Abstract
Chronic thromboembolic pulmonary hypertension still remains an under-recognized and misdiagnosed condition. If left untreated, it can progress to right heart failure. Diagnosis and referral to a specialized center is paramount since surgical treatment offers a life-long cure to these patients and reversal of their right heart failure. Although new medications have been developed to treat pulmonary hypertension, they remain significantly inferior to surgical treatment in both exercise capacity and pulmonary vascular resistance improvement. The UCSD surgical classification describes the level of disease and corresponds to the difficulty of the endarterectomy. Pulmonary thromboendarterectomy remains the gold standard treatment for patients with chronic thromboembolic pulmonary hypertension. In experienced hands, pulmonary thromboendarterectomy can be performed with excellent long-term results and very low mortality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pulmonary thromboendarterectomy
- PTE
- Chronic thromboembolic pulmonary hypertension
- CTEPH
- Right heart failure
- Pulmonary embolism
Introduction
Pulmonary thromboendarterectomy (PTE) is the definitive treatment for chronic pulmonary hypertension as the result of thromboembolic disease and is now widely recognized as the treatment of choice for patients suffering from right heart failure as the result of chronic thromboembolic pulmonary hypertension (CTEPH). Although the procedure is curative and in experienced hands carries a low morbidity and mortality, it is only rarely performed. The main problem is the fact that CTEPH remains significantly under-recognized. It is not uncommon for the patients suffering from this disease to be misdiagnosed and mistreated for a variety of other conditions. Furthermore, of those who are truly diagnosed, the majority will be at later stages of the disease. However, once correctly diagnosed and referred, pulmonary thromboendarterectomy can be highly effective and promises to provide a lifetime cure for such patients.
Unlike other forms of heart failure, patients with CTEPH often have full recovery of their right heart function and size, as long as the pulmonary hypertension has resolved. This is an interesting and unexplained phenomenon that is a unique feature of the right heart failure associated with CTEPH. In most other causes of heart failure especially left ventricular failure, even when the obstruction has been removed, the ventricle does not fully recover and certainly does not normalize in size. In contrast, in the setting of CTEPH, the right ventricle does so. The improvement in right heart failure and its associated tricuspid regurgitation is proportional to the degree of resolution achieved in the patient’s pulmonary hypertension. Therefore one can expect full recovery of the right heart and normal tricuspid function in patients who have had successful outcomes with normal post-operative pulmonary pressures.
Pulmonary hypertension and subsequent right heart failure secondary to chronic thromboembolic disease is a relatively uncommon condition occurring in 1–5 % of adult patients who survive an acute pulmonary embolic event [1, 2]. More recent studies however suggest a higher incidence in patients with an acute episode of pulmonary embolism; 3.1 % at 1 year and 3.8 % at 2 years [2, 3]. It is extremely hard to accurately determine the true incidence of chronic thromboembolic pulmonary hypertension for obvious reasons, but one can come up with educated estimates of this disease. The estimated incidence of acute pulmonary embolism is approximately 630,000 per year in the United States, based on clinical data [4, 5], and is related to approximately 235,000 deaths per year, based on autopsy data [6]. Calculations extrapolated from mortality rates and the random incidence of major thrombotic occlusion of pulmonary vessels at autopsy support an estimate that more than 100,000 people in the United States currently suffer from pulmonary hypertension that could be relieved by operation [7]. Given an incidence of about 4 % after an episode of acute PE, one can estimate that there are about 25,000 new patients annually in the US alone suffering from this disease, yet the number of pulmonary endarterectomies performed remains low at about 250–300 cases annually in the US, the majority of which are performed at the authors’ institution, University of California San Diego.
Once chronic pulmonary hypertension develops, the prognosis is poor, and this prognosis is even worse in patients without an intracardiac shunt. As a rule patients with pulmonary hypertension caused by pulmonary emboli fall into a higher risk category than those with Eisenmenger’s syndrome and encounter a higher mortality rate. In fact, survival of patients with chronic thromboembolic pulmonary hypertension is inversely related to the magnitude of pulmonary artery systolic pressure and pulmonary vascular resistance [8]. When the mean pulmonary artery pressure in patients with thromboembolic disease exceeds 50 mmHg, the 5-year mortality approaches 90 % [9].
Regardless of the exact incidence or the circumstances, it is clear that acute embolism and its chronic relation, fixed chronic thromboembolic occlusive disease, are both much more common than generally appreciated and are seriously underdiagnosed. Houk and colleagues [10] in 1963 reviewed the literature of 240 reported cases of chronic thromboembolic obstruction of major pulmonary arteries but found that only 6 cases had been diagnosed correctly before death. Calculations extrapolated from mortality rates and the random incidence of major thrombotic occlusion found at autopsy would support a postulate that more than 100,000 people in the United States currently have pulmonary hypertension that could be relieved by operation. Therefore, despite an improved understanding of pathogenesis, diagnosis, and management, pulmonary emboli and the long-term sequelae of thromboembolic pulmonary hypertension, remain frequent and often fatal disorders.
