Abstract
Aortic valve disease commonly presents with symptoms of congestive heart failure. The medical management of these patients is associated with poor outcomes. Aortic valve replacement improves outcomes. However, patient selection and preoperative evaluation is challenging. Advance age, associated comorbidities, and frailty may limit their life expectancy in addition to the cardiac disease. These factors should be carefully considered to risk stratify and guide the decision to perform surgery. This chapter reviews the evaluation, management, and outcomes of patients with congestive heart failure secondary to aortic valve disease with normal and low left ventricular ejection fraction.
The advent of transcatheter aortic valve replacement promises to revolutionize the way these patients are managed, offering an alternative treatment to patients who previously were considered not candidates for surgery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Congestive heart failure
- Aortic valve disorders
- Aortic stenosis
- Aortic regurgitation
- Surgery
- Transcatheter aortic valve replacement
- Preoperative evaluation
- Outcomes
Introduction
Congestive heart failure (CHF) is a common manifestation of aortic stenosis and aortic regurgitation [1, 2]. CHF in the setting of aortic valve disease carries a dismal prognosis. Patients with aortic stenosis (AS) and CHF have an expected survival of less than 2 years when treated medically [3]. More contemporary data demonstrated that 1-year mortality is 50 % and at 10 years 98 % of patients are death [4, 10] (Table 10.1). Patients with heart failure symptoms secondary to aortic regurgitation (AR) also have a dismal prognosis. The expected 3-year survival is only 50 % [11].
Aortic valve surgery is a well-established and reproducible procedure that is associated with low peri-procedure morbidity and mortality, symptomatic improvement, and improvement in long-term survival [1, 12]. In spite of its safety and benefits, a large proportion of patients with CHF secondary to aortic valve disorders don’t have surgery. Reasons for no intervention include too advanced cardiac disease, advanced age, presence of comorbidities, and short life expectancy [2, 13]. The notion that surgery is associated with prohibitively high operative risk and no significant clinical improvement in patients with advanced heart failure secondary to aortic valve disease dissuade many practitioners to recommend aortic valve replacement (AVR). In this chapter we review the indications for surgical management and the outcomes of patients with advance heart failure symptoms (NYHA class III–IV) and left ventricular dysfunction (LVEF ≤35 %) secondary to aortic valve stenosis and regurgitation. Notwithstanding their high operative risk, most of these patients benefit form AVR. AVR improve their symptoms, cardiac function, and long-term survival compared to medical management.
Aortic Stenosis and Congestive Heart Failure
Aortic stenosis is a disease of the elderly [14, 15]. It is estimated that 2.8 % of the population older than 70 years have aortic stenosis [14, 15]. Of them, 40–60 % have class III–IV symptoms and only one third of patients with LVEF ≤35 % have AVR [2, 8, 16].
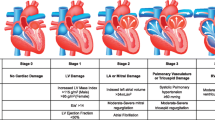
Aortic stenosis leads to left ventricular outflow obstruction and chronic pressure overload of the left ventricle. The LV hypertrophies in order to decrease wall stress. The magnitude and adequacy of that hypertrophy and the associated changes in systolic ventricular function determine the clinical presentation, hemodynamic characteristics, response to treatment, and prognosis [17–20] (Fig. 10.1). Aortic stenosis can lead to heart failure symptom by several mechanisms: (1) Diastolic dysfunction: it is the result of LV hypertrophy, increased wall thickness and decreased LV volume to mass ratio. LV end diastolic pressure (LVEDP) is increases from diminished compliance and not from systolic failure [21–24]. (2) Systolic dysfunction secondary to afterload mismatch: if the hypertrophic process is inadequate to compensate for the increased afterload, wall stress increases and the ejection fraction falls. This condition is called “afterload mismatch” and limits fiber shortening [18–21]. There are two subgroups in this category: (a) patients that preserve their stroke volume and therefore their transaortic gradients are elevated and (b) patients on whom the stroke volume diminishes and therefore the transaortic gradient is low. This last group is difficult to differentiate from the next one. (3) Systolic dysfunction secondary to intrinsic myocardial dysfunction: Persistently elevated wall stress, inadequate blood supply, and superimposed ischemia or infarction, myocardial fibrosis, and abnormalities of calcium handling further depress myocardial contractility. As before, these patients have diminished stroke volume and low transvalvular gradients but the benefits of surgery are less well established [20, 21]. If myocardial dysfunction is secondary to afterload mismatch, AVR is associated with good outcomes. If intrinsic myocardial dysfunction predominates, the response to AVR is less favorable with higher operative mortality and less LVEF improvement after AVR [18, 20, 25, 26]. Nevertheless their less favorable outcome with AVR, these patients have a significantly better prognosis with surgery that with medical management.
CHF Secondary to Aortic Stenosis with Normal Left Ventricular Function and Normal Stroke Volume
If the LV hypertrophy is adequate, the wall stress normalizes and the left ventricular function is maintained (Fig. 10.1) [18, 19, 21]. These patients have normal left ventricular function as evidenced by a normal stroke volume and ejection fraction. The transvalvular gradient is elevated. LVEDP is elevated secondary to decreased compliance from diastolic dysfunction and increased afterload.
They respond very well to aortic valve replacement. The surgical risk is low [1, 12]. Risk adjusted operative mortality is 2.3 % and has steadily declined over the last 10 years [1]. The operative mortality increases with the severity of the symptoms and lower LVEF [1, 12]. Patients with congestive heart failure symptoms have an operative mortality of 4.4 % vs. 1.6 % on those without [1]. Operative mortality in patients with a LVEF ≥30 % is 2.4 % vs. 5.2 % if LVEF <30 % [1].
AVR effectively relieves symptoms and improves quality of life [27]. Long-term survival is similar to that expected for an age and sex matched population for patients with normal LVEF, but there is an excess mortality for patients with NYHA class III–IV symptoms [27–29]. Contemporary series have demonstrated that AVR can be performed with no operative mortality and 1 and 3-year survival of 97 and 94 % respectively [30]. Mihaljevic demonstrated in 3,049 patients operated for aortic stenosis that 5-year survival for patients with no LV dysfunction was 80 %. However, for those in NYHA class III–IV, 5-year survival was 65–70 %[28]. The New York State database demonstrated that 30-month survival for patients with EF >40 % was 87.5 % and with CHF was 83.4 % [31].
