be applied onto metallic interconnects to minimize scale growth, electrical resistance, and Cr volatility.
Volatility of Cr species from the metallic interconnect plays a key role in the degradation of the electrochemical performance of SOFCs. Metallic interconnects with Cr2O3 scale give out Cr-containing volatile species that deposit on the electrolyte and electrode surfaces and interfaces, and lead to rapid deterioration of the cathode’s oxygen reduction reaction rate and thus to a significant decline in cell performance. The mechanism of Cr poisoning is not clear, although reactions of the cathode with CrO2(OH)2 are considered the likely cause. Possible chromium poisoning mitigation strategies include the use of suitable surface layers on alloy interconnects to reduce Cr evaporation; La-chromite, Mn-chromite, and non-Cr-containing spinels such as (Mn,Co)3O4 can reduce Cr flux during the operation of cell or stack. Such layers could potentially be applied either in a separate fabrication step (e.g., by thermal spray) or grown in situ through suitable modification of the alloy bulk or surface.
3 SOFC Designs
Over the years, several SOFC designs have been investigated and developed by configuring the three active components of the cell, the electrolyte and the two electrodes, in different geometric configurations. The two most common designs are planar and tubular, and their many variants. These are reviewed below.
3.1 Planar SOFC Design
In the planar design, a series of cell components are configured as thin, flat plates, then electrically connected to build up desirable electrochemical performance. A schematic of a generic planar SOFC design is shown in Fig. 3. The planar cells can be either electrolyte-supported, electrode-supported, or metal-supported. Each of these designs can also have a number of interesting variants; for example, the planar SOFC may be in the form of a circular disk fed with fuel from the central axis, or it may be in the form of a square or rectangular plate fed from the edges. Planar designs offer several potential advantages, including simpler and less expensive manufacturing processes and higher power densities than tubular cells described in the next section. However, planar designs need high-temperature gas-tight seals between the components in the SOFC stack; such seals are not necessary with tubular cells. Seal development remains as the most challenging area in successfully commercializing planar SOFCs. An important feature of the planar design resides in the configuration of gas flow and gas manifold, which play a crucial role in minimizing the use of seals, improving fuel utilization, managing uniform distribution of temperature and current to reduce thermal stresses, and improving the stability of the stack. Generally, the interconnect is ribbed on both sides to allow cross-flow, co-flow, or counter-flow configurations.
The initial planar SOFC configurations employed a thick electrolyte as the support, which required an operating temperature often higher than 900 °C. Advances in ceramic processing have allowed fabrication of thin electrolytes, 10 μm or thinner, by low-cost conventional ceramic processing techniques such as tape casting, tape calendaring, slurry sintering, screen printing, or by plasma spraying. As a consequence, anode-supported planar cell stacks have been extensively fabricated and tested by a number of developers for long-term operation. Electrochemical performance of an anode-supported single planar cell at 600, 700, and 800 °C is shown in Fig. 4; the cell was optimized with an anode thickness of 0.5 mm and porosity ~57 %. The anode interlayer was ~20 μm. The electrolyte was ~8 μm and cathode interlayer ~20 μm. The flow rates of humidified hydrogen and air were 300 and 550 ml/min, respectively. Zhao and Virkar [31] have shown that the electrochemical performance of planar SOFCs is highly dependent on cell materials, electrode microstructures, and cell geometric parameters, with power densities as high as 1.8 W/cm2 at 800 °C being possible as shown in Fig. 4.
Electrochemical performance for an optimized anode-supported single cell measured at 600, 700, and 800 °C [31]
3.2 Tubular SOFC Design
Tubular SOFCs may be of a large diameter (>15 mm), or of much smaller diameter (<5 mm), the so-called microtubular cells. Also, the tubes may be flat and joined together to give higher power density and easily printable surfaces for depositing the electrode layers. In a typical tubular SOFC, as illustrated by the Siemens Westinghouse design shown in Fig. 5, the cell tube is porous doped lanthanum manganite fabricated by extrusion/sintering and is closed at one end. The cell components, dense YSZ electrolyte, porous Ni-YSZ anode, and doped lanthanum chromite interconnect, are deposited in the form of thin layers by atmospheric plasma spraying.