Clinical Presentation
There are no signs or symptoms specific for chronic thromboembolism. The most common symptom associated with thromboembolic pulmonary hypertension, as with all other causes of pulmonary hypertension and right heart failure is exertional dyspnea. This dyspnea is out of proportion to any abnormalities found on clinical examination. Like complaints of easy fatigability, dyspnea that initially occurs only with exertion is often attributed to anxiety or being “out of shape”. Syncope, or presyncope (light-headedness during exertion) is another common symptom in pulmonary hypertension. Generally, it occurs in patients with more advanced disease and higher pulmonary arterial pressures.
Non-specific chest pains or tightness occur in approximately 50 % of patients with more severe pulmonary hypertension. Hemoptysis can occur in all forms of pulmonary hypertension and probably results from abnormally dilated vessels distended by increased intravascular pressures. Peripheral edema, early satiety, and epigastric or right upper quadrant fullness or discomfort will develop as the right heart failure progresses. Some patients with chronic pulmonary thromboembolic disease present after a small acute pulmonary embolus that may produce acute symptoms of right heart failure. A careful history brings out symptoms of dyspnea on minimal exertion, easy fatigability, diminishing activities, and episodes or angina-like pain or light-headedness. Further examination reveals the signs of pulmonary hypertension and right heart failure.
The physical signs of pulmonary hypertension are the same no matter what the underlying pathophysiology. Initially the jugular venous pulse is characterized by a large A-wave. As the right heart fails, the V-wave becomes predominant. The right ventricle is usually palpable near the lower left sternal border, and pulmonary valve closure may be audible in the second intercostal space. Occasional patients with advanced disease are hypoxic and slightly cyanotic. Clubbing is an uncommon finding.
The second heart sound is often narrowly split and varies normally with respiration; P2 is accentuated. A sharp systolic ejection click may be heard over the pulmonary artery. As the right heart fails, a right atrial gallop usually is present, and tricuspid insufficiency develops. Because of the large pressure gradient across the tricuspid valve in pulmonary hypertension, the murmur is high pitched and may not exhibit respiratory variation. These findings are quite different from those usually observed in tricuspid valvular disease. A murmur of pulmonic regurgitation may also be detected.
Diagnosis
To ensure diagnosis in patients with right heart failure secondary to chronic pulmonary thromboembolism, a standardized evaluation is recommended for all patients who present with unexplained pulmonary hypertension. This workup includes a chest radiograph, which may show either apparent vessel cutoffs of the lobar or segmental pulmonary arteries or regions of oligemia suggesting vascular occlusion. Central pulmonary arteries are generally enlarged, and the right ventricle may also be enlarged without any enlargement of the left atrium or ventricle. However, one should keep in mind that despite these classic findings, a large number of patients might present with a relatively normal chest radiograph, even in the setting of high degrees of pulmonary hypertension or right heart failure. The electrocardiogram demonstrates findings of right ventricular hypertrophy (right axis deviation, dominant R-wave in V1). Pulmonary function tests are necessary to exclude obstructive or restrictive intrinsic pulmonary parenchymal disease as the cause or the hypertension.
The most useful screening studies are two-dimensional surface echocardiography with Doppler imaging and ventilation-perfusion (V/Q) scanning. The standard echo helps to define the presence and severity of right heart failure, tricuspid regurgitation, and severity of pulmonary hypertension. In addition it is also helpful to rule out other causes, such as Eisenmenger’s Syndrome. The echocardiogram rapidly demonstrates right sided chamber enlargement and right ventricular hypertrophy (Fig. 15.1). The main pulmonary artery is usually enlarged; the intraventricular septum may appear flattened and often exhibits paradoxical motion, with encroachment of the right ventricular septum in the left ventricle. Varying degrees of tricuspid regurgitation are usually present. Continuous wave Doppler scanning of the tricuspid regurgitation jet is helpful in the estimation of the pulmonary artery systolic pressure. In addition, because exercise characteristically increases the pulmonary hypertension, echocardiography with exercise should always be applied whenever the disease is suspected but when the resting echocardiogram demonstrates only subtle abnormalities.
