Abstract
Tumor hypoxia, or the condition of low oxygen, is a key factor for tumor progression and treatment resistance. Hypoxic areas arise as a result of an imbalance between the supply and consumption of oxygen. Cellular responses to hypoxia are orchestrated through activation of the hypoxia-inducible factor family of transcription factors (HIFs). There are several approaches for detecting tumor hypoxia in head and neck cancers (HNC). Direct oxygen measurements in tissues with Eppendorf-pO2 histography have been used, but this method is invasive. Recent studies have focused on molecular markers of hypoxia, such as HIF-1 and carbonic anhydrase isozyme IX (CA-IX), and on developing noninvasive imaging techniques. Hypoxia appears to be prognostic for outcome in HNC. Several studies have shown that low pO2 in tumor, high HIF-1, Glut-1 and CA-IX expression, serum level of osteopontin correlated with treatment outcomes in HNC patients treated with RT or chemoradiotherapy. Several strategies have been used to overcome hypoxia-induced treatment resistance in HNC, such as hyperbaric oxygen treatment, accelerated radiotherapy with carbogen and nicotinamide, hypoxic cell radiosensitizers: nitroimi-dazoles, erythropoietin manipulation, and hypoxic cell cytotoxin. More recently, Micro-Environment-Vascular Normalization, HIF-1 Targeting and 18F-FMISO positron emission tomography-based intensity-modulated radiotherapy are promising methods.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Tumor hypoxia, or the condition of low oxygen, is a key factor for tumor progression and treatment resistance. Hypoxia develops in solid tumors due to aberrant blood vessel formation, fluctuation in blood flow, and increasing oxygen demands for tumor growth. Because hypoxic tumor cells are more resistant to ionizing radiation, tumor hypoxia has been recognized as a potential cause of failure when treating human solid tumors with ionizing radiation, both in experimental models and in patients with several type of cancer including head and neck cancers (HNC). The importance of hypoxia as a potential mechanism limiting the probability of cure rate in patients with HNC treated with radiation has been recognized [1].
Description of Factors Associated with Hypoxia and Potential Mechanisms of Resistance Related to Hypoxia
Hypoxic areas arise as a result of an imbalance between the supply and consumption of oxygen. In locally advanced solid tumors, the O2 consumption rate of neoplastic cells may outweigh a restricted oxygen supply and results in the development of tissue areas with low or very low O2 levels [2]. Other mechanisms are involved in the development of hypoxia in solid tumors: severe structural and functional abnormalities of tumor microvessels induce perfusion limited O2 delivery; deterioration of diffusion geometry limits oxygen penetration; tumor-associated and/or therapy-induced anemia could lead to a reduced O2 transport capacity [2].
As a consequence of these mechanisms tumor hypoxia is associated with the production of fewer radiation-induced cytotoxic free radicals, less radiation-induced DNA damage, and decreased tumor cell kill. This is called as oxygen enhancement effect. Damage to DNA is mainly induced by interaction with oxygen reacting free radicals formed by the ionization of water surrounding the DNA [3]. Typically, DNA strand breaks that are not repaired can lead to fatal chromosomal aberrations. It has been shown that oxygenated cells are 2.5–3 times more radiosensitive than hypoxic cells [3]. Hypoxic cells are also considered to be resistant to most anticancer drugs for several reasons [4]: first, hypoxic cells are distant from blood vessels and, as a result, may not be adequately exposed to some types of anticancer drugs [5]; second, cellular proliferation decreases as a function of distance from blood vessels, an effect that is at least partially due to hypoxia; third, hypoxia can select for cells that have lost sensitivity to p53-mediated apoptosis, which might lessen sensitivity to some anticancer agents; fourth; hypoxia can upregulate genes involved in drug resistance, including genes encoding P-170 glycoprotein.
Hypoxia is not only an important cause of treatment resistance but also a powerful stimulus of several critical tumor phenotypes. These discoveries have prompted to question whether the link between hypoxia and radioresistance is completely explainable by the oxygen enhancement effect as described above or, whether hypoxia also influences radiosensitivity through biological effects.
Molecular Pathways Involved in Hypoxia
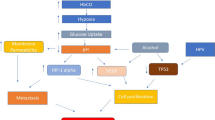
Cellular responses to hypoxia are orchestrated through activation of the hypoxia-inducible factor family of transcription factors (HIFs) [6]. HIF-1 is a heterodimer that consists of the hypoxic response factor HIF-1α and the constitutively expressed HIF-1β [7]. The level of HIF-1α expression is determined by the rates of protein synthesis and protein degradation. HIF-1α protein synthesis is regulated via O2-independent mechanisms, by the activation of the phosphatidylinositol 3-kinase (PI3K) and ERK-mitogen-activated protein kinase (MAPK) pathways [8]. These pathways can be activated by signaling via receptor tyrosine kinases, nonreceptor tyrosine kinases, or G-protein-coupled receptors.
HIF-1α protein degradation is regulated by O2-dependent prolyl hydroxylation, which targets the protein for ubiquitylation by E3 ubiquitin-protein ligases. These ligases contain the Von Hippel–Lindau (VHL) tumor-suppressor protein, which binds specifically to hydroxylated HIF-1α. Ubiquitylated HIF-1α is rapidly degraded by the proteasome. In the absence of oxygen, HIF-1 binds to hypoxia-response elements (HREs), thereby activating the expression of numerous hypoxia-response genes, such as the proangiogenic growth factor vascular endothelial growth factor (VEGF). The redox active apurinic/apyrimidinic endonuclease-1 has been shown to keep HIF-1α in a reduced state that is necessary for its transcriptional function. HIF-1 can affect several intracellular processes, including glycolysis, cell proliferation, apoptosis, angiogenesis, and invasion/metastasis – which have been shown to influence the response to radiation and might, therefore, serve as a link between HIF-1 activity and tumor radiosensitivity.
