Abstract
Bioprosthetic surgical valves have shorter durability compared to mechanical devices and the risk of reoperation is often prohibitively high. Contemporary transcatheter valves have been, and will continue to be, successfully deployed within failing surgical prostheses utilizing a valve-in-valve technique. Detailed knowledge of the physical characteristics of the in situ surgical prosthesis is crucial for procedural success. Currently, the internal diameter of the surgical valve is the most important dimension used to size transcatheter valves. Unfortunately, there is significant variability in both labeling and reported dimensions between surgical valve manufacturers with no accepted gold standard. Multiple novel transcatheter valve systems are currently being developed for valve-in-valve applications that utilize a variety of innovative sizing criteria. Imaging plays a key role in all aspects of valve-in-valve implantation. Multidetector computed tomography (MDCT) can help size existing surgical prosthesis and is proving invaluable for next-generation device development and refinement. Although echocardiography provides important periprocedural guidance for radiolucent surgical prostheses, a detailed understanding of the radiopaque surgical landmarks is crucial for correct valve-in-valve positioning and thus successful implantation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Bioprosthetic valves are commonly utilized when there is need to reduce thromboembolic risk and avoid bleeding associated with anticoagulation [1]. However the durability of bioprosthetic valves is less than mechanical prostheses [2, 3] and reoperation for failing bioprostheses is the current standard of care [4–6] (Fig. 32.1). Unfortunately, patients requiring reoperation are at higher risk for adverse events with rates in reported series ranging from 4 to 20 % [7–11]. The use of transcatheter valve technology to treat degenerating surgical bioprostheses (the “valve-in-valve” procedure) has paralleled the growth of transcatheter aortic valve replacement (TAVR) in native valve disease. Multiple case series have reported excellent 30-day outcomes [12–27] and, more recently, the Global Valve-In-Valve Registry reported stable hemodynamics and persistent improvement in functional status at 12 months (Fig. 32.2).
It is recognized that the valve-in-valve procedure is technically challenging and requires appropriate case and device selection. Imaging plays a key role in all aspects of valve-in-valve implantation. Multidetector computed tomography (MDCT), echocardiography, and periprocedural angiography to evaluate the radiopaque surgical landmarks are all crucial for correct valve-in-valve sizing and positioning and thus, successful implantation. We will review imaging requirements for valve-in-valve implantation for failed surgical bioprostheses. Valve-in-valve implantation for failed or malpositioned transcatheter valves is a separate topic and not discussed.
Surgical Valves
Bioprosthetic surgical valves have consistent, reproducible leaflet kinetics as the leaflets are mounted in a rigid framework. The wide variety of surgical valves available can be broadly classified into stented and stentless devices (Tables 32.1 and 32.2). These groups have unique features that must be considered for valve-in-valve sizing and positioning. Conventional bioprosthetic valves incorporate bovine or porcine pericardium incorporated into a stent frame or stentless conduit.
Stented valves have a support structure composed of various alloys and plastics attached to a basal ring covered by a fabric sewing cuff (Fig. 32.3). The basal ring may be circular or scallop shaped and newer designs incorporate low profiles or supra-aortic design features. The support structures define the internal, outer, and external diameter of the valve. The support structures are generally radiopaque and well visualized on computed tomography during assessment of the internal diameter of the in vivo surgical prosthesis as well as on angiography during positioning and implantation. The rigid frame of most bioprostheses facilitates transcatheter valve positioning and paravalvular sealing while reducing the risk of atrioventricular block, annular rupture, and coronary obstruction. It is important to recognize two important exceptions: the Sorin Mitroflow valve (Sorin, Vancouver, BC, Canada) and the St. Jude Trifecta valve (St. Jude, St. Paul, MN). In contrast to other stented valves, these valves have leaflets mounted outside the stent to maximize the orifice area. During valve-in-valve implantation the leaflets are not constrained by the stent frame and thus may extend to the aortic wall causing obstruction of the coronary ostia.
Stented bioprosthetic surgical valves. Top row (left-right): Soprano (Sorin Biomedica), Magna (Edwards Lifesciences), Perimount (Edwards Lifesciences), Mosaic (Medtronic). Bottom row (left-right): Hancock Ultra (Medtronic), Mitroflow (Sorin Biomedica), Trifecta (St. Jude Medical), Epic (St. Jude Medical)
Stentless valves may be autograft, heterograft, or homograft and are sutured into the aortic root in the position of the native valve, thus eliminating the need for the support structures (Fig. 32.4). However, the stentless valve also lacks radiopaque elements to guide positioning and anchor the new transcatheter valve. Positioning with fluoroscopy relies on the presence of leaflet calcification.
Transcatheter Valves
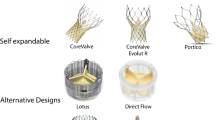
Currently three transcatheter device systems have been used for valve-in-valve intervention (Fig. 32.5). Importantly these valves differ fundamentally from surgical valves as the leaflet kinematics is determined by valve expansion.
SAPIEN/XT Balloon Expandable Valves
The Edwards SAPIEN and SAPIEN XT valve is a balloon expandable valve with bovine pericardium leaflets on a radiopaque cobalt chromium frame. The current SAPIEN XT device is available in 20, 23, 26, and 29 mm sizes and can be implanted in valves with internal diameters of 16–27 mm; however, caution is warranted when implanting within small surgical prostheses as the residual transvalvular gradient can be significant. Small-diameter surgical bioprostheses (particularly ≤19 mm) may not allow for optimal expansion of current transcatheter implants. The strut height of the SAPIEN XT valve post-deployment ranges from 13.5 to 19.1 mm making this device suitable for implantation in all four valve positions.
CoreValve Prostheses
The Medtronic CoreValve has three porcine leaflets mounted on a self-expandable nitinol stent. It is currently available in two sizes: 26 mm (accommodates 20–23 mm diameter) and 29 mm (accommodates 23–27 mm diameter). The valve can only be mounted one way in the restraining sheath, hence the device can only be deployed retrograde. The length of this device makes it unsuitable for implantation in the mitral position.
Melody Valve
The Melody transcatheter valve (Medtronic Inc) is a bovine jugular venous valve attached to a platinum iridium scaffold. Originally designed to treat dysfunctional right ventricular outflow tract conduits, this valve has been deployed in the pulmonic and tricuspid position.
Imaging for Valve Sizing
Surgical stented valves have labeled sizes from 18 to 29 mm and stentless 19–25 mm depending upon the manufacturer. However these sizings are not standardized [28, 29] and may often refer to the internal or outer diameter for stented valves, or external diameter for stentless valves (Fig. 32.6). In this regard, operative reports and reference tables of surgical valve dimensions are crucial for planning. In cases where the type of prosthesis is unknown, computed tomography or transesophageal echocardiography may help clarify valve geometry.
The principle of oversizing used in transcatheter intervention of native valve aortic stenosis [30] also applies to valve-in-valve intervention. It is the internal diameter that is most relevant for valve-in-valve therapy as an implant is chosen with a nominal external diameter matching the internal diameter of the failed valve. Importantly, the internal diameter of surgical valves can vary by up to 10 % from the labeled size. Within stented prostheses the sewing ring and stent posts can constrain an oversized transcatheter valve, while stentless valves often need 2–3 mm oversizing to achieve stability. Currently, optimal transcatheter valve function requires expansion of the prosthesis to its nominal dimensions. A transcatheter valve implanted within a smaller surgical prosthesis can be expected to function suboptimally with increased transvalvular gradient, impaired leaflet coaptation, and reduced durability. Matching of an appropriate size transcatheter valve to the surgical valve is thus crucial as excessive oversizing, especially in small-diameter surgical prostheses (<19 mm), may lead to lack of expansion of the transcatheter valve resulting in high-residual transvalvular gradients [31, 32]. While the acceptable boundaries of under-expansion remain undefined, a recent case report utilizing a 20 mm SAPIEN XT valve within a 16 mm internal diameter surgical prosthesis for severe regurgitation resulted in only a modest residual transvalvular gradient. Further study is warranted as there remains limited long-term evidence documenting durability of valve-in-valve implantations.