Echocardiographic appearances of the heart before (pre-PTE; top) and after (post-PTE; bottom) operation. Note the shift of the intraventricular septum toward the left in systole before the operation (top left), together with the relatively small left atrial and left ventricular chambers. After the operation, the septum is restored to its normal geometry, and the massive enlargement of the right atrium and right ventricle has resolved
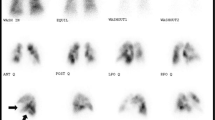
The ventilation-perfusion lung scan is the fundamental test for establishing the diagnosis of unresolved pulmonary thromboembolism. An entirely normal lung scan excludes the diagnosis of both acute or chronic, unresolved thromboembolism. The usual lung scan pattern in most patients with primary pulmonary hypertension either is relatively normal or shows a diffuse non-uniform perfusion. When subsegmental or larger perfusion defects are noted on the scan, even when matched with ventilatory defects, pulmonary angiography is appropriate to confirm or rule out thromboembolic disease. It is important to note that any patient with unexplained dyspnea should be worked up for pulmonary hypertension, and any patient with a diagnosis of pulmonary hypertension should undergo a V/Q scan.
Currently, pulmonary angiography still remains the gold standard for diagnosis of CTEPH, however with the advent of high resolution scans, and magnetic resonance imaging, more and more centers rely on the diagnostic power and the non-invasive nature of these tests to confirm the diagnosis. Organized thromboembolic lesions do not have the appearance of the intravascular filling defects seen with acute pulmonary emboli, and experience is essential for the proper interpretation of pulmonary angiograms in patients with unresolved, chronic embolic disease. Organized thrombi appear as unusual filling defects, webs, or bands, or completely thrombosed vessels that may resemble congenital absence of the vessel [11] (Fig. 15.2). In addition to pulmonary angiography, patients over 45 undergo coronary arteriography and other cardiac investigation as necessary. If significant disease is found, additional cardiac surgery is performed at the time of pulmonary thromboendarterectomy.
In recent years higher resolution helical computed tomography (CT) scans of the chest have been used more frequently in diagnosis of pulmonary thromboembolic disease. Presence of large clots in lobar or segmental vessels generally confirms the diagnosis. CT features of chronic thromboembolic pulmonary hypertension include evidence of organized thrombus lining the pulmonary vessels in an eccentric fashion, enlargement of the right ventricle and the central arteries, variation in size of segmental arteries, and parenchymal changes characteristic of pulmonary infarction. In addition, in rare situations where there are concerns of external compression, or occlusion of main pulmonary arteries are present, CT scans can be helpful in differentiating thromboembolic disease from other causes such as mediastinal fibrosis, lymph nodes, or tumors. In the current era of multi-detector CT scans, the resolution of the pulmonary arteries is much better and it is possible that this diagnostic modality may eventually replace pulmonary angiography as the gold standard in diagnosing CTEPH and in planning the surgical treatment once the diagnosis is made. In addition, the volumetric assessment of the right ventricle can be more accurately performed with multi-detector CT. It is important to remember, however, that the diagnosis of CTEPH by CT scans is difficult and requires experienced radiologists with expertise in this area and currently remains an adjunct to pre-operative planning.
Medical Treatment
There is no curative role for medical management of these patients and at best it is palliative. There are a number of new pulmonary vasodilators that are now available for the treatment of the pulmonary hypertension and right heart failure in these patients; but considering the fact that the primary pathology is the physical obstruction of pulmonary vasculature, there is no surprise that their effects are only transient at best. Right ventricular failure may show some improvement with combination of diuretics and vasodilators, but because the failure is due to a mechanical obstruction it will not resolve until the obstruction is removed. Similarly, the prognosis is unaffected by medical therapy [12, 13], which should be regarded as only supportive. Because of the bronchial circulation, pulmonary embolization seldom results in tissue necrosis. Surgical endarterectomy therefore will allow distal pulmonary tissue to be used once more in gas exchange.
Currently there is only one FDA approved drug for the treatment of CTEPH in patients deemed to have inoperable disease and also patients who continue to have residual pulmonary hypertension following pulmonary thromboendarterectomy. Riociguat is a new drug in the class of soluble guanylate cyclase stimulators that has been shown to increase 6 min walk distance and decrease PVR in patients with CTEPH [20]. The observed improvements in exercise capacity and PVR of the patients in this study continue to remain significantly inferior to surgery and their durability is not proven. Pulmonary thromboendarterectomy remains the gold standard treatment and determination of inoperable disease should be made by an experienced center. Initiation of medical treatment in a patient with potentially operable disease may prevent someone from a curative procedure [21], or result in delay of referral.
Chronic anticoagulation represents the mainstay of the medical regimen. Anticoagulation is primarily used to prevent future embolic episodes, but it also serves to limit the development of thrombus in regions of low flow within the pulmonary vasculature. Inferior vena caval filters are used routinely to prevent recurrent embolization.
