Abstract
All diurnal primates live in social groups but with a great range of variation in the types of groups they form. Primate societies vary in group size, composition, dispersal patterns, levels of cohesion, and the extent of overt social interaction or differentiation of relationships within the group. They also vary in the flexibility seen in these aspects of social organization. For example, cross-population studies as well as diachronic studies suggest that black howlers are constrained to live in highly cohesive groups of no more than 10 individuals, despite considerable variation in group composition (Pavelka and Chapman, 2006; Van Belle and Estrada, 2006). Other species, such as Muriquis, reveal considerable flexibility in group size and cohesion; an increase in population size over a 25-year period produced significantly larger social groups and a shift from cohesive to flexible fission–fusion grouping pattern in which members regularly fission and fuse into parties of ever-changing size and composition (Dias and Strier, 2003).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
All diurnal primates live in social groups but with a great range of variation in the types of groups they form. Primate societies vary in group size, composition, dispersal patterns, levels of cohesion, and the extent of overt social interaction or differentiation of relationships within the group. They also vary in the flexibility seen in these aspects of social organization. For example, cross-population studies as well as diachronic studies suggest that black howlers are constrained to live in highly cohesive groups of no more than 10 individuals, despite considerable variation in group composition (Pavelka and Chapman, 2006; Van Belle and Estrada, 2006). Other species, such as Muriquis, reveal considerable flexibility in group size and cohesion; an increase in population size over a 25-year period produced significantly larger social groups and a shift from cohesive to flexible fission–fusion grouping pattern in which members regularly fission and fuse into parties of ever-changing size and composition (Dias and Strier, 2003).
The focus of this chapter is on cohesion and the question of what holds social groups together. I also suggest two possible mechanisms of cohesion not previously considered for primates. It is traditionally assumed that primate groups are held together by the social relationships among group members. These relationships are built and maintained by affiliative interactions such as social grooming (Dunbar, 1999; Cooper and Bernstein, 2000; Seyfarth, 1983). In the 1980s Robert Hinde offered a framework for social groups in which interactions among and between individuals, over time, developed into relationships among those individuals, and the web of these relationships essentially formed the social group. This framework began with the assumption that individuals are different, and their differences shape their interactions and the relationships they form. In Hinde’s words “a first requirement for understanding the causal basis of social behavior is an understanding of how individuals differ in their propensities to behave and in their behavior with particular others” (Hinde, 1983:4). He describes a continuous dialectic between the natures of individual animals and the interactions and relationships in which they participate, and the need for us, as researchers, to know how individuals are shaped by their social experiences. “A second requirement for understanding the causal basis of social behavior is a set of principles concerned with how interactions affect subsequent interactions within the same relationship, and, more generally, with the development of relationships” (Hinde, 1983:5). In Hinde’s model, “each relationship is set in a nexus of other relationships, which mutually affect each other” and the social group is “constituted by those relationships”. In other words, interactions among individuals with different natures and life experiences lead, over time, to relationships (specifically differentiated relationships) which themselves constitute the fabric of the social group. These social relationships hold the group together; they are the mechanism of cohesion.
For many well-studied species, Robert Hinde’s framework for the foundation of social groups seems particularly apt. Japanese monkeys are a classic example of a female-bonded or resident nepotistic society in which females remain throughout their lives in their natal groups, and their social relationships are highly differentiated by kinship, dominance, personality, and individual social and reproductive history (Pavelka, 1993). Social groups are comprised of, and appear to be held together by, an array of complex interactions and relationships. Large provisioned groups with artificially enhanced and locally provided food supplies are probably somewhat inflated in terms of matriline size and social complexity, but even smaller unprovisioned Japanese monkey groups on Yakushima Island have distinctly differentiated individuals and relationships (personal observation). This type of social group, described by many who studied female-bonded cercopithecines, and characterized by Hinde, was for some time regarded as typical of primates.
It is clear now that there is no “typical” primate (Strier, 1994) and that many primate species are not female bonded. Some form groups in which females are not related, and have undifferentiated, egalitarian relationships with weak social bonds (van Schaik, 1989). However, from the perspective of Hinde’s framework, it is unclear that how undifferentiated relationships, and weak social bonds, can hold a group together. If unrelated females in a group interact little, what is the basis of the relationships? And without strong relationships among group members, what is the basis of the social group? How are groups comprised of unrelated, undifferentiated individuals held together?
This question led me to switch, over 10 years ago, from the terrestrial, provisioned, female-bonded old-world Japanese monkeys to wild, arboreal, bisexually dispersing Central American black howler monkeys in Monkey River, Belize. Compared to Japanese macaques, black howlers engage in almost no overt social interactions (within the group). Other than juvenile play (with other juveniles and with subadult males), they interact very little. They rarely groom or fight or displace each other and exchange few if any visual or vocal social signals that are detectable to observers. While they do regularly have intergroup interactions in which they roar at the neighbors (see more below), it is rare for anything to happen within the group. There are no apparent dominance hierarchies, since there are few interactions of any kind. On very rare occasions, such as when a new monkey is trying to join the group, some fighting does occur among group members, but these are rare enough to prove the general rule that they do not usually do much that is social.
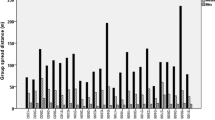
But there is the following paradox: while they engage in extremely low levels of social interaction, and thus have no visible relationships, they live in perhaps the most cohesive of primate societies. Social groups are consistently small (<10) with small group spread (rarely greater than 10 m). While resting (and they are “resting” or inactive for 60–80% of the time), it is not uncommon for the entire group to be in the same tree. They show very low levels of what are now called fission–fusion dynamics (Aureli et al., 2008) and do not form subgroups unless group size increases to beyond 10 individuals, which it rarely does. Considering them from the Hinde framework and Japanese monkey (and baboon) perspective, they beg the question – what is holding the group together in the absence of overt social interactions or social signals? What is the mechanism of group cohesion in the absence of active affiliation or coalitionary support? How do they manage extremely high within group cohesion with extremely low levels of social interaction?
I suggest the following two possible alternative mechanisms for social cohesion in species such as black howlers in which intragroup social interaction and opportunities for reciprocity and altruism are rare: behavioral synchrony and intergroup encounters. I also present data supporting a climate-related constraint on group size.
In addition to the small group size and spread, black howlers are highly synchronous in their behavior and highly coordinated in their activity. Our data show that in over 85% of scan samples, all group members are engaged in the same activity (inactive, forage, travel, social). The group almost gives the impression of being an organism in itself. Members sit tight in their sleeping tree until 9 am. Then the whole group defecates, one after another, and the whole group moves off to find food. They travel and forage together, within 10 m of one another, until they all settle, often in the same tree, for several hours of inactivity. Two individuals doing something social, such as playing, is the source of the non-synchronous scans.
Activity synchrony has not been considered as a possible mechanism of social cohesion in primates; however, studies of other mammals (e.g., Muskoxen; Cote et al., 1997; sheep, Michelena et al., 2006; Rook and Penning, 1991; and Ibex, Ruckstuhl and Neuhaus, 2001) suggest that activity synchrony, the tendency for all group members to engage in the same activity at the same time, may function to maintain cohesion. Reproductive synchrony, the widespread tendency for individuals to carry out some stage of their reproductive cycle simultaneously with other members of the population, has long been viewed as a possible antipredator strategy (Darling, 1938; Ims, 1990). Activity synchrony might function to increase cohesion and reduce predation (Ruckstuhl and Neuhaus, 2001). Synchronizing activities with other group members may be an adaptation to maintain contact with the group (Jarman, 1974) and decrease predation risk by dilution and detection effects (Dehn, 1990). Activity synchrony helps to keep members of the group in close proximity since it prevents individuals being left behind when the others become active (Dunbar and Dunbar, 1980). However, individuals of dissimilar body size to the other group members might not be able to follow their optimal activity budget if they are synchronized with other group members (Ruckstuhl, 1998, 1999). This may explain in part why males and females in sexually dimorphic social ungulates segregate into single sex groups outside the breeding season (Main et al., 1996). Within these groups, individual activity budgets are similar and synchrony of activities is generally high (Conradt, 1998; Ruckstuhl, 1998, 1999; Conradt and Roper, 2000).
In terms of cohesion, black howlers may be contrasted with the highly dispersed fission–fusion societies of chimpanzees and spider monkeys, in which community members form subgroups of changing size and composition in response to food availability and predation risk. Black howlers and spider monkeys represent the ends of a continuum, with the highly cohesive howlers at one end and the highly dispersed spiders at the other, with much variation in between. Recognition of flexibility in intragroup cohesion over time (e.g., Muriquis above) and of variation in grouping patterns in other primate species (e.g., Hamadryas baboons range by day in stable cohesive one-male units that fuse into “super-troops” at night) is leading to a rethinking of the dichotomous treatment of cohesive versus fluid groups and to increased interest in the range of fission–fusion dynamics (FFD) that may exist within and between species and the conditions that produce them. In a recent paper in Current Anthropology, Filipo Aureli et al. (2008) have suggested that social systems characterized by fluid (high FFD) versus cohesive (low FFD) grouping patterns may be qualitatively different in their socioecology, social interactions, and cognitive abilities. Different levels of cohesion, or fission–fusion dynamics, may play an important role in determining the kinds of inter-individual behaviors seen in different groups.
