Abstract
Pulmonary hypertension due to chronic thromboembolic disease is potentially curable with pulmonary thromboendarterectomy surgery. As a result, it is important for clinicians to recognize and appropriately diagnose this form of pulmonary hypertension. Advances in this field with changes in surgical technique, the availability of PH-targeted medical therapy for select patient subgroups, and the development of balloon pulmonary angioplasty have broadened therapeutic options for patients. This review will examine what is known about the epidemiology and medical conditions placing patients at risk of developing this disease, will present an approach to evaluation of patients with suspected chronic thromboembolic disease, and will describe the surgical and non-surgical management of this unique patient population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prior to the mid-1950s, the development of cor pulmonale from organized thrombus involving the pulmonary arteries was merely an autopsy observation and the subject of scattered case reports [1]. However, subsequent technological developments in cardiac catheterization and imaging modalities not only allowed accurate pre-mortem diagnosis of this entity, but cardiothoracic surgical advancements established a surgical technique to remove the organized thromboembolic material from the pulmonary arteries. This thromboendarterectomy procedure restored blood flow to the lungs and successfully relieved the right heart strain and pulmonary hypertension that afflicted these patients. The past five decades have witnessed tremendous forward strides in our understanding of the natural history, pathophysiology, and management of chronic thromboembolic pulmonary hypertension (CTEPH) [2]. These advances have resulted in a greater recognition of this disease, and the appreciation that CTEPH is a potentially curable form of pulmonary hypertension with pulmonary thromboendarterectomy (PTE) also sometimes referred to as pulmonary endarterectomy (PEA). Most recently, modifications of surgical techniques have allowed distal resection of chronic thrombotic lesions from the pulmonary vascular bed, providing an opportunity for a surgical cure to more patients. And for those patients with inoperable CTEPH, pulmonary hypertension (PH)-targeted medical therapy and, in some specialized centers, balloon pulmonary angioplasty now present opportunities for effective therapies in this select patient group. An update on the epidemiology and medical conditions placing patients at risk of developing CTEPH, an approach to an evaluation of patients with suspected chronic thromboembolic disease, and a review of the state-of-the-art surgical and non-surgical management of this unique patient population will be examined.
Epidemiology and risk factors
Chronic thromboembolic pulmonary hypertension (CTEPH), currently categorized as World Health Organization (WHO) Group 4 [3], is defined as resting pulmonary hypertension with a mean pulmonary artery pressure (mPAp) ≥ 25 mm Hg in the presence of organized thrombus within the pulmonary arteries after at least 3 months of anticoagulant therapy. While no inciting thromboembolic event can be identified in up to 25 % of CTEPH patients, this is more likely due to underdiagnosis of the initial pulmonary embolic event rather than an alternative pathologic process [4]. The reported incidence of CTEPH following an acute thromboembolic event varies widely from 0.57 to 8.8 % [5–10]. The variability in reported incidence is based on differences in study method, patient population, method of pulmonary hypertension detection—right heart catheterization versus echocardiography—and values used to define pulmonary hypertension.
One of the first studies addressing this question followed 223 patients prospectively following an acute pulmonary embolism (PE) for a median of 93.4 months. Patients who remained symptomatic following the initial PE were screened with echocardiography and those with a pulmonary artery systolic pressure (PASP) estimate ≥40 mmHg underwent confirmation right heart catheterization to confirm the diagnosis of CTEPH. The incidence of CTEPH was 3.8 %. No patients developed CTEPH greater than 2 years after the initial episode of PE [5].
Dentali and colleagues reported the highest incidence of CTEPH following acute PE at 8.8 %. This finding is likely a result of a study design that screened all patients, even those without symptoms, following an acute PE. There was a 4.4 % incidence of patients who presented with symptoms of shortness of breath [8]. Though study limitations included small patient numbers and the fact that right heart catheterization was not performed to confirm the diagnosis of CTEPH, this study highlights the difficulty with epidemiologic studies in CTEPH which focus only on patients who experience cardiopulmonary symptoms following a limited time period after surviving an acute pulmonary embolic event.
Reported in 2014, a study examining the incidence of CTEPH prospectively followed 158 patients for a median of 26 months following acute PE and confirmed the diagnosis of CTEPH in seven patients or 4.8 %. Of note, on retrospective review of the CT angiogram diagnostic for the index PE event, all seven patients already had findings, suggesting the presence of CTEPH [11]. This observation has raised concerns that a diagnosed “initial acute PE event” might actually be the presentation of CTEPH in some patients.
Risk factors for the development of CTEPH following an acute PE include multiple episodes of pulmonary embolism, younger age, larger perfusion defects, and idiopathic pulmonary embolism CTEPH [5, 11]. One study has also suggested that echocardiogram estimates of sPAP > 50 mm Hg at the time of acute PE increases the risk of developing CTEPH 10-fold [10]. Whether this represents a misdiagnosis of CTEPH as an acute PE is unknown.
Additional risk factors for the development of CTEPH include the presence of the thrombophilic disorders antiphospholipid antibody syndrome (APS), elevated factor VIII levels and factor V Leiden mutation. APS has been observed in 10–20 % of patients with CTEPH, and in a study of 122 patients with CTEPH, factor VIII levels were significantly higher than a control group and remained elevated after PTE surgery [5, 12]. In a smaller study of 45 patients with CTEPH, nine of 31 (29 %) white patients were heterozygous for factor V Leiden compared with 7.8 % of controls [13]. The prevalence of other known thrombophilic disorders is not higher in patients with CTEPH compared to the general population.
A number of medical conditions have been associated with the development of CTEPH including the presence of a ventriculo-atrial shunts, infected pacemaker wires, absence of a spleen, blood groups other than O, thyroid replacement therapy, and a history of malignancy [14]. The mechanism by which thyroid replacement therapy and blood groups other than O contribute to the development of CTEPH may be through their effects on factor VIII levels. Patients with blood group O have been shown to have 25 % lower plasma levels of factor VIII [15] and patients exposed to levothyroxine have increases von Willebrand factor (vWF) levels and increased platelet function [16].
Pathobiology of CTEPH
The majority of CTEPH cases are thought to have evolved from a prior episode of acute pulmonary embolism (PE), although the latter may have been asymptomatic and never diagnosed [4]. Though efforts at identifying the pathophysiologic mechanisms of CTEPH have been focused on the transition from an acute thromboembolism to a chronic, endothelialized, endovascular “scar,” the process is incompletely understood. In addition to a genetic predisposition for inadequate thrombus dissolution, for which there is limited published data, explanatory hypotheses for the transition from acute to chronic thromboembolism within the pulmonary arteries include mechanisms of localized inflammation, ineffective angiogenesis, and endothelial dysfunction [17, 18].
Inflammatory infiltrates within the thrombus/vessel-wall complex may result in a chemokine cascade that promotes smooth muscle cell proliferation, fibroblast migration and proliferation, apoptosis inhibition, and endothelin-1 production. Although specifics remain uncertain, it appears that the inflammatory milieu results in maladaptive vessel-wall remodeling with poor thrombus dissolution and lumen recanalization. This theory is supported by published data on inflammatory markers and endarterectomized material analyzed from CTEPH patients [17–20].