For cell operation, oxidant (air or oxygen) is introduced through an alumina injector tube positioned inside the cell. The oxidant is discharged near the closed end of the cell and flows through the annular space formed by the cell and the coaxial injector tube. Fuel flows on the outside of the cell from the closed end and is electrochemically oxidized while flowing to the open end of the cell generating electricity. At the open end of the cell, the oxygen-depleted air exits the cell and is combusted with the partially depleted fuel. Typically, 50–90 % of the fuel is utilized in the electrochemical cell reaction. Part of the depleted fuel is recirculated in the fuel stream and the rest combusted to preheat incoming air and/or fuel. The electrochemical performance of a tubular cell (2.2 cm diameter, 150 cm active length) at 800, 900, and 1000 °C with 89 % H2 + 11 % H2O fuel (85 % fuel utilization) and air as oxidant is shown in Fig. 6. Such tubular cells have a power density at 1000 °C of about 0.2 W/cm2.
The single biggest advantage of tubular cells over planar cells is that they do not require any high-temperature seals to isolate oxidant from the fuel, and this makes performance of tubular cell stacks very stable over long periods of times (several years). However, their areal power density is much lower (about 0.2 W/cm2) compared to planar cells (up to 2 W/cm2 for single cells and at least 0.5 W/cm2 for stacks), and manufacturing costs higher. The volumetric power density is also lower for tubular cells than for planar cells. To increase the power density and reduce the physical size and the cost of tubular SOFC stacks, alternate tubular geometry cells have been investigated. Such alternate geometry cells combine all the advantages of the tubular SOFCs, such as not requiring high-temperature seals, while providing higher areal and volumetric power densities. Figure 7 shows several of such designs investigated by Siemens; the performance of these new design cells is higher than that of cylindrical tubular cells, but still lower than that of anode-supported planar cells.
A single SOFC, whether planar or tubular, generally produces a voltage of less than about 1 V. A series connection of single cells, a stack, is therefore formed to obtain higher power. Unlike a single cell, which deals with mostly material aspects of the components, cell stacks must consider many other issues to generate maximum stable power. The design of SOFC stacks must take into account the electrochemical performance to generate more power from the stack; structural and mechanical integrity to allow the stack to be operated at high temperature for sufficiently long duration and possibly under thermal cycles; gas manifolding for supplying reactant gas to a large cell area, removing reaction products and providing gas hermeticity; and ease of fabrication.
4 SOFC Applications and Power Systems
SOFCs have high efficiency and fuel flexibility because of the high operation temperature (550–1000 °C). On one hand, operating SOFCs at elevated temperatures produces high quality exhaust, which can be used to provide heat sources for stationary applications, or to heat the reformer for endothermic steam reforming reactions, or even to fire a secondary gas turbine. Hence, relative to other fuel cells, SOFCs have a high electrical efficiency, even in small size systems. On the other hand, over the operating temperature regime (550–1000 °C), CO can be used as the fuel, rather than poisoning the anode. Therefore, a variety of fuels can be reformed within the cell stack (internal reforming) or through a separate fuel reformer (external reforming). This flexibility allows use of fuels, such as biogas, liquid hydrocarbon fuels, and landfill gas, etc., and enables SOFCs to be well-suited especially for stand-alone and remote applications. These fuels can be reformed to a mixture of hydrogen and carbon monoxide. The direct internal reforming represents reformation of the fuel directly on the fuel cell anode, thus electrochemical reaction and fuel reformation simultaneously take place at the anode. This is a simple and very efficient design with least loss of energy; however, carbon deposition and temperature inhomogeneity due to endothermic cooling may present problems in direct internal reforming. Indirect reforming uses a separate fuel reforming catalyst which is integrated within the SOFC stack upstream of the anode side and typically, utilizes heat and water from the SOFC stack. Indirect reforming may not be as efficient as direct reforming, but it provides more stable cell performance. Any external reformer is physically separated from the fuel cell stack; hence it can be operated at different pressure and temperature if necessary. This is of particular importance because it eliminates the problem of carbon deposition from fuel decomposition that deactivates the cell anode.
