Abstract
Mycetoma is a chronic subcutaneous infection that develops after one of the multiple etiologic microorganisms is inoculated into a site of skin trauma. Although mycetoma is primarily a subcutaneous disease, it can involve bone and lymph nodes by contiguous spread. Mycetoma shows three clinical characteristics: tumor, sinuses, and grains. The tumor results as a consequence of a progressive and relatively painless swelling. Sinuses are a characteristic of the disorder; they can be absent in early stages, but later develop and drain purulent material and grains. Grains are colonies of the causative agent and can be black, white, or red. Mycetoma can be caused by a variety of fungal agents (eumycetoma), or filamentous gram-positive branching bacteria belonging to the aerobic Actinomycetales (actinomycetoma).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Mycetoma is a chronic subcutaneous infection that develops after one of the multiple etiologic microorganisms is inoculated into a site of skin trauma. Although mycetoma is primarily a subcutaneous disease, it can involve bone and lymph nodes by contiguous spread. Mycetoma shows three clinical characteristics: tumor, sinuses, and grains. The tumor results as a consequence of a progressive and relatively painless swelling. Sinuses are a characteristic of the disorder; they can be absent in early stages, but later develop and drain purulent material and grains. Grains are colonies of the causative agent and can be black, white, or red. Mycetoma can be caused by a variety of fungal agents (eumycetoma), or filamentous gram-positive branching bacteria belonging to the aerobic Actinomycetales (actinomycetoma).
Gill first described mycetoma while working in Madura, India, in 1842, and this was subsequently documented by Godfrey in Madras [1]. Gill reported this entity as “foot tumor,” and Colenbrook introduced the term “Madura foot” in 1846. Ballingal described the microscopic details of the disease for the first time in 1855; however, he did not define its etiology. In 1860, Carter described a disease principally affecting the foot and assigned a fungal origin to this disease in 1861 [2]. He also introduced the term mycetoma, meaning “fungus tumor,” and extended the concept to include infections with grains that had colors other than black. During the second half of the nineteenth century, mycetomas were reported throughout the world: in Europe in 1888, in Africa in 1894, and in the USA in 1896.
The hyphomycete isolated from a black grain was given the generic name Madurella by Brumpt [3]. In 1913, Pinoy subclassified this disease into two categories: “actinomycosis” and “true mycetoma” according to the type of etiologic agent [4]. In 1916, Chalmers and colleagues coined the term maduromycoses for the first time to refer to mycetomas of fungal etiology, rejecting the term “Madura foot” to include extrapedal forms of this disease [5,6]. Even though distinction between eumycetomas and actinomycetomas was achieved at this time, the term mycetoma is still used to refer to both entities, and most of the published literature has mixed these terms, making it difficult to draw clear conclusions about these distinct disorders.
Despite the acquisition of considerable new knowledge concerning this disease during the last century, including the identification of new agents by the use of novel molