Abstract
Atrial fibrillation (AF), the most common sustained form of cardiac arrhythmia, is an endemic disease with an increasing prevalence [14, 32]. Its polymorphic dynamical nature severely hampers the development of a single therapy effective in all individual patients [23, 24]. The limited understanding of the AF mechanisms indicates a clear medical need for improving current therapies, adapting them to various AF dynamics that can be found in different patient populations, and selecting the therapy that is optimal for an individual patient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Atrial fibrillation (AF), the most common sustained form of cardiac arrhythmia, is an endemic disease with an increasing prevalence [14, 32]. Its polymorphic dynamical nature severely hampers the development of a single therapy effective in all individual patients [23, 24]. The limited understanding of the AF mechanisms indicates a clear medical need for improving current therapies, adapting them to various AF dynamics that can be found in different patient populations, and selecting the therapy that is optimal for an individual patient.
Several guidelines for the management of idiopathic AF have been proposed and provide a basis for the treatment of AF in clinical settings [19]. For paroxysmal and persistent AF, ventricular rate control and/or rhythm control (reduction of paroxysms) strategies are proposed, while for patients with permanent AF, the objective is sinus rhythm restoration. Pharmacological therapy with an antiarrhythmic drug is an important part of any rhythm control strategy. Restoration of sinus rhythm can also be achieved by electrical cardioversion, delivering an intracardiac or external electrical shock, a difficult task being the maintenance of sinus rhythm after successful cardioversion. Other treatments have been directed at eliminating the triggers and modifying the AF electrophysiological substrate. Catheter ablation of AF has now become almost a standard procedure for patients with paroxysmal or idiopathic AF, especially for patient with focal triggers. Surgical ablation procedures, such as the maze procedure, are currently performed in patients undergoing concomitant open heart surgery. In addition to pharmacological or ablation therapies, some of the recent pacemakers and defibrillators have incorporated different features to prevent or terminate AF, such as overdrive pacing or antitachycardia pacing (ATP). However, no clinical study has yet proven the long-term clinical benefit.
Computer modeling of biophysical phenomena has gained increasing importance for research in physiology and biology, and the recent improvements in computational speed have enabled in silico experiments in highly detailed and realistic biophysical models. The use of such models overcomes some of the limitations encountered in in vivo experimental or clinical research, by providing an access to all variables of interest at any temporal or spatial scales and by allowing the possibility of performing and repeating the experiments under controlled conditions. Existing biophysical models differ in the trade-off made between accuracy of the representation and the computational load: the more detailed the model, the greater the computational complexity. The choice of a specific model is dependent on the question to be answered. For instance, a model can be modified to selectively reproduce the physiological parameters of a specific type of patient and, likewise, targeted modeling research can be conducted to find a dedicated treatment for the patient. The ability to accurately reproduce a specific physiological response in a model will, as a result, depend on the ability to implement all features that are pertinent to the application considered.
This chapter describes how a biophysical model of human atria can be used to simulate various types of AF dynamics and, on the basis of this, to evaluate and develop therapeutic strategies. Integrated functional computer modeling is considered today as a potentially effective solution for translational research in which the research results can be translated into clinically applicable therapeutic options [14].
The elements of the biophysical model of AF are described in Sect. 4.2. Next, in Sect. 4.3, specific variants of the model are presented, aimed at modeling different AF dynamics based on different tissue properties. These models were developed to reproduce the various dynamical mechanisms of AF suggested as being responsible for its polymorphic nature [23]. This section also describes the database of simulated episodes of AF used in the various applications. Different therapies were subsequently tested on the specific AF dynamics. With this multifaceted approach, a large series of experiments could be conducted on a substrate corresponding to the physiological settings of a specific patient. The examples of model-based analysis and applications included in this chapter are: a study of the conditions leading to the spontaneous termination of AF (Sect. 4.4), the optimization of ablation patterns (Sect. 4.5), and the preliminary results of a study on the feasibility of therapeutic pacing for AF (Sect. 4.6).
4.2 Computer Modeling of AF
Several computer models of the human atria have been developed over the last decades. Moe et al. developed the first model of AF in 1964 using cellular automata [22]. About 40 years later, more sophisticated models that take into account a detailed description of the atrial cellular membrane kinetics together with several aspects of the complex atrial anatomy were conceived [8, 28, 36, 38]. In 2000, Harrild and Henriquez presented the first membrane-based model of 3D conduction in a realistic human atrial geometry [8]. However, the model’s heavy computational demand precluded its use in studies on abnormal activation sequences. The design of simplified models that retain the salient features of atrial anatomy and electrophysiology has allowed the large-scale simulation of abnormal reentrant processes such as atrial arrythmias [36, 38] and associated therapies such as AF ablation [5, 28].
4.2.1 Biophysical Model of Human Atria
The biophysical model used in the material presented in this chapter was developed by the Lausanne Heart Group (http://www.lausanneheart.ch) with the aim of simulating several types of dynamics of AF and associated therapies such as ablation and pacing. This objective required the simulations of long periods of AF on a computer (several minutes or even hours of real-time AF); hence, it was designed such that the main aspects of a human atrial geometry were accounted for, while keeping computational load tractable [5, 38]. The model consists of three main components (Fig. 4.1): (1) realistic atrial geometry, (2) electrical propagation in the atrial tissue represented by a grid of atrial units, interconnected via resistors representing the interconnections of the myocytes at the gap junctions, and (3) atrial membrane kinetic model.
4.2.1.1 Atrial Geometry
Existing computer models of the atria extract the information about anatomy from several sources: commercially available, published atrial datasets [8, 28] or basic considerations from anatomy textbooks or devoted literature on atrial dimensions, atrial muscle bundles and strands, and propagation velocities [8, 36]. The geometry used in our model was derived from magnetic resonance images of the human atria segmented slice by slice, with 1 mm spacing [38]. These images formed the basis for a 3D atrial surface on which surface smoothing was applied to construct a mesh of 100,000 triangular elements (400 mm resolution). The model was given a realistic size and geometry, but the thickness of the atrial wall was taken to be infinitesimal (monolayer model). The resulting atrial geometry with the orifices for the valves and veins is presented in Fig. 4.1, illustrating the tricuspid valve, the two venae cavae and the coronary sinus in the right atrium (RA), the mitral valve and the four pulmonary veins in the left atrium (LA).
A thick-walled variant of this geometry was also constructed [35]. However, the increased computational load of this variant precluded its use in large-scale evaluations such as the ones presented in this chapter. This variant was used during incidental checks on the nature of the results obtained using the monolayer geometry.
4.2.1.2 Electrical Propagation in Atrial Tissue
The mathematical formulation of the electrical propagation on the grid of atrial units interconnected via resistors has been described previously [38]. In the simplest version of the model, these resistors were all given an equal value, resulting in a surface with intrinsic homogeneous, isotropic properties. More sophisticated versions including anisotropy and heterogeneities in conductivity were also developed [11]. Fast conduction systems such as the Bachmann’s bundle, the crista terminalis, and the pectinate muscles were also included if deemed essential for a specific experiment [35].
4.2.1.3 Atrial Cellular Model
A dynamical activation model, based on membrane channel ion kinetics, was assigned to each atrial unit of the grid. In a first step, a variant of the Luo and Rudy ventricular model (LR) [20] was used, adapted to atrial cellular properties [18]. In a second step, the kinetics of a dedicated atrial cellular model, the Courtemanche–Ramirez–Nattel model (CRN) [3], was implemented. This model has a much heavier computational load than the LR-model. In these two models, channel conductances could be adjusted to mimic specific substrate conditions present during AF. In the simplest variant, all cells were assigned homogeneous intrinsic kinetics properties. More sophisticated versions included heterogeneities in the membrane properties [12].
4.2.2 Modeling Different Types of AF
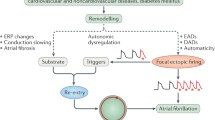
Atrial arrhythmias were initiated in the biophysical model of AF in a similar way as in clinical experiments, i.e., using a programmed stimulation protocol or a burst-pacing protocol. Burst pacing at 20 Hz near the sinoatrial node was eventually selected because it does not require any adjustment of timing of the stimulation protocol. In our baseline model of healthy atria, most of the attempts to initiate arrhythmias either failed or produced unstable reentrant waves that terminated after a few seconds. Conditions favorable for the perpetuation of AF were created in a model of pathologic atrial tissue. While using the same atrial geometry, different types of sustained AF dynamics could be obtained by using the basic atrial kinetics models modified to take into consideration the electrical remodeling observed during AF and by varying the cellular or conduction properties to modify the atrial substrate, as summarized in Fig. 4.1. These models correspond to the different AF pathophysiologies proposed as being the cause of AF in the human heart [14].
4.2.2.1 Multiple Wavelet AF
The first AF model was based on a homogeneous atrial tissue using the LR model adjusted to mimic electrical remodeling as observed in patients suffering from permanent AF, such as a shortening of the action potential duration [13]. The resulting AF dynamics revealed multiple reentrant wavelets (three to eight), continuously changing in size and direction due to functional or anatomical reentries [13]. This reentry can be described by the multiple wavelet hypothesis formulated by Moe et al. [22]. It corresponds to the observations made in human mapping experiments, classified as type III AF, by Konings et al. [17]. In this variant of our model, AF was sustained for more than 10 min, which is the longest simulation ever performed in a biophysical model of AF. These conditions were chosen as a basis to test therapeutic interventions.
4.2.2.2 Meandering Wavelet AF
The second AF model was based on a homogeneous atrial tissue having an overall slower propagation. It uses the CRN model adjusted to reproduce the restitution properties measured in human atrial cells during permanent AF [13, 34]. The resulting AF dynamics revealed several meandering wavelets (one to four), commonly accompanied by shifting leading circles. This dynamics corresponds to type I or type II AF as described by Konings et al. [17]. AF was not sustained for more than 40 s. These model specifications were chosen as a basis for studying spontaneous AF termination.
4.2.2.3 Heterogeneities
Other AF models were developed in which different types of heterogeneities were introduced. Patchy heterogeneities in the cellular membrane properties (with a characteristic length scale of 2 cm) were introduced, which led to different AF dynamics [12]. A cholinergic AF model resulted in dominant mother rotors, supporting the hypothesis in which a single source of stable reentrant wave front maintains a fibrillatory activity [15, 37]. Similarly, a model variant was created including patchy heterogeneities in action potential duration [7].
4.2.2.4 Focal AF
The last AF model presented here was based on a mechanism different from that of functional reentry: the multiple source hypothesis, which postulates that a small number of foci is needed to maintain fibrillatory activity. It is now known that these foci of rapid ectopic activity, often located inside the pulmonary veins, play a pivotal role in the initiation of AF in humans [9]. In order to study this particular situation, a model variant was developed that included several focal sources of rapid discharge (placed mainly in the pulmonary veins region) [10]. This model was used to study the mechanisms of ablation of focal sources.
4.2.3 Link to Clinical Data
The link between computer simulations and clinical data is crucial for the validation of computer modeling assumptions and for the translation of research results into clinically relevant applications. First, in the computer modeling experiments, direct access to transmembrane potentials was available. This transmembrane potential was color-coded, red representing a value of 20 mV, and blue the resting potential of −80 mV. The complete distribution of instantaneous transmembrane potentials over the atrial surface was depicted as potential maps.
Electrical activity of the atria can also be recorded by electrodes in contact with the atrial wall (endocardial electrograms). This is the type of signals recorded during electrical mapping, either by catheter-guided electrodes during an electrophysiogical study or an ablation procedure or by the electrodes of an implantable device (pacemaker or defibrillator). In our simulations, local endocardial electrograms at virtual electrodes positioned at 1 mm from the atrial surface were computed using a current source approximation [11]. These simulated electrograms were in agreement with measured electrical mapping data during AF. The advantage of simulations like these is that high-resolution maps over the entire atrial surface can be produced, with maps that are unperturbed by any ongoing ventricular activity, movement artifacts, or recording noise.
Finally, the standard 12-lead surface electrocardiogram is the most commonly used noninvasive tool for diagnosing cardiac arrhythmias. In our study, it was simulated using a thorax model involving the geometries of a healthy subject’s torso, lungs, heart, and blood cavities derived from magnetic resonance images and using an equivalent double layer source model to compute the atrial components of the electrocardiograms [35]. The inclusion of the volume conduction model resulted in electrocardiographic signals that are in all aspects similar to those observed clinically.
4.3 Therapeutic Strategies for AF
4.3.1 Modeling AF Therapies
A computer model able to simulate sustained AF with realistic properties offers the possibility of evaluating different therapies for AF. In atrial models, pharmacological interventions were simulated by modulating the ion kinetics of the atrial units [16]. The effectiveness of surgical or catheter ablation line patterns was simulated by modifying the conduction (via the resistivity) of the cardiac cells located on the ablation line [5]. Therapeutic pacing, as in overdrive or ATP protocols, was simulated by injecting intracellular current in 3 mm2 areas at different locations [33]. Model-based studies of defibrillation have been mostly developed in ventricular models [31].