Do high or low cohesion levels favor the evolution of complex social signals and intragroup dynamics, including, for example, the evolution of altruism or reciprocity? On the one hand, high fission–fusion dynamics would presumably require complex social skills as group members are constantly negotiating and renegotiating (sub)group entrance and membership. Individuals have to be able to keep track of a large number of group members and relationships without the benefit of regular contact. On the other hand, where fission–fusion dynamics are high, individuals may not need to develop such high levels of social skill and complexity since they can use subgroup formation to get away from one another. Fission–fusion dynamics are seen first and foremost as a means to avoid direct competition for food and would likewise make it possible to avoid many kinds of social interactions. Animals in captivity often display patterns, such as reconciliation, that are absent in their wild conspecifics. In this vein, tightly cohesive groups such as black howlers might be expected to show fairly complex intragroup dynamics and social signaling. But they do not.
To address the question of what kinds of grouping require what kind of interactions and signals, Aureli et al. (2008) have envisioned a social landscape with two axes (degree of cohesiveness and degree of differentiation of social relationships) and four quadrants. From the examples given in each of the quadrants, it seems that the most socially and cognitively complex primates fall into the category of least cohesive (high FFD) with highly differentiated relationships (quadrant IV). Chimpanzees, spider monkeys, and humans fit this description and thus fall into this quadrant. Humans, for example, have highly differentiated social relationships and social signals, yet are highly dispersed in terms of spatial cohesion. Whether in traditional foraging or modern societies, group members split up and spread out during the daytime, although they may regroup regularly at night when the risk of predation is highest. Primates characterized by high cohesion (lower FFD) and highly differentiated relationships also exist (quadrant III). Many of the classic female-bonded or resident nepotistic species, such as macaques, baboons, and cebus monkeys, fall into this category. But what of the situation described in this chapter for black howlers: high cohesion combined with undifferentiated relationships and few social signals? In the social landscape of Aureli et al., this category exists but no primate examples are given. The only example in the most cohesive and least differentiated quadrant is schooling fish. Are black howler monkeys the primate equivalent of schooling fish? High levels of synchrony maintain cohesion and act as a predator avoidance strategy in schooling fish. If there are many biological and behavioral mechanisms that humans and nonhumans primates use to reinforce social and cooperative behavior, then behavioral and activity synchrony could be such a mechanism, operating in the absence of strong bonds, differentiated relationships, and complex social signals within the group.
Model of social landscapes related to amount of cohesion (from Aureli et al. 2008)
There is also no real evidence of predation on the black howler monkey groups at Monkey River. The monkeys exhibit very low levels of vigilance and despite the presence of jaguars in the area, seem completely undisturbed by and even uninterested in researchers moving around on the forest floor beneath them. Furthermore, disappearances of individuals in these very stable social groups are rare. The apparent absence of predation combined with the very low levels of social interactions and relationships begs the question as to why the monkeys do not wander off to forage on their own. What is holding them together? The absence of within-group feeding competition may reduce the costs of living in a group but in itself does not explain what holds them together. Here one may need to look to the deep at neurophysiological adaptations for sociality that are the subject of other chapters in this volume. It may be as “simple” as the monkeys feeling anxious when alone and thus preferring the company of known others (Louise Barrett, personal communication).
Another of the multiple mechanisms for sociality may be, as social and cultural anthropologists have long known, a common foe. The low levels of intragroup interaction of any kind in black howlers contrast with the quite high levels of intergroup interaction. On an almost daily basis, group members engage in howling bouts with adjacent groups. These can be quite dramatic and they are certainly loud. Primarily adult males, but often adult females as well, will lunge and roar at the neighbors, who are doing the same to them. Many researchers have attempted to identify the cause or function of intergroup encounters, all basically assuming that these interactions relate first and foremost to between group feeding and reproductive competition. Results are mixed and many hypotheses exist to explain what might be the motivation for individuals to engage in such energetically costly behavior, which also brings the risk of injury. Observations of intergroup encounters in the black howlers of Monkey River are particularly puzzling in that they do not appear to result in any clear winners or losers. These dramatic encounters are generally followed by both groups settling down to a period of peaceful inactivity, often in adjacent trees. This may last for several hours after which one or both groups moves off without incident. They occur in areas of home range overlap, which are quite large, and thus within the normal home range of both groups. Notably, the members of each group gather in a small area with small group spread. In other words, the encounter appears to function to increase bonding and cohesion within each group and to have few if any consequences in terms of territory holding, access to food supplies, or changes in group membership. In this regard, apparently agonistic intergroup encounters in the highly cohesive yet highly undifferentiated black howlers may have more in common with sporting events than with warfare in humans.