Recently, two other pathways that independently influence gene expression and processes of importance for tumor cell behavior have proved to be O2-sensitive [9]. The first occurs through regulation of an important integrator of metabolic signals, the kinase mammalian target of rapamycin (mTOR, also known as FRAP1), and its downstream effectors that orchestrate the initiation of protein synthesis, autophagy, and apoptosis sensitivity. The second is through activation of the unfolded protein response (UPR), a program of transcriptional and translational changes that occurs as a consequence of endoplasmic reticulum (ER) stress. The UPR controls multiple downstream processes, including protein production, protein maturation and degradation, cell metabolism, and cell death. HIF-, mTOR- and UPR-dependent responses to hypoxia act in an integrated way, influencing each other and common downstream pathways that affect gene expression, metabolism, cell survival, tumorigenesis, and tumor growth.
Increased HIF-1α protein synthesis was inhibited by treatment with rapamycin – a macrolide antibiotic inhibits mTOR. However, it is not known whether phosphorylation of these proteins by mTOR is necessary or sufficient for increased HIF-1α synthesis. In addition to effects on HIF-1α synthesis, activation of the RAF–MEK–ERK signaling pathway has also been shown to stimulate HIF-1α transactivation-domain function. This effect is due at least in part to phosphorylation by ERK of the co-activator p300.
A recently characterized hypoxia-induced protein, that regulated in development and DNA damage 1 (REDD1), could negatively control mTOR activity. In head and neck squamous cell carcinoma (HNSCC) cell lines, the expression of the phosphorylated forms of the mTOR downstream targets S6 kinase and S6 (pS6) decreased after hypoxia. These events were associated with REDD1 upregulation. Inhibition of AMP-activated protein kinase (AMPK) before prolonged hypoxia prevented REDD1 expression, thereby sustaining mTOR activity. Reduced mTOR activity in response to hypoxia through AMPK/REDD1 was deregulated, which hence might contribute to the persistent activation of the mTOR pathway in HNSCC cells [10].
How to Detect Hypoxia in the Tumors: Techniques to Measure Tumor Hypoxia
There are several approaches for detecting tumor hypoxia in HNC. In a recent hypoxia workshop, convened by Cancer Imaging Program of the National Cancer Institute (NCI) [11], the conclusion was that, although hypoxia is an important aspect of tumor physiology and response to treatment, there is a lack of simple and efficient methods to measure hypoxia and image oxygenation hampers further understanding and limits their prognostic usefulness. There is no gold standard for measuring hypoxia. Briefly, techniques for measuring tumor oxygenation can be categorized into two groups: direct invasive and indirect noninvasive methods. Direct oxygen measurements in tissues with Eppendorf-pO2 histography have been used, but this method is invasive. Recent studies have focused on molecular markers of hypoxia, such as HIF-1 and carbonic anhydrase isozyme IX (CA-IX), and on developing noninvasive imaging techniques. The workshop report also presented a comprehensive review of different approaches for measuring tumor hypoxia.
Electrode pO2 measurements have been used in several normal tissues, such as brain, breast, subcutis, and skeletal muscle, and these measurements have been used to develop profiles that can be illustrated by pO2 histograms reflecting the oxygenation status of a given tissue. These pO2 distribution profiles may reflect the efficacy of several oxygen supply determinants, such as blood flow rate, the blood’s oxygen transport capacity, the availability of oxygen to the cells, rate of oxygen extraction from the blood, oxygen diffusion distances, microvascular density, and oxygen diffusion coefficients within the tissue, as well as the rate of oxygen consumption by the cells. Although the microelectrode technique directly measures tumor pO2, it suffers from several drawbacks that make it difficult for general use. These include invasiveness, tumor inaccessibility, pressure dependence, interobserver variability, failure to distinguish necrosis from hypoxia, and the lack of spatial information [12].
Endogenous and secreted molecular markers for tumor hypoxia represent proteins and genes whose expressions are induced by hypoxic exposure. One of the most studied oxygen response pathways is HIF-1 pathway, HIF-1 and several of its downstream targets, including Glut-1 (glucose transporter-1), CA-IX, and VEGF, have been widely investigated as prognostic markers in HNC patients with mixed results. One advantage of endogenous markers is that levels of these proteins can be assessed on archival materials, thereby allowing possible correlation with treatment outcomes. In addition, it requires neither the injection of a hypoxic marker drug used as an exogenous nor any additional invasive procedure except the need of a biopsy at diagnosis. A possible drawback of these approaches is that these proteins can be regulated by factors other than hypoxia. Another hypoxia-related marker, the serum level of osteopontin (OPN) has also been reported recently. Le et al. [13] investigated the relationship between OPN, tumor pO2, and prognosis in patients with HNSCC, and it has been shown that Plasma OPN levels appeared to correlate with tumor hypoxia in HNSCC patients and may serve as noninvasive tests to identify patients at high risk for tumor recurrence.
Indirect approaches use injectable molecular reporters of oxygen (exogenous marker), which include 2-nitroimidazole compounds, such as misonidazole (MISO), pimonidazole (1-(2-nitro-1-imidazolyl)-3-N-piperidino-2-propanolol) [14], and EF5 (nitroimidazole [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide]) [15]. These compounds form stable adducts with intracellular macromolecules only in hypoxic regions (pO2 < 10 mmHg) [16]. Detection of these adducts with antibodies can provide information on the relative oxygenation at the cellular level [17, 18]. In general, 2-nitroimidazole markers stain for areas of chronic hypoxia and are more sensitive at severe hypoxic conditions than the microelectrode [19]. This approach is limited by the requirement for exogenous drug administration, additional biopsies for staining, and quantification of staining [20].
MRI can provide a useful way to measure hypoxia. Absolute pO2 can be measured on the basis of fluorocarbon reporter molecules. These may be introduced by direct intratumoral injection and they provide measurements consistent with electrodes (interstitial pO2). A major advantage over electrodes is that maps of regional pO2 may be measured at 50–150 individual locations simultaneously. MRI methods for interrogating tumor oxygenation are attractive since they avoid the complication of short-lived radioactivity and MRI equipment is widely available. Blood oxygen level dependent (BOLD) MRI is an imaging technique that distinguishes paramagnetic deoxy-Hb from O2Hb. BOLD MRI signal is related to vascular oxygenation and may allow direct estimates of pO2. However, the correlation becomes difficult for small blood vessels where partial-volume effects combine vessel and tissue in individual voxels and BOLD may also be confounded by flow effects [21]. Oxygen-sensitive MR reporter molecules have also been developed, generally based on perfluorocarbons (PFCs). Other MRI-based imaging such as, FREDOM (fluorocarbon relaxometry using echoplanar imaging for dynamic oxygen mapping) and PISTOL (proton imaging of silanes for tissue oxygen levels) are also under investigation [21].
Positron emission tomography (PET)-based hypoxia imaging has also been widely over the past 15 years. 18F-fluoro-misonidazole [1-(2-nitroimidazolyl)-2-hydroxy-3-fluoropropane; 18F-FMISO], is the most widely used PET agent for mapping regional hypoxia [21]. Because 18F-FMISO partitions nearly equally between octanol and water, normoxic tissues have tissue-to-blood ratio (T/B) pixel values of almost 1.0. When the O2 supply is adequate, most tissues have relatively similar levels of 18F as in the blood. The hypoxic part of a tumor can be characterized by the maximum T/B value or by the total number of pixels with T/B greater than same threshold. 18F-FMISO PET could identify hypoxic tissue that is heterogeneously distributed within human tumors and can help to facilitate image-directed radiotherapy. 18F-FMISO imaging has also been used to identify postradiotherapy tumor recurrence by differential uptake of tracer. A significant correlation was found between hypoxic tissue identified by 18F-FMISO and both pimonidazole and CA-IX, detected by immunohistochemical staining techniques. Several other compounds have also been evaluated as imaging agents [21]. 18F-fluoro-erythro-nitroimidazole (18F-FETNIM) has been evaluated in HNC. A derivative that is more hydrophilic than 18F-FMISO, 18F-fluoro-azomycin-arabinofuranoside (18F-FAZA), has been shown to be promising for clinical use, as it is the 18F-fluoro-etanidazole (18F-FETA) and EF5. An alternative PET agent for hypoxia is based on a metal complex of radioactive copper with ATSM, diacetyl-bis(N4-methylthiosemicarbazone) [21]. Dithiosemicarbazones have antitumor properties that are enhanced when they are complexed with Cu(II). Because there are several advantageous imaging radionuclides of copper, this has led several laboratories to develop substituted ligands of dithiosemicarbazones as potential imaging agents. Cu-ATSM uptake is more rapid than 18F-FMISO uptake, and the reported hypoxic to normoxic ratio is greater. One concern is that, because of its lipophilicity, the early uptake and washout of Cu-ATSM is probably influenced by regional blood flow, which is a major confounder with hypoxia [21]. Nevertheless, Cu-ATSM is finding a useful role in several clinical settings.
Hypoxia and Clinical Outcomes in Head and Neck Cancers
Hypoxia appears to be prognostic for outcome in HNC, with data suggesting that hypoxia is prognostic for survival and local control. Several studies have shown that low pO2 in tumor, defined by either the median value or the hypoxic fraction, correlated with treatment outcomes in HNC patients treated with RT or chemoradiotherapy [22–24]. Brizel et al. [24] reported 63 HNC patients with pretreatment tumor oxygen assessment, including primary site and lymph nodes. Hypoxia (tumor median pO2, 10 mmHg) adversely affected 2 year local–regional control, disease-free survival, and overall survival (35% vs. 83%). It was also found that tumor pO2 predicted for pathologically persistent neck nodes in patients undergoing a neck dissection for clinical N2–3 necks after chemoradiation treatment [25]. In another study by Terris [26], only a small number of patients were assessed and hypoxia did not appear to be a prognostic factor. A multicenter study by Nordsmark et al. [27] involving 397 patients with HNC provided further evidence that tumor pO2 was an independent predictor for survival and tumor hypoxia was associated with a poor prognosis in patients with advanced HNC following primary radiotherapy. In HNC, hypoxia not only predicts for disease-free survival and overall survival but also for local control, suggesting hypoxia-induced radiation resistance as an important factor for local failure.
The prognostic impact of HIF-1α and HIF-2α expression has been the subject of numerous studies [28–31]. High HIF-1 expression has been correlated with a poorer survival in HNC treated with radiation with or without chemotherapy [28, 32]. Similar trends are observed in nasopharyngeal tumors [33]. Koukourakis et al. [28] assessed the expression of HIF-1α and HIF-2α in 75 cancer specimens from patients with HNSCC treated with concurrent chemoradiotherapy. HIF-1α and HIF-2α overexpression were shown in 52 and 33% of cancer samples, respectively. Bone/cartilage involvement was more frequent in tumors with high HIF-1α expression. HIF-1α and HIF-2α overexpression were significantly associated with high microvessel density and with VEGF expression. High levels of HIF-1α and HIF-2α expression were associated with incomplete response to chemoradiation, poor local relapse-free survival, and poor overall survival. HIF-2α expression was an independent prognostic factor in multivariate models. Aebersold et al. [32] explored the predictive potential of HIF-1α expression in 98 patients with oropharyngeal cancer treated by curative radiation therapy in which 94% of the primary tumors showed overexpression of HIF-1α. The degree of HIF-1α immuno reactivity correlated inversely with both the rate of complete remission of the primary tumor and lymph node metastases as well as with local failure, and overall survival. Winter et al. [34] investigated the role of expression of HIF-1α and HIF-2α in a series of 151 patients who underwent surgery for HNSCC. High HIF-1α was expressed in 45 of 140 tumors (30%) and HIF-2α was expressed in 21 of 139 tumors (14%). HIF-1α alone was associated with a worse disease-free survival, and high HIF-1α/high HIF-2α expression was also an independent prognostic factor. The immunohistochemical detection of the HIF-1α target gene Glut-1 has been shown to be correlated with a poorer survival in HNC [35]. Oliver et al. [36] investigated the relationship between Glut-1 expression and clinical outcome of a retrospective series of 54 cases of oral squamous cell carcinomas. There was a significant relationship between those tumors which demonstrated intense staining of Glut-1 and loco-regional recurrence. Kunkel et al. [37] analyzed retrospectively Glut-1 expression in 118 patients with oral squamous cell carcinoma. The survival rate of patients with a low Glut-1 labeling index was significantly longer compared with patients with a high Glut-1 labeling index (138 months vs. 60 months), and Glut-1 expression was found to be an independent prognostic marker.