4.3.1.1 AF Database
The evolving nature of AF was taken into consideration by performing multiple simulations for each of the therapeutic strategies studied. After the initiation of AF by means of rapid pacing, the evolving nature was accounted for by randomly selecting several moments in the ongoing sustained simulated AF (Fig. 4.2) and storing the subsequent episodes in a database. This methodology adds a statistical dimension to the analysis. In our studies on AF ablation [5, 30] and pacing of AF [33], up to 50 different instantaneous transmembrane potentials maps were taken as initial conditions for the application of the therapies. These snapshots correspond to different states of activity in the tissue, such as the number of wavelets and their spatial distribution. A similar approach was also used for the study of spontaneous termination of AF, where the elements of the database were chosen as initial conditions from which the system evolved freely until termination [34].
Creation of an AF database for the simulation of spontaneous termination and therapeutic strategies for AF. AF was initiated with a rapid pacing at the sinoatrial node (SAN) region at 20 Hz during 3 s. When pacing was stopped, sustained AF was obtained, and the AF database was constructed by randomly selecting instantaneous transmembrane potential maps at different time instants. A black-and-white scale was used here, black representing a value of 20 mV and white the resting potential of −80 mV
4.4 Spontaneous Termination of AF
4.4.1 Simulation of Spontaneously Terminated Episodes
Spontaneous termination of AF is frequently observed in patients, and this also took place in our computer simulations. In patients, within the first 24 h, up to 50% of cases with a new onset of AF revert to sinus rhythm. However, if the AF episode lasted for more than 7 days, the chance of spontaneous cardioversion was greatly reduced [19]. So far the mechanisms of this termination are not fully understood. Identification of the spontaneous termination mechanisms could lead to a better understanding of AF and therefore to the development of more effective therapies. It is, however, difficult to study spontaneous AF termination episodes in any detail since they are transient and sometimes very short. Clinical studies have been conducted mostly on paroxysmal AF observed from 24-h Holter recordings [27], mapping experiments [25], or from data collected from implantable devices. The use of a biophysical model allows the generation of a high number of spontaneously terminated AF episodes in a spatially more complete way than is possible in clinical settings [34]. For the study presented below, 50 episodes of spontaneous termination in both the LR and the CRN models were observed, and the mechanisms of termination could be studied in detail during the 8 s preceding termination.
4.4.2 Temporal Scales of Termination
To assess the time scales involved in the AF termination process, the following parameters were assessed: the duration of the AF episode, the temporal evolution of AF cycle length (AFCL), and the AF dynamics characterized by the number of wave fronts (#WF) present in the atria [34]. Each measure was systematically performed globally in both atria, and separately in the RA and the LA (Fig. 4.3). In the LR model, mean AF episode duration was 58.5 ± 56.2 s. AFCL and #WF started to increase on a global scale at about 1,600 ms prior to termination, for both the RA and the LA. However, a consistently higher #WF was found in the RA, while instants with faster AF (shorter AFCL) were observed in the LA. In the CRN model, mean AF episode duration was 8.5 ± 7.1 s and therefore shorter than for the LR model. The AFCL started to increase about 3 s before termination on a global scale but 800 ms earlier in the LA than in the RA. In a similar way, #WF started to decrease 1,800 ms earlier in the LA than in the RA. An asymmetry in dynamics between atria, even for a homogeneous substrate, was observed in the CRN model. Clinical observations in humans indicate that distinct frequency changes in the process of spontaneous termination occur during the last few seconds before termination [27]. Ndrepepa et al. showed that the earliest detectable event prior to AF termination occurred on average 4 s before termination with a significant increase of cycle length in the LA first of all, followed by an increase in the RA about 1 s later [25]. In our simulations, a similar observation was made when using the CRN kinetics model. However, when using a model with a different AF dynamics, the LR model, the temporal dynamics of the spontaneous termination was different. Further investigations will be devoted to the precise description and understanding of these mechanisms.