AVR decreases ventricular afterload and is associated with improved LVEF, regression of LV hypertrophy, and LV mass [25, 32, 33]. Sharma described that LVEF improved by 6.8 EF points after AVR. The improvement was evident at 6 months and was maintained for up to 10 years after surgery (EF 56 ± 4 % preoperatively, 63 ± 3 % at 0–6 months, 63 ± 5 % at 7–24 months, and 63 ± 4 at 25–120 months) [34]. Some studies showed no change in LVEF after AVR in patients with normal LVEF. LV mass regression was more marked in the first 6 months after surgery and maintained for up to 10 years (181 ± 26 g/m2 preoperative vs. 124 ± 27 g/m2 at 6 months, 117 ± 15 g/m2 at 24 months, and 113 ± 14 g/m2 at 120 months after AVR) [34].
CHF Secondary to Aortic Stenosis with Normal Left Ventricular Function and Low Transvalvular Gradient (AVA ≤0.8 cm2, EF ≥50 %, Mean Aortic Valve Gradient <40 mmHg) (Table 10.2)
These patients have more hypertrophy than the necessary to compensate for the increased afterload and wall stress (Fig. 10.1) [21]. This group represents 9–35 % of patients with severe AS and normal LVEF [8, 35–38]. They are commonly overlooked in clinical practice. Since they have preserved LVEF and low transvalvular gradient, the small AVA is often attributed to calculation error [35]. The severity of their stenosis is erroneously underestimated [35]. Therefore, they are 40–50 % less likely to be referred to surgery [35, 38].
These patients are often elderly females, have severe left ventricular hypertrophy, thicker ventricles, smaller left ventricular cavities with a restrictive filling pattern (diastolic dysfunction) and intrinsic myocardial dysfunction secondary to myocardial fibrosis [8, 35, 37, 39, 40]. The low transvalvular gradient results from decreased flow across the aortic valve secondary to low stroke volume or prolonged systolic ejection period [36]. These patients are in more advanced stages of their disease and have worse prognosis than patients with normal EF and high gradient aortic stenosis [38, 39].
Symptomatically the majority of these patients are in NYHA functional class III–IV [36, 37].
Several studies have demonstrated that these patients have better survival when treated with AVR compared to medical management (Table 10.2). The operative mortality is between 2.7 % and 18 %. These patients are predisposed to low cardiac output postoperatively given their severe left ventricular hypertrophy and diastolic dysfunction, and decreased systemic arterial compliance [39]. Aggressive volume resuscitation and beta blockade is often necessary.
Pai studied 52 patients with severe aortic stenosis, EF ≥55 % and a mean transvalvular gradient <30 mmHg [8]. By propensity score matching 18 patients who had AVR were compared with 14 patients without AVR. One and 5-year survival were 92 % and 88 % in the AVR group compared with 82 % and 10 % in the non-AVR group. Series from Tarantini and Hachicha also confirmed those findings (Table 10.2) [36, 38]. LVEF an NYHA functional class improved after surgery [36].
CHF Secondary to Aortic Stenosis with Low Left Ventricular Ejection Fraction
Poor preoperative left ventricular function is the major predictor of outcomes in patients with aortic stenosis [25, 28, 29, 31].
The incidence of left ventricular dysfunction in patients with severe aortic stenosis is difficult to precise. It varies with the definition used and the population investigated. 5.4 % of patients in the Society of Thoracic Surgeons database who had isolated AVR between 1997 and 2006 had LVEF <30 % [1]. The Euro Heart Survey of Valvular Heart Disease showed that 2.9 % of the patients who underwent AVR had LVEF <30 % and 16.4 % had LVEF between 30 % and 50 % [2]. In AVR series, the incidence ranges from 12 % to 21 % depending on the LVEF threshold used [18, 41]. In a study from an echocardiography database, 26 % of patients with severe aortic stenosis had LVEF ≤35 % and 23 % had a mean transvalvular gradient ≤30 mmHg [8]. Only one third of them had AVR [8].
CHF Secondary to Aortic Stenosis with Low Left Ventricular Ejection Fraction and High Transvalvular Gradients (Table 10.3)
These patients with CHF secondary to severe AS and depressed LVEF but able to generate transaortic gradients ≥40 mmHg, benefit significantly from AVR [8, 25, 41–45] (Fig. 10.1, Table 10.3). They represent 20 % of the patients with severe AS and low LVEF [41].
Thirty-day mortality ranged from 9 % to 19.5 %. Predictors of operative mortality were preoperative myocardial infarction, coronary artery disease, and cardiomegaly.
Symptomatic improvement occurred in the majority of patients after AVR. Most patients were in functional class I or II at late follow-up. LVEF improved early after AVR and continued to improve at late follow-up [34, 44]. The improvement in LVEF was usually more pronounced than in patients with preserved LVEF and severe AS [34]. Improvement in LVEF was associated with greater AS severity as determined by smaller aortic valve area and higher mean gradients, better preoperative ejection fraction, less remodeled ventricles, and the absence of coronary artery disease or previous myocardial infarction [25, 42, 46].
Aggregated long-term survival ranged from 77 % to >90 % at 1 year and from 58 % to 71 % at 5 years. In the absence of coronary artery disease survival of patients with severe aortic and reduced left ventricular function with elevated gradients was similar to the expected survival of the overall population [25]. Independent predictors of long-term survival by multivariate analysis are listed in Table 10.4.