Imaging the In Vivo Valve
Understanding the mechanism of surgical valve failure is of significant relevance to planning a valve-in-valve procedure. Bioprosthetic valves may fail due to leaflet or non-leaflet pathologies. Leaflet failure is most commonly due to degeneration or calcification. Often as valves degenerate, calcific deposits form within the leaflet tissue at sites of high stress (commissural and leaflet attachment points). Valves with significant calcification or pannus may have reduced internal diameters relative to ex vivo sizing and imaging of the valve to assess leaflet bulkiness, as well as the extent and location of pannus and calcification can be used when considering sizing of the transcatheter valve. Cardiac CT and transesophageal echo are both useful modalities for assessing leaflet morphology (Fig. 32.7).
In addition to stenosis, bioprosthetic valves may develop significant regurgitation due to leaflet degeneration, endocarditis, or paravalvular leaks. The latter must be distinguished from other causes of valvular regurgitation as it cannot be ameliorated by valve-in-valve intervention (Fig. 32.8).
Anatomic Suitability for Valve-in-Valve Procedures
Assessment of the in vivo valve is important for sizing and predicting leaflet function. Adequate expansion, symmetry, and circularity is needed for optimal function [33], and the presence of significant bulkiness or calcification could lead to a suboptimal result. The geometry of the aortic root, the relationship of the prosthesis, and leaflet height in relation to the coronary ostia as well as the size of the sinotubular junction are relevant in pre-procedure planning (Fig. 32.9). Typically, however, as the transcatheter valve is constrained within the structure of the surgical prosthesis, there is less concern with coronary obstruction, annular rupture, and heart block with valve-in-valve implantation compared to native valve intervention (Fig. 32.10).
Imaging for Access and Delivery
Access routes for valve-in-valve intervention are the same as those for conventional TAVR. Both transapical and transfemoral access are currently used for aortic valve-in-valve implantation. Transapical access currently remains the preferred route for mitral valve-in-valve interventions as it provides direct coaxial alignment. A variety of percutaneous and surgical routes have been used for tricuspid and pulmonic valve-in-valve procedures both within native valves and surgically implanted right heart conduits. Regardless of the route selected, coaxial alignment is critical for valve-in-valve implantation success. Surgical bioprosthetic valves have the benefit of radiopaque annular and strut frames [34] making the optimal angle for deployment easier to ascertain than with native valve disease [35, 36] (Figs. 32.11 and 32.12).
Positioning of the new prosthesis is dependent on the model used (Fig. 32.13). The CoreValve may be deployed in a supra-annular position to optimize the hemodynamics. Conversely, the SAPIEN XT must overlap to extend below the bioprosthetic annulus to splay the struts and prevent embolization. As the height of the deployed SAPIEN XT valve may be up to 3 mm shorter than when crimped on the balloon, the positioning must account for the transcatheter valve struts engaging the tissue valve and concertina shortening occurring on the ventricular side. Adequacy of deployment can be determined by intraprocedural TEE, and subsequent TTE and CT (Fig. 32.14).