Endothelial activation is an important component in the process of thrombus dissolution, mostly through vessel recanalization. A dysfunctional endothelium does not allow for effective angiogenesis, including fibrinolysis and thrombus contraction and absorption, resulting instead in fibroblast proliferation and deregulation of myofibroblast differentiation. This hypothesis, taken together with “localized inflammation” and reports of abnormal fragmentation of fibrinogen variants [21], provide a plausible mechanism for abnormal thrombus dissolution and maladaptive vessel-wall remodeling, bridging the gap between acute pulmonary embolus and chronic thromboembolic disease [17, 22–24].
An essential aspect of the pathophysiologic pathway leading to CTEPH is the development of a distal pulmonary vasculopathy, which occurs in varying degrees in addition to the upstream large vessel occlusion or narrowing with chronic thrombus. Early observations revealed small-vessel disease with pathologic changes similar to PAH, including vessel-wall muscularization, intimal fibrosis, medial hypertrophy, and even plexiform lesions [25]. However, a recent study reports post-capillary remodeling in CTEPH patients, associated with bronchial-to-pulmonary venous shunting and collateral blood flow, suggesting a much more complex distal vasculopathy than previously appreciated [26].
Uncertain is whether this downstream vascular pathology evolves as a function of the duration of established chronic thromboembolic disease or provoked by a relative high flow state in the vascular bed uninvolved with obstructing thrombus. Whether influenced by a particular genetic predisposition, the presence of a chronic infectious or inflammatory state, or other comorbid medical conditions remain areas of investigation [17].
Clinical presentation and evaluation
The clinical presentation of CTEPH is almost universally marked by the development of exertional dyspnea and an unexpected decline in exercise tolerance. In some instances, the onset of dyspnea clearly starts at the time of a known venous thromboembolic event and fails to resolve despite therapeutic anticoagulation. Often however, the onset of dyspnea is insidious and a delay in diagnosis is common, similar to the delay in diagnosis observed in Group I pulmonary arterial hypertension (PAH). Atypical chest pain, palpitations, and a dry cough are more rarely observed in the early stages of the disease. As the disease progresses and the right heart fails, exercise limitation becomes more severe and patients develop symptoms of lower extremity edema, ascites, and syncope. Hemoptysis can occur in the course of the disease and is likely related to lung infarction when it occurs in the setting of acute pulmonary embolism and bleeding from dilated bronchial arterial collaterals in the setting of chronic pulmonary artery obstruction.
The physical examination findings of CTEPH can be subtle early in the course of the disease and become more notable as right heart function deteriorates. Cardiac examination is notable for a loud, split second heart sound with accentuation of the pulmonic component, a tricuspid regurgitation murmur heart best at the left sternal border, the presence of a right-sided S4, and the development of a right-sided S3 when heart failure becomes more severe. Pulmonary flow murmurs can be heard in up to 30 % of patients with CTEPH as a result of turbulent flow across incompletely obstructed medium to large pulmonary arteries. While cyanosis can be observed, especially in patients with significant right-to-left shunt through a patent foramen ovale, clubbing is not a feature typical of CTEPH.
The chest radiograph is of limited utility in the diagnosis of CTEPH and is mostly helpful to rule out significant parenchymal lung disease or major anatomic deformities that would complicate PTE surgery. Enlargement—and occasionally, asymmetric enlargement—of the central pulmonary arteries and a decrease in peripheral vascular markings can be seen in CTEPH. In the setting of significant pulmonary hypertension, cardiomegaly and loss of the retrosternal air space from right heart chamber enlargement can be observed. However, these radiographic findings of pulmonary hypertension are nonspecific and can be also be chest radiographic findings in PAH [27].
Basic pulmonary function studies do not provide specific clues to the diagnosis of CTEPH, and primarily useful in evaluating for coexisting parenchymal lung disease or airflow obstruction. Twenty percent of CTEPH patients will exhibit a mild to moderate restrictive defect, typically on the basis of parenchymal scarring from prior lung infarction [28]. Similarly, a modest reduction in single breath diffusing capacity for carbon monoxide (DLco) may be present in some CTEPH patients. A normal value does not exclude the diagnosis [29], and severe reduction in DLco is indicative that the distal pulmonary vascular bed is significantly compromised, making an alternative diagnosis other than CTEPH more likely. CTEPH patients will frequently exhibit some degree of hypoxemia, often worsening with exercise. This is a result of moderate ventilation–perfusion mismatch and an inadequate cardiac output response to exercise which results in a low mixed venous oxygen saturation [30]. Marked hypoxemia at rest implies severe right heart dysfunction or the presence of a considerable right-to-left shunt, typically through a patent foramen ovale. More rigorous cardiopulmonary exercise testing (CPET) can be valuable in assessment of the degree of cardiopulmonary compromise and dead space ventilation in symptomatic patients with chronic thromboembolic disease who exhibit normal echocardiographic findings at rest [31]. In a longitudinal study following patients after an acute pulmonary embolism, Held and colleagues found CPET a useful complementary screening tool for the early detection of possible CTEPH where resting echocardiographic findings were unremarkable [32].
Often the first objective indication of the presence of elevated pulmonary pressures or right ventricular compromise is provided with transthoracic echocardiography. Estimates of pulmonary artery systolic pressure (PASP) by Doppler are a common tool used to screen all groups of pulmonary hypertension including CTEPH. In addition to elevated PASP, common findings on echocardiogram include right atrial enlargement, right ventricular enlargement, right ventricular hypertrophy, and reduced systolic function [33]. In CTEPH, as in other forms of pulmonary hypertension, accurate assessment of right ventricular function, right ventricular work load, right ventricular ejection fraction, and ultimately disease severity by ECHO is challenging. Various ECHO measurements have been used to make this assessment including the tricuspid annular plane systolic velocity (S′), RV fractional area change (RVFAC), RV myocardial performance index (RVMPI), RV strain patterns, and tricuspid annular plane systolic excursion (TAPSE). While some of these measurements have been shown to correlate with invasive measurements of PVR, other measurements such as TAPSE lose their utility postoperatively [34–36].
CTEPH: diagnostic imaging
Ventilation–perfusion (V/Q) scintigraphy plays a pivotal role in the diagnostic algorithm of pulmonary hypertension and remains the most widely available, sensitive test for the detection of CTEPH [37]. The combination of an abnormal V/Q scan and confirmatory digital subtraction pulmonary angiography (DSPA) is the current gold standard for the detection of CTEPH and for operability assessment. A normal V/Q essentially rules out the presence of CTEPH. Although previous studies demonstrated a wide gap between the sensitivity of V/Q and computed tomography pulmonary angiography (CTPA)—96–97 versus 51 %, respectively [38]—this discrepancy has diminished as CTPA imaging has advanced and interpretive experience has grown. In a study of 114 consecutive patients being evaluated for CTEPH, the sensitivity of V/Q scan was 96–100 % with a specificity of 95–94 % compared with a sensitivity of 92 % and specificity of 95 % for CTPA [39]. Additional data supporting the increasing sensitivity of CTPA were found in a study comparing the sensitivity of 320-slice CTPA to DSPA. The sensitivity of CTPA to detect chronic thromboembolic findings was 97.0 % at the main/lobar level though dropped to 85.8 % at the segmental level [40]. Nonetheless, the simplicity of interpretation inherent with a V/Q scan—that is either normal or abnormal—along with the difficult-to-recognize findings of chronic thromboembolic disease on CTPA makes perfusion scintigraphy the ideal screening study in the evaluation of patients for CTEPH.
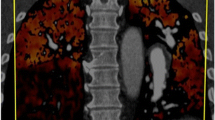
In chronic thromboembolic disease, at least one and more commonly several segmental or larger mismatched perfusion defect are present (Fig. 1). In those patients with small-vessel pulmonary vascular disease, perfusion scans either are normal or exhibit a “mottled” appearance characterized by non-segmental defects [41, 42]. It has also been observed that the magnitude of perfusion defects exhibited by CTEPH patients with operable disease may understate the degree of pulmonary vascular obstruction determined by angiography, a result of radiolabeled macroaggregated albumin traversing recanalized thrombus [43]. Therefore, CTEPH should be considered, and an evaluation for operable disease is warranted even if the V/Q scan demonstrates a limited number of mismatched perfusion defects, or regions of relative hypo-perfusion (“gray zones”). And though an abnormal perfusion scan is observed in patients with CTEPH, this finding lacks specificity. A number of other disorders affecting the proximal pulmonary vessels [44–46] may result in scan findings similar to those in chronic thromboembolic disease, and as such, further diagnostic imaging is necessary.
Catheter-based pulmonary angiography has traditionally been considered the “gold standard” for confirming the diagnosis of CTEPH and assessing the proximal extent of chronic thromboembolic disease. It has been the diagnostic study against which other modalities have been compared, especially in providing an anatomic “map” for surgery. In most circumstances, a properly performed pulmonary angiogram, including lateral projections, will provide sufficient information on which to base a decision regarding chronic thrombus location and, as a result, surgical accessibility. The angiographic appearance of chronic thromboembolic disease is distinct from the well-defined, intraluminal filling defects of acute pulmonary embolism and reflects the complex patterns of organization and recanalization after an acute event. Several angiographic patterns have been described in chronic thromboembolic disease, including “pouch defects,” pulmonary artery webs or bands, intimal irregularities, abrupt, frequently angular narrowing of the major pulmonary arteries, and complete obstruction of main, lobar, or segmental vessels at their point of origin [47]. In the majority of CTEPH patients, two or more of these angiographic findings are present, typically involving both lungs (Fig. 2).
Pulmonary angiogram demonstrating features of chronic thromboembolic disease; study obtained in the patient whose V/Q scan is pictured in Fig. 1. a Solid white arrow depicts a “pouch defect” and proximal vessel arrow in the artery leading to the apical right upper lobe; the distal descending PA narrows and is accompanied by poor perfusion of several lower lobe vessels (open white arrow). This finding is better demonstrated on lateral view (open white arrow). b The left pulmonary angiogram in the same patient demonstrates marked vessel narrowing and hypoperfusion of the posterior lower lobe artery (solid white arrow)
With the advances in computed tomography, CT angiography of the chest has played an increasing role in the evaluation for CTEPH. Findings on CTPA suggesting the presence of CTEPH include mosaic perfusion of the lung parenchyma; enlargement of the central pulmonary arteries; variability in the size of lobar and segmental-level vessels with a reduction in vessel caliber of those involved with chronic thrombi; and peripheral, scar-like lesions in poorly perfused lung regions [48]. With contrast enhancement of the pulmonary vasculature during CT imaging, organized thrombus can be seen to line the pulmonary vessels, often in an eccentric manner. Associated narrowing of pulmonary arteries, web strictures or “bands”, post-stenotic vessel dilatation, and other irregularities of the intima may also be appreciated [49, 50] (Fig. 3). These radiologic signs are distinct from the isolated finding of intraluminal filling defects observed with acute thromboemboli [48, 49]. And with appropriate timing of the intravenous contrast bolus for CTA, opacification of both the pulmonary and systemic circulation is possible. In addition to the pulmonary vascular bed, this allows examination of a number of cardiac features including cardiac chamber size, position and shape of the interventricular septum (deviated toward the left ventricle in the setting of significant RV pressure overload), the presence of congenital cardiac abnormalities, anomalous pulmonary venous drainage, and the size and distribution of collateral vessels arising from the systemic arterial circulation, such as bronchial arteries off the aorta and collaterals arising from coronary vessels [48, 51, 52]. CTPA has the additional benefit of providing information about the lung parenchyma and mediastinum. This allows a clearer definition of the extent of coexisting lung disease (such as emphysema and interstitial lung disease) which will affect operability, and the identification of mediastinal adenopathy and fibrosis, which might account for the perfusion abnormalities observed on V/Q scan, would be essential information, thereby excluding a patient from surgical consideration [48].
CT angiogram of the patient in Figs. 1 and 2. a A vascular “web” in the proximal right middle lobe (solid white arrow), and eccentric lining thrombus involving the right descending PA (open white arrow) are CT findings consistent with chronic thromboembolic disease. b Farther caudad in the same study, the irregular lining thrombus in the descending PA is associated with vessel narrowing (open white arrow). This is more pronounced in the posterior vessel to the left lower lobe (solid white arrow), the CT finding correlating with what was observed on the pulmonary angiogram in Fig. 2
Dual-energy CT (DECT) is a newer CT technique that generates a lung perfusion map and traditional CTPA images in a single acquisition. In a small single-center study evaluating the utility of DECT, 14 out of 40 patients were diagnosed with CTEPH. DECT had a sensitivity of 100 % and specificity of 92 % [53]. The main limitation to this technique is a streaking artifact that renders some studies difficult to interpret and may impact inter-observer agreement. Pulmonary blood volumes on DECT have also been shown to inversely correlate with mPAp and PVR [54] and can provide information regarding the severity of pulmonary hypertension. The utility of DECT, as well as other perfusion-enhanced CT modalities such as single-photon emission CT (SPECT), still must be confirmed in larger multicenter study to better elucidate its role in the diagnosis of CTEPH and operability assessment [53, 55].
Magnetic resonance techniques have been evaluated in the diagnosis and operability assessment of CTEPH. MR angiography has a high sensitivity for CTEPH occurring in the proximal pulmonary arteries, but the sensitivity drops as low as 57 % at the segmental and subsegmental level [56, 57]. MR perfusion imaging techniques have demonstrated better promise in the diagnosis of CTEPH. Recent data from the ASPIRE registry used MR perfusion to screen 132 patients suspected of having CTEPH. Seventy-eight patients were diagnosed with CTEPH and MRI perfusion demonstrated an overall sensitivity of 97 %, specificity 92 %, positive predictive value 95 %, and negative predictive value 96 % compared with V/Q scan (sensitivity 96 %, specificity 90 %) and CTPA (sensitivity 94 %, specificity 98 %) [58].