Why do black howlers have such undifferentiated social relationships? Possibly because they are folivores, with leaves comprising 60% of their annual diet. Socioecological models of primate social groups (Wrangham, 1980; van Schaik, 1989; Sterck et al., 1997) have predicted that folivores should be characterized by female dispersal and subsequent undifferentiated relationships between and among the unrelated adults in the group, such as we see in the bisexually dispersing and egalitarian black howlers. The evenly distributed food supply also permits the spatial cohesion that we see, there being no reason for the animals to spread out to find food. Even during periods of high frugivory in our study groups, small group size means that all members can feed together in a patch. So the consistently small groups make it much easier to be spatially cohesive. But the small group size is hard to explain, since we have found no evidence of feeding competition even in the largest of our study groups (Knopff and Pavelka, 2006). The small group size makes it possible for them to remain tightly cohesive, but what enforces the small group size if not feeding competition?
The consistently small yet unexplained group size in Alouatta pigra, compared with its geographic neighbor and close relative Alouatta palliata (that live in groups of up to 40 individuals), was one of the main factors leading to its designation as a separate species (Smith, 1970). A. palliata is found throughout Central America while A. pigra has a very limited distribution on the eastern side of the Yucatan peninsula in Belize, Mexico, and Guatemala. It is not clear why there are two such closely related species living side by side in such a small geographic area.
In October of 2001, 3 years after we began the black howler study in southeastern Belize, the study site was devastated by Hurricane Iris.
The population was immediately reduced by 40% and continued to decline for 3 years before stabilizing (Pavelka et al., 2003; Pavelka et al., 2007; Pavelka and Behie, 2008). Prior to the storm, eight stable social groups (53 monkeys) lived in adjacent overlapping home ranges within a 52-ha study area. The storm devastated the forest and the monkey population. The period of 12 weeks after the storm is best described as a period of social chaos with solitary monkeys and small unstable groups wandering throughout and in and out of the area. All food sources and known arboreal pathways were gone and many individuals were gone. After 12 weeks, social groups began to coalesce. We do not know to what extent these groups were new or contained fragments of the pre-hurricane groups. Interestingly, while social groups had reestablished themselves after 12 weeks, these groups did not settle into stable home ranges for almost a year.
The effect of the storm on social behavior was harder to document, since for several months we were unable to enter the forest. All trails had been destroyed and deadfall, including many large fallen trees, and made access impossible. Procuring local assistance to open new trails proved difficult since the local human population was dealing with their own homelessness and lack of power or water. Our observations for the first 3–4 months were limited to what we could see from the river and the road that border the site, and did not permit scrutiny of intragroup dynamics, although we did observe a severe fight between two adult males that led to the death of one. For the remainder of that next year, we did have good focal animal data and these show no significant change in the amount of time spent in social contact. The primary change was a significant increase in the time spent inactive (Behie and Pavelka, 2005) which may have been due to the changes in the food supply (Pavelka and Behie, 2005), which led to complete folivory where the monkeys had previously consumed almost 40% fruit annually. Furthermore, the low population density and unstable home ranges lead to less contact between social groups, if frequency of howling bouts is taken as an indication of these. Certainly the forest became a comparatively quiet place compared to before the storm. It may be that without stable home ranges, intergroup encounters were more risky, with outcomes less predictable. The newly formed groups may have opted to avoid each other in the forest for that first year before home ranges stabilized.