The second target gene of HIF-1α which has been extensively studied with regard to its prognostic significance is CA-IX [38]. As with HIF-1α and Glut-1, most studies found a negative impact of high CA-IX expression in patient with HNC. In one study by Koukourakis et al. [39], HIF-2α and CA-IX were assessed in a series of patients treated with radiotherapy in the frame of the continuous hyperfractionated accelerated radiotherapy (CHART) randomized trial (54 Gy in only 12 days compared with conventional radiotherapy, 66 Gy in 6.5 weeks). Both high levels of HIF-2α and CA-IX were correlated with loco-regional control and survival, suggesting the importance of tumor hypoxia in HNC. However, no benefit was found with the accelerated regimen in the group of hypoxic tumors. In another study [40], tumors with a nonhypoxic profile, as defined as low HIF-1α and low CA-IX expression had significantly better local control.
A recent work by Overgaard et al. [41] used another hypoxia-related marker, the serum level of OPN, in a randomized trial that compared patients’ radiotherapy with and without an hypoxic sensitizer (nimorazole). The patients who benefited the most from the hypoxic modification were in the group with high levels of serum OPN, strongly suggesting that measuring tumor hypoxia before radiotherapy help to individualize irradiation in a more rational way. Studies showing a prognostic significance of 2-nitroimidazole markers have also been reported for HNC [19].
Strategies to Overcome Hypoxia-Induced Treatment Resistance in Head and Neck Cancers
Hyperbaric Oxygen Treatment
The most straightforward strategy to overcome hypoxia is to administer oxygen at a pressure higher than room air (usually 3 atm), i.e., hyperbaric oxygen treatment. The largest clinical trial with hyperbaric oxygen has been conducted by the British Medical Research Council, which randomized 1,669 patients between radiotherapy with or without hyperbaric oxygen [42]. Hyperbaric oxygen significantly improved both survival and local control after radiotherapy for head-and-neck tumors and showed promising results in HNC patients. Some of the earliest work toward this end was done using hyperbaric oxygen to radiosensitize cervical [43] or HNC [44]. Though there was some initial success with this technique, recent studies have indicated that combining radiation with hyperbaric oxygen results in significant increase of normal tissue toxicities [45]. A meta-analysis of randomized trials suggests that the use of hyperbaric oxygen breathing during RT can improve local control by 10% and also improve 5-year survival for irradiated head and neck tumors, however, it has not gained general acceptance for clinical use due to inconsistent responses, safety issues, and the high cost for implementation and especially due to the increased incidence of severe radiation toxicity [46].
Accelerated Radiotherapy with Carbogen and Nicotinamide
Another promising approach to decrease hypoxia in HNC is to combine radiotherapy with both the vasodilator nicotinamide and carbogen breathing (95%O2, 5%CO2) to increase tumor pO2. This strategy, so-called accelerated radiotherapy with carbogen and nicotinamide (ARCON) has produced excellent 3-year local control rates >80% for locally advanced stage T3–4 laryngeal and oropharyngeal cancers in a phase II study [47]. Following this promising result, a large randomized phase III clinical trial testing the efficacy of ARCON in laryngeal cancer patients has been performed in the Netherlands and should be presented in 2009 [48].
Improving Hemoglobin with Erythropoietin Manipulation
Early studies were also done using blood transfusion to increase the oxygen transport and, thereby, increase the tumor tissue pO2. Despite some initial success [49] with this method transfusion failed to improve the local control in a randomized trial performed by the Danish Head and Neck Cancer (DAHANCA) group. Recently, blood transfusion has been supplanted by the administration of erythropoiesis-stimulating factors. Unfortunately, the combination of erythropoietin and radiotherapy is proved to be detrimental in several large randomized studies. Anemia is associated with a poorer outcome in patients treated with radiotherapy [50], possibly because it leads to low oxygen levels in tumors. Correction of anemia has been suggested to reverse this hemoglobin effect, thereby improving cancer control [51]. Recombinant human erythropoietin (EPO) can correct anemia and improve the quality of life in anemic patients with cancer. A phase III trial (ENHANCE study, 351 patients) was conducted to address the question whether anemia correction with erythropoietin could improve the outcome of curative radiotherapy among patients with HNSCC [52]. It showed that EPO corrected anemia, but tumor control, survival, and disease control rates were significantly worse when using EPO. This detrimental effect associated with EPO, when combined with RT in HNSCC was confirmed by the results of RTOG 99-03 [53] and DAHANCA-10 randomized studies (14th European Cancer Conference ECCO). In this later study, in a series of 515 patients eligible for analysis, a significantly poorer loco-regional control rates was observed for the patients who received erythropoietin compared to the control group in HNSCC patients treated with radiotherapy. However, the target hemoglobin range in that study was 14.0–15.5 g/dl, which is beyond the optimal range for tumor oxygenation.