Operative Procedure
Pulmonary thromboendarterectomy is a technically demanding operation that is performed only in select centers around the world. Proper patient selection, meticulous surgical technique, and vigilant postoperative management have contributed to the success of this operation. A true endarterectomy (not an embolectomy) of all affected parts of the lung is essential to clear all affected areas of the pulmonary vasculature. It is clear that pulmonary endarterectomy relieves pulmonary hypertension by improving lung ventilation-perfusion match, improving right ventricular function and tricuspid regurgitation, limiting retrograde extension of clot obstruction, and preventing arteriopathic changes in the remaining patent small pulmonary vessels [14, 15]. Furthermore with resolving pulmonary hypertension, the right ventricle will regress to a normal size and improve its overall function.
The description of a surgical procedure for removal of thromboembolic material dates back to 1908, when Trendelenburg [16] first illustrated an approach in a dying patient. However it was not until the introduction and development of cardiopulmonary bypass when more procedures with better outcomes were performed. By the mid 1980s there were a total of 85 reported cases that were managed surgically but still carried a high mortality rate of about 22 % [17]. Although there have been other reports of surgical treatment of CTEPH, most of the surgical experience in pulmonary endarterectomy has been reported from the UCSD Medical Center [7, 11], and it is this experience that forms the basis of this chapter.
With our growing experience now accounting for over 3300 of these procedures, we know that there are certain principles of this procedure that have to be adhered to. Although an endarterectomy is possible even if one deviates from these principles, a successful and complete endarterectomy is not, and such outcomes are questionable. What follows is a description of the techniques of this procedure highlighting the fundamental points.
Technical Principles of the Procedure
There are several guiding principles for this operation. First and foremost the approach must be bilateral; because, for pulmonary hypertension to be a major factor, both pulmonary arteries must be substantially involved. Furthermore, it is extremely unlikely to have unilateral disease as the result of thromboembolism. In fact we believe that a small subgroup of our patients who truly do have unilateral disease, perhaps suffer from an underlying pulmonary vascular pathology with subsequent thrombosis, rather than true thromboembolism. The only reasonable approach to both pulmonary arteries is through a median sternotomy incision. Historically, there have been many reports of unilateral operation, and occasionally this is still performed with various results in inexperienced centers, through a thoracotomy. However, the unilateral approach ignores the disease on the contralateral side, subjects the patient to hemodynamic jeopardy during the clamping of the pulmonary artery, and does not allow good visibility because of the continued presence of bronchial blood flow. In addition, collateral channels develop in chronic thrombotic hypertension not only through the bronchial arteries but also from diaphragmatic, intercostal, and pleural vessels. The dissection of the lung in the pleural space via a thoracotomy incision can therefore be extremely bloody. The median sternotomy incision, apart from providing bilateral access, avoids entry into the pleural cavities, and allows the ready institution of cardiopulmonary bypass.
Cardiopulmonary bypass is an essential part of this operation and integral to ensure cardiovascular stability during the procedure. In addition cardiopulmonary bypass allows cooling the patient in preparation of circulatory arrest. Given the extent and the location of thromboembolic material, which have now transformed into a scar like fibrotic tissue adherent to the pulmonary vasculature, superior visibility is required. This is only achievable in a bloodless field so the surgeon can define an adequate endarterectomy plane and then can follow the pulmonary endarterectomy specimen deep into the subsegmental vessels. Because of the copious bronchial blood flow usually present in these patients, periods of circulatory arrest are necessary to ensure perfect visibility. Again, there continue to be sporadic reports of performing this operation without circulatory arrest with various outcomes. However, it should be emphasized that although endarterectomy is clearly possible without circulatory arrest, a complete endarterectomy is not. Surgeons claiming success with a complete endarterectomy without circulatory arrest are likely to leave behind distal disease in the subsegmental branches without ever recognizing it. We always initiate the procedure without circulatory arrest, and depending on the collateral flow through the bronchial arteries and other channels, a variable amount of dissection is possible before the circulation has to be stopped, but never a complete dissection. The circulatory arrest periods are typically limited to 20 min, with restoration of flow between each arrest. With experience, a complete endarterectomy usually can be performed within a single period of circulatory arrest on each side.
The next principle of this operation relies mainly on the experience of the operator in recognizing the true endarterectomy plane of the media, and following the specimen to its feathered tail end in each branch. It is essential to appreciate that the removal of visible thrombus is largely incidental to this operation. Indeed, in most patients, no free thrombus is present; and on initial direct examination, the pulmonary vascular bed may appear normal. The early literature on this procedure indicates that thrombectomy was often performed without endarterectomy, and in these cases the pulmonary artery pressures did not improve, often with the resultant death of the patient.
