Abstract
A substantial proportion of patients with severe aortic stenosis may paradoxically have low transvalvular flow and a low gradient, despite the presence of normal left ventricular (LV) ejection fraction. These patients are characterized by pronounced LV concentric remodeling with small LV cavity size, impaired LV filling, altered myocardial function, and worse prognosis. This frequent clinical entity is often misdiagnosed, which may lead to an underestimation of aortic stenosis severity and thereby to underutilization or inappropriate delay of surgery. It is important to recognize this entity so we do not deny surgery to a symptomatic patient with small aortic valve area and low gradient. Thus, when there is a discordance between the valve area (in the severe range) and the gradient (in the moderate range) in patients with preserved LV ejection fraction, a more comprehensive Doppler echocardiographic evaluation and potentially other diagnostic tests may be required to confirm disease severity and guide therapeutic management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology (ESC) guidelines for the management of patients with valvular heart disease, only patients having severe aortic stenosis (AS) associated with symptoms and/or left ventricular (LV) ejection fraction (LVEF) less than 50% have a class I indication for aortic valve replacement (AVR) [1, 2]. Severe AS is generally defined as an aortic valve area (AVA) less than 1.0 cm2 and a mean transvalvular gradient greater than 40 mm Hg (or >50 mm Hg in the ESC guidelines). However, the clinician is often confronted with patients who have discordant findings (eg, an AVA = 0.8 cm2 consistent with the presence of a severe AS but a mean gradient less than 40 mm Hg, indicating that the disease is only moderate in severity). This situation raises uncertainty regarding the actual severity of the stenosis as well as the potential indication of AVR if the patient is symptomatic.
The transvalvular pressure gradient is inversely related to the square of AVA and directly related to the square of flow. Thus, a patient with severe AS may nonetheless present with a low gradient if his or her LV output is reduced; this situation is classically observed in patients with low LVEF, low-flow, low-gradient AS, who represent approximately 5% to 10% of the AS population. These patients have a poor prognosis if treated medically but a high operative mortality if treated surgically [3, 4]. A low-dose (up to 20-µg/kg/min) dobutamine stress echocardiography is particularly helpful in this subset of patients 1) to assess the presence of myocardial contractile reserve, which provides important information for operative risk stratification; and 2) to differentiate pseudo-severe from a true severe stenosis. Pseudo-severe stenosis refers to the situation of a weakened ventricle that is not able to completely open a mildly or moderately stenotic valve. From the resting Doppler-echocardiography or catheterization, it is difficult or impossible to differentiate pseudo-severe from true severe AS because in low-flow state conditions, the AVA is small and the gradient is low regardless of the severity of the stenosis. When flow rate is increased by dobutamine stress, there is no or minimal change in AVA and a marked increase in gradient in the case of true severe AS, whereas there is a substantial increase in AVA and no or minimal increase in gradient in the case of pseudo-severe AS [5].
Patients with low LVEF, low-gradient, true severe AS and contractile reserve are good candidates for AVR. In these patients, it is particularly important to implant a prosthesis with superior hemodynamic performance to avoid prosthesis-patient mismatch. A failing ventricle is highly sensitive to an increase in afterload. Thus, patients with depressed LV function tolerate the residual transvalvular pressure gradient associated with prosthesis-patient mismatch poorly [6, 7]. Patients with true severe AS but no contractile reserve may also benefit from AVR but they have a high operative risk [8]. In these patients, transcatheter valve implantation may provide a potential alternative to surgical AVR because it may reduce the operative risk and the occurrence of prosthesis-patient mismatch. Patients with pseudo-severe AS may not necessarily benefit from AVR but also have a poor prognosis if managed conservatively; future studies are necessary to determine the optimal strategy in these patients, including consideration of cardiac transplantation.