The reason of the observed negative effect of EPO on tumor control could be that tumor oxygenation is decreased by both anemia and inappropriately high-hemoglobin levels. The latter are associated with an increased blood viscosity and a drop in nutritive perfusion. Hemoglobin concentrations between 12 and 14 g/dl could be optimal for maximum tumor oxygenation [51]. Thus, it is important to keep the hemoglobin concentrations within this range during radiotherapy. In addition, a retrospective analysis of a subset of patients from the ENHANCE study suggested that the expression of erythropoietin receptors on cancer cells can play an important role in HNSCC patients receiving erythropoietin during radiotherapy [54]. Loco-regional progression-free survival was substantially poorer if erythropoietin was administered to patients positive for the receptor expression compared with placebo, however, erythropoietin did not impair outcome in receptor-negative patients. Given these results, the use of EPO in HNC patients should not be considered outside controlled clinical trials [55].
Hypoxic Cell Radiosensitizers: Nitroimidazoles
A widely investigated hypoxia-targeted strategy is to use electron-affinic drugs (nitroimidazoles) to sensitize tumors to the effect of radiation. Xenograft studies showed significant radiosensitization with nitroimidazole compounds in tumors without enhancing normal tissue toxicity. These encouraging results led to the realization of several of clinical trials exploring the clinical radiosensitizing potential of misonidazole in the late 1970s. However, the results of these clinical trials have been generally disappointing. One of the possible factor to explain the failure of misonidazole to provide significant advantage is the low plasma concentrations achievable with the permitted dose of this neurotoxic drug. Nevertheless, some was seen in one randomized trial. In the DAHANCA 2 trial [56], 626 patients with head and neck carcinoma were randomized to two different split-course radiation regimens and given either misonidazole or placebo during the initial 4 weeks of treatment. Overall, the misonidazole-treated group did not have a significantly better control rate than the placebo group. However, some benefit was found in patients with pharynx carcinomas. Misonidazole induced significant peripheral neuropathy in 26% of the treated patients, whereas other drug-related side effects were minimal. In the DAHANCA 5 trial [57], a less toxic nitroimidazole compound, nimorazole (Naxogin®), was used. Four hundred and twenty-two patients with carcinoma of the supraglottic larynx and pharynx were randomized to receive nimorazole or placebo, in association with conven-tional primary radiotherapy. With a median follow-up of 112 months, the nimorazole group showed a significantly better loco-regional control rate than the placebo group and a lower cancer-related death rate, without increasing the major side-effects.
Hypoxic Cell Cytotoxin: Bioreductive Drugs
Bioreductive agents can selectively kill hypoxic cells. The first bioreductive drug used in clinical trials was mitomycin-C [58]. Haffty et al. [59] showed that the addition of mitomycin-C to RT resulted in statistically significant improvement in loco-regional control and cause-specific survival in HNC. Another study by Dobrowsky et al. [60, 61] comparing conventional fractionated RT to the Vienna continuous hyperfractionated accelerated RT regimen (V-CHART) or to V-CHART plus mitomycin-C showed that the best survival and loco-regional control rates were observed for the V-CHART and mitomycin-C group. However, the use of mitomycin-C is limited by its significant toxicity making it unlikely to be the ideal drug for exploiting tumor hypoxia.
Recently, a new approach to tumor hypoxia has been developed using drugs that are preferentially cytotoxic to hypoxic cells [4], such as tirapazamine (TPZ). Preclinical studies have demonstrated that TPZ results in potentiation of both radiation and CDDP cytotoxicity [62]. In a phase I trial of TPZ, CDDP, and radiation (TPZ/CIS), impressive results were seen in locally advanced HNSCC [63]. This drug was then further evaluated in a randomized phase II trial Trans-Tasman Radiation Oncology Group (TROG) 98.02 [64], 122 previously untreated advanced HNSCC patients were randomized to receive RT concurrently with either CDDP plus TPZ (TPZ/CIS), or CDDP and 5-FU. The striking observation of this study was that tumor control probability was strongly related to the pretreatment level of hypoxia, as measured by PET misonidazole. Hypoxic tumors treated with tirapazamine had an excellent control rate (>90%) while hypoxic tumors receiving 5-FU instead of tirapazamine had a very poor control rate (<25%) [65]. On the other hand, Rischin et al. reported results of a phase III trial HeadSTART of TROG during ASCO 2008. Eight hundred and sixty-one patients with previously untreated Stage III or IV HNSCC were randomized to receive RT concurrently with either CDDP (100 mg/m2 every 3 weeks) or CDDP (75 mg/m2 every 3 weeks + tirapazamine). No benefit was found due to the addition of TPZ to CT–RT in the absence of selection for the presence of hypoxia. All together, these two randomized studies suggest that a key issue in this area is to detect hypoxia and adapt the treatment to the characteristics of each individual tumor.
In another phase II trial [66], 62 patients with lymph node-positive, resectable, stage IV HNSCC were randomized to receive either two cycles of induction chemotherapy (TPZ, cisplatin, and 5-FU) followed by simultaneous chemoradiotherapy (TPZ, cisplatin, and 5-FU) or to receive the same regimen without TPZ. Patients who did not achieve a complete response at 50 Gy underwent surgical treatment. The addition of TPZ increased hematologic toxicity but did not improve outcomes in the small series of patients with resectable HNSCC.
Micro-Environment-Vascular Normalization
Jain [67] has proposed the concept of normalization of tumor vasculature through antiangiogenesis and antivasculature targeted therapy [68]. Owing to high levels of proangiogenic molecules produced locally, such as VEGF, tumors become hypervascular, but the vessels are leaky and the blood flow is spatially and temporally heterogeneous. This leads to increased interstitial fluid pressure (IFP) and focal hypoxia, creating barriers to delivery and efficacy of therapeutics. The proposed mechanism of action of the VEGF-specific inhibitors, such as bevacizumab and sorafenib, is the inhibition of new-vessel formation and killing of immature tumor vessels, transient normalization of the remaining vasculature by decrease in macromolecular permeability (and thus the IFP) and hypoxia, and improvement of blood perfusion. The lowered IFP can lead to improved delivery of chemotherapeutics and molecularly targeted agents; the improved oxygenation sensitizes cancer cells to cytotoxic therapeutics and reduces the selection of more-malignant phenotype; and, finally, increased cellular proliferation around normalized vessels might increase the cytotoxicity of chemotherapeutics [69]. Normalization of the vasculature might also benefit the direct killing of cancer cells by bevacizumab, in synergy with the chemotherapeutics.