In addition to the dramatic reduction in population and group density, another effect of Hurricane Iris was a significant reduction in the size of the already small groups (from a modal group size of eight before the storm to a maximum group size of five for several years after). In 1999, Pat Wright suggested that there might be a connection between cyclones in the north of Madagascar and the consistently small group sizes found in lemur species in this area (Wright, 1999). This led us to ask the following question: is group size and species distribution in A. pigra related to the weather patterns in their range? We conducted a preliminary investigation of hurricane tracks in and around Central America over the past 150 years (NOAA, 2007), which shows a concentration of hurricanes crossing onto land in precisely the range of A. pigra (see maps below). In fact, since 1858, 315 Atlantic hurricanes and tropical storms have crossed into coastal regions populated by A. pigra (825 km of coastline), where only 120 have crossed in the range of A. palliata (1360 km of coastline), producing a hurricane to coastline ratio of 0.382 for A. pigra and only 0.088 for A. palliata.
These data raise the intriguing possibility that natural disasters play a role in determining, or constraining, group size and perhaps even in explaining speciation. We do not know of a mechanism by which storm frequency in an area might constrain group size; however, if group size is constrained by such an external factor, high levels of cohesion might be permitted even in a folivore and in the absence of social interactions among group members. In the case of black howlers, the small groups permit high levels of cohesion, with all group members able to feed together in a patch, and the absence of feeding competition within the group favors undifferentiated relationships. To the question of what holds such a group together under conditions of low levels of social interaction and indifferent “relationships”, activity synchrony and intergroup encounters are suggested alternate mechanisms of cohesion, working in concert with the evolved neurophysiological adaptations for sociality which are the subject of much of this volume.
References
Aureli, F., Schaffner, C.M., Boesch, C., Bearder, S.K., Call, J., Chapman, C.A., Connor, R., Di Fiore, A., Dunbar, R.I.M., Henzi, S.P., Holekamp, K., Korstjens, A.H., Layton, R., Lee, P., Lehmann, J., Manson, J.H., Ramos-Fernandez, G., Strier, K.B., and van Schaik, C.P. 2008. Fission-fusion dynamics: New research frameworks. Curr. Anthropol. 49(4):627–646.
Behie, A.M. and Pavelka, M.S.M. 2005. The short term effect of hurricane iris on the diet and activity budget of black howlers (Alouatta pigra) in monkey river, Belize. Folia Primatologica. 76(1):1–9.
Conradt, L. 1998. Could asynchrony in activity between the sexes cause intersexual social segregation in ruminants. Proc. Roy. Soc. Lond. 265:1359–1363.
Conradt, L. and Roper, T.J. 2000. Activity synchrony and social cohesion: a fisson-fusion model. Proc. Roy. Soc. Lond. 267: 2213–2218.
Cooper, M.A. and Bernstein, S.I. 2000. Social grooming in Assamese macaques (Macaca assamensis) Am. J. Primatol. 50:77–85.
Cote, S.D., Schaefer, J.A., and Messier, F. 1997. Time budgets and synchrony of activities in muskoxen: The influence of sex, age and season. Can. J. Zool. 75(10):1628–1635.
Darling, F.F. 1938. Bird flocks and breeding cycle. Cambridge University Press: Cambridge.
Dias, L.G. and Strier, K.B. 2003. Effects of group size on ranging patterns in Brachyteles arachnoides hypoxanthus. Int. J. Primatol. 24:209–221.
Dehn, M.M. 1990. Vigilance for predators: detection and dilution effects. Behav. Ecol. Sociobiol. 26:337–342.
Dunbar, R.I.M. 1999. Co-evolution of neocortx size, group size and language in humans. Biomed. Life Sci. 40(1):47–59.
Dunbar, R.I.M. and Dunbar E.P. 1980. The pairbond in klipspringer. Anim. Behav. 28:219–229.
Hinde, R.A. Ed. 1983. Primate social relationships: An integrated approach. Blackwell Scientific Publications: Boston, MA, pp. 182–190.
Ims, R.A. 1990. On the adaptive value of reproductive synchrony as a predator-swamping strategy. Am. Naturalist 136(4):485–498.
Jarman, P.J. 1974. The social organisation of antelope in relation to their ecology. Behaviour 48:215–267.
Knopff, K.H. and Pavelka, M.S.M. 2006. Feeding competition and group size in Alouatta pigra. Int. J. Primatol. 27(4):1059–1078.
Main, M.B., Weckerly, F.W., & Bleich, V.C. 1996. Sexual segregation in ungulates: new directions for research. J. Mammal. 77:449–461.
Michelena, P., Noel, S., Gautrais, J., Gerard, J.F., Deneubourg, J.L., and Bon, R. 2006. Sexual dimorphism, activity budget and synchrony in groups of sheep. Oecologia 148:170–180.