Surgical Technique
The surgical approach for this procedure is through a median sternotomy to gain access to both sides. Typically the right heart is severely enlarged, with a tense right atrium and a variable degree of tricuspid regurgitation. There is usually significant right ventricular hypertrophy and associated right heart failure, and with critical degrees of obstruction, the patient’s condition may become unstable with the manipulation of the heart. Care must be taken to avoid any unnecessary manipulation of the heart while the patient is safely placed on cardiopulmonary bypass.
Anticoagulation is achieved with the use of heparin sodium (400 units/kg, intravenously) administered to prolong the activated clotting time beyond 400 s. Full cardiopulmonary bypass is instituted with high ascending aortic cannulation and bi-caval cannulation. The heart is emptied on bypass, and a temporary pulmonary artery vent is placed in the midline of the main pulmonary artery about 1 cm distal to the pulmonary valve. The insertion site can then be used for the beginning of the left pulmonary arteriotomy. The patient is then actively cooled to a core temperature of about 18–20 °C.
Initially, it is most convenient for the primary surgeon to stand on the patient’s left side, and perform the endarterectomy on the right side. The superior vena cava is also fully mobilized. The approach to the right pulmonary artery is made medial, not lateral, to the superior vena cava. Once the superior vena cava is fully mobilized, and the core temperature has reached 20 °C, an aortic cross clamp is applied and myocardial protection is provided through a single dose of antegrade cold blood cardioplegia (1 L). The entire procedure is now performed with a single aortic cross-clamp period with no further administration of cardioplegic solution. Additional myocardial protection is provided by using a cooling jacket surrounding the heart throughout the remainder of the procedure. Both tourniquets are now secured around the superior and inferior vena cavae to ensure complete drainage and to avoid any air entry in the venous cannulae during circulatory arrest.
A modified cerebellar retractor is then used to expose the pulmonary artery between the aorta and the superior vena cavae. An incision is made in the right pulmonary artery from beneath the ascending aorta out under the superior vena cava and entering the lower lobe branch of the pulmonary artery just after the take-off of the middle lobe artery (Fig. 15.3). It is important that the incision stays in the center of the vessel and continues in the middle of the descending pulmonary artery into the lower, rather than the middle lobe artery. The incision is carried past the take-off of the middle lobe artery.
Exposure of the right pulmonary artery, as viewed by the surgeon standing on the left side. The incision is placed between the superior vena cava (SVC) and the Aorta, as shown in the insert. It is imperative that the incision towards the right lower lobe artery is made in the middle of the vessel. During the dissection the edges of this incision is left intact for easier and more hemostatic closure
Any loose thrombus, if present, is now removed. This is necessary to obtain good visualization. It is most important to recognize, however, that first, an embolectomy without subsequent endarterectomy is quite ineffective and, second, that in most patients with chronic thromboembolic hypertension, direct examination of the pulmonary vascular bed at operation generally shows no obvious embolic material. Therefore, to the inexperienced or cursory glance, the pulmonary vascular bed may well appear normal even in patients with severe chronic embolic pulmonary hypertension.
If the bronchial circulation is not excessive, the endarterectomy plane can be found during this early dissection. However, although a small amount of dissection can be performed before the initiation of circulatory arrest, it is unwise to proceed unless perfect visibility is obtained because the development of a correct plane is essential.
The correct plane appears pearly white, which is smooth and silky in appearance and lies between the intima and media. A microtome knife is used to develop the endarterectomy plane posteriorly, because any inadvertent egress in this site could be repaired readily, or simply left alone. Dissection in the correct plane is critical because if the plane is too deep the pulmonary artery may perforate, with fatal results, and if the dissection plane is not deep enough, inadequate amounts of the chronically thromboembolic material will be removed. When the proper plane is entered, the layer will strip easily, and the material left with the outer layers of the pulmonary artery will appear somewhat yellow, but there should be no residual yellow plaque.
If the dissection is too deep, a reddish or pinkish color indicates the adventitia has been reached. As a general rule while developing the plane of dissection a non-smooth light purplish or pinkish color is an indication that the plane of dissection is too deep and care must be taken to immediately get back into the more superficial correct plane before the vessel wall is injured. Once the correct plane is recognized the dissection is carried into each one of the lobar, segmental, and subsegmental branches until a feathered tail is obtained.
There are five categories of pulmonary occlusive disease related to thrombus that can be appreciated, and we use the UCSD classification system which describes the different levels of the thromboembolic specimen [22], and corresponds to the degree of difficulty of the endarterectomy. Level 0 is no evidence of chronic thromboembolic disease present, in other words there has been a misdiagnosis or perhaps one lung is completely unaffected by thromboembolic disease, both of which are rare. In this entity there is intrinsic small vessel disease, although secondary thrombus may occur as a result of stasis. Small-vessel disease may be unrelated to thromboembolic events (“primary” pulmonary hypertension) or occur in relation to thromboembolic hypertension as a result of a high flow or high pressure state in previously unaffected vessels similar to the generation of Eisenmenger’s syndrome. We believe that there may also be sympathetic “cross-talk” from an affected contralateral side or stenotic areas in the same lung.