Because of their superior electrical efficiency and fuel flexibility, SOFC-based power systems, compared to other fuel cell systems, enable numerous applications at various power levels, from a few-watt to megawatt size systems. The following sections describe various applications of SOFC-based power systems that have been demonstrated or are under development.
4.1 Small SOFC Systems for Residential CHP Applications
A major application for SOFCs is at 1–5 kW level to supply combined heat and power (CHP) to residential buildings utilizing natural gas as the fuel. Early SOFC CHP units were designed, produced, and tested by Hexis (Switzerland). These units are based on a planar SOFC system, with an inner round aperture in the center serving as a channel for the supply of fuel. Fuel flows toward outside in a radial manner. The air is preheated and flows from outside through four channels onto the metallic interconnect inside the cell stack, then is diverted there and flows toward outside in a radial manner. The excess fuel that has not electrochemically converted is afterburned at the edges of the cell stack. Based on this design, a system, named “Galileo 1000”, has been developed to supply an electrical power of 1 kW and a thermal capacity of about 2.5 kW; over 20 such units are presently under demonstration. Ceres Power (UK), in partnership with British Gas, has developed a metal-supported planar SOFC system, utilizing ceria-based electrolyte, for generating electricity and central heating requirement (including hot water) for a typical home. Metallic supported SOFC stack operates at relatively low temperatures (~550 °C) and tends to be lightweight. As a result, Ceres Power’s unit is compact and wall-mountable, which may provide access to both the boiler replacement and new residential applications. Ceramic Fuel Cells Ltd. (Australia) utilizes planar SOFCs for residential CHP units; over 50 of its 1 kW units have been or are being demonstrated in various parts of the world.
Tubular SOFC design is also attractive for residential units because of its reliable and stable performance over long periods. Kyocera (Japan) has developed anode-supported flat tubular cells, and these cells are being utilized by several organizations in Japan (Osaka Gas, Tokyo Gas, etc.) to fabricate and test SOFC-based residential CHP systems. The first trial operation of such a 1 kW unit in a residential house was conducted in 2005–2006 for a period of 2000 h; the average electric efficiency and hot water heat recovery efficiency were 44.1 % (LHV) and 34 % (LHV), respectively. Over 75 such units, illustrated in Fig. 8, are now installed and operating in homes throughout Japan, and are expected to be commercialized in the next two years.
Toto Ltd. (Japan) is producing and testing 2 kW size CHP units using cathode-supported tubular cells. Siemens (USA) working with Fuel Cell Technologies (Canada) also produced and tested about a dozen 3–5 kW size CHP units using cathode-supported tubular cells; these units performed well with very stable performance for up to one year; unfortunately, both of these organizations are now out of the SOFC business.
Several other organizations are also developing residential CHP units using either planar or tubular SOFCs. Development of SOFC systems for residential CHP applications is accelerating, particularly in Japan and Europe, through formation of partnerships with appliance makers. Residential CHP units will probably be the first commercial application of SOFCs.
4.2 Large SOFC Systems for Distributed Power Generation
Starting in 1986, Westinghouse (whose fossil energy business was acquired by Siemens in 1998) successfully fabricated about 15 integrated SOFC systems with a power range from 0.4 to 220 kW and tested them on customer sites. The longest test was conducted on a 100 kW atmospheric power generation system, shown in Fig. 9, using tubular SOFCs. This 100 kW cogeneration system was the first demonstration of the tubular cells in a full scale SOFC module. The stack consisted of 1152 cells (2.2 cm diameter and 150 cm active length) in 48 cell bundles of 24 cells each. This system operated for over 36,750 h in USA, Netherlands, Germany, and Italy on desulfurized natural gas without any detectable performance degradation at an efficiency of ~46 %. This was the first successful demonstration of the solid oxide fuel cells for large-scale power generation.
Siemens also produced a 220 kW pressurized SOFC/gas turbine hybrid system, which was installed and tested at the National Fuel Cell Research Center on the campus of the University of California-Irvine (USA). This system was the world’s first demonstration of an SOFC coupled with a microturbine generator and the first demonstration of a pressurized SOFC generator. The system accumulated nearly 3400 h of runtime, while operating at a calculated net AC electrical efficiency of 53 %. Analysis indicates that with such pressurized SOFC/gas turbine hybrids, an electrical efficiency of ~70 % is achievable in large MW sized systems.
As mentioned previously, Siemens is no longer in the SOFC business. However, other organizations are continuing work on the utilization of SOFCs for large-scale power generation. Chief among these are Versa Power Systems (in collaboration with FuelCell Energy, USA), United Technologies (in collaboration with Delphi Corporation, USA), and Rolls Royce Fuel Cell Systems (UK/USA). These organizations are supported by the US Department of Energy’s Solid State Energy Conversion Alliance (SECA) program that was initiated in the year 2000 to reduce the cost of SOFC power generation systems. Another company, Bloom Energy (USA), started in the year 2001 with venture capital, recently announced the sale, delivery, and installation of several 100 kW sized SOFC power systems, known as Bloom Boxes shown in Fig. 10, to commercial customers such as Adobe Systems, Bank of America, Cox Enterprises, Coca Cola Company, eBay, FedEx, Google, Safeway, Staples, Walmart, etc.
Extensive research is presently being conducted worldwide to decrease the cost of such power systems, particularly in the areas of cell and stack materials, DC to AC power conditioning systems, and other balance-of-plant components. With decreased cost, large-scale SOFC power systems are expected to provide a low- or no-pollution technology to produce electricity.
4.3 Portable SOFC Power Systems
The portable applications generally require power in the range from milliwatts to a few hundred watts. Proton exchange membrane fuel cells (including direct methanol fuel cells) are fuel cells of choice for such portable applications due to their lightweight and low operating temperature; however, SOFC-based units are also being developed, primarily for certain military, leisure, emergency, and transportation applications because of their superior fuel flexibility, not requiring hydrogen. SOFCs can be successfully operated on fuels such as propane, gasoline, diesel, kerosene, JP-8 military fuel, ethanol, and other biofuels. Challenges arising for SOFCs in portable applications are that stacks must be light, have a short start-up time, and be thermally sustaining. Quick start-up time is particularly crucial for portable applications; however, it is very difficult to achieve because of relatively low thermal shock resistance of ceramic components. One approach is to use microtubular SOFCs, which are capable of tolerating thermal shock resistance. Moreover, microtubular design can give reasonable volumetric power densities because the power density increases inversely proportional to the tube diameter.
Microtubular SOFCs have been successfully integrated into portable power units, primarily by companies such as Adaptive Materials Inc. (USA) and Protonex Technology Corporation (USA). Figure 11 shows Adaptive Materials (AMI) 50 W and 250 W systems that use propane as the fuel to produce continuous power. The 50 W system, running on propane, provides power for ground sensors, unmanned aerial vehicles, and robots. The 250 W system is fueled by propane or LPG and is used to extend military mission durations and delivers off-grid power for electronics, radios, and computers. The use of globally available fuels in such portable SOFC systems eliminates complicated logistics.