National Oceanic and Atmosheric Administration. United States Department of Commerce. Accessed 2007. http://maps.csc.noaa.gov/hurricanes/download.jsp
Pavelka, M.S.M. 1993. Monkeys of the Mesquite: The social life of the South Texas snow monkey. Kendall/Hunt Publishing Co: Dubuque Iowa.
Pavelka, M.S.M. and Behie, A.M. 2005. The effect of hurricane iris on the food supply of black howlers (Alouatta pigra) in Southern Belize. Biotropica. 37(1):102–108.
Pavelka, M.S.M. and Behie A.M. 2008. Reduction and recovery of a black howler monkey population in response to a major hurricane. International Primatological Society, Edinburgh. Primate Eye. 96:88.
Pavelka, M.S.M., Brusselers, O., Nowak, D., and Behie, A.M. 2003. Population reduction and social disorganization in Alouatta pigra following a hurricane. Int. J. Primatol. 24(2):1037–1055.
Pavelka, M.S.M. and Chapman, C., 2006. Population structure of black howlers (Alouatta pigra) in Southern Belize and responses to Hurricane Iris. In New perspectives in the study of Mesoamerican primates: Distribution, ecology, behavior, and conservation, A. Estrada, P. Garber, M.S.M. Pavelka, and L. Lueke (eds.), Developments in Primatology: Progress and Prospects, Springer, New York, pp. 143–163.
Pavelka, M.S.M., McGoogan, K., and Steffens, T. 2007. Population size and characteristics of Alouatta pigra before and after a major hurricane. Int. J. Primatol. 28(4):919–929.
Rook, A.J. and Penning, P.D. 1991. Synchronisation of eating, ruminating and idling activity by grazing sheep. Appl. Anim. Behav. Sci. 32:157–166.
Ruckstuhl, K.E. 1998. Foraging behaviour and sexual segregation in bighorn sheep. Anim. Behav. 56:99–106.
Ruckstuhl, K.E. 1999. To synchronise or not to synchronise: A dilemma in young bighorn males? Behaviour 136:805–818.
Ruckstuhl, K. and Neuhaus, P. 2001. Behavioral synchrony in IBEX groups: Effects of age, sex and habitat. Behaviour 138(8):1103–1046.
Seyfarth, R.M. 1983. Grooming and social competition in primates. In Primate social relationships: An integrated approach, R.A. Hinde (ed.), Blackwell Scientific Publications, Cambridge, MA, pp. 182–190.
Smith, J.D. 1970. The systematic status of the black howler monkey, Alouatta pigra lawrence. J. Mammal. 51:358–369.
Sterck, E.H.M., Watts, D.P., and van Schaik, C.P. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41(5):291–309.
Strier, K. 1994. The myth of the typical primate. Yearbook Phys. Anthropol. 37:233–271.
Van Belle, S. and Estrada, A. 2006. Demographic features of Alouatta pigra populations in extensive and fragmented forests. In New perspectives in the study of Mesoamerican primates, A. Estrada, P.A. Garber, M. Pavelka, and L. Luecke (eds.), Springer, New York, pp. 121–142.
Van Schaik, C.P. 1989. The ecology of social relationships amongst female primates. In Comparative sociobiology. The behavioural ecology of humans and other mammals, V. Staden R.A. Foley (eds.), Blackwell Scientific Publications, Oxford, pp. 195–218.
Wrangham, R.W. 1980. An ecological model of female-bonded primate groups. Behaviour 75:262–300.
Wright, P.C. 1999. Lemur traits and Madagascar ecology: Coping with an island environment. Yearbook Phys. Anthropol. 42:31–72.
Acknowledgments
I would like to thank Bob Sussman for inviting me to be part of this very interesting project. Also many thanks to the graduate and undergraduate students who have worked on the Monkey River project, as well the people of Monkey River for their many years of support. In particular I acknowledge Travis Steffens and Tracy Wyman who are working with me on the hurricane to coastline study mentioned briefly in this paper, and Dr. Alison Behie of Australia National University, who spent several years in the forest observing the black howler monkeys. The Monkey River project was supported by the Natural Sciences and Engineering Council of Canada (NSERC), The Province of Alberta, National Geographic, and the University of Calgary.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Pavelka, M.S.M. (2011). Mechanisms of Cohesion in Black Howler Monkeys. In: Sussman, R., Cloninger, C. (eds) Origins of Altruism and Cooperation. Developments in Primatology: Progress and Prospects, vol 36. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-9520-9_9
Download citation
DOI: https://doi.org/10.1007/978-1-4419-9520-9_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-9519-3
Online ISBN: 978-1-4419-9520-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)