Level I disease (Fig. 15.4) refers to the situation in which thromboembolic material is present and readily visible on the opening of the main left and right pulmonary arteries. A subset of level I disease, level Ic, is complete occlusion of either the left or right pulmonary artery and non-perfusion of that lung. Complete occlusion may present an entirely different disease, especially when it is unilateral and on the left side. This group of patients, typically a young female with complete occlusion of the left pulmonary artery, may not reperfuse their affected lung despite a complete endarterectomy, indicating a different intrinsic pulmonary vascular disease, unrelated to thromboembolic disease. In level II (Fig. 15.5), the disease starts at the lobar or intermediate level arteries and the main pulmonary arteries are unaffected. Level III disease is limited to thromboembolic disease originating in the segmental vessels only (Fig. 15.6). Level IV is disease of the subsegmental vessels (Fig. 15.7), with no other disease appreciated at more proximal levels. Level III and level IV disease present the most challenging surgical situation. The disease is very distal and confined to the segmental and subsegmental branches. These levels are most often associated with presumed repetitive thrombi from upper extremity sources, indwelling catheters (such as pacemaker wires) or ventriculoatrial shunts.
Surgical specimen removed from right and left pulmonary arteries in a patient with level III disease. Note that in this patient the dissection plane has to be developed and raised at each segmental level. The center of the pictures shows a remnant of a chronic indwelling catheter. The ruler measures 15 cm
With the modified cerebellar retractor in place and the artery well exposed the dissection is then carried on. When blood obscures direct vision of the pulmonary vascular bed, circulatory arrest is then initiated, and the patient undergoes exsanguination. It is rare that one 20-min period for each side is exceeded.
The endarterectomy is then performed with an eversion technique. Because the vessel is everted and subsegmental branches are being worked on, a perforation here will become completely inaccessible and invisible later. This is why the absolute visualization in a completely bloodless field provided by circulatory arrest is essential. It is important that each subsegmental branch is followed and freed individually until it ends in a “tail,” beyond which there is no further obstruction. Residual material should never be cut free; the entire specimen should “tail off” and come free spontaneously.
Once the right-sided endarterectomy is completed, circulation is re-started, and the arteriotomy is repaired with a continuous 6-0 polypropylene suture. The hemostatic nature of this closure is aided by the nature of the initial dissection, with the full thickness of the pulmonary artery being preserved immediately adjacent to the incision.
After the completion of the repair of the right arteriotomy, the surgeon moves to the patient’s right side.
The left-sided dissection is virtually analogous in all respects to that accomplished on the right. The duration of circulatory arrest intervals during the performance of the left-sided dissection is subject to the same restriction as the right.
After the completion of the endarterectomy, cardiopulmonary bypass is reinstituted and warming is commenced. The rewarming period generally takes approximately 90–120 min but varies according to the body mass of the patient.
Based on the results of the pre-operative echo and the intra-operative TEE, we would then decide if a right atrial exploration is necessary. In general if either one of the two test is positive for a bubble test we would explore the right atrium. The right atrium is then opened through a small atriotomy located over the site of fossa ovalis. Any intra-atrial communication is then closed. Although tricuspid valve regurgitation is invariable in these patients and is often severe, tricuspid valve repair is not performed. Right ventricular remodeling occurs within a few days, with the return of tricuspid competence.
If other cardiac procedures are required, such as coronary artery or mitral or aortic valve surgery, these are conveniently performed during the systemic rewarming period [14].
When the patient has fully rewarmed, cardiopulmonary bypass is discontinued. Dopamine is routinely administered at low doses, and other inotropic agents and vasodilators are titrated as necessary to sustain acceptable hemodynamics. With a successful endarterectomy, the cardiac output is generally high, with a low systemic vascular resistance. Temporary atrial and ventricular epicardial pacing wires are placed.
Despite the duration of extracorporeal circulation, hemostasis is readily achieved, and the administration of platelets or coagulation factors is rarely necessary. Wound closure is routine. Given the degree of pre-operative volume overload, the relief of pulmonary obstruction, in addition to the previous systemic hypothermia, a vigorous diuresis is usual for the following few hours.
Results
In our most recent series involving about 2700 patients, the trend shows improving mortality rates [18]. There were no mortalities in the last 260 patients. Currently our overall mortality rate is about 1 % for patients who undergo isolated pulmonary endarterectomy. A similar number of men and women were referred for operation. With the exception of closure of patent foramen ovale, in 10 % of cases, at least one additional cardiac procedure was performed at the time of operation. Most commonly, the adjunct procedures were coronary revascularization, aortic valve replacement, or mitral valve repair/replacement. There was no significant difference between patients undergoing pulmonary endarterectomy alone or combined with other cardiac operations with respect to cardiopulmonary bypass time, crossclamp time, or circulatory arrest time. In general total cardiopulmonary bypass time correlates with body mass and cooling-rewarming intervals.
With this operation, a reduction in pulmonary artery pressures and pulmonary vascular resistance to normal levels and corresponding improvement in pulmonary blood flow and cardiac output are generally immediate and sustained. Improvement in the right ventricular function correlates with the decrease in the pulmonary pressures and the pulmonary vascular resistance. Generally the improvement is evident on the post-operative echocardiogram performed before discharge. This is in contrast to other conditions causing ventricular failure, and especially in disparity to the left ventricular failure caused as a result of outflow obstruction. In those patients, even when the insulting lesion or obstruction has been resolved, the ventricular dysfunction will not normalize. A good example is left ventricular failure as the result of aortic valve stenosis. After a vavular replacement, neither the LV dysfunction nor its hypertrophy will improve much. It is true that the failure will not progress, the patients will be much improved, and there maybe some subtle enhancement in the LV function, but very rarely the function and size will normalize.
Before the operation, more than 85 % of the patients were in New York Heart Association (NYHA) functional class III or IV; at the time of discharge, 80.2 % were re-classified as NYHA functional class I or II. Echocardiographic studies on this patient cohort and other previous studies from our institution [19] demonstrated that, with the elimination of chronic pressure overload, right ventricular geometry rapidly reverted to normal. Tricuspid valve function (as measured by tricuspid regurgitant velocity) returned to normal within a few days as a result of restoration of tricuspid annular geometry after the remodeling of the right ventricle. Tricuspid valve annuloplasty was not performed, even when severe tricuspid regurgitation was documented preoperatively.
Reperfusion edema is the single most frequent complication after pulmonary endarterectomy, occurring in up to 11 % of patients. In most patients with reperfusion injury, the problem resolved with avoidance of hypercarbia, and a short period of ventilatory support and aggressive diuresis. A minority of patients with severe lung reperfusion injury required long periods of ventilatory support, and extreme cases (approximately 1 %) required veno-venous extracorporeal support for blood carbon dioxide removal and oxygenation. Neurologic complications from circulatory arrest largely have been eliminated by shorter circulatory arrest periods and the use of a direct cooling jacket placed around the head. Rates of perioperative confusion and stroke for pulmonary endarterectomy were similar to those seen with conventional open heart surgery.
Conclusion
It is increasingly apparent that pulmonary hypertension caused by chronic pulmonary embolism is a condition which is under-recognized, and carries a poor prognosis. Because of the obstructive nature of this disease, medical therapy remains ineffective in prolonging life and at best only transiently improves the symptoms. The only therapeutic alternative to pulmonary thromboendarterectomy is lung transplantation. The advantages of thromboendarterectomy include a lower operative mortality and excellent long-term results without the risks associated with chronic immunosuppression and chronic allograft rejection.
Technical advances of the procedure over the last four decades, and in particular in the last 15 years have significantly improved outcomes. Newly designed instruments allow better visualization and more complete endarterectomy in the distal segmental and subsegmental branches. Attention to the surgical principles of this procedure is of paramount importance. The procedure is performed through a median sternotomy for bilateral exposure and exploration. It should be performed with the use of circulatory arrest for excellent exposure of the distal branches. The correct plane is recognized, developed, and should then be followed all the way to the distal feathered tails in each branch. With careful and meticulous intra-operative techniques as well as vigilant post-operative care, the mortality for thromboendarterectomy at our institution is now in the range of 1 %, with sustained benefit. These results are clearly superior to those for transplantation both in the short and long term.
Although PTE is technically demanding for the surgeon, and requires careful dissection of the pulmonary artery planes and the use of circulatory arrest; excellent short- and long-term results can be achieved. It is the successive improvements in operative technique that allow pulmonary endarterectomy to be offered to patients with an acceptable mortality rate and excellent anticipation of clinical improvement. With this growing experience, we are now offering this procedure to all patients including some very high risk candidates, as long as there is evidence of thromboembolic disease, regardless of the degree of pulmonary hypertension or right ventricular failure.