CHF Secondary to Aortic Stenosis with Low Left Ventricular Ejection Fraction and Low Transvalvular Gradients (Table 10.5)
These are the most challenging patients with CHF and AS. They have more advanced myocardial dysfunction secondary to (a) afterload mismatch and therefore reversible or (b) to the combination of afterload mismatch and intrinsic myocardial dysfunction that will not reverse with AVR [20, 50]. While most patients will benefit form AVR, the ones that would benefit the most are those with reversible myocardial dysfunction. The determination of contractile reserve (defined as an increase in the stroke volume ≥20 % by the infusion of low dose of dobutamine) is useful to determine the presence of reversible or irreversible myocardial dysfunction [4, 21, 49, 51, 52] (Fig. 10.2). It is believed that the myocardial dysfunction in patients with AS, low LVEF, and low transvalvular gradient (mean aortic valve gradient <40 mmHg) who have contractile reserve is primarily due to afterload mismatch and therefore reversible, while patients without contractile reserve are believe to have intrinsic myocardial dysfunction [4, 21, 49, 51, 52]. The determination of irreversible myocardial dysfunction by the absence of contractile reserve is not perfect since a large number of patients without contractile reserve will benefit from AVR [4, 9, 53].
The dobutamine challenge also helps to differentiate patients with low cardiac output and true severe aortic stenosis from those with low cardiac output and mild aortic stenosis or pseudo aortic stenosis by examining the changes in aortic valve area, stroke volume, and mean aortic valve gradient (Fig. 10.2). Patients with true severe aortic stenosis respond to the dobutamine induced increase in stroke volume with an increase in the mean gradient while the calculated aortic valve area remains low. Patients with pseudo aortic stenosis increase their aortic valve area. They do not benefit from AVR [4, 49, 51, 52].
The presence of contractile reserve has prognostic implications for patients treated either with AVR or with medical management. It predicts the operative risk as well as the long-term survival of patients with low EF low gradient aortic stenosis. Early mortality in patients with contractile reserve ranges from 5 % to 7 % while in those without ranges from 26 % to 33 % [4, 9, 52].
Long-term survival and functional status after AVR is also influenced by contractile reserve. Monin in a multicenter study demonstrated that patients with contractile reserve had improved 1-year (90 vs. 60 %) and a 5-year (74 vs. 37 %) survival after AVR compared to those without contractile reserve. In the same study, NYHA functional class improvement occurred in 84 % of patients with contractile reserve vs. in 45 % of patients without [4]. In a subsequent sub study, Quere analyzed the outcomes of patients who survived AVR and showed that contractile reserve did not influence long-term survival suggesting that the contractile reserve is only a primary determinant of surgical risk [53].
In spite of the high operative mortality and limited long-term survival associated with AVR in patients without contractile reserve, AVR significantly improve their prognosis compared to medical management. Monin demonstrated that 1 and 5-year survival with or without contractile reserve was better for patients treated with AVR than for patients who received medical therapy (Tables 10.1 and 10.5) [4].
Tribouilloy demonstrated on 81 patients with low-flow/low-gradient AS without contractile reserve that AVR was associated with lower 1-year (75 vs. 35 %) and 5-year (54 vs. 13 %) mortality than medical therapy. In addition only 9 % of the AVR patients had heart failure symptoms at follow-up compared with 81 % of the medically managed patients [9].
Clavel (2003) demonstrated in the same group of patients that AVR was only associated with improved overall survival compared with medical management in the subset of patients with more severe stenosis (AVA <1.0 cm2). This lack of improvement was likely due to the high operative mortality (18 %) [7]. Once operative mortality was excluded, patients who survived AVR had excellent late survival compared with patients treated medically (70 vs. 50 %) (Table 10.5) [7].
Early postoperative improvement in ejection fraction is associated with improved long-term survival and functional status [53, 55]. Connolly demonstrated that LVEF improved in 74 % of the survivors. The mean improvement was an increase of 10 ± 14 EF units. Positive change in LVEF was associated with female sex and smaller preoperative aortic valve area [26]. Those who did not improve probably had intrinsic myocardial dysfunction from previous myocardial infarction or myocardial fibrosis (see above).
Quarre demonstrated that LVEF improvement can be observed in patients without contractile reserve after AVR [53]. 83 % of patients with and 65 % of the patients without contractile reserve obtained ≥10 % improvement in LVEF after AVR. The magnitude of the improvement was similar regardless the presence of contractile reserve. Contractile reserve was not a predictor of improvement in LVEF after AVR. Therefore absence of contractile reserve does not always predict irreversible myocardial dysfunction. Higher preoperative mean aortic valve gradient and absence of multivessel coronary artery disease were associated with improvement in LVEF after AVR [53].
Predictors of Outcomes
Since contractile reserve alone does not accurately predict long-term outcomes in severe aortic stenosis with low LVEF and low transvalvular gradients, other factors should be considered to risk stratify patients before AVR [53]. Several studies have identified independent predictors of early mortality, long-term survival and improvement in LVEF and functional class (Table 10.4).
Predictors of 30-day mortality include coronary artery disease (CAD) (as defined by previous MI, multivessel CAD, and concomitant CABG), which likely indicates the presence of intrinsic and probably irreversible myocardial dysfunction secondary to fibrosis or myocardial infarction [9, 25, 42, 43, 47, 48]. In addition, concomitant CABG increases the operative time and complexity of the surgery. Surrogates for more advanced left ventricular dysfunction are low mean transaortic gradients, absence of contractile reserve, and dilated left ventricle [4, 9, 42, 44, 47–49]. The increased mortality with small prosthesis size may be the result of high residual aortic valve gradient and incomplete relieve of the LV outflow obstruction [26]. Patients with LV dysfunction tolerate poorly residual aortic gradient and patient prosthesis mismatch since they are highly sensitive to increased afterload [26, 41, 56, 57]. Patient prosthesis mismatch results in decreased survival, lower freedom from heart failure, and incomplete left ventricular mass regression [41, 57]. Patient prosthesis mismatch should be avoided by implanting prosthesis with superior hemodynamic performance and considering the prosthetic effective orifice area indexed to body surface area at the time of AVR. Some have advocated the use of stentless valves [58]. Percutaneous aortic valves may have an advantage in this group since they have better hemodynamic performance with larger postoperative aortic valve area and lower transvalvular gradient than surgically placed valves [41, 59].
Other factors associated with early mortality are common predictors of increased surgical risk (age, presence of comorbidities, advanced functional status, emergency surgery, and female gender) (Table 10.4).