Spontaneous termination in the Luo-Rudy (LR) and the Courtemanche–Ramirez–Nattel (CRN) models. The mean temporal evolution of AFCL and #WF during the 8 s preceding termination are shown for the two different models. For each model, instantaneous transmembrane potential maps for an example of AF termination episode are also presented. A color scale was used here, red representing a value of 20 mV and blue the resting potential of −80 mV
4.4.3 Spatial Scales of Termination
The spatial aspect of termination was assessed through a visual inspection of the extinction of the last active reentrant wave front before AF termination. In most cases, this last wave front was annihilated by one or several collisions, generating a larger wave front resembling normal activation, which died out in one of the extremities of the geometry. Therefore, the extinction site was the location where AF terminated, but not necessarily where electrical activity died.
In the LR model, no significant difference (p = 0.1096) was found between the number of episodes with an extinction site in the left atrium (21/50, 42%) and the number of episodes terminating in the right atrium (29/50, 58%). Therefore, AF based on multiple wavelets reentries did not terminate predominantly at a specific anatomic location on the atrium.
In the CRN model, significantly fewer episodes (p < 0.001) terminated in the LA (9/50, 18%) compared to the RA (41/50, 82%). This is very different from what has been observed in the LR model using the same atrial geometry. The role of atrial geometry still needs to be analyzed further. Model-based analysis is essential for understanding the spatial patterns involved in AF termination and its dependence on the underlying dynamics. This is a field where computer modeling offers unique possibilities since these detailed observations cannot be easily performed in clinical studies based on surface electrocardiograms or mapping data with a limited number of electrodes.
4.5 Ablation of AF
Surgical ablation of AF aims at creating lines of block to interrupt electrical conduction and to prevent the AF reentrant process, the gold standard being the Maze III procedure developed by Cox et al. [4]. This complex ablation procedure has proven to be effective in treating chronic AF, but it can be time-consuming and associated with a risk of serious complications. Therefore, less-invasive radiofrequency catheter ablation alternatives were developed and investigated clinically. However, the ideal location and number of ablation lines, their best connection, and appropriate length still remain to be determined.
Different ablation patterns were systematically studied in the biophysical model of chronic AF. For each pattern evaluated, results were averaged over 10–40 AF initial conditions on which a specific ablation pattern was applied instantaneously. The simulation results confirmed that the most complex ablation patterns led both to the best success rate and shortest time to AF termination. Ablation patterns involving lines in the right or left atrium only led to success ranges of 20–60% and 55–80%, respectively, while those combining lines in both atria showed an increased rate in the range 80–100% [30]. The best results were obtained with the Maze III pattern: 100% success rate in the biophysical model, which is in the upper range of clinical data showing a long-term success rate ranging from 80 to 99% [4]. Figure 4.4 shows an example of AF termination using this ablation pattern. Simulations studies were also directed at finding ablation patterns reproducing the maximum conversion rate of 100% obtained with the Maze III procedure while using a minimum number of lesions [30]. This was the case for the patterns combining an isolation of the pulmonary veins, the left isthmus line, and the line between vena cavae in the right atrium.
Example of a successful ablation in the biophysical model of AF with the Maze III pattern (represented in the upper left box). t = 0 ms: AF initial condition, t = 965 ms: last reentry in the LA, t = 1,105 ms: last reentry in anterior RA, t = 1,400 ms: last reentry in posterior RA, t = 1,565 ms: uniform propagation wave fronts, t = 1,800 ms: uniform propagation wave front with prolonged action potential duration, t = 2,015 ms: final activity just before termination
A comparison between in silico ablation results obtained in the biophysical model and in vivo data from patients who underwent radiofrequency ablation showed a positive correlation for conversion rates to sinus rhythm and residual atrial flutter [29]. Computer modeling offers the possibility to test ablation line patterns in a reversible way in a human model, to test patterns not generally performed in clinical experiments, to observe the AF termination process in detail, and to study the impact of imperfections that may be present in the ablation lines.
4.6 Pacing of AF
Several ATP techniques such as burst or ramp pacing are currently used clinically to terminate atrial tachycardia or atrial flutter. Pacing treatment has the advantage over electrical cardioversion in that the therapy is painless and the energy cost negligible. However, no ATP therapy has proved to be effective in terminating AF, probably due to its more complex dynamics, the variable number of wavelets and the smaller variable excitable gap [21]. On the other hand, the possibility of local atrial capture by rapid pacing has been shown in animal and human experiments during electrically induced or spontaneous AF [1, 6, 26]. The resulting paced AF cycle length was smaller than the original one and did not lead to termination but sometimes to a loss of capture.