MR techniques have also shown significant promise in CTEPH, just as in the other WHO groups of pulmonary hypertension, to evaluate the severity of right ventricular dysfunction, right ventricular workload, and response to therapy, whether surgical, minimally invasive or medical [59–63]. Newer MR techniques in development include four-dimensional flow studies that map the direction and speed of blood flow. Mapping the blood flow vortices in the right ventricle can provide information about right ventricular diastolic dysfunction, and changes in the vortices of flow within the pulmonary arteries have been shown to change in response to therapy with balloon pulmonary angioplasty [64, 65]. Many of these newer MR techniques offer the promise of providing greater insight into disease severity and treatment selection especially in instances when symptoms are disproportionate to the severity of resting hemodynamic impairment or the degree of pulmonary vascular obstruction.
Pulmonary thromboendarterectomy
Operability assessment
Once a diagnosis of CTEPH is established, an assessment of operability is the next, and often most difficult, next step. This decision has even greater implications in a new era with approved PH-targeted medical therapy for inoperable CTEPH and an expanding role of balloon pulmonary angioplasty (BPA). PTE has been established as the treatment of choice for CTEPH patients deemed operable because of the low mortality, excellent outcomes, and potential for cure at experienced CTEPH centers [37]. In large patient series published from the USA and Europe, the mean and median PVR achieved after surgery is usually <300 dyn s cm−5 [66, 67]. As a result, every effort should be made to have patients with CTEPH evaluated at a center experienced with performing PTE surgery (Fig. 4). However, even at experienced CTEPH centers, operability assessment is often subjective, and a decision to proceed with PTE in borderline cases is accompanied by a frank discussion with patients weighing expectations of a favorable surgical outcome against associated risks.
CTEPH treatment algorithm proposed at the 5th World Symposium on Pulmonary Hypertension, Nice France, March 2013. With permission, reference [37]
Surgical success owes as much to appropriate patient selection as it does to surgical technique and postoperative management. The determination of operability relies on several key assessments: (1) the chronic thrombotic lesions are surgically accessible based on the experience of the surgical team; (2) the thrombus burden observed on imaging correlates with degree of hemodynamic impairment observed during right heart catheterization. This is particularly difficult for patients with primarily segmental and subsegmental chronic thromboembolic disease; (3) there is the anticipation that an endarterectomy will result in pulmonary hemodynamic and cardiopulmonary symptom improvement; and (4) patient comorbidities do not preclude surgical consideration.
While centers have previously reported higher mortality in patients with preoperative PVR > 1000–1200 dyn s cm−5, this finding is not a contraindication to PTE and should not limit referral to a CTEPH center for surgical evaluation. And although over the past decade overall perioperative mortality rates have declined worldwide, patients with more severe pulmonary hypertension and those with decompensated right heart failure from their chronic thromboembolic disease remain at greater risk [67]. In a recent report, the San Diego group reported a declining overall operative mortality risk of 2.2 % following thromboendarterectomy surgery in 500 patients operated between 2006 and 2010; in this same group, those patients with a preoperative PVR > 1000 dyn/s/cm−5 experienced a mortality rate of 4.1 % compared to 1.6 % in those patients with a PVR less than 1000 dyn/s/cm−5 [66]. A more recent report from de Perrot and colleagues compared post-endarterectomy outcomes in those CTEPH patients with a preoperative total pulmonary vascular resistance of greater than (N = 26) or less than (N = 78) 1200 dyn s cm−5. Overall in-hospital mortality after pulmonary endarterectomy was 4 %, with all deaths occurring in patients with a TPR > 1200 dyn s cm−5 and decompensated right heart failure on presentation [68].
As perioperative mortality rates have declined, PTE surgery is now more routinely being performed in symptomatic patients with chronic thromboembolic disease (CTED) but without pulmonary hypertension at rest [69]. Patients with CTED are thought to improve from PTE surgery through a reduction in dead space ventilation [70]. Data supporting the role of PTE surgery in CTED is limited. Recently, a CTEPH center in the UK published data from 42 patients with symptomatic CTEPH and a baseline mean PA pressure <25 mmHg who underwent PTE surgery. PTE resulted in a significant improvement in functional status and quality of life with 95 % of patients remaining NYHA class I or II at 1-year follow-up. While there was no in-hospital mortality, complications occurred in 40 % of patients [69].
The presence of comorbidities further impacts the decision to proceed with surgery. There should be the anticipation that a PTE will have a meaningful outcome for the patient undergoing this complex and technically challenging procedure. Patients with significant comorbid conditions such as severe emphysema, or those with a life-limiting malignancy, are not only at considerable perioperative risk, but are unlikely to realize the functional status benefit that would be achieved with a PTE. Based on data from the European CTEPH registry, coronary artery disease increases the in-hospital and 1-year mortality associated with the surgery from 2.1 to 10 % and 5.1 to 15 %, respectively [67]. Advanced age by itself is not a contraindication to surgery. Over a 5-year period, Berman and colleagues reported a comparable in-hospital mortality rate of 4.6 % (14 of 308) for patients <70 years old compared to 7.8 % (8 of 103) for those patients >70 years. However, total hospital days and ICU days were greater in the older patient group [71]. Other factors that make the surgery technically more difficult but have not been shown to increase mortality include elevated BMI [72], taller patients, and the presence of prior sternotomy.
Outcomes: pulmonary thromboendarterectomy surgery
Since its inception, pulmonary thromboendarterectomy surgery has evolved significantly [1]. However, the fundamental elements of the procedure have remained little changed over the past two decades. Currently, a thromboendarterectomy necessitates a median sternotomy, cardiopulmonary bypass, and deep hypothermia (for tissue protection) with circulatory arrest periods in order to provide a bloodless surgical field. This allows for an optimal visual field to facilitate as complete a dissection as possible [73].
Recent reports of in-hospital mortality rates for PTE surgery are 2.2 % [66] at a single US referral center and 4.7 % [67] across multiple European centers. Long-term survival following PTE is excellent. For patients who survive to hospital discharge, survival rates of 92.5 % at 5 years and 88.3 % at 10 years have been reported in the UK. More recent data from the same center showed similar results with a 5-year survival rate of 90.0 % [74]. Other centers have demonstrated similar long-term survival results with reports from the Netherlands demonstrating 1-, 3-, and 5-year survival rates of 93.1, 91.2, and 88.7 %, respectively [75].
PTE performed at experienced centers usually results in an immediate and sustained improvement in hemodynamics. Data published from CTEPH centers around the world have reported similar improvements in hemodynamics with improvements of PVR from >800 dyn to values below 400 [76]. Results are similarly robust for improvements in CO/CI and mPAp. Most importantly, the hemodynamic improvement is sustained on long-term follow-up. Data from the UK have shown that prior to PTE, 66 % of patients were functional class III or IV and 88 % of patients had improved to functional class I or II at 12-month follow-up [77]. Mean improvement in 6-min walk test following PTE of 103 ± 22.7 m was also sustained at 12-month follow-up. Data from Italy have shown similar results with a sustained functional improvement over a 4-year period with 97 % of patients NYHA III or IV prior to PTE and 74 % improved to functional class I at 4-year follow-up [78].