Deployment of a SAPIEN XT inside a Hancock II surgical prosthesis. Injection in the aortic root demonstrates a coaxial angle for deployment. The radiopaque markers of the prosthesis and the cusps of the aortic root are aligned (a). The SAPIEN XT is positioned using the markers and aortic cusps as a guide (b). The transcatheter valve is deployed (c) and repeat injection confirms optimal positioning (d). Note the splaying of the frame on the ventricular side
Assessing optimal transcatheter valve-in-valve deployment. Intraprocedural TEE shows good valve orifice (a) with no aortic regurgitation (b). Subsequent CT shows a well expanded circular frame within the surgical struts (c) and transthoracic echocardiography demonstrates mild late paravalvular regurgitation (d)
Novel Transcatheter Systems for Valve-in-valve Implantation
Multiple novel transcatheter valve systems are currently being developed for valve-in-valve applications that utilize a variety of innovative sizing criteria. Although still being refined, these systems will need to be easily deliverable and repositionable while maximizing the effective orifice area and minimizing both the residual gradient and inter- or transvalvular regurgitation. As with existing transcatheter systems, sizing will still be individualized for each patient, taking into consideration the reported internal diameter, patient size, bulkiness of the degenerated valve, nature of the valve failure, and the location of calcification or pannus. MDCT is proving invaluable for next-generation device development and refinement.
Conclusion
Transcatheter valve-in-valve intervention is becoming an acceptable alternative to standard surgical reoperation in select high-risk patients with degenerated surgical bioprostheses. Valve-in-valve procedures can be performed using current transcatheter valve devices using similar access and imaging to native valve interventions. The structural elements of the surgical prosthesis often limit the risk of complications due to coronary obstruction, annular rupture, and atrioventricular heart block. However, optimal transcatheter valve function requires expansion of the prosthesis to its nominal dimensions. Knowledge of the in situ surgical prosthesis is therefore crucial for procedural success. MDCT imaging is useful for identifying and sizing the in vivo surgical prostheses. MDCT also clearly defines adjacent anatomical structures important for valve-in-valve positioning. Echocardiography and angiography provide crucial periprocedural guidance during valve-in-valve implantation within both radiolucent and radiopaque surgical prostheses. Further study is warranted to assess the long-term durability of valve-in-valve implantation within degenerated surgical prostheses in all four valve positions.
References
Ruel M, Chan V, Bedard P, Kulik A, Ressler L, Lam BK, Rubens FD, Goldstein W, Hendry PJ, Masters RG, Mesana TG. Very long-term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults <60 years of age. Circulation. 2007;116(11 Suppl):I294–300.
Chikwe J, Filsoufi F. Durability of tissue valves. Semin Thorac Cardiovasc Surg. 2011;23(1):18–23.
Grunkemeier GL, Jamieson WR, Miller DC, Starr A. Actuarial versus actual risk of porcine structural valve deterioration. J Thorac Cardiovasc Surg. 1994;108(4):709–18.
Balsam LB, Grossi EA, Greenhouse DG, Ursomanno P, Deanda A, Ribakove GH, Culliford AT, Galloway AC. Reoperative valve surgery in the elderly: predictors of risk and long-term survival. Ann Thorac Surg. 2010;90(4):1195–200; discussion 1201.
Jones JM, O’Kane H, Gladstone DJ, Sarsam MA, Campalani G, MacGowan SW, Cleland J, Cran GW. Repeat heart valve surgery: risk factors for operative mortality. J Thorac Cardiovasc Surg. 2001;122(5):913–8.
Maganti M, Rao V, Armstrong S, Feindel CM, Scully HE, David TE. Redo valvular surgery in elderly patients. Ann Thorac Surg. 2009;87(2):521–5.
Leontyev S, Borger MA, Davierwala P, Walther T, Lehmann S, Kempfert J, Mohr FW. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg. 2011;91(4):1120–6.
Beurtheret S, Gariboldi V, Feier H, Grisoli D, Riberi A, Mouly-Bandini A, Metras D, Kerbaul F, Collart F. Short-term results of repeat valve replacement: a predictive factor analysis. J Heart Valve Dis. 2010;19(3):326–32.
Lytle BW, Cosgrove DM, Taylor PC, Gill CC, Goormastic M, Golding LR, Stewart RW, Loop FD. Reoperations for valve surgery: perioperative mortality and determinants of risk for 1,000 patients, 1958–1984. Ann Thorac Surg. 1986;42(6):632–43.
Piehler JM, Blackstone EH, Bailey KR, Sullivan ME, Pluth JR, Weiss NS, Brookmeyer RS, Chandler JG. Reoperation on prosthetic heart values. Patient-specific estimates of in-hospital events. J Thorac Cardiovasc Surg. 1995;109(1):30–48.
Kirsch M, Nakashima K, Kubota S, Houel R, Hillion ML, Loisance D. The risk of reoperative heart valve procedures in Octogenarian patients. J Heart Valve Dis. 2004;13(6):991–6; discussion 996.
Boone RH, Webb JG, Horlick E, Benson L, Cao QL, Nadeem N, Kiess M, Hijazi ZM. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheter Cardiovasc Interv. 2010;75(2):286–94.
Cerillo AG, Chiaramonti F, Murzi M, Bevilacqua S, Cerone E, Palmieri C, Del Sarto P, Mariani M, Berti S, Glauber M. Transcatheter valve-in-valve implantation for failed mitral and tricuspid bioprosthesis. Catheter Cardiovasc Interv. 2011;78(7):987–95.
Cheung AW, Gurvitch R, Ye J, Wood D, Lichtenstein SV, Thompson C, Webb JG. Transcatheter transapical mitral valve-in-valve implantations for a failed bioprosthesis: a case series. J Thorac Cardiovasc Surg. 2011;141(3):711–5.
de Weger A, Tavilla G, Ng AC, Delgado V, van der Kley F, Schuijf JD, Bax JJ, Klautz RJ. Successful transapical transcatheter valve implantation within a dysfunctional mitral bioprosthesis. JACC Cardiovasc Imaging. 2010;3(2):222–3.
Gotzmann M, Mugge A, Bojara W. Transcatheter aortic valve implantation for treatment of patients with degenerated aortic bioprostheses–valve-in-valve technique. Catheter Cardiovasc Interv. 2010;76(7):1000–6.
Kempfert J, Van Linden A, Linke A, Borger MA, Rastan A, Mukherjee C, Ender J, Schuler G, Mohr FW, Walther T. Transapical off-pump valve-in-valve implantation in patients with degenerated aortic xenografts. Ann Thorac Surg. 2010;89(6):1934–41.
Khambadkone S, Coats L, Taylor A, Boudjemline Y, Derrick G, Tsang V, Cooper J, Muthurangu V, Hegde SR, Razavi RS, Pellerin D, Deanfield J, Bonhoeffer P. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112(8):1189–97.
Khawaja MZ, Haworth P, Ghuran A, Lee L, de Belder A, Hutchinson N, Trivedi U, Laborde JC, Hildick-Smith D. Transcatheter aortic valve implantation for stenosed and regurgitant aortic valve bioprostheses CoreValve for failed bioprosthetic aortic valve replacements. J Am Coll Cardiol. 2010;55(2):97–101.
Maroto LC, Rodriguez JE, Cobiella J, Marcos P. Transapical off-pump aortic valve-in-a-valve implantation in two elderly patients with a degenerated porcine bioprosthesis. Eur J Cardiothorac Surg. 2010;37(3):738–40.
McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, Vincent JA. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507–16.
Montorfano M, Latib A, Chieffo A, Moshiri S, Franco A, Grimaldi A, Alfieri O, Colombo A. Successful percutaneous anterograde transcatheter valve-in-valve implantation in the mitral position. JACC Cardiovasc Interv. 2011;4(11):1246–7.
Pasic M, Unbehaun A, Dreysse S, Buz S, Drews T, Kukucka M, Hetzer R. Transapical aortic valve implantation after previous aortic valve replacement: clinical proof of the “valve-in-valve” concept. J Thorac Cardiovasc Surg. 2011;142(2):270–7.