To study these processes, we implemented and tested an ATP algorithm currently used in pacemakers, namely, burst pacing, and determined the optimal pacing sites and pacing periods leading to local capture of AF. We followed a methodology similar to that used in our previous studies on AF ablation, and for each pacing protocol tested, the results were the average values obtained following three different initial AF conditions [33].
4.6.1 Pacing Protocol and Assessment of AF Capture
The pacing protocol is presented in Fig. 4.5. Burst pacing at constant cycle length was applied during 30 s, at 20–110% AFCL with 5% increments. Five pacing sites were evaluated: two sites in the RA, two sites in the LA, and one on the septum between both atria. Capture was defined as the ability of the pacing burst to take control over an area with a radius greater than 2 cm around the pacing site for a minimum of five consecutive beats. This definition was related to local capture around the pacing site, not to the generalized capture of both atria. Three different measures were used to describe the results. The capture interval was the pacing period interval (expressed in percentage of AFCL) for which capture was observed during more than 50% of the time. Within this capture interval, time to capture was defined as the duration from the start of ATP pacing to the onset of the first capture episode, thus providing an indication of how rapidly capture was achieved. Finally, capture robustness was used as a measure of the ability to sustain capture, computed as the percentage of time spent in capture between the first capture episode and the end of the pacing protocol.
Protocol for the simulation of AF pacing in the biophysical model of AF. Three initial conditions were extracted from the AF database. The burst pacing protocol was then tested at five different pacing sites. Local capture was assessed 2 cm away from the pacing site. Results were averaged on the different AF initial conditions and three measures describing the capture were computed
4.6.2 AF Pacing Results
Capture results for the different pacing sites and the burst pacing protocol are summarized in Fig. 4.6. The optimal pacing period was similar for all pacing sites when computed as a percentage of the AFCL measured at the pacing location (68–83%), except for the isthmus pacing where a very narrow capture interval with only brief episodes of captures was observed. The expression of the capture interval as a percentage of AFCL allowed us to be independent of a specific AF cycle length and to compare results for the different pacing sites, even if longer AFCL were observed at some locations such as the septum. No significant difference was observed between the times to capture at the different pacing locations. Higher capture robustness was found in the right atrial free wall, the left atrial appendage, and the pulmonary veins compared with the isthmus or the septum. However, when pacing only in one atrium, control in both atria was not observed. Obtaining capture in both atria was found possible only when pacing in the septum, although being far less robust. Figure 4.6 presents the spatial repartition of the wave fronts following capture for the different pacing sites. The wave fronts induced by pacing encompassed for some locations the major part of the paced atrium. However, this was often accompanied by residual waves outside the area of capture. AF termination was not possible due to the fact that a slowing down or an abrupt stop of the pacing protocol allowed these residual wavelets to penetrate the captured area and to reinitiate AF. These observations of capture loss are consistent with experimental data [6]. Taken together, these results suggest that modeling studies can be helpful in the optimization of pacing protocols for AF treatment. We found that ATP algorithms working well for slower atrial tachyarrhythmias cannot be directly transposed to terminate AF, which is faster and less organized. Nevertheless, parameters such as pacing sites and frequencies can be optimized to maximize pacing efficacy. This explorative study was based on three initial conditions. More simulations will be needed to define precisely optimal pacing protocols for AF, and to explain the underlying mechanisms of AF control and pace termination.
Simulations results for AF pacing. For each of the five pacing sites, capture results are expressed with the capture interval, the time to capture, and the capture robustness. Three instantaneous transmembrane potential maps of AF activity during burst pacing at optimal frequency are also shown. The instantaneous transmembrane potential map on the right shows a time instant where the most favorable capture was achieved with the captured wave fronts induced by pacing highlighted in red
4.7 Conclusion
This chapter shows how a biophysical model can be used to increase our understanding of AF. It permits the simulation of different AF dynamics, the evaluation of the mechanisms of spontaneous termination, and the study of currently used therapies for AF. The advantage of a computer modeling approach over clinical experiments lies in the possibility of performing systematic studies at detailed temporal and spatial scales, and thus offering a deeper insight into the underlying pathophysiological processes. While the biophysical model presented here is simplified in many aspects, the simulations are complex enough to reproduce observations made in humans, both in terms of obtained AF dynamics and the effectiveness of therapies. Today, with the emergence of mathematical models of increasing complexity, the challenge remains to reproduce phenomena with the least possible number of relevant parameters and, most importantly, to be able to have a close match to clinical observations [2]. The ability to reproduce a specific AF dynamics corresponding to a single patient makes this modeling framework attractive for dedicated experiments. Hopefully, such an approach will make a better translation of research results into therapeutic options possible. An improved understanding of the initiating and perpetuating factors of AF in individual patients will enhance the development of mechanism-based therapies.