And as evolving surgical techniques have allowed more distal resection of chronic thromboembolic disease, immediate and long-term results have been similarly impressive (Fig. 5). In a 2014 report from D’Armini and colleagues, of 331 endarterectomies performed, 110 were classified as distal resection. Overall in-hospital mortality rate was 6.9 % with no significant difference between groups, immediate pulmonary hemodynamic improvements were comparable (PVR 876 ± 392 to 251 +146 dyn s cm−5 in the proximal group vs. 926 ± 337 to 295 ± 161 dyn s cm−5 in the distal resection group), and the sustained hemodynamic benefit at 12-month follow-up was similar in both patient groups [79]. This report supports the benefit of PTE surgery for distal (segmental) resection; however, it should be emphasized that an endarterectomy performed at this level requires considerable surgical expertise.
Endarterectomy specimen obtained from the patient whose studies are pictured in Figs. 1, 2, and 3. Semi-organized thrombus was obtained from the right descending PA in addition to the extensive chronic thrombotic material pictured. On the left, the endarterectomized material from the upper lobe, lingula, and lower lobe originated primarily from the segmental level
Though the definition of residual pulmonary hypertension varies between reporting centers, there are patients who do not achieve normal pulmonary pressures and right heart function following PTE surgery. Occurrence estimates vary between 5 and 35 % of operated patients [74, 77, 78, 80, 81], though long-term information is lacking as to what level of residual pulmonary hypertension negatively impacts functional status and survivorship, and therefore requires PH-targeted medical therapy. In a recent report, residual PH after PTE surgery negatively impacted in-hospital mortality. In 500 consecutive cases performed at UCSD between 2006 and 2010, mortality was 10.3 % for patients with a postoperative PVR > 500 dyn s cm−5 compared with 0.9 % for patients with a postoperative PVR < 500 dyn s cm−5 [66].
Non-surgical approach to CTEPH
PH-targeted medical therapy
Despite the evolution of PTE surgery, for patients who are not surgical candidates and not candidates for balloon pulmonary angioplasty, there is a role for pulmonary hypertension-targeted medical therapies [82]. The use of pulmonary hypertension-targeted medical therapy in CTEPH is widespread and generally occurs in one of three categories of patients: (1) patients who are deemed inoperable or not a suitable candidate for PTE surgery; (2) patients with residual pulmonary hypertension following PTE surgery; and (3) patients being “bridged” with therapy until they can be evaluated for PTE at a CTEPH center.
Up to 37 % of patients in a European and Canadian registry of CTEPH centers were deemed inoperable and were not offered PTE surgery [4]. There was a wide variation among centers however ranging from 12 to 61 %, and fewer patients were declined surgery at more experienced centers. The most common reason (48 %) for not offering surgery was clot deemed inaccessible to surgery. Other reasons included comorbidities (13 %) and discordance between clot burden and hemodynamic impairment.
Despite widespread use, only three PH-targeted therapies, sildenafil, riociguat, and bosentan, have been studied in randomized controlled fashion. In addition, large-scale trials on the impacts of therapy prior to PTE surgery, or “bridge” therapy, have not yet been completed. Smaller trials examining this treatment strategy have shown acute improvements in hemodynamics and measurements of right ventricular function in the preoperative period but no effects on postoperative hemodynamics or other outcome measures.
Of the PH-targeted therapies, only the guanylate cyclase stimulator, riociguat, is approved for patients with CTEPH and only for patients with inoperable disease or residual pulmonary hypertension following PTE surgery. In the 16-week randomized, placebo-controlled trial CHEST-1, 261 patients with inoperable CTEPH or residual pulmonary hypertension following PTE surgery were treated over a 16-week period with riociguat or placebo [83]. Patients treated with riociguat had an improvement in 6-min walk distance of 39 m compared to a 6-m decrease in the placebo arm. There was also an improvement in hemodynamics, WHO functional status, and NT-proBNP levels. Data from the open-label, long-term extension of this trial confirmed the durable exercise and functional status benefit of this medication for up to a year, with a similar safety profile achieved in CHEST-1 [84].
The use of endothelin receptor antagonists (ERA) in CTEPH is common and occurs in up to 20 % of patients at the time of PTE evaluation according to data from a single US center. In the BENEFiT trial, 157 inoperable patients or patients with residual pulmonary hypertension following PTE were randomized to bosentan or placebo. The study failed to meet the primary end point, an improvement in 6-min walk distance, but did demonstrate a 24 % reduction in PVR and improvement in functional class in the treatment arm [85]. The MERIT study, examining the effects of newer ERA, macitentan, in patients with inoperable CTEPH, is currently underway.
The phosphodiesterase inhibitor (PDE5) sildenafil was studied in a small, randomized controlled study of 19 patients and in a larger open-label study of 104 patients. The effect on 6-min walk distance was mixed, but improvements in PVR and quality of life were observed [86]. In small, uncontrolled studies, all of the currently available prostacylin (PGI2)-based therapies including epoprostenol, iloprost, and trepsotinil have demonstrated improvements in hemodynamics and functional status [87–89].
The improvements achieved with medical therapy are more modest than what are achieved with PTE surgery, and medical therapy should not be used as an alternative to surgery in patients felt to have operable CTEPH or delay a referral to a CTEPH center for surgical evaluation [90].
Balloon pulmonary angioplasty
Balloon pulmonary angioplasty (BPA) has emerged recently as a potentially effective therapy to treat CTEPH. In simplest terms, this procedure identifies areas of vascular obstruction in the pulmonary arteries using selective angiography, and balloon dilates these lesions with similar well-established angioplasty techniques used in other vascular locations. In practice, it has taken more than 20 years to refine the technique, optimize patient selection and vessel selection to match the safety and efficacy achieved with PTE surgery at experienced centers.
The first case report of BPA was published in 1988 [91], and it was not until 13 years later in 2001 that a larger case series was published describing 18 patients who underwent BPA [92]. Patients required a median of three procedures and achieved a reduction in mPAp from 43.0 ± 12.1 to 33.7 ± 10.2 mmHg (P = 0.007), an improvement in 6-MW distance from 209 to 497 yards (P < 0.0001) and an improvement in functional class from 3.3 to 1.8 (P < 0.001). However, 11 (61 %) patients suffered lung injury in the treated lung segments, three (16.7 %) of which required mechanical ventilation and one (5.6 %) patient died in the immediate post-procedure period. While benefits of the procedure were clearly demonstrated in this series, the complication rate and mortality compared to PTE surgery was too high to justify widespread adoption of the procedure.
For this reason, refinement of the BPA technique has occurred in locations around the world where access to experienced PTE surgical centers capable of distal endarterectomy has been limited. In 2012, three publications from medical centers in Japan generated a renewed enthusiasm for the role of BPA in the treatment for CTEPH [93–95]. The largest of the three series included 68 patients who were deemed inoperable for PTE surgery based on comorbidities and lesion location. Following a median of four sessions, mPAp decreased from 45.4 ± 9.6 to 24.0 ± 6.4 mmHg (P < 0.01), and improvements in 6MWD and NYHA functional class were also observed [93]. In all three reports, lung injury complications including reperfusion edema, vascular rupture, and hemoptysis were observed in 50–70 % of patients.