We recently reported that about one tier of the patients with severe AS on the basis of AVA (<1.0 cm2; indexed AVA <0.6 cm2/m2) who paradoxically have a low transvalvular flow rate (stroke volume index [SVi] <35 mL/m2) despite the presence of a preserved LVEF (≥ 50%) [9••]; we named this clinical entity “paradoxical low-flow AS” [9••]. The reduction in LV output may, in turn, result in lower than expected transvalvular gradients in a large proportion of these patients [9••, 10•, 11, 12•]. Clinically, this situation is highly insidious because AS may appear less severe on the basis of the transvalvular gradients, when in fact these patients often have a higher global hemodynamic load and a more pronounced impairment of intrinsic myocardial function, consistent with a more advanced stage of the disease.
Pathophysiology of Paradoxical Low-Flow AS
Mechanisms of Reduced LV Outflow in the Setting of Preserved LVEF
When compared to patients with severe AS and normal LV outflow, the patients with paradoxical low-flow AS (ie, low flow with preserved LVEF) are characterized by higher prevalence in women, older age, higher degree of LV concentric remodeling, higher degree of myocardial fibrosis, impaired LV filling, smaller end-diastolic volume, and reduced mid-wall and longitudinal shortening (Table 1) [9••, 10•, 11, 12•, 13•, 14••].
There is an important interindividual variability in the type (concentric vs eccentric) and magnitude of LV remodeling in response to pressure overload. An important proportion of patients with AS and/or hypertension develop pronounced LV concentric remodeling with reduced LV cavity size and impaired LV filling. Several potential factors may predispose to the development of this adverse remodeling pattern and thereby to paradoxical low-flow AS including older age, female gender, etiology (AS vs hypertension), magnitude, and chronicity of the pressure overload, as well as metabolic factors such as insulin resistance [10•, 15, 16•, 17, 18]. A recent study demonstrated that insulin resistance linked to visceral obesity is associated with more pronounced concentric remodeling and altered myocardial diastolic and systolic function in patients with AS and preserved LVEF [18]. Thus, AS patients with metabolic syndrome or type 2 diabetes might be at higher risk to develop a paradoxical low-flow pattern.
Paradoxical low-flow AS shares many pathophysiologic and clinical similarities with normal LVEF heart failure. Both entities are characterized by a restrictive physiology pattern, in which the LV pump function and thus the stroke volume are markedly reduced despite preserved LVEF (Fig. 1, Table 1). Several factors contribute to the reduction in LV pump function in these patients. First, the pronounced/exaggerated myocardial concentric remodeling contributes to reduce the size, compliance, and filling of the LV cavity. Moreover, the LV systolic function, which is apparently normal when examining the LVEF is, in fact, substantially reduced when looking at other indices (eg, LV mid-wall or longitudinal shortening, velocity, or strain) that are more sensitive to detect alterations of intrinsic myocardial systolic function [9••, 10•, 14••, 19]. It is important to emphasize that LVEF markedly underestimates the extent of myocardial systolic dysfunction in the presence of LV concentric remodeling [9••, 10•, 14••, 19], which is a predominant feature of paradoxical low-flow AS. To this effect, previous studies reported that 30% to 35% of asymptomatic patients with AS and preserved LVEF have abnormally low mid-wall or longitudinal shortening [9••, 10•, 19].
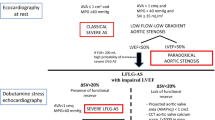
Comparison of typical left ventricular geometry and Doppler echocardiographic findings in normal versus paradoxical low-flow aortic stenosis (AS). AVA—aortic valve area; LVEDV—left ventricular end-diastolic volume; LVEF—left ventricular ejection fraction; SV—stroke volume; SVi—stroke volume index; Zva—valvuloarterial impedance; ∆Pmean—mean transvalvular gradient. (From Pibarot and Dumesnil [11]; with permission)
Consequences of Reduced LV Outflow on Clinical Presentation
Although patients with paradoxical low-flow AS often have similar AVA, dimensionless velocity index, and valvular resistance compared to patients with normal flow, the gradient is lower than one would expect from the severity of stenosis because the flow across the stenotic orifice is reduced (Fig. 1) [11]. This pseudo-normalization phenomenon observed for the gradient also applies to blood pressure, which is often within normal range in patients with paradoxical low-flow AS despite the presence of increased arterial rigidity [9••, 20]. These patients generally have reduced arterial compliance and/or increased vascular resistance but these abnormalities of the arterial hemodynamics are often masked because of the pseudo-normalization of blood pressure that may occur as a result of the reduced LV output. Thus, the clinical presentation of paradoxical low-flow AS is highly insidious because, on the basis of gradient and blood pressure (ie, the parameters that are the most often relied upon by clinicians), both the valvular and arterial hemodynamic burdens may appear less severe than they are in reality.
Patients with paradoxical low-flow AS generally have a markedly higher level of global LV hemodynamic load as reflected by a higher valvuloarterial impedance (Zva), compared to patients with normal-flow severe AS (Fig. 1) [9••, 10•, 16•]. The Zva is easily measurable by Doppler-echocardiography with the use of the formula: Zva = (ΔPmean + SBP)/SVi, where ΔPmean and SBP are the mean transvalvular gradient and systolic blood pressure, respectively [16•, 20]. This index of global (valvular + arterial) LV hemodynamic load provides an estimate of the energy cost in millimeters of mercury per milliliter of blood flow indexed to body size pumped by the heart during systole. One of the main advantages of Zva is to allow the clinician to unmask the pseudo-normalization of gradient and blood pressure, and thereby to better identify the patients with paradoxical low-flow AS.
Not all patients with paradoxical low-flow AS have a low gradient (<40 mm Hg). According to recent studies [9••, 10•, 12•, 13•], approximately 30% to 35% of patients have paradoxical low-flow AS; and among these patients, 65% have a low gradient. Thus, approximately 20% to 25% of the total AS population has low-flow, low-gradient severe AS (on the basis of AVA) with preserved LVEF. These patients with normal LVEF, low-flow, low-gradient AS represent a highly challenging subset of patients in terms of diagnosis and clinical decision making, especially if they are symptomatic. Their AVA suggests the presence of a severe AS and thus a class I indication for AVR, whereas their gradient is consistent with moderate stenosis and thus a conservative therapy. This situation is particularly puzzling for the clinician because these patients with paradoxical low-flow AS have, by definition, a normal LVEF and it is often believed that a normal LVEF necessarily implies a normal stroke volume and transvalvular flow rate. With this false premise in mind, the clinician may conclude that a low gradient (<40 mm Hg) is incompatible with a severe stenosis in a patient with normal LVEF even if the AVA is less than 1.0 cm2 and he or she will be reluctant to refer such patients to surgery. Several recent studies demonstrated that this perception is wrong and that an important proportion of patients with severe AS and normal LVEF may have a relatively low gradient [9••, 10•, 13•, 14••].
Illustrative Case
This is the case of a 75-year-old woman (body surface area, 1.8 m2) with a history of calcific AS and progressive deterioration of her New York Heart Association functional class during the past 6 months. The Doppler-echocardiographic examination shows a high degree of LV concentric remodeling with a small cavity size (relative wall-thickness ratio: 0.5; LV end-diastolic diameter: 43 mm; LV end-diastolic volume: 85 mL), and a preserved LV systolic function (LVEF, 60%) but a low stroke volume (50 mL; indexed: 28 mL/m2) (Fig. 1, right panel). Because of the low-flow state, the transvalvular gradient is only moderately elevated (peak: 42 mm Hg, mean: 25 mm Hg) despite the presence of a severe stenosis, as documented by a severely calcified aortic valve with restricted opening (aortic valve area 0.7 cm2 by continuity equation and 0.8 cm2 by planimetry with calcification score of 3/3). This is a typical case of paradoxical low-flow, low-gradient severe AS despite preserved LVEF.
Therapeutic Management and Outcome
In our previous study of 512 patients with AVA less than 0.6 cm2/m2 and normal LVEF, only 47% of patients with paradoxical low-flow AS were referred to AVR during the 5-year follow-up compared with 65% in the normal-flow AS group [16•]. This finding is not surprising given that clinicians generally put much more weight on the gradient than on other stenotic indices (ie, AVA, dimensionless index) to make their decision to refer a patient to surgery. Although fewer patients with paradoxical low-flow AS were referred to surgery, they nonetheless had a much better outcome with AVR than with medical treatment, even after adjusting for differences in baseline risk profile. Furthermore, the benefit of surgery was observed in this subset of patients regardless of their gradient (> or <40 mm Hg).
These data were independently corroborated by Barasch et al. [13•] who reported that, in patients with severe AS defined as an indexed AVA less than 0.6 cm2/m2, the presence of a mean gradient less than 30 mm Hg is associated with almost a 50% lower referral rate to surgery, which led to a twofold increase in mortality compared to patients with higher gradients. Additionally, Pai et al. [21] recently reported that patients with severe AS on the basis of AVA, low gradient (<30 mm Hg), and preserved LVEF had significantly better survival when treated surgically than when treated medically (5-year survival, 90% vs 20%; P < 0.0001). Further studies are needed to compare the prognosis of patients with paradoxical low-flow AS with or without low gradient versus patients with normal-flow AS. However, in doing so, the investigators will have to pay attention to select an appropriate end point. It would be inadequate to include AVR in the end points, given that this end point is essentially determined by the clinician’s perception of disease severity, which is, in turn, highly influenced by the magnitude of the gradient. Thus, the most appropriate and robust end point for future studies would be occurrence of heart failure and cardiovascular mortality regardless of the type of treatment.
Overall, these findings confirm that paradoxical low-flow, low-gradient AS is often misdiagnosed, which leads to underestimation of stenosis severity and symptoms and therefore underutilization or inappropriate delay of AVR.
Pitfalls and Differential Diagnosis
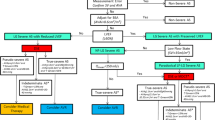
Besides paradoxical low-flow AS, there are other potential causes of discordance between AVA (eg, 0.8 cm2) and gradient (eg, 30 mm Hg) in patients with preserved LVEF: 1) measurement errors (ie, underestimation of stroke volume and AVA); 2) small body size; and 3) inconsistency in guidelines criteria. It is important to make the distinction between paradoxical low-flow AS versus these three other potential situations given they have markedly different implications in terms of therapeutic management (Fig. 2).
Proposition of an algorithm for differential diagnosis in patients with aortic stenosis (AS) and preserved left ventricular ejection fraction presenting with a small aortic valve area (<1.0 cm2) but a low mean gradient (<40 mm Hg). This algorithm will need to be validated in future studies. AVA—aortic valve area; AVAi—indexed aortic valve area; CT—multislice computed tomography; ∆Pmean—mean transvalvular gradient
Measurement Errors
The stroke volume may be underestimated because of underestimation of LV outflow tract diameter and/or misplacement of pulsed-wave Doppler sample volume. An underestimation of stroke volume will translate into an underestimation of AVA and may thus lead the clinician to conclude that the patient has paradoxical low-flow, low-gradient, severe AS, whereas, in fact, he or she has a moderate AS with normal flow. Several methods can be used to corroborate the Doppler-echocardiographic measurements of stroke volume and AVA, and to confirm the presence of paradoxical low-flow AS (Fig. 2).
When paradoxical low-flow AS is suspected, LV geometry measurements should first be reviewed with the expectation of finding a small LV cavity (LV end-diastolic internal diameter <50 mm and/ or LV end-diastolic volume index <60 mL/m2) and a noticeable increase in relative wall-thickness ratio (ie, >0. 45) (Table 1). Moreover, the clinician can easily estimate the stroke volume by multiplying the LVEF by the LV end-diastolic volume obtained by the Teichholz formula [22]. If the stroke volume measured by this independent method is consistent with the stroke volume measured in the LV outflow tract, the clinician can be reassured about the accuracy of the measurement of stroke volume.
The measurement of the valve orifice area by transthoracic or transesophageal planimetry may also be used to corroborate the value of AVA obtained by the continuity equation method. However, this method has important limitations: it measures the anatomic orifice area at peak systole, whereas the continuity equation method provides an estimate of the effective orifice area averaged over the whole of systole [23]. As a consequence, the AVA obtained by planimetry tends to overestimate by 10% to 50% the AVA obtained by continuity. Moreover, optimal image quality is essential to ensure reliable measurement of the anatomic orifice area by planimetry, and transesophageal echocardiography is often necessary to achieve such quality.
In patients having persistent ambiguities or discrepancies on their echocardiograms, cardiac MRI or invasive hemodynamic studies may also be used to validate LV geometry, stroke volume, and AVA measurements.
Small Body Size
The body surface area is an important determinant of resting cardiac output. Thus, a patient with a small body surface area may have a relatively low flow and thus a low gradient despite the presence of a small AVA (<1.0 cm2). An AVA of 0.9 cm2 represents severe AS in a patient with a body surface area of 1.9 cm2, but the same AVA represents a moderate AS in a patient with a body surface area of 1.4 m2. This situation can simply be ruled out by calculating the indexed AVA. A value greater than 0.6 cm2/m2 often indicates the presence of moderate AS (Fig. 2).
Inconsistency in Guidelines Criteria
Minners et al. [24••] recently reported that there is a discrepancy in the criteria of AVA (<1.0 cm2) and mean gradient (>40 mm Hg) proposed in the guidelines to define severe AS. When examining the relationship between AVA and gradient data obtained from their echocardiographic laboratory in a large series of patients, these investigators showed that the AVA cutoff value of 1.0 cm2 corresponds to a value of mean gradient of 30 to 35 mm Hg, which is lower than the value proposed in the guidelines. However, there were some limitations in this study, including the fact that they did not account for the effect of body size and that they had no outcome data to support their recommendation to lower the cutoff value of AVA (to 0.8 cm2) for severe AS in the guidelines definition.
To this effect, a recent study from the Mayo Clinic including 360 patients from community cardiology practice demonstrated that an AVA less than 1.0 cm2 predicts excess mortality and heart failure, irrespective of symptoms or gradient (Maurice Enriquez-Sarano, Personal communication). These findings are also consistent with those reported by other previous studies [9••, 12•, 13•, 21]. Overall, these findings suggest that the present AVA (<1.0 cm2) and indexed AVA (<0.6 cm2/m2) cutoff values for severe stenosis appear to be adequate regardless of gradients.
Paradoxical low-flow AS
The differential diagnosis among the potential causes of discordance of AVA versus gradient in patients with normal LVEF can thus be made by calculating the indexed AVA, by corroborating the estimation of stroke volume and AVA by several independent methods, and by identifying the typical features of paradoxical low-flow AS (Fig. 2).
If this process confirms the existence of paradoxical low-flow AS, it is then important to rule out the presence of a pseudo-severe stenosis (Fig. 2). Given that transvalvular flow rate is reduced in these patients, it cannot be excluded that the stenosis may be “pseudo-severe” in certain patients (ie, the flow may not be high enough to fully open a valve that is only moderately stenotic). In this regard, exercise stress echocardiography may eventually prove useful to assess the response of AVA and gradient with increasing flow rate in patients with no or equivocal symptoms. A low-dose dobutamine stress echocardiography might also be considered, but it should be used with caution. There should be close monitoring of blood pressure and LV outflow tract velocity because dobutamine stimulation may reduce LV preload and thereby cause a marked reduction in stroke volume and blood pressure in these patients who often have a restrictive physiology pattern. Further studies are needed to confirm the safety and clinical usefulness of stress echocardiography in this specific population. Plasma natriuretic peptides may also prove helpful to assess the impact of the valvular hemodynamic burden on myocardial function and to enhance risk stratification in patients with paradoxical low-flow AS, but confirmation of this concept awaits further data.
The measurement of the extent of aortic valve calcification by multislice CT may provide another key to disease severity in patients with low-flow, low-gradient AS who are potentially considered for surgery. Paradoxical low-flow AS is associated with several factors (ie, pronounced concentric remodeling, myocardial fibrosis, impaired myocardial function) that may also increase the operative risk [12•, 25, 26]. Given that patients with low-flow, low-gradient (with normal or reduced LVEF) AS have higher operative risk and greater potential for technical pitfalls, measurement errors, and overestimation of AVA, it is important to further confirm the stenosis severity with the use of a flow-independent method prior to referring the patient to surgery. If echocardiographic assessment of valve morphology and calcification is inconclusive, multislice CT may be used for this purpose and a valve calcium score greater than 1500 to 1600 Agatston units would support the presence of true severe stenosis and the indication for AVR [27]. If on the other hand, CT reveals a low calcium score, the echocardiographic data should be re-assessed and additional diagnostic tests should be considered.
Conclusions
Normal LVEF, low-flow, low-gradient, severe AS is a frequent clinical entity that is often misdiagnosed, which may lead to an underestimation of disease severity and thus to underutilization or inappropriate delay of surgery. This paradoxical low-flow pattern may generate some ambiguity in the interpretation of the echocardiographic findings within the framework of the ACC/AHA/ESC guidelines. It is thus important to recognize this entity and to rule out other potential confounding situations, such as measurement errors and pseudo-severe AS, so we do not deny surgery to a symptomatic patient with small AVA and low gradient. Thus, when there is a discordance between the AVA (in the severe range) and the gradient (in the moderate range) in patients with preserved LVEF, a more comprehensive Doppler echocardiographic evaluation and potentially other diagnostic tests (exercise stress echocardiography, CT, MRI, plasma natriuretic peptides, and invasive studies) may be required to confirm disease severity and guide therapeutic management.
References
Papers of particular interest, published recently, have been highlighted as follows: •Of importance ••Of major importance
Vahanian A, Baumgartner H, Bax J, et al.: Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007, 28:230–268.
Bonow RO, Carabello BA, Kanu C, et al.: ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2006, 114:e84–e231.
Monin JL, Quere JP, Monchi M, et al.: Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003, 108:319–324.
Clavel MA, Fuchs C, Burwash IG, et al.: Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS Study. Circulation 2008, 118:S234–S242.
deFilippi CR, Willett DL, Brickner ME, et al.: Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. Am J Cardiol 1995, 75:191–194.
Blais C, Dumesnil JG, Baillot R, et al.: Impact of prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation 2003, 108:983–988.
Kulik A, Burwash IG, Kapila V, et al.: Long-term outcomes after valve replacement for low-gradient aortic stenosis: impact of prosthesis-patient mismatch. Circulation 2006, 114(Suppl I):I5553–I5558.
Tribouilloy C, Levy F, Rusinaru D, et al.: Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009, 53:1865–1873.
•• Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P: Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007, 115:2856–2864. This is the first study to describe this new clinical entity of paradoxical low-flow, low-gradient severe AS with preserved LVEF. This is probably the article in this list that should be read in priority when one wants to learn more about this particular topic.
• Cramariuc D, Cioffi G, Rieck AE, et al.: Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc Imaging 2009, 2:390–399. This is an important study that confirms the results of Hachicha et al. [9••] in a large prospective series of asymptomatic patients.
Pibarot P, Dumesnil JG: Aortic stenosis: look globally, think globally. JACC Cardiovasc Imaging 2009, 2:400–403.