4.4 SOFC-Based Transportation Auxiliary Power Units
Another application of SOFC systems is in the transportation sector. As for portable applications, the proton exchange membrane fuel cell is generally regarded as the fuel cell of choice for transportation applications, particularly for propulsion to replace the internal combustion engine. Proton exchange membrane fuel cells require pure hydrogen, with no carbon monoxide, as the fuel to operate successfully. However, presently no hydrogen infrastructure exists, and onboard reformer systems to produce hydrogen from existing fuel base (gasoline, diesel) are technically challenging, complex, and expensive. Furthermore, it is difficult to eliminate all carbon monoxide from the reformate stream. In contrast, SOFCs can use carbon monoxide along with hydrogen as fuel, and their higher operating temperature and availability of water on the anode side make on-cell or in-stack reformation of hydrocarbon fuels feasible. Also, no noble metal catalysts are used in SOFCs, reducing the cost of the cells. Although not practical for propulsion, the application of SOFCs in the transportation sector will be for onboard auxiliary power units (APUs). Such APUs, operating on existing fuel base, will supply the ever-increasing electrical power demands of luxury automobiles, recreational vehicles, and heavy-duty trucks for various comfort items, such as refrigerators, televisions, stereos, even computers, and microwaves. The challenges for SOFC in APUs, similar to the aforementioned portable applications, reside in achieving SOFC systems with compact size, light weight, short start-up time, mechanical robustness, and capability for thermal cycling.
Delphi Cooperation has developed a 3–5 kW size SOFC APU system using anode-supported planar cells. This unit is intended to operate on gasoline or diesel, which is reformed through partial oxidation within the APU unit. The building blocks of such an APU consist of an SOFC stack, fuel reformation subsystem, energy recovery unit, thermal management subsystem, process air supply subsystem, control subsystem, and power electronics and energy storage (battery) subsystem, arranged as illustrated in Fig. 12. In 2008, Delphi Corporation (USA) and Peterbilt Motors Co. (USA) successfully demonstrated the operation of a Delphi’s SOFC APU in powering a Peterbilt Model 386 truck’s “hotel” loads (Fig. 13). The Delphi SOFC APU provided power for the Model 386′s electrical system and air conditioning and maintained the truck’s batteries, all while the truck’s diesel engine was turned off. Delphi hopes to commercialize such SOFC APUs in the next few years. Several other companies are also developing similar SOFC APUs.
5 Summary
Fuel cells have been known for over 150 years. Solid oxide fuel cells, in particular, utilize an electrolyte material that was first mentioned by Nernst in the late 1890s. Research, development, and demonstration of SOFCs accelerated in the last 30 years and they are now on the verge of commercialization for at least certain applications. SOFCs offer potential for various applications with a wide power range from milli-watts to mega-watts because of fuel flexibility and combined high quality heat and power. Novel SOFC materials and their structures are being investigated and developed to reduce polarization and thus improve electrochemical performance, especially in the intermediate temperature regime. Stable performance over long durations sometimes is overlooked, but remains a crucial factor in SOFC commercialization. With respect to planar SOFCs, successful development of high-temperature sealing materials and concepts is of paramount importance for the long-term performance stability and lifetime of stacks and hence for their eventual commercialization at competitive costs. On the contrary, a number of tubular SOFC systems have been successfully demonstrated up to 220 kW size for long-term stable performance. Extensive research is being conducted to decrease the cost of these power systems, particularly in the areas of cell and stack materials, DC to AC power conditioning systems, and other balance-of-plant components. With decreased cost, SOFC power systems are expected to provide a widespread low- or no-pollution technology to produce electricity. The first SOFC commercialization is expected to be for residential CHP for which many successful demonstrations by several companies have been accomplished. Large-scale distributed power generation also got a recent boost this year when Bloom Energy (USA) sold, delivered and installed many 100 kW sized SOFC systems for commercial customers.