The data from Japan were followed by a case series from Norway in 2013 of 21 patients deemed poor candidates for PTE who underwent BPA. As observed in the series from Japan, there were significant improvements in hemodynamics, NYHA functional class, pro-BNP levels, and cardiopulmonary exercise testing after BPA [96]. While rates of reperfusion lung injury were lower at 35 %, mortality was 10 %.
In a relatively short period of time, the procedure has been further refined with improved techniques to image diseased segments, size balloons, measure pressure gradients, and predict the likelihood of lung injury. In a series from Japan, 140 consecutive BPA procedures in 54 patients were used to generate a scoring index to predict lung injury [97]. Notably, the incidence of clinically significant lung injury following BPA was only 6 %, and the mortality was 1.9 %. While the specific BPA techniques still vary between centers, the complication rate and mortality mirrors this trend with routine complication and mortality rates now <5 % [98].
BPA has also been performed and proven safe and effective in patients with residual pulmonary hypertension following PTE surgery [99]. While interest in BPA grows, so does the need to better define its role in the treatment for CTEPH, particularly in locations where PTE surgery is routinely performed. Additionally, little is known about how BPA affects the ability to perform successful PTE surgery. Concerns center around the potential disruption of the surgical plane during BPA, thereby increasing the difficulty of surgery should PTE be necessary following this procedure.
References
Daily PO, Auger WR (1999) Historical perspective: surgery for chronic thromboembolic disease. Sem Thorac Cardiovasc Surg 11(2):143–151
Pepke-Zaba J, Hoeper MM, Humbert M (2013) Chronic thromboembolic pulmonary hypertension: advances from bench to patient management. Eur Resp J 41:8–9
Simonneau G, Gatzoulis MA, Adatia I et al (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62:D34–D41
Pepke-Zaba J, Delcroix M, Lang I et al (2011) Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 124:1973–1981
Pengo V, Lensing ASA, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P (2004) Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 350:2257–2264
Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, Dainelli A, Giuntini C (2006) Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine 85:253–262
Becattini C, Agnelli G, Pesavento R, Silingardi M, Poggio R, Taliani MR, Ageno W (2006) Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 130:172–175
Dentali F, Donadini M, Gianni M, Bertolini A, Squizzato A, Venco A, Ageno W (2009) Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res 124:256–258
Klok FA, van Kralingen KW, van Dijk APJ, Heyning FH, Vliegen HW (2010) Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acutepulmonary embolism. Haematologica 95:970–975
Korkmaz A, Ozlu T, Ozsu S, Kazaz Z, Bulbul Y (2012) Long-term outcomes in acute pulmonary thromboembolism: the incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost 18:281–288
Guerin L, Couturaud F, Parent F et al (2014) Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Haemost 112:598–605
Bonderman D, Turecek PL, Jakowitsch J, Weltermann A, Adlbrecht C, Schneider B, Kneussl M, Rubun LJ, Kyrle PA, Klepetko W, Maurer G, Lang IM (2003) High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost 90:372–376
Wong CL, Szydio R, Gibbs S, Laffan M (2010) Hereditary and acquired thrombotic risk factors for chronic thromboembolic pulmonary hypertension. Blood Coagul Fibrinolysis 21:201–206
Bonderman D, Wilkens H, Wakounig S, Schafers H-J, Jansa P, Linder J, Simkova I, Martischnig AM, Dudczak J, Sadushi R, Skoro-Sajer N, Klepetko W, Lang IM (2009) Risk factors for chronic thromboembolic pulmonary hypertension. Eur Resp J 33:325–331
O’Donnell J, Laffan MA (2001) The relationship between ABO histo-blood group, factor VIII and von Willebrand factor. Transfus Med 11:343–351
Homoncik M, Gessl A, Ferlitsch A, Jilma B, Vierhapper H (2007) Altered platelet plug formation in hyperthyroidism and hypothyroidism. J Clin Endocrinol Metab 92:3006–3012
Lang IM, Pesavento R, Bonderman D, Yuan JX-J (2013) Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Resp J 41:462–468
Quarck R, Wynants M, Verbeken E, Meyns B, Delcroix M (2015) Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur Resp J 46:431–443
Bernard J, Yi ES (2007) Pulmonary thromboendarterectomy: a clinicopathologic study of 200 consecutive pulmonary thromboendarterectomy cases in one institution. Hum Pathol 38:871–877
Wynants M, Quarck R, Ronisz A, Alfaro-Moreno E, Van Raemdonck D, Meyns B, Delcroix M (2012) Effects of C-reactive protein on human pulmonary vascular cells in chronic thromboembolic pulmonary hypertension. Eur Resp J 40:886–894
Morris TA, Marsh JJ, Chiles PG, Magana MM, Liang NC, Soler X, Desantis DJ, Ngo D, Woods VL Jr (2009) High prevalence of dysfibrinogenemia among patients with chronic thromboembolic pulmonary hypertension. Blood 114:1929–1936
Zabini D, Nagaraj C, Stacher E, Lang IM, Nierlich P, Klepetko W, Heinemann A, Olschewski H, Balint Z, Olschewski A (2012) Angiostatic factors in the pulmonary endarterectomy material from chronic thromboembolic pulmonary hypertension patients cause endothelial dysfunction. PLoS One 7(8):e43793
Sakao S, Hao H, Tanabe N, Kasahara Y, Kurosu K, Tatsumi K (2011) Endothelial-like cells in chronic thromboembolic pulmonary hypertension: crosstalk with myofibroblast-like cells. Resp Res 12:109
Firth AL, Yao W, Ogawa A, Madani MM, Lin GY, Yuan JX (2010) Multipotent mesenchymal progenitor cells are present in endarterectomized tissues from patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Cell Physiol 298:C1217–C1225
Moser KM, Bloor CM (1993) Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 103:685–692
Dorfmūller P, Gūnther S, Ghigna M-R, de Montpréville BoulateD, Paul J-F, Jaïs X, Decante B, Simonneau G, Darteville P, Humbert M, Fadel E, Mercier O (2014) Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Resp J 44(5):1275–1288
McCann C, Gopalan D, Sheares K, Screaton N (2012) Imaging in pulmonary hypertension, part 2: large vessel diseases. Postgrad Med J 88:317–325
Morris TA, Auger WR, Ysrael MZ, Olson LK, Channick RN, Fedullo PF, Moser KM (1996) Parenchymal scarring is associated with restrictive spirometric defects in patients with chronic thromboembolic pulmonary hypertension. Chest 110:399–403
Steenhuis LH, Groen HJM, Koeter GH, van der Mark TW (2000) Diffusion capacity and haemodynamics in primary and chronic thromboembolic pulmonary hypertension. Eur Respir J 16:276–281
Kapitan KS, Buchbinder M, Wagner PD, Moser KM (1989) Mechanisms of hypoxemia in chronic thromboembolic pulmonary hypertension. Am Rev Respir Dis 139:1149–1154