References
Allessie M, Kirchof C, Scheffer GJ, Chorro F, and Brugada J. Regional control of atrial fibrillation by rapid pacing in conscious dogs. Circulation 1991;84:1689–1697.
Coronel R. Myths, metaphors, and mathematical models. Heart Rhythm 2007;4:1046–1047.
Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol 1998;275:H301–H321.
Cox JL, Schuessler RB, D’Agostino HJ Jr, Stone CM, Chang B-C, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation: III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569–583.
Dang L, Virag N, Ihara Z, Jacquemet V, Vesin J-M, Schlaepfer J, Ruchat P, Kappenberger L. Evaluation of ablation patterns using a biophysical model of atrial fibrillation. Ann Biomed Eng 2005;33:465–474.
Daoud E, Pariseau B, Niebauer M, Bogun F, Goyal R, Harvey M, Man C, Strickberger SA, Morady F. Response of type I atrial fibrillation to atrial pacing in humans. Circulation 1996;94:1036–1040.
Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation 1998;98:2202–2209.
Harrild D, Henriquez C. A computer model of normal conduction in the human atria. Circ Res 2000;87:e25–e36.
Haissaguerre M, Jais P, Shah S, Takashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339:649–666.
Haissaguerre M, Lim K-T, Jacquemet V, Rotter M, Dang L, Hocini M, Matsuo S, Knecht S, Jais P, Virag N. Atrial fibrillatory cycle length: computer simulation and potential clinical importance. Europace 2007;9:vi64–vi70.
Jacquemet V, Virag N, Ihara Z, Dang L, Blanc O, Zozor S, Vesin J-M, Kappenberger K, Henriquez C. Study of unipolar electrogram morphology in a computer model of atrial fibrillation. J Cardiovasc Electrophysiol 2003;14:S172–S179.
Jacquemet V, Virag N, Kappenberger L. Wavelength and vulnerability to atrial fibrillation: insights from a computer model of human atria. Europace 2005;7:S83–S92.
Kim B-S, Kim Y-H, Hwang G-S, Pak HN, Lee SC, Shim WJ, Oh DJ, Ru YM. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol 2002;39:1329–1336.
Kirchhof P, Bax J, Blomstrom-Lundquiest C, Calkins H, Camm AJ, Cappato R, Cosio F, Crijns H, Diener H-C, Goette A, Israel CW, Kuck K-H, Lip GYH, Nattel S, Page RL, Ravens U, Schotten U, Steinbeck G, Vardas P, Waldo A, Wegscheider K, Willems S, Breithardt G. Early and comprehensive management of atrial fibrillation: proceedings from the 2nd AFNET/EHRA concensus conference on atrial fibrillation entitled ‘research perspective in atrial fibrillation’. Europace 2009;11:860–885.
Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res 2002;90(9):E73–E87.
Kneller J, Kalifa J, Zou R, Zaitsev AV, Warren M, Berenfeld O, Vigmond EJ, Leon J, Nattel S, Jalife J. Mechanisms of atrial fibrillation termination by pure sodium channel blockade in an ionically-realistic mathematical model. Circ Res 2005;96:35–47.
Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation 1994;89:1665–1680.
Li D, Zhang L, Kneller J, Nattel S. Potential ionic mechanism for repolarization differences between canine right and left atrium. Circ Res 2001;88:1168–1175.
Lip GYH, Tse H-F. Management of atrial fibrillation. Lancet 2007;370:604–618.
Luo C-H, Rudy Y. A model of the ventricular cardiac action potential. Circ Res 1991;68:1501–1526.
Mitchell ARJ, Spurrell PAR, Cheatle L, Sulke N. Effect of atrial antitachycardia pacing treatment in patients with an atrial defibrillator: randomized study comparing subthreshold and nominal pacing outputs. Heart 2002;87:433–437.