Combined effects of bevacizumab with erlotinib and irradiation have been observed using a preclinical study on a HNC orthotopic model [70]. A phase I dose escalation study [71] has been conducted to evaluate the combination of bevacizumab and chemoradiotherapy (5-FU, hydroxyurea, radiation) in a series of 44 poor-prognosis HNC patients. Bevacizumab was integrated with chemoradiotherapy at a dose of 10 mg/m2 every 2 weeks. Some fistula formation/tissue necrosis were observed that could be bevacizumab-related. Erlotinib and bevacizumab combination has been investigated in 58 patients with recurrent or metastatic HNSCC in a phase I/II study [72]. The most common side effects of any grade were rash and diarrhea. A few patients could have benefit from this approach especially when the ratio of tumor-cell phosphorylated VEGF receptor-2 (pVEGFR2) over total VEGFR2 and endothelial-cell pEGFR over total EGFR in pretreatment biopsies were associated with complete response.
A phase II trial of sorafenib has been conducted in a small series of 27 patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. Sorafenib was well tolerated with a few grade 3 and no grade 4 toxicities but had modest anticancer activity comparable to monotherapy with other targeted agents in this group of patients [73].
Targeting HIF-1
Given the role of HIF-1α in response to hypoxia, there is a major interest to develop specific HIF-1 inhibition. In xenograft assays, manipulation of HIF-1 activity by genetic or pharmacological means has marked effects on tumor growth along with some effects on angiogenesis, glucose metabolism, and/or cell survival [74].
Topotecan, a topoisomerase I poison that reversibly binds to and stabilizes the topoisomerase I enzyme, inhibited HIF-1 protein translation by a proteasome- and DNA damage-independent mechanism. Currently, topotecan is being tested in a clinical trial at the National Cancer Institute for its ability to inhibit HIF-1α protein expression and function in patients with advanced malignancies refractory to standard therapy [74].
Inhibitors of several upstream signaling pathways of HIF-1, such as EGFR and mTOR, have been extensively investigated in clinical trials in these recent years [7]. The mTOR inhibitors (everolimus and temsirolimus) that can suppress mTOR-dependent HIF-1 translation, and EGFR inhibitors (gefitinib, erlotinib) or antibodies (cetuximab, panitumumab) could inhibit HIF-1 induction by EGFR-dependent pathways [8].
Hsp90 is a molecular chaperone associated with a number of proteins, which include transcription factors (AhR, glucocorticoid receptor, p53) and signaling kinases (Akt, ErbB2, Raf-1, v-Src), and ensures the proper conformation, localization, and function of these client proteins. Hsp90 inhibitors were found to induce ubiquitination and proteasome-mediated degradation of HIF-1α in a VHL-independent fashion, under both normoxic and hypoxic conditions [74].
Histone deacetylase (HDAC) inhibitors have also been tested recently [74]. The dynamic process of reversible acetylation of the lysine residue of histone, and nonhistone proteins is controlled by HDAC and histone acetyltransferases. Acetylation of histone proteins is important for DNA chromatin conformation and regulation of gene expression. Acetylation of nonhistone proteins has been implicated in protein stability and function and direct acetylation of HIF-1α has been suggested.
PET-Based Intensity-Modulated Radiotherapy
Image fusion techniques and the use of intensity-modulated and image-guided radiotherapy can allow to delineate hypoxic radioresistant subtarget volumes for delivering a partial tumor boost. PET could detect hypoxia in tumors and a higher dose could be given to the hypoxic areas, using intensity-modulated radiotherapy (IMRT). The MSKCC experience with microboosts on hypoxic areas up to 100 Gy. This approach requires that PET imaging be sensitive and specific enough to image hypoxia. In this framework, a validation of PET imaging used for adaptive radiotherapy was undertaken in animal models by comparing small-animal PET images (2.7 mm resolution) with autoradiography (AR) (100 μm resolution) in various tumors under various physiological situations [75]. Discrepancies were found between the PET images and the underlying microscopic reality represented by AR images. These differences, attributed to the finite resolution of PET, were important when considering small regions of the tumors. Dose painting based on PET images should be carefully considered and should take these limitations into account.
The feasibility of a Cu-ATSM-guided IMRT approach through coregistering hypoxia (60)Cu-ATSM PET to the corresponding CT images for IMRT planning has been reported in HNC patients [76]. Radiation dose to the hGTV could be escalated without compromising normal tissue sparing (parotid glands and spinal cord). The plan delivered 80 Gy in 35 fractions to the ATSM-avid tumor subvolume and the GTV simultaneously receives 70 Gy in 35 fractions while more than one-half of the parotid glands were spared to less than 30 Gy.
Thorwarth et al. [77] investigated the feasibility of different hypoxia dose painting strategies in radiotherapy of 13 head and neck cancer patients. For each patient, three different treatment plans were created: a conventional IMRT plan, an additional uniform dose escalation (uniDE) of 10% to the fluorodeoxyglucose (FDG)-positive volume, and a plan in which dose painting by numbers (DPBN) was implemented. DPBN was realized according to a map of dose-escalation factors calculated from dynamic 18F-FMISO PET data. For DPBN, the prescriptions could be fulfilled in larger regions of the target than for uniDE. DPBN seems to result in higher benefits for the patients regarding tumor control probability. If hypoxia could be adequately quantified with a simple imaging technique such as FMISO positron emission tomography, DPBN in head-and-neck cancer could substantially increase tumor control.