The primary problem remains that this is an under-recognized condition, and unfortunately there are still a large number of patients with CTEPH who carry other diagnoses and are mistreated as such. Increased understanding of both the prevalence of this condition and the opportunity and availability of a surgical cure should benefit more patients. Surgical removal of the thromboembolic material by means of a complete endarterectomy provides these patients an opportunity for relief from this debilitating and ultimately fatal disease.
Editor’s Note
While the bulk of the experience has been at UCSD, other selected centers worldwide have adopted this technique with reasonable results. An alternative to circulatory arrest is antegrade cerebral perfusion at low temperatures with low flow cardiopulmonary bypass, with acceptable visualization. One presumes that with increased use of multi-detector CT scanning, we will see more of these patients being referred for further management and surgery.
Key Points to Remember
Chronic thrombo-embolic pulmonary hypertension is a potentially treatable condition. The prognosis is best before the mean PA pressure crosses 50 mmHg and is good in patients with proximal thrombo-emboli.
Diagnosis of this condition requires a high index of suspicion and this is a large underdiagnosed clinical entity.
Treatment is almost always surgical. This entails complete dissection and removal of thrombo-emboli from both sides. Visualization of the plane between the lining of pulmonary arterial branches and thrombo-emboli is best under conditions of circulatory arrest. However, in selected patients and instances, antegrade cerebral perfusion with low flow cardiopulmonary bypass may be provide adequate visualization. The hallmark of success is the fall in pulmonary artery pressures immediately and gradual resolution of tricuspid regurgitation.
References
Ribeiro A, Lindmarker P, Johnsson H, et al. Pulmonary embolism: one-year follow-up with echocardiography, Doppler, and five-year survival analysis. Circulation. 1999;99:1325–30.
Pengo V, Anthonie WA, Lensing MD, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64.
Becattini C, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130(1):172–5.
Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17:259–70.
Goldhaber SZ, Hennekens CH, Evans DA, et al. Factors associated with correct antemortem diagnosis of major pulmonary embolism. Am J Med. 1982;73:822–6.
Landefeld CS, Chren MM, Myers A, et al. Diagnostic yield of the autopsy in a university hospital and a community hospital. N Engl J Med. 1988;318:1249–54.
Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lesions learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–64.
Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011–20.
Riedel M, Stanek V, Widimsky J, et al. Longterm follow-up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151–8.
Houk VN, Hufnnagel CA, McClenathan JE, Moser KM. Chronic thrombosis obstruction of major pulmonary arteries: report of a case successfully treated by thromboendarterectomy and review of the literature. Am J Med. 1963;35:269–82.
Jamieson SW, Kapalanski DP. Pulmonary endarterectomy. Curr Probl Surg. 2000;37(3):165–252.
Dantzker DR, Bower JS. Partial reversibility of chronic pulmonary hypertension caused by pulmonary thromboembolic disease. Am Rev Respir Dis. 1981;124:129–31.
Dash H, Ballentine N, Zelis R. Vasodilators ineffective in secondary pulmonary hypertension. N Engl J Med. 1980;303:1062–3.
Thistlethwaite PA, Auger WR, Madani MM, et al. Pulmonary thromboendarterectomy combined with other cardiac operations: indications, surgical approach, and outcome. Ann Thorac Surg. 2001;72:13–9.
Thistlethwaite PA, Kemp A, Du L, Madani MM, Jamieson SW. Outcomes of pulmonary endarterectomy for treatment of extreme thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2006;131:307–13.
Trendelenberg F. Uber die operative behandlung der embolie derlungarterie. Arch Klin Chir. 1908;86:686–700.
Chitwood WR, Sabiston DC, Wechsler AS. Surgical treatment of unresolved pulmonary embolism. Clin Chest Med. 1984;5:507–36.
Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94(1):97–103; discussion 103. doi:10.1016/j.athoracsur.2012.04.004. Epub 2012 May 23.
Thistlethwaite PA, Madani MM, Jamieson SW. Pulmonary thromboendarterectomy surgery. Cardiol Clin. 2004;22:467–78.
Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29.
Auger WR, Jamieson SW. Riociguat for pulmonary hypertension. N Engl J Med. 2013;369:2266–8.
Madani MM, Jamieson SW, Pretorius V, et al. Subsegmental pulmonary endarterectomy: time for a new surgical classification. International CTEPH Conference, Paris. 2014: Abstract presentation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag London Ltd.
About this chapter
Cite this chapter
Pollema, T.L., Madani, M.M. (2016). Other Techniques in Special Circumstances: Pulmonary Thromboendarterectomy in Right Heart Failure. In: Raman, J. (eds) Management of Heart Failure. Springer, London. https://doi.org/10.1007/978-1-4471-4279-9_15
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4279-9_15
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4278-2
Online ISBN: 978-1-4471-4279-9
eBook Packages: MedicineMedicine (R0)