References
W. Nernst, Electrical glow-light. U.S. Patent 623,811 (1899)
W. Nernst, Uber die elektrolvtische Leitung Fester Korper bei sehr hohen Temperaturen. Z. Electrochem. 6, 41–43 (1900)
E. Bauer, H. Preis, Über Brennstoff-Ketten mit Festleitern. Z. Electrochem. 43, 727–732 (1937)
C. Wagner, Über den mechanismus der elektrischen Stromleitung im Nernststift. Naturwissenschaften 31, 265–268 (1943)
J. Weissbart, R. Ruka, A solid electrolyte fuel cell. J. Electrochem. Soc. 109, 723–726 (1962)
S.C. Singhal, K. Kendall, High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications Oxford, Elsevier (2003)
K. Huang, J.B. Goodenough, Solid oxide fuel cell Technology. Woodhead Publishing Ltd, Cambridge (2009)
S.C. Singhal (ed.), SOFC-I, PV89-11, The Electrochemical Society Proceedings Series, Pennington (1989)
F. Grosz, P. Zegers, S.C. Singhal, O. Yamamoto (eds.) SOFC-II, Commission of the European Communities, Luxembourg (1991)
S.C. Singhal, H. Iwahara (eds.), SOFC-III, PV93-4, The Electrochemical Society Proceedings Series, Pennington (1993)
M. Dokiya, O. Yamamoto, H. Tagawa, S.C. Singhal (eds.), SOFC-IV, PV95-1, The Electrochemical Society Proceedings Series, Pennington (1995)
U. Stimming, S.C. Singhal, H. Tagawa, W. Lehnert (eds.), SOFC-V, PV97-40, The Electrochemical Society Proceedings Series, Pennington (1997)
S.C. Singhal, M. Dokiya (eds.), SOFC-VI, PV99-19, The Electrochemical Society Proceedings Series, Pennington (1999)
H. Yokokawa, S.C. Singhal (eds.), SOFC-VII, PV2001-16, The Electrochemical Society Proceedings Series, Pennington (2001)
S.C. Singhal, M. Dokiya (eds.), SOFC-VIII, PV2003-07, The Electrochemical Society Proceedings Series, Pennington (2003)
S.C. Singhal, J. Mizusaki (eds.), SOFC-IX, PV2005-07, The Electrochemical Society Proceedings Series, Pennington (2005)
K. Eguchi, S.C Singhal, H. Yokokawa, J. Mizusaki (eds.), SOFC-X, the Electrochemical Society Transactions, vol. 7 (2007)
S.C. Singhal, H. Yokokawa (eds.), SOFC-XI, the Electrochemical Society Transactions, vol. 25 (2009)
U. Bossel (ed.), First European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 1994)
B. Thorstensen (ed.), Second European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 1996)
P. Stevens (ed.), Third European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 1998)
A.J. McEvoy (ed.), Fourth European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 2000)
J. Huijsmans (ed.), Fifth European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 2002)
M. Mogensen (ed.), Sixth European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 2004)
J. Kilner (ed.), Seventh European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 2006)
R. Steinberger-Wilckens (ed.), Eighth European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 2008)
J.T.S. Irvine (ed.), Ninth European Solid Oxide Fuel Cell Forum Proceedings (European Fuel Cell Forum, Oberrohrdorf, 2010)
X.-D. Zhou, S.C. Singhal, Fuel cell: solid oxide fuel cells: overview. In: Garche J, Dyer C, Moseley P, Ogumi Z, Rand D, Scrosati B (eds.). Encyclopedia of electrochemical power sources, Amsterdam, Elsevier 3, 1–16 (2009)
B.C.H. Steele, Appraisal of Ce1-yGdyO2−y/2 electrolytes for IT-SOFC operation at 500°C. Solid State Ionics 129, 95–110 (2000)
Z.P. Shao, S.M. Haile, A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431, 170–173 (2004)
F. Zhao, A.V. Virkar, Dependence of polarization in anode-supported solid oxide fuel cells on various cell parameters. J. Power Sources 141, 79–95 (2005)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag London
About this chapter
Cite this chapter
Singhal, S.C. (2013). Solid Oxide Fuel Cells: Past, Present and Future. In: Irvine, J., Connor, P. (eds) Solid Oxide Fuels Cells: Facts and Figures. Green Energy and Technology. Springer, London. https://doi.org/10.1007/978-1-4471-4456-4_1
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4456-4_1
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4455-7
Online ISBN: 978-1-4471-4456-4
eBook Packages: EnergyEnergy (R0)