Aortic valve replacement is the main factor associated with long-term survival, LVEF improvement, and improved functional status in patients with low LVEF and low gradient aortic stenosis [4, 8, 9, 54]. This reinforces the notion that even risky, AVR provides a significant survival advantage to these patient compared to medical management alone.
Other factor negatively associated with long-term survival was presence of coronary artery disease [8, 9, 25, 47]. As in early mortality, CAD indicates intrinsic myocardial dysfunction. Factors associated with ventricular dysfunction (contractile reserve, low preoperative mean gradient, pre and postoperative low cardiac output, remodeled ventricles, and atrial fibrillation) also negatively affected long-term survival [4, 9, 25, 36, 42, 44, 45, 47, 49]. Early improvement in LVEF [44] predicted long-term survival indicating that most of myocardial dysfunction was reversible secondary to afterload mismatch. Advance age, comorbidities, and elevated EuroSCORE also predicted decreased survival [8, 44, 45, 47, 54].
Improvement in LVEF was associated with similar factor as those that predict early and late survival [25, 43, 44, 46, 53, 54]. Coronary artery disease, myocardial dysfunction, less ventricular remodeling. In addition the presence of systemic hypertension was negatively associated with improvement in LVEF. It was presumed that systemic hypertension was associated with myocardial fibrosis.
The only independent factor associated with improved functional status at late follow-up was the early improvement in LVEF >10 ejection fraction units [44].
Many of the variables associated with adverse outcomes are similar to those associated with adverse outcomes in the general population of aortic stenosis patients. Hannan identified age >60 years, LVEF <50 %, CHF, myocardial infarction less than 24 h before surgery, lower body surface area, previous cardiac operation, and several comorbidities as independent predictors of 30 day mortality [31]. Factors associated with increased long-term mortality were concomitant CABG, age >60, small body surface area, emergency status, and comorbidities [31]. Mihaljevic identified the following risk factors as associated with early death: older age, LV dilatation, and smaller prosthetic size. Risk factors for late death were older age, greater degree of aortic stenosis, greater LV mass index, smaller prosthetic size, LV dysfunction, and advanced symptoms. Risk factors associated with advanced symptoms include calcific aortic stenosis and severe LV dysfunction [28].
Emerging Therapies for Aortic Stenosis: Role of TAVR and Percutaneous Valvuloplasty
Transcatheter Aortic Valve Replacement (TAVR)
Transcatheter aortic valve replacement (TAVR): has become an alternative to surgical AVR for high surgical risk or inoperable patients with aortic stenosis [10, 59]. AVR for patients with severe aortic stenosis with low ejection fraction and low gradient is associated with significant operative mortality and morbidity (Table 10.5). It is expected that TAVR would decrease operative mortality due to the less invasive nature of the procedure and the avoidance of cardiopulmonary bypass. Clavel compared TAVR to AVR in this group of patients [60]. Mean LVEF was 34 ± 10 %. Aortic valve area was 0.72 ± 0.17 cm2 in AVR and 0.64 ± 0.18 cm2 in the TAVR group. Mean aortic valve gradient was 36 ± 14 mmHg. Operative mortality was higher in the TAVR group (19 vs. 12 %) partially related to the high-risk profile in this group. TAVR was also associated with a better improvement in aortic valve area and transvalvular gradient compared to AVR and with a lower incidence of patient prosthesis mismatch. As a consequence, TAVR patients had a faster and more complete recovery of their LVEF. At 1 year follow-up 58 % of the TAVR patients had normalized their LVEF compared with 28 % of the AVR patients. Unbenhaum reported transapical aortic vale replacement in 21 patients with advanced heart failure and severe ventricular dysfunction (LVEF 20 ± 5 %) secondary to aortic stenosis (AVA 0.8 ± 0.3 cm2, mean gradient 33 ± 13 mmHg) [61]. Operative mortality was 4.8 %. One and 2-year survival was 76 and 62 %. There was early improvement of LVEF to 38 ± 11 %. This study demonstrates the feasibility of treating patients with low gradient low EF aortic stenosis with a percutaneous transapical approach. TAVR may be an alternative to AVR in these high-risk patients as long as 30-day mortality is lower than AVR.
Percutaneous Aortic Valvuloplasty
Percutaneous aortic valvuloplasty is an alternative for patients with severe acquired aortic stenosis and LV dysfunction who are not candidates for surgery. Percutaneous aortic valvuloplasty was associated with temporary reduction of transvalvular gradients (from 55 to 29 mmHg), increase in the aortic valve area (mean increase 0.3 cm2), improvement in left ventricular performance, and symptomatic improvement [62]. However, it had several disadvantages: (1) it was associated with a 25 % risk of complications, (2) the improvement was short lived with recurrence of the symptoms within a few months and (3) there was no survival benefit [63–66]. With the advent of transcatheter aortic valve replacement, aortic valvuloplasty has reemerged as a procedure for the treatment of aortic stenosis [67]. It is usually utilized in patients with congestive heart failure or cardiogenic shock to stabilize them and bridge them to TAVR or to a high-risk aortic valve replacement [66, 67].
Aortic Regurgitation and CHF
Aortic insufficiency leads to both pressure and volume overload on the left ventricle [18, 21, 68]. Volume overload (increased preload) results from the diastolic regurgitant volume. Increased afterload is the result of the increased aortic stroke volume (regurgitant volume plus forward stroke volume) that leads to systolic arterial hypertension. The increase in wall stress leads to compensatory LV dilatation and eccentric hypertrophy. These changes decrease wall stress and preserve ejection fraction. Progressive LV dilatation overcomes those compensatory mechanisms leading to myocardial dysfunction and decreased EF. At this point, LV function will improve after AVR. Persistent regurgitation and further increase in wall stress lead to further systolic dysfunction secondary to ischemia and myocardial fibrosis [68, 69]. At this stage, when the ventricle is severely dilated, intrinsic myocardial dysfunction becomes the predominant mechanism responsible for LV dysfunction and AVR is less likely to improve LV function. However, even without LVEF improvement, AVR will improve loading conditions and facilitate CHF management [21].
Congestive heart failure secondary to severe aortic regurgitation (AR) has several commonalities with the one secondary to severe aortic stenosis. It is common, its medical management results in poor outcomes, and it is undertreated.