Lee et al. reported the results from a prospective study of pre-/midtreatment 18F-FMISO PET scans in a series of loco-regionally advanced HNC patients treated with concomitant chemotherapy and IMRT [78]. Each patient underwent four PET scans: one pretreatment FDG PET/CT scan, two pretreatment 18F-FMISO PET/CT scans, and a final 18F-FMISO PET (midtreatment) scan performed 4 weeks after the start of chemoradiotherapy. An heterogeneous distribution of 18F-FMISO was noted in the primary and/or nodal disease in 90% of the patients. The positive 18F-FMISO findings of the midtreatment PET scan was not correlated with patient outcome.
Another study has evaluated the influence of changes in tumor hypoxia on the efficacy of IMRT dose painting, according to serial 18F-FMISO PET imaging [79]. Seven patients with HNC were imaged twice with FMISO PET, separated by 3 days, before radiotherapy. IMRT plans were designed, on the basis of the first FMISO scan, to deliver a boost dose of 14 Gy to the hypoxic volume, in addition to the 70-Gy prescription dose. The changes in spatial distribution of tumor hypoxia, as detected in serial FMISO PET imaging added some complexity to define an adequate, coverage of hypoxic tumor volumes achievable by dose-painting IMRT and, dose painting potentially increased the EUD of the hypoxic volumes.
Other Methods
Hyperfractionation radiotherapy (HFRT) [80] was designed to improve radiotherapy effectiveness by delivering two to three fractions daily with a reduced dose per fraction, which may reduce late toxicity despite an increased total dose. In addition, hyperfractionation could induce reoxygenation and its use was associated with an 8% improvement in survival at 5 years [81]. Other radiotherapy techniques can be of interest to overcome tumor hypoxia, such as high linear-energy transfer (LET) radiation which is less oxygen dependent. For example, carbon ions could be used to decrease the radiation resistance induced by hypoxia, and is currently under investigation.
In conclusion, tumor hypoxia continues to be a therapeutic challenge in HNC. Nonetheless, the prospect of reducing its impact is looking brighter with improved ability of detecting hypoxia and better understanding of its molecular targets for therapeutic exploitation. Testing new leads from the laboratory will require clinical trials with innovative designs that incorporate serial novel noninvasive surrogate end points for hypoxia, such as molecular makers or imaging methods.
References
Bourhis J. Hypoxia response pathways and radiotherapy for head and neck cancer. J Clin Oncol. 2006;24:725–6.
Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39.
Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007;26:241–8.
Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–47.
Tannock IF. Conventional cancer therapy: promise broken or promise delayed? Lancet. 1998;351 Suppl 2:SII9–16.
Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32.
Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–11.
Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64.
Schneider A, Younis RH, Gutkind JS. Hypoxia-induced energy stress inhibits the mTOR pathway by activating an AMPK/REDD1 signaling axis in head and neck squamous cell carcinoma. Neoplasia. 2008;10:1295–302.
Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757.
Stone HB, Brown JM, Phillips TL, et al. Oxygen in human tumors: correlations between methods of measurement and response to therapy. Summary of a workshop held November 19–20, 1992, at the National Cancer Institute, Bethesda, Maryland. Radiat Res. 1993;136:422–34.
Le QT, Sutphin PD, Raychaudhuri S, et al. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res. 2003;9:59–67.
Raleigh JA, Calkins-Adams DP, Rinker LH, et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765–8.
Evans SM, Hahn S, Pook DR, et al. Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer Res. 2000;60:2018–24.
Varghese AJ, Gulyas S, Mohindra JK. Hypoxia-dependent reduction of 1-(2-nitro-1-imidazolyl)-3-methoxy-2-propanol by Chinese hamster ovary cells and KHT tumor cells in vitro and in vivo. Cancer Res. 1976;36:3761–5.
Ljungkvist AS, Bussink J, Kaanders JH, et al. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167:127–45.
Evans SM, Koch CJ. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003;195:1–16.
Kaanders JH, Wijffels KI, Marres HA, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–74.
Lee NY, Le QT. New developments in radiation therapy for head and neck cancer: intensity-modulated radiation therapy and hypoxia targeting. Semin Oncol. 2008;35:236–50.
Krohn KA, Link JM, Mason RP. Molecular imaging of hypoxia. J Nucl Med. 2008;49(2):129S–48.
Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol. 2000;57:39–43.
Rudat V, Stadler P, Becker A, et al. Predictive value of the tumor oxygenation by means of pO2 histography in patients with advanced head and neck cancer. Strahlenther Onkol. 2001;177:462–8.
Brizel DM, Dodge RK, Clough RW, et al. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–7.
Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:1418–23.
Terris DJ. Head and neck cancer: the importance of oxygen. Laryngoscope. 2000;110:697–707.
Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24.
Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192–202.
Kyzas PA, Stefanou D, Batistatou A, et al. Hypoxia-induced tumor angiogenic pathway in head and neck cancer: an in vivo study. Cancer Lett. 2005;225:297–304.
Beasley NJ, Leek R, Alam M, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–7.
Silva P, Slevin NJ, Sloan P, et al. Prognostic significance of tumor hypoxia inducible factor-1alpha expression for outcome after radiotherapy in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2008;72:1551–9.
Aebersold DM, Burri P, Beer KT, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–6.
Hui EP, Chan AT, Pezzella F, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–604.
Winter SC, Shah KA, Han C, et al. The relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer. 2006;107:757–66.
Jonathan RA, Wijffels KI, Peeters W, et al. The prognostic value of endogenous hypoxia-related markers for head and neck squamous cell carcinomas treated with ARCON. Radiother Oncol. 2006;79:288–97.