• Dumesnil JG, Pibarot P, Carabello B: Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010, 31:281–289. This is an interesting viewpoint article of paradoxical low-flow, low-gradient AS. It also provides important additional results of the study by Hachicha et al. [9••].
• Barasch E, Fan D, Chukwu EO, et al.: Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis 2008, 17:81–88. This is an important study showing that patients with normal LVEF, low-flow, low-gradient. AS have a 50% lower referral to surgery but a twofold increase in mortality.
•• Weidemann F, Herrmann S, Stork S, et al.: Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009, 120:577–584. This is a very important and comprehensive study showing that patients with small AVA, normal LVEF, and low gradient have more myocardial fibrosis and reduced myocardial systolic function.
Strotmann JM, Lengenfelder B, Blondelot J, et al.: Functional differences of left ventricular hypertrophy induced by either arterial hypertension or aortic valve stenosis. Am J Cardiol 2008, 101:1493–1497.
• Hachicha Z, Dumesnil JG, Pibarot P: Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol 2009, 54:1003–1011. This is an important study that demonstrates the usefulness of the valvuloarterial impedance to estimate the global hemodynamic load and to predict outcomes in patients with AS.
Gerdts E: Left ventricular structure in different types of chronic pressure overload. Eur Heart J 2008, 10(Suppl. E):E23–E30.
Pagé A, Dumesnil JG, Clavel MA, et al.: Metabolic syndrome is associated with more pronounced impairment of LV geometry and function in patients with calcific aortic stenosis: a substudy of the ASTRONOMER trial. J Am Coll Cardiol 2009 (in press).
Dumesnil JG, Shoucri RM: Quantitative relationships between left ventricular ejection and wall thickening and geometry. J Appl Physiol 1991, 70:48–54.
Briand M, Dumesnil JG, Kadem L, et al.: Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol 2005, 46:291–298.
Pai RG, Varadarajan P, Razzouk A: Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008, 86:1781–1789.
Dumesnil JG, Dion D, Yvorchuk K, et al.: A new, simple and accurate method for determining ejection fraction by Doppler echocardiography. Can J Cardiol 1995, 11:1007–1014.
Garcia D, Kadem L: What do you mean by aortic valve area: geometric orifice area, effective orifice area, or gorlin area? J Heart Valve Dis 2006, 15:601–608.
•• Minners J, Allgeier M, Gohlke-Baerwolf C, et al.: Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J 2008, 29:1043–1048. This is a very important study showing that there are inconsistencies in the criteria of AVA and mean gradient proposed in the guidelines to grade severe AS.
Orsinelli DA, Aurigemma GP, Battista S, et al.: Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. A high risk subgroup identified by preoperative relative wall thickness. J Am Coll Cardiol 1993, 22:1679–1683.
Duncan AI, Lowe BS, Garcia MJ, et al.: Influence of concentric left ventricular remodeling on early mortality after aortic valve replacement. Ann Thorac Surg 2008, 85:2030–2039.
Aortic valve calcification measured by multislice computed tomography in aortic stenosis - correlation with hemodynamic severity and clinical implication for patients with low ejection fraction. Cueff C, Serfaty JM, Cimadevilla C, et al. Eurecho 2009. Madrid December 10, 2009. Eur J Echocardiogr 2009, 10: ii45-ii73 [Abstract].
Acknowledgment
Dr. Philippe Pibarot holds the Canada Research Chair in Valvular Heart Disease, Canadian Institutes of Health Research (CIHR), Ottawa, Ontario, Canada. This work is funded by a research grant (#MOP 57445) from CIHR.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pibarot, P., Dumesnil, J.G. Low-Flow, Low-Gradient, Normal Ejection Fraction Aortic Stenosis. Curr Cardiol Rep 12, 108–115 (2010). https://doi.org/10.1007/s11886-010-0090-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-010-0090-0