In the Euro Heart Survey, AR was the third most common valve pathology after aortic stenosis and mitral regurgitation. Only one third of those patients were treated surgically. That proportion is even lower in patients with left ventricular dysfunction: only 22 % of patients with LVEF between 30 % and 50 % and 3 % of patients with LVEF <30 % had AVR [2, 11, 70].
In the STS database, 47 % of isolated AVR patients had some degree of AR. Fifty-two percent were in NYHA class III–IV. However, only 5 % had LVEF <30 % [1].
Twenty percent of patients with severe AR from and echocardiography database have LVEF ≤35 % [11]. In other series, they represent 11 % of patients who received AVR [18].
The natural history of asymptomatic severe AR with normal LVEF and normal ventricular dimensions is benign [21, 68, 72, 73]. However, once congestive heart failure, ventricular dilatation, or LV dysfunction develops, the prognosis of medically treated patients is poor. Survival ranges from 20 % to 50 % at 5 years and the majority of patients are in NYHA class III–IV [11, 68, 74, 75]. Thus, surgery is recommended when (a) patients become symptomatic (ACC-AHA guidelines class I and ESC guidelines class IB), (b) the LVEF is ≤50 % independently of symptoms (ACC-AHA guidelines class I and ESC class IB) or (c) the LV dilates (LVEDD >75 mm or LVESD >55 mm,ACC-AHA guidelines class IIa) [21, 75]. The ESC guidelines recommends surgery with lesser degree of LV dilatation (LVEDD >70 mm or LVESD >50 mm, class IIaC) [75].
The surgical outcomes of patients with advanced CHF (NYHA class III–IV) and severe LV dysfunction (LVEF ≤35 %) secondary to chronic AR has only been studied in a few series (Table 10.6).
The operative mortality in this group was high (Table 10.6). Operative mortality was four time higher in low ejection fraction patients than in patients with normal EF (14 vs. 3.7 %) [78]. Concomitant procedures and advanced NYHA class increased operative mortality [78]. Klodas demonstrated that patients in NYHA class III–IV had a six time higher operative mortality (7.8 vs. 1.2 %) [77].
Aortic valve replacement improves long-term survival compared to medical management. One-year survival ranged from 80 % to 99 %. Five-year survival ranged from 60 % to 80 %. The only study that compared medical management with AVR showed that AVR improved 1-year survival from 65 % to 88 % and 5-year survival from 37 % to 70 % (Table 10.6) [11]. After adjusting for baseline variables AVR was associated with a significantly lower hazard of mortality (HR 0.59, 95 % CI 0.42–0.98, P < 0.04) [11].
Other independent predictors of long-term survival were preoperative LVEF, NYHA class, and age [78]. Five year survival for patients with LVEF <35 % was 60 % compared with 85 % for patients with normal EF. This survival, although better than with medical management, was 65 % lower than the expected survival of an age and sex matched population [78]. This suggests that AVR does not completely reverse the myocardial dysfunction induced by long-standing AR.
Other factor associated with poor long-term survival was dilated left ventricle as identified by indexed LV dimensions. Patients with LVESDi ≥20 mm/m2 and LVEDDi ≥30 mm/m2 had worse long-term survival independently of their preoperative LVEF and NYHA functional class [79]. Previous studies have shown that extreme LV dilatation (LVEDD≥80 mm) did not prevent improvement in LV function after AVR [80].
Even patient with extremely reduced LVEF (LVEF <20 %) achieved a survival advantage with AVR compared to medical management [11]. Severe pulmonary hypertension (systolic pulmonary pressure >60 mmHg) and functional mitral regurgitation adversely affect long-term survival [75, 81]. AVR and mitral valve repair were also associated with a better survival than medical management [75, 81].
AVR result in symptomatic improvement. The majority of patients remain free of CHF symptoms after AVR [48, 78].
Similarly to aortic stenosis the impairment in LVEF in AR is related to a combination of (a) afterload mismatch and (b) intrinsic myocardial dysfunction. LVEF improvement after AVR will depend of the relative contribution of each mechanism. AVR resulted in LVEF improvement in the majority of patients with severe LV dysfunction [78, 79]. The improvement is more pronounced in patients with lower preoperative EF [78, 79]. The time course of LVEF improvement show a modest initial decrement in EF followed by a gradual improvement over the course of the next 6 months [69, 79]. Late improvement in LVEF was associated with the presence of early LV reverse remodeling defined as a 10 % reduction in LVEDD [69]. LVEF decreased significantly late after AVR in patients with no early LV reverse remodeling. Preoperative LV stroke volume >97 ml was the best independent predictor of early reverse remodeling [69].
Heart transplantation and mechanical circulatory support should be considered as an alternative treatment in this group of patients [82]. However, in spite of the high operative mortality most patients with congestive heart failure secondary to severe AR with severely reduced LVEF greatly benefit from AVR [78]. Most patients achieved lasting symptomatic improvement and improvement in their ejection fraction. Long-term survival is better than the one after heart transplantation without the side effects and complications of immunosuppression and rejection.
Conclusions
Patients with aortic valve disorders and associated left ventricular dysfunction usually present with congestive heart failure. Their prognosis with medical management is extremely poor. Aortic valve replacement, although risky is associated with improved long-term survival, improved ventricular function, and functional class compared to medical management. The decision to perform aortic valve surgery in these patients is challenging. Associated comorbidities, frailty and other factors may limit their life expectancy in addition to the cardiac disease [2, 84]. These factors should be carefully considered to risk stratify and guide the decision to perform surgery. The use of risk calculators (EuroSCORE and STS risk calculator) is a helpful tool to guide therapy [85, 86]. However, they may over or underestimate the risk [87]. Surgery should be considered on those patients whose life expectancy is not limited by their comorbidities or frailty. Transcatheter aortic valve implantation may benefit these patients if their use results in lower procedural mortality than surgery.
Abbreviations
- ACC:
-
American College of Cardiology
- AHA:
-
American Heart Association
- AS:
-
Aortic stenosis
- AR:
-
Aortic regurgitation
- AVA:
-
Aortic valve area
- AVR:
-
Aortic valve replacement
- CABG:
-
Coronary arteries bypass graft surgery
- CAD:
-
Coronary artery disease
- CHF:
-
Congestive heart failure
- DST:
-
Dobutamine stress test
- ESC:
-
European Society of Cardiology
- LV:
-
Left ventricle
- LVEDD:
-
Left ventricle end diastolic diameter
- LVEF:
-
Left ventricular ejection fraction
- LVESD:
-
Left ventricle end systolic diameter
- NYHA:
-
New York Heart Association
- SV:
-
Stroke volume
References
Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137(1):82–90. PubMed.
Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24(13):1231–43. PubMed.
Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38 Suppl 1:61–7.
Monin JL, Quéré JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Guéret P. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108(3):319–24. Epub 2003 Jun 30. PubMed.
Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82(6):2111–5. PubMed.
Brown ML, Pellikka PA, Schaff HV, Scott CG, Mullany CJ, Sundt TM, Dearani JA, Daly RC, Orszulak TA. The benefits of early valve replacement in asymptomatic patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2008;135(2):308–15. Epub 2007 Dec 26. PubMed.
Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Beanlands RS, Mathieu P, Magne J, Pibarot P. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS Study. Circulation. 2008;118(14 Suppl):S234–42. PubMed.
Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg. 2008;86(6):1781–9. PubMed.
Tribouilloy C, Lévy F, Rusinaru D, Guéret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A, Chauvel C, Metz D, Quéré JP, Monin JL. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53(20):1865–73. PubMed.
Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD,Williams MR, Fontana GP, Miller DC, Anderson WN, Akin JJ, Davidson MJ, Smith CR; PARTNER trial investigators. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;20;385(9986):2485–91. doi: 10.1016/S0140-6736(15)60290-2. Epub 2015. PubMed PMID: 25788231.
Kamath AR, Varadarajan P, Turk R, Sampat U, Patel R, Khandhar S, Pai RG. Survival in patients with severe aortic regurgitation and severe left ventricular dysfunction is improved by aortic valve replacement: results from a cohort of 166 patients with an ejection fraction < or =35 %. Circulation. 2009;120(11 Suppl):S134–8. PubMed.
The Society of Thoracic Surgeons Executive Summary Adult Cardiac Surgery Database 2011 Harvest 2. Available on line at http://www.sts.org/sites/default/files/documents/20112ndHarvestExecutiveSummary.pdf. Accessed on 20 Aug 2011.
Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111(24):3290–5. Epub 2005 Jun 13. PubMed.
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–11. PubMed.
Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21(5):1220–5.
Bach DS. Prevalence and characteristics of unoperated patients with severe aortic stenosis. J Heart Valve Dis. 2011;20(3):284–91. PubMed.
Brogan 3rd WC, Grayburn PA, Lange RA, Hillis LD. Prognosis after valve replacement in patients with severe aortic stenosis and a low transvalvular pressure gradient. J Am Coll Cardiol. 1993;21(7):1657–60. PubMed.
Chukwuemeka A, Rao V, Armstrong S, Ivanov J, David T. Aortic valve replacement: a safe and durable option in patients with impaired left ventricular systolic function. Eur J Cardiothorac Surg. 2006;29(2):133–8. PubMed.
Tajik AJ. Aortic valve stenosis: etiology, pathophysiology, evaluation and management. Curr Probl Cardiol. 1987;8:458–508.
Carabello BA. Aortic stenosis: from pressure overload to heart failure. Heart Fail Clin. 2006;2(4):435–42. Review. PubMed.
Bonow RO, Carabello BA, Chatterjee K, de Leon Jr AC, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52(13):e1–142. PubMed.
Gaasch WH, Levine HJ, Quinones MA, Alexander JK. Left ventricular compliance: mechanisms and clinical implications. Am J Cardiol. 1976;38:645–53.
Hess OM, Ritter M, Schneider J, Grimm J, Turina M, Krayenbuehl HP. Diastolic stiffness and myocardial structure in aortic valve disease before and after valve replacement. Circulation. 1984;69:855–65.
Murakami T, Hess OM, Gage JE, Grimm J, Krayenbuehl HP. Diastolic filling dynamics in patients with aortic stenosis. Circulation. 1986;73:1162–74.
Connolly HM, Oh JK, Orszulak TA, Osborn SL, Roger VL, Hodge DO, Bailey KR, Seward JB, Tajik AJ. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation. 1997;95(10):2395–400. PubMed.
Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation. 2000;101(16):1940–6. PubMed.
Vahanian A, Otto CM. Risk stratification of patients with aortic stenosis. Eur Heart J. 2010;31(4):416–23. Epub 2010 Jan 4. Review. PubMed.
Mihaljevic T, Nowicki ER, Rajeswaran J, Blackstone EH, Lagazzi L, Thomas J, Lytle BW, Cosgrove DM. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135(6):1270–8; discussion 1278–9. Epub 2008 May 23. PubMed.
Kvidal P, Bergström R, Hörte LG, Ståhle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35(3):747–56. PubMed.
Malaisrie SC, McCarthy PM, McGee EC, Lee R, Rigolin VH, Davidson CJ, Beohar N, Lapin B, Subacius H, Bonow RO. Contemporary perioperative results of isolated aortic valve replacement for aortic stenosis. Ann Thorac Surg. 2010;89(3):751–6. PubMed.
Hannan EL, Samadashvili Z, Lahey SJ, Smith CR, Culliford AT, Higgins RS, Gold JP, Jones RH. Aortic valve replacement for patients with severe aortic stenosis: risk factors and their impact on 30-month mortality. Ann Thorac Surg. 2009;87(6):1741–9. PubMed.
Pantely G, Morton M, Rahimtoola SH. Effects of successful, uncomplicated valve replacement on ventricular hypertrophy, volume, and performance in aortic stenosis and in aortic incompetence. J Thorac Cardiovasc Surg. 1978;75:383–91.
Kennedy JW, Doces J, Stewart DK. Left ventricular function before and following aortic valve replacement. Circulation. 1977;56:944–50.
Sharma UC, Barenbrug P, Pokharel S, Dassen WR, Pinto YM, Maessen JG. Systematic review of the outcome of aortic valve replacement in patients with aortic stenosis. Ann Thorac Surg. 2004;78(1):90–5. Review. PubMed.
Pibarot P, Dumesnil JG. Paradoxical low-flow, low-gradient aortic stenosis adding new pieces to the puzzle. J Am Coll Cardiol. 2011;58(4):413–5. PubMed.
Tarantini G, Covolo E, Razzolini R, Bilato C, Frigo AC, Napodano M, Favaretto E, Fraccaro C, Isabella G, Gerosa G, Iliceto S, Cribier A. Valve replacement for severe aortic stenosis with low transvalvular gradient and left ventricular ejection fraction exceeding 0.50. Ann Thorac Surg. 2011;91(6):1808–15. PubMed.
Herrmann S, Störk S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlöhner S, Voelker W, Ertl G, Weidemann F. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58(4):402–12. PubMed.
Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115(22):2856–64. Epub 2007 May 28. PubMed.
Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31(3):281–9. Epub 2009 Sep 8. Review. PubMed PMID: 19737801; PubMed Central PMCID: PMC2814220.
Pibarot P, Dumesnil JG. Low-flow, low-gradient, normal ejection fraction aortic stenosis. Curr Cardiol Rep. 2010;12(2):108–15. Review. PubMed.
Kulik A, Burwash IG, Kapila V, Mesana TG, Ruel M. Long-term outcomes after valve replacement for low-gradient aortic stenosis: impact of prosthesis-patient mismatch. Circulation. 2006;114(1 Suppl):I553–8. PubMed.
Flores-Marín A, Gómez-Doblas JJ, Caballero-Borrego J, Cabrera-Bueno F, Rodríguez-Bailón I, Melero JM, Porras C, Sánchez-Espín G, Such M, Olalla E, de Teresa E. Long-term predictors of mortality and functional recovery after aortic valve replacement for severe aortic stenosis with left ventricular dysfunction. Rev Esp Cardiol. 2010;63(1):36–45. PubMed.
Powell DE, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Perez JL, Kronzon I. Aortic valve replacement in patients with aortic stenosis and severe left ventricular dysfunction. Arch Intern Med. 2000;160(9):1337–41. PubMed.
Vaquette B, Corbineau H, Laurent M, Lelong B, Langanay T, de Place C, Froger-Bompas C, Leclercq C, Daubert C, Leguerrier A. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long-term outcome. Heart. 2005;91(10):1324–9. PubMed PMID: 16162627; PubMed Central PMCID: PMC1769144.
Halkos ME, Chen EP, Sarin EL, Kilgo P, Thourani VH, Lattouf OM, Vega JD, Morris CD, Vassiliades T, Cooper WA, Guyton RA, Puskas JD. Aortic valve replacement for aortic stenosis in patients with left ventricular dysfunction. Ann Thorac Surg. 2009;88(3):746–51. PubMed.
Matsumura Y, Gillinov AM, Toyono M, Wada N, Yamano T, Thomas JD, Shiota T. Usefulness of left ventricular shape to predict the early recovery of left ventricular function after isolated aortic valve replacement for aortic valve stenosis. Am J Cardiol. 2008;102(11):1530–4. Epub 2008 Sep 12. PubMed.
Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, Petit-Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: a European multicenter study. J Am Coll Cardiol. 2008;51(15):1466–72. PubMed.
Rothenburger M, Drebber K, Tjan TD, Schmidt C, Schmid C, Wichter T, Scheld HH, Deiwick M. Aortic valve replacement for aortic regurgitation and stenosis, in patients with severe left ventricular dysfunction. Eur J Cardiothorac Surg. 2003;23(5):703–9; discussion 709. PubMed.
Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol. 2001;37(8):2101–7. PubMed.
Hwang MH, Hammermeister KE, Oprian C, Henderson W, Bousvaros G, Wong M, Miller DC, Folland E, Sethi G. Preoperative identification of patients likely to have left ventricular dysfunction after aortic valve replacement: participants in the Veterans Administration Cooperative Study on Valvular Heart Disease. Circulation. 1989;80(Suppl I):I-65–76.
Steinhauser ML, Stone PH. Risk stratification and management of aortic stenosis with concomitant left ventricular dysfunction. Curr Treat Options Cardiovasc Med. 2007;9(6):490–500. PubMed.
Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes Jr DR. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: the clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation. 2002;106(7):809–13. PubMed.
Quere JP, Monin JL, Levy F, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Gueret P, Tribouilloy C. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation. 2006;113(14):1738–44. Epub 2006 Apr 3. PubMed.
Pereira JJ, Lauer MS, Bashir M, Afridi I, Blackstone EH, Stewart WJ, McCarthy PM, Thomas JD, Asher CR. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol. 2002;39(8):1356–63. PubMed.
Morris JJ, Schaff HV, Mullany CJ, Rastogi A, McGregor CG, Daly RC, Frye RL, Orszulak TA. Determinants of survival and recovery of left ventricular function after aortic valve replacement. Ann Thorac Surg. 1993;56:22–9; discussion 29–30.
Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation. 2003;108:983–8.
Ruel M, Al-Faleh H, Kulik A, Chan K, Mesana TG, Burwash IG. Prosthesis-patient mismatch after aortic valve replacement primarily affects patients with pre-existing left ventricular dysfunction: impact on survival, freedom from heart failure, and left ventricular mass regression. J Thorac Cardiovasc Surg. 2006;131:1036–44.
Bevilacqua S, Gianetti J, Ripoli A, Paradossi U, Cerillo AG, Glauber M, Matteucci ML, Senni M, Gamba A, Quaini E, Ferrazzi P. Aortic valve disease with severe ventricular dysfunction: stentless valve for better recovery. Ann Thorac Surg. 2002;74(6):2016–21. PubMed.
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–98. Epub 2011 Jun 5. PubMed.
Clavel MA, Webb JG, Rodés-Cabau J, Masson JB, Dumont E, De Larochellière R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122(19):1928–36. Epub 2010 Oct 25. PubMed.
Unbehaun A, Pasic M, Buz S, Dreysse S, Kukucka M, Hetzer R, Drews T. Transapical aortic valve implantation in patients with severely depressed left ventricular function. J Thorac Cardiovasc Surg. 2011. [Epub ahead of print] PubMed.
Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry [no authors listed]. Circulation. 1991; 84:2383–97.
Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation. 1994;89:642–50.
Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol. 1995;26:1522–8.
Nishimura RA, Holmes Jr DR, Michela MA. Follow-up of patients with low output, low gradient hemodynamics after percutaneous balloon aortic valvuloplasty: the Mansfield Scientific Aortic Valvuloplasty Registry. J Am Coll Cardiol. 1991;17:828–33.
Kapadia SR, Goel SS, Yuksel U, Agarwal S, Pettersson G, Svensson LG, Smedira NG, Whitlow PL, Lytle BW, Tuzcu EM. Lessons learned from balloon aortic valvuloplasty experience from the pre-transcatheter aortic valve implantation era. J Interv Cardiol. 2010;23(5):499–508. doi:10.1111/j.1540-8183.2010.00577.x. PubMed.
Tissot CM, Attias D, Himbert D, Ducrocq G, Iung B, Dilly MP, Juliard JM, Lepage L, Détaint D, Messika-Zeitoun D, Nataf P, Vahanian A. Reappraisal of percutaneous aortic balloon valvuloplasty as a preliminary treatment strategy in the transcatheter aortic valve implantation era. Eur Interv. 2011;7(1):49–56. doi:10.4244/EIJV7I1A11. PubMed.
Bekeredjian R, Grayburn PA. Valvular heart disease: aortic regurgitation. Circulation. 2005;112(1):125–34. Review. Erratum in: Circulation. 2005 Aug 30;112(9):e124. PubMed.
Sénéchal M, Bernier M, Dagenais F, Dubois M, Dubois-Sénéchal IN, Voisine P. Usefulness of preoperative stroke volume as strong predictor of left ventricular remodeling and outcomes after aortic valve replacement in patients with severe pure aortic regurgitation. Am J Cardiol. 2011. [Epub ahead of print] PubMed.
Supino PG, Borer JS, Preibisz J, Bornstein A. The epidemiology of valvular heart disease: a growing public health problem. Heart Fail Clin. 2006;2(4):379–93. Review. PubMed.
Dujardin KS, Enriquez-Sarano M, Schaff HV, Bailey KR, Seward JB, Tajik AJ. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation. 1999;99(14):1851–7. PubMed.
Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991;84:1625–35.
Borer JS, Hochreiter C, Herrold EM, Supino P, Aschermann M, Wencker D, Devereux RB, Roman MJ, Szulc M, Kligfield P, Isom OW. Prediction of indications for valve replacement among asymptomatic or minimallysymptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation. 1998;97:525–34.
Sionis A, García-Alvarez A, Castel MA, Cordero M, Josa M, Pérez-Villa F, Roig E. Severe aortic regurgitation and reduced left ventricular ejection fraction: outcomes after isolated aortic valve replacement and combined surgery. J Heart Lung Transplant. 2010;29(4):445–8. Epub 2009 Dec 24. PubMed.
Khandhar S, Varadarajan P, Turk R, Sampat U, Patel R, Kamath A, Pai RG. Survival benefit of aortic valve replacement in patients with severe aortic regurgitation and pulmonary hypertension. Ann Thorac Surg. 2009;88(3):752–6. PubMed.
Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A, Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology, ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28(2):230–68. Epub 2007 Jan 26. PubMed.
Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Optimizing timing of surgical correction in patients with severe aortic regurgitation: role of symptoms. J Am Coll Cardiol. 1997;30(3):746–52. PubMed.
Chaliki HP, Mohty D, Avierinos JF, Scott CG, Schaff HV, Tajik AJ, Enriquez-Sarano M. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002;106(21):2687–93. PubMed.
Brown ML, Schaff HV, Suri RM, Li Z, Sundt TM, Dearani JA, Daly RC, Orszulak TA. Indexed left ventricular dimensions best predict survival after aortic valve replacement in patients with aortic valve regurgitation. Ann Thorac Surg. 2009;87(4):1170–5; discussion 1175–6. Erratum in: Ann Thorac Surg. 2009 Aug; 88(2):710. Zhuo, Li [corrected to Li, Zhuo]. PubMed.
Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Aortic regurgitation complicated by extreme left ventricular dilation: long-term outcome after surgical correction. J Am Coll Cardiol. 1996;27(3):670–7. PubMed.
Pai RG, Varadarajan P. Prognostic implications of mitral regurgitation in patients with severe aortic regurgitation. Circulation. 2010;122(11 Suppl):S43–7. PubMed.
Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report – 2010. J Heart Lung Transplant. 2010;29(10):1104–18. PubMed.
Tarantini G, Buja P, Scognamiglio R, Razzolini R, Gerosa G, Isabella G, Ramondo A, Iliceto S. Aortic valve replacement in severe aortic stenosis with left ventricular dysfunction: determinants of cardiac mortality and ventricular function recovery. Eur J Cardiothorac Surg. 2003;24(6):879–85. PubMed.
Chikwe J, Adams DH. Frailty: the missing element in predicting operative mortality. Semin Thorac Cardiovasc Surg. 2010;22(2):109–10. Review. PubMed.
Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24(9):882–3.
The Society of Thoracic Surgeons Risk Calculator. Available online at http://www.sts.org/quality-research-patient-safety/quality/risk-calculator-and-models/risk-calculator. Accessed on line 20 Aug 2011.
Mack MJ. Risk scores for predicting outcomes in valvular heart disease: how useful? Curr Cardiol Rep. 2011;13(2):107–12. Review. PubMed.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag London Ltd.
About this chapter
Cite this chapter
Crestanello, J.A. (2016). Aortic Valve Surgery in Patients with Congestive Heart Failure. In: Raman, J. (eds) Management of Heart Failure. Springer, London. https://doi.org/10.1007/978-1-4471-4279-9_10
Download citation
DOI: https://doi.org/10.1007/978-1-4471-4279-9_10
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-4278-2
Online ISBN: 978-1-4471-4279-9
eBook Packages: MedicineMedicine (R0)