Oliver RJ, Woodwards RT, Sloan P, et al. Prognostic value of facilitative glucose transporter Glut-1 in oral squamous cell carcinomas treated by surgical resection; results of EORTC Translational Research Fund studies. Eur J Cancer. 2004;40:503–7.
Kunkel M, Reichert TE, Benz P, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–24.
De Schutter H, Landuyt W, Verbeken E, et al. The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy +/− chemotherapy. BMC Cancer. 2005;5:42.
Koukourakis MI, Bentzen SM, Giatromanolaki A, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol. 2006;24:727–35.
Schrijvers ML, van der Laan BF, de Bock GH, et al. Overexpression of intrinsic hypoxia markers HIF1alpha and CA-IX predict for local recurrence in stage T1–T2 glottic laryngeal carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:161–9.
Overgaard J, Eriksen JG, Nordsmark M, et al. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005;6:757–64.
Report of a Medical Research Council Working Party. Radiotherapy and hyperbaric oxygen. Lancet. 1978;2:881–4.
Dische S, Anderson PJ, Sealy R, et al. Carcinoma of the cervix – anaemia, radiotherapy and hyperbaric oxygen. Br J Radiol. 1983;56:251–5.
Henk JM, Kunkler PB, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Final report of first controlled clinical trial. Lancet. 1977;2:101–3.
Bennett M, Feldmeier J, Smee R et al. Hyperbaric oxygenation for tumour sensitisation to radiotherapy. Cochrane Database Syst Rev. 2005;4:CD005007.
Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6:10–21.
Kaanders JH, Pop LA, Marres HA, et al. ARCON: experience in 215 patients with advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;52:769–78.
Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol. 2002;3:728–37.
Evans JC, Bergsjo P. The influence of anemia on the results of radiotherapy in carcinoma of the cervix. Radiology. 1965;84:709–17.
Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76.
Rades D. Erythropoietin administration during radiotherapy in anaemic head-and-neck cancer patients: is it still a reasonable option or too dangerous? Oral Oncol. 2009;45:91–3.
Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–60.
Machtay M, Pajak TF, Suntharalingam M, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Onco-logy Group (RTOG 99-03). Int J Radiat Oncol Biol Phys. 2007;69:1008–17.
Henke M, Mattern D, Pepe M, et al. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol. 2006;24:4708–13.
Bokemeyer C, Aapro MS, Courdi A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43:258–70.
Overgaard J, Hansen HS, Andersen AP, et al. Misonidazole combined with split-course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys. 1989;16:1065–8.
Overgaard J, Hansen HS, Overgaard M, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol. 1998;46:135–46.
De Ridder M, Van Esch G, Engels B, et al. Hypoxic tumor cell radiosensitization: role of the iNOS/NO pathway. Bull Cancer. 2008;95:282–91.
Haffty BG, Son YH, Papac R, et al. Chemotherapy as an adjunct to radiation in the treatment of squamous cell carcinoma of the head and neck: results of the Yale Mitomycin Randomized Trials. J Clin Oncol. 1007;15:268–76.
Dobrowsky W, Naude J, Widder J, et al. Continuous hyperfractionated accelerated radiotherapy with/without mitomycin C in head and neck cancer. Int J Radiat Oncol Biol Phys. 1998;42:803–6.
Dobrowsky W, Naude J. Continuous hyperfractionated accelerated radiotherapy with/without mitomycin C in head and neck cancers. Radiother Oncol. 2000;57:119–24.
Gandara DR, Lara Jr PN, Goldberg Z, et al. Tirapazamine: prototype for a novel class of therapeutic agents targeting tumor hypoxia. Semin Oncol. 2002;29:102–9.
Rischin D, Peters L, Hicks R, et al. Phase I trial of concurrent tirapazamine, cisplatin, and radiotherapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:535–42.
Rischin D, Peters L, Fisher R, et al. Tirapazamine, Cisplatin, and Radiation versus Fluorouracil, Cisplatin, and Radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol. 2005;23:79–87.
Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24:2098–104.
Le QT, Taira A, Budenz S, et al. Mature results from a randomized Phase II trial of cisplatin plus 5-fluorouracil and radiothe-rapy with or without tirapazamine in patients with resectable Stage IV head and neck squamous cell carcinomas. Cancer. 2006;106:1940–9.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63.
Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40.
Bozec A, Sudaka A, Fischel JL, et al. Combined effects of bevacizumab with erlotinib and irradiation: a preclinical study on a head and neck cancer orthotopic model. Br J Cancer. 2008;99:93–9.
Seiwert TY, Haraf DJ, Cohen EE, et al. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J Clin Oncol. 2008;26:1732–41.
Cohen EE, Davis DW, Karrison TG, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–57.
Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25:3766–73.
Melillo G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev. 2007;26:341–52.
Christian N, Lee JA, Bol A, et al. The limitation of PET imaging for biological adaptive-IMRT assessed in animal models. Radiother Oncol. 2009;91:101–6.
Chao KS, Bosch WR, Mutic S, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–82.
Thorwarth D, Eschmann SM, Paulsen F, et al. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys. 2007;68:291–300.
Lee N, Nehmeh S, Schoder H, et al. Prospective trial incorpo-rating pre-/mid-treatment [(18)F]-misonidazole positron emission tomography for head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:101–8.
Lin Z, Mechalakos J, Nehmeh S, et al. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys. 2008;70:1219–28.
Bourhis J, Lapeyre M, Tortochaux J, et al. Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer: a GORTEC trial. J Clin Oncol. 2006;24:2873–8.
Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Tao, Y., Bourhis, J. (2011). Hypoxia in Head and Neck Cancers: Clinical Relevance and Treatment. In: Bernier, J. (eds) Head and Neck Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-9464-6_10
Download citation
DOI: https://doi.org/10.1007/978-1-4419-9464-6_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-9463-9
Online ISBN: 978-1-4419-9464-6
eBook Packages: MedicineMedicine (R0)