Roberts PA, Boudjemline Y, Cheatham JP, Eicken A, Ewert P, McElhinney DB, Hill SL, Berger F, Khan D, Schranz D, Hess J, Ezekowitz MD, Celermajer D, Zahn E. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011;58(2):117–22.
Seiffert M, Franzen O, Conradi L, Baldus S, Schirmer J, Meinertz T, Reichenspurner H, Treede H. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv. 2010;76(4):608–15.
Van Garsse LA, Ter Bekke RM, van Ommen VG. Percutaneous transcatheter valve-in-valve implantation in stenosed tricuspid valve bioprosthesis. Circulation. 2011;123(5):e219–21.
Webb JG, Wood DA, Ye J, Gurvitch R, Masson JB, Rodes-Cabau J, Osten M, Horlick E, Wendler O, Dumont E, Carere RG, Wijesinghe N, Nietlispach F, Johnson M, Thompson CR, Moss R, Leipsic J, Munt B, Lichtenstein SV, Cheung A. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121(16):1848–57.
Christakis GT, Buth KJ, Goldman BS, Fremes SE, Rao V, Cohen G, Borger MA, Weisel RD. Inaccurate and misleading valve sizing: a proposed standard for valve size nomenclature. Ann Thorac Surg. 1998;66(4):1198–203.
Shibata T, Inoue K, Ikuta T, Bito Y, Yoshioka Y, Mizoguchi H. Which valve and which size should we use in the valve-on-valve technique for re-do mitral valve surgery? Interact Cardiovasc Thorac Surg. 2009;8(2):206–10.
Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Nercolini D, Tapiero S, Litzler PY, Bessou JP, Babaliaros V. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47(6):1214–23.
Azadani AN, Jaussaud N, Matthews PB, Chuter TA, Ge L, Guy TS, Guccione J, Tseng EE. Aortic valve-in-valve implantation: impact of transcatheter- bioprosthesis size mismatch. J Heart Valve Dis. 2009;18(4):367–73.
Azadani AN, Jaussaud N, Matthews PB, Ge L, Chuter TA, Tseng EE. Transcatheter aortic valves inadequately relieve stenosis in small degenerated bioprostheses. Interact Cardiovasc Thorac Surg. 2010;11(1):70–7.
Azadani AN, Tseng EE. Transcatheter heart valves for failing bioprostheses: state-of-the-art review of valve-in-valve implantation. Circ Cardiovasc Interv. 2011;4(6):621–8.
Bapat V, Mydin I, Chadalavada S, Tehrani H, Attia R, Thomas M. A guide to fluoroscopic identification and design of bioprosthetic valves: a reference for valve-in-valve procedure. Catheter Cardiovasc Interv. 2013;81:853–61.
Binder RK, Leipsic J, Wood D, Moore T, Toggweiler S, Willson A, Gurvitch R, Freeman M, Webb JG. Prediction of optimal deployment projection for transcatheter aortic valve replacement: angiographic 3-dimensional reconstruction of the aortic root versus multidetector computed tomography. Circ Cardiovasc Interv. 2012;5:247–52.
Gurvitch R, Wood DA, Leipsic J, Tay E, Johnson M, Ye J, Nietlispach F, Wijesinghe N, Cheung A, Webb JG. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3(11):1157–65.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Poulter, R., Bapat, V., Wood, D.A. (2014). Valve-in-Valve for Transcatheter Aortic Valve Replacement: Do Imaging Requirements Change?. In: Min, J., Berman, D., Leipsic, J. (eds) Multimodality Imaging for Transcatheter Aortic Valve Replacement. Springer, London. https://doi.org/10.1007/978-1-4471-2798-7_32
Download citation
DOI: https://doi.org/10.1007/978-1-4471-2798-7_32
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-2797-0
Online ISBN: 978-1-4471-2798-7
eBook Packages: MedicineMedicine (R0)