Moe GK, Rheinbold WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J 1964;67:200–220.
Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002;415:219–226.
Nattel S, Opie LH. Controversies in atrial fibrillation. Lancet 2006;367:262–272.
Ndrepepa G, Weber S, Karch MR, Schneider MAE, Schreieck J, Schomig A, Schmitt C. Electrophysiologic characteristics of the spontaneous onset and termination of atrial fibrillation. Am J Cardiol 2002;90:1215–1220.
Pandozi C, Bianconi L, Villani M, Castro A, Altamura G, Toscano S, Jesi AP, Gentilucci G, Ammirati F, Lo Bianco F, Santini M. Local capture by atrial pacing in spontaneous chronic atrial fibrillation. Circulation 1997;95:2416–2422.
Petrutiu S, Sahakian AV, Swiryn S. Abrupt changes in fibrillatory wave characteristics at the termination of paroxysmal atrial fibrillation in humans. Europace 2007;9:466–470.
Reumann M, Bohnert J, Seeman G, Osswals B, Doössel O. Preventive ablation strategies in a biophysical model of atrial fibrillation based on realistic anatomical data. IEEE Trans Biomed Eng 2008;55:399–406.
Ruchat P, Dang L, Schlaepfer J, Virag N, von Segesser LK, Kappenberger L. Use of a biophysical model of atrial fibrillation in the interpretation of the outcome of surgical ablation procedures. Eur J Cardiothorac Surg 2007;32:90–95.
Ruchat P, Virag N, Dang L, Schlaepfer J, Pruvot E, Kappenberger L. A biophysical model of atrial fibrillation ablation: what can a surgeon learn from a computer model? Europace 2007;9:vi71–vi76.
Skouibine K, Trayanova NA, Moore P. Success and failure of the defibrillation shocks: insights from a simulation study. J Cardiovasc Electrophysiol 2000;11:785–796.
Steinberg JS. Atrial fibrillation: an emerging epidemic? Heart 2004;90:239–240.
Uldry L, Virag N, Kappenberger L, Vesin J-M. Optimization of antitachycardia pacing protocols applied to atrial fibrillation: insights from a biophysical model. Proceedings of the 31st Annual International Conference of IEEE EMBC 2009:3024–3027, September 2009.
Uldry L, Virag N, Jacquemet V, Vesin J-M, Kappenberger L. Spontaneous termination of atrial fibrillation: study of the effect of atrial geometry in a biophysical model. Proceedings of the 31st Annual International Conference of IEEE EMBC 2009:4504–4507, September 2009.
Van Oosterom A, Jacquemet V. Genesis of the P wave: atrial signal as generated by the equivalent double layer source model. Europace 2005;7:S21–S29.
Vigmond EJ, Ruckdeschel R, Trayanova N. Reentry in a morphologically realistic atrial model. J Cardiovasc Electrophysiol 2001;12:1046–1054.
Vigmond EJ, Tsoi V, Kuo S, Arvalo H, Kneller J, Nattel S, Trayanova N. The effect of vagally induced dispersion of action potential duration on atrial arrhythmogenesis. Heart Rhythm 2004;1:334–344.
Virag N, Jacquemet V, Henriquez CS, Zozor S, Blanc O, Vesin J-M, Pruvot E, Kappenberger L. Study of atrial arrhythmias in a computer model based on magnetic resonance images of human atria. Chaos 2002;12:754–763.
Acknowledgements
This study was made possible through grants from the Theo-Rossi-Di-Montelera Foundation, Medtronic Europe, and the Swiss Governmental Commission of Innovative Technologies (CTI). The authors wish to thank Ryan Lahm, Drs. Josée Morisette, and Arthur Stillman who kindly furnished the atrial geometry surface model. The authors also would like to thank Prof. Adriaan van Oosterom for helpful discussions and suggestions regarding the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Uldry, L., Virag, N., Vesin, JM., Kappenberger, L. (2010). Studies of Therapeutic Strategies for Atrial Fibrillation Based on a Biophysical Model of the Human Atria. In: Kerckhoffs, R. (eds) Patient-Specific Modeling of the Cardiovascular System. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6691-9_4
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6691-9_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6690-2
Online ISBN: 978-1-4419-6691-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)