Held M, Grün M, Kaiser R, Wilkens H, Jany BH (2014) Cardiopulmonary exercise testing to detect pulmonary hypertension in patients with suspected chronic thromboembolic pulmonary hypertension and normal echocardiography. Respiration 87:379–387
Held M, Hesse A, Gött F et al (2014) A symptom-related monitoring program following pulmonary embolism for the early detection of CTEPH: a prospective observational registry study. Pulm Med 14:141–148
Raisinghani A, Ben-Yehuda O (2006) Echocardiography in chronic thromboembolic pulmonary hypertension. Semin Thorac Cardiovasc Surg 18:230–235
Blanchard DG, Malouf PJ, Gurudevan SV et al (2009) Utility of right ventricular Tei index in the noninvasive evaluation of chronic thromboembolicpulmonary hypertension before and after pulmonary thromboendarterectomy. JACC Cardiovasc Imaging 2(2):143–149
Xie Y, Burke BM, Kopelnik A et al (2014) Echocardiographic estimation of pulmonary vascular resistance in chronic thromboembolic pulmonary hypertension: utility of right heart doppler measurements. Echocardiography 31:29–33
Marston N, Brown JP, Olson N et al (2015) Right ventricular strain before and after pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Echocardiography 32:1115–1121
Kim NH, Delcroix M, Jenkins DP, Channick R, Darteville P, Jansa P, Lang I, Madani MM, Ogino H, Pengo V, Mayer E (2013) Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 62:D92–D99
Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P, Al-Nahhas A (2007) Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic disease as a treatable cause of pulmonary hypertension. J Nucl Med 48:680–684
He J, Fang W, Lv B, He JG, Xiong CM, Liu ZH, He ZX (2012) Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun 33(5):459–463
Sugiura T, Tanabe N, Matsuura Y, Shigeta A, Kawata N, Jujo T, Yanagawa N, Sakao S, Kasahara Y, Tatsumi K (2013) Role of 320-slice computed tomography in the diagnostic workup of patients with chronic thromboembolic pulmonary hypertension. Chest 143:1070–1077
Lisbona R, Kreisman H, Novales-Diaz J, Derbekyan V (1985) Perfusion lung scanning: differentiation of primary from thromboembolic pulmonary hypertension. Am J Roentgenol 144:27–30
Powe JE, Palevsky HI, McCarthy KE, Alavi A (1987) Pulmonary arterial hypertension: value of perfusion scintigraphy. Radiology 164:727–730
Ryan KL, Fedullo PF, Davis GB, Vasquez TE, Moser KM (1998) Perfusion scan findings understate the severity of angiographic and hemodynamic compromise in chronic thromboembolic pulmonary hypertension. Chest 93:1180–1185
Kerr KM, Auger WR, Fedullo PF, Channick RN, Yi ES, Moser KM (1995) Large vessel pulmonary arteritis mimicking chronic thromboembolic disease. Am J Respir Crit Care Med 152:367–373
Rossi SE, McAdams HP, Rosado-de-Christenson ML, Franks TJ, Galvin JR (2001) Fibrosing mediastinitis. Radiographics 21:737–757
Kerr KM (2005) Pulmonary artery sarcoma masquerading as chronic thromboembolic pulmonary hypertension. Nat Clin Pract Cardiovasc Med 2:108–112
Auger WR, Fedullo PF, Moser KM, Buchbinder M, Peterson KL (1992) Chronic major- vessel chronic thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology 183:393–398
Wijesuriya S, Chandratreya L, Medford AR (2013) Chronic pulmonary emboli and radiologic mimics on CT pulmonary angiography. A diagnostic challenge. Chest 143:1460–1471
Doǧan H, de Roos A, Geleijins J, Huisman MV, Kroft LJM (2015) The role of computed tomography in the diagnosis of acute and chronic pulmonary embolism. Diagn Interv Radiol 21:307–316
Willemink MJ, van Es HW, Koobs L, Morshuis WJ, Snijder RJ, van Heesewijk JPM (2012) CT evaluation of chronic thromboembolic pulmonary hypertension. Clin Radiol 67:277–285
McKie SJ, Hardwick DJ, Reid JH, Murchison JT (2005) Features of cardiac disease demonstrated on CT angiography. Clin Radiol 60:31–38
Lee NS, Blanchard DG, Knowlton KU, McDivitt AM, Pretorius V, Madani MM, Fedullo PF, Kerr KM, Kim NH, Poch DS, Auger WR, Daniels LB (2015) Prevalence of coronary artery-pulmonary artery collaterals in patients with chronic thromboembolic pulmonary hypertension. Pulm Circ 5(2):313–321
Dournes G, Verdier D, Montaudon M et al (2014) Dual-energy CT perfusion and angiography in chronic thromboembolic pulmonary hypertension: diagnostic accuracy and concordance with radionuclide scintigraphy. Eur Radiol 24:42–51
Meinel FG, Graef A, Thierfelder KM et al (2013) Automated quantification of pulmonary perfused blood volume by dual-energy CTPA in chronic thromboembolic pulmonary hypertension. Rofo 186:151–156
Grgic A, Miodek F, Schäfers H-J et al (2016) Assessment of operability by means of CTPA and perfusion SPECT in patients with chronic thromboembolic pulmonary hypertension. Acta Radiol 57(1):33–40
Ley S, Ley-Zaporozhan J, Pitton MB, Schneider J, Wirth GM, Mayer E, Duber C, Kreitner K-F (2012) Diagnostic performance of state-of-the-art imaging techniques for morphological assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol 22:607–616
Nikolaou K, Schoenberg SO, Attenberger U, Scheidler J, Dietrich O, Kuehn B, Rosa F, Huber A, Leuchte H, Baumgartner R, Behr J, Reiser MF (2005) Pulmonary arterial hypertension: diagnosis with fast perfusion imaging and high-spatial- resolution MR angiography—preliminary experience. Radiology 236:694–703
Rajaram S, Swift AJ, Capener D, Telfer A et al (2013) 3D contrast-enhanced ling perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: results from the ASPIRE Registry. Thorax 68:677–678
Rolf A, Rixe J, Kim WK et al (2014) Right ventricular adaptation to pulmonary pressure load in patients with chronic thromboembolic pulmonary hypertension before and after successful pulmonary endarterectomy—a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 16:96
Fukui S, Ogo T, Morita Y et al (2014) Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J 43:1394–1402
Kreitner K-F, Ley S, Kauczor H-U, Mayer E, Kramm T, Pitton MB, Krummenauer F, Thelen M (2004) Chronic thromboembolic pulmonary hypertension: pre- and postoperative assessment with breath-hold magnetic resonance techniques. Radiology 32:535–543
Claessen G, La Gerche A, Dymarkowski S et al (2015) Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc 4(3):e001601–e001602. doi:10.1161/JAHA.114.001602
Ohno Y, Koyama H, Yoshikawa T et al (2012) Contrast-enhanced multidetector-row computed tomography vs. time-resolved magnetic resonance angiography vs. contrast-enhanced perfusion MRI: assessment of treatment response by patients with inoperable chronic thromboembolic pulmonary hypertension. J Magn Reson Imaging 36:612–623
Ota H, Sugimura K, Miura M, Shimokawa H (2015) Four-dimensional flow magnetic resonance imaging visualizes drastic change in vortex flow in the main pulmonary artery after percutaneous transluminal pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Eur Heart J 36(25):1630. doi:10.1093/eurheartj/ehv054
Fenster BE, Browning J, Schroeder JD et al (2015) Vorticity is a marker of right ventricular diastolic dysfunction. Am J Physiol Heart Circ Physiol. doi:10.1152/ajpheart.00278.2015
Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NK, Fedullo PF, Jamieson SW (2012) Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2700 patients. Ann Thorac Surg 94:97–103
Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G, Darteville P (2011) Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 141:702–710
de Perrot M, Thenganatt J, McRae K, Moric J, Mercier O, Pierre A, Mak S, Granton J (2015) Pulmonary endarterectomy in severe chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 34:369–375
Taboada D, Pepke-Zaba J, Jenkins DP, Berman M, Treacy CM, Cannon JE, Toshner M, Dunning JJ, Ng C, Tsui SS, Sheares KK (2014) Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Resp J 44(6):1635–1645
van der Plas MN, Reesink HJ, Roos CM (2010) Pulmonary endarterectomy improves dyspnea by the relief of dead space ventilation. Ann Thorac Surg 89:347–352
Berman M, Hardman G, Sharples L, Pepke-Zaba J, Sheares K, Tsui S, Dunning J, Jenkins DP (2012) Pulmonary endarterectomy: outcomes in patients aged >70. Eur J Cardio-Thorac Surg 41:e154–e160
Fernandes TM, Auger WR, Fedullo PF, Kim NH, Poch DS, Madani MM, Pretorius VG, Jamieson SW, Kerr KM (2014) Baseline body mass index does not significantly affect outcomes after pulmonary thromboendarterectomy. Ann Thorac Surg 98:1776–1781
Madani MM, Jamieson SW (2006) Technical advances of pulmonary endarterectomy for chronic thromboembolic disease. Semin Cardiovasc Thorac Surg 18:243–249
Freed DH, Thomson BM, Berman M, Tsui SSL, Dunning J, Sheares KK, Pepke-Zaba J, Jenkins DP (2011) Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg 141:383–387
Saouti N, Morshuis WJ, Heijmen RH, Snijder RJ (2009) Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: a single institution experience. Eur J Cardiothorac Surg 35:947–952. doi:10.1016/j.ejcts.2009.01.023 (discussion 952)
Rahnavardi M, Yan TD, Cao C, Vallely MP, Bannon PG, Wilson MK (2011) Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: a systematic review. Ann Thorac Cardiovasc Surg 17:435–445
Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, Hodgkins D, Goldsmith K, Hughes RJ, Sheares K, Tsui SSL, Armstrong IJ, Torpy C, Crackett R, Carlin CM, Das C, Coghlan JG, Pepke-Zaba J (2008) Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 177:1122–1127
Corsico AG, D’Armini AM, Cerveri I, Klersy C, Ansaldo E, Niniano R, Gatto E, Monterosso C, Morsolini M, Nicolardi S, Tramontin C, Pozzi E, Vigano M (2008) Long term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med 178:419–424
D’Armini AM, Morsolini M, Mattiucci G, Grazioli V, Pin M, Valentini A, Silvaggio G, Klersy C, Dore R (2014) Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 148(3):1005–1011
Bonderman D, Skoro-Sajer N, Jakowitsch J, Adlbrecht C, Dunkler D, Taghavi S, Klepetko W, Kneussl M, Lang IM (2007) Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 115:2153–2158
Thistlethwaite PA, Kemp A, Du L, Madani MM, Jamieson SW (2006) Outcomes of pulmonary thromboendarterectomy for treatment of extreme thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 131:307–313
Bresser P, Pepke-Zaba J, Jaïs X, Humbert M, Hoeper MM (2006) Medical therapies for chronic thromboembolic pulmonary hypertension. An evolving paradigm. Proc Am Thorac Soc 3:594–600
Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C (2013) Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 369:319–329
Simonneau G, D’Armini AM, Ghofrani H-A, Grimminger F, Hoeper MM, Jansa P, Kim NH, Wang C, Wilkins M, Fritsch A, Davie N, Colorado P, Mayer E (2015) Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: along-term extension study (CHEST-2). Eur Respir J 45:1293–1302
Jais X, D’Armini A, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zapa J, Perchenet L, Morganti A, Simonneau G, Rubin LJ (2008) Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 52:2127–2134
Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, Sheares KK, Hughes R, Morrell NW, Pepke-Zaba J (2008) Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 134:229–236
Cabrol S, Souza R, Jais X, Fadel E, Ali RHS, Humbert M, Darteville P, Simonneau G, Sitbon O (2007) Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 26:357–362
Krug S, Hammerschmidt S, Pankau H, Wirtz H, Seyfarth HJ (2008) Acute improved hemodynamics following inhaled iloprost in chronic thromboembolic pulmonary hypertension. Respiration 76:154–159
Skoro-Sajer N, Bonderman D, Wiesbauer F, Harja E, Jakowitsch J, Klepetko W, Kneussl MP, Lang IM (2007) Treprostinil for severe inoperable chronic thromboembolic pulmonary hypertension. J Thromb Haemost 5:483–489
Jensen KW, Kerr KM, Fedullo PF, Kim NH, Test VJ, Ben-Yehuda O, Auger WR (2009) Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation 120:1248–1254
Voorburg JAI, Cats VM, Buis B, Bruschke AVG (1988) Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest 94:1249–1253
Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ (2001) Balloon angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 103:10–13
Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H (2012) Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 5:748–755
Katoaka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, Tamura Y, Ando M, Fukuda K, Yoshino H, Satoh T (2012) Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 5:756–762
Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, Tatebe S, Miyamichi-Yamamoto S, Shimokawa H (2012) Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 76:485–488
Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R (2013) Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 99:1415–1420
Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Taguchi H, Fukuda K, Yoshino H, Satoh T (2013) Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc Interv 7:725–736
Taniguchi Y, Miyagawa K, Nakayama K, Kinutani H, Shinke T, Okada K, Okita Y, Hirata KI, Emoto N (2014) Ballon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. Eurointervention 10:518–525
Shimura N, Kataoka M, Inami T, Yanagisawa R, Ishiguro H, Kawakami T, Higuchi Y, Ando M, Fukuda K, Yoshino H, Satoh T (2015) Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 183:138–142
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. David Poch has served as a speaker on behalf of Bayer Pharmaceuticals.
Additional information
Drs. David S. Poch and William R. Auger have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Poch, D.S., Auger, W.R. Chronic thromboembolic pulmonary hypertension: detection, medical and surgical treatment approach, and current outcomes. Heart Fail Rev 21, 309–322 (2016). https://doi.org/10.1007/s10741-015-9518-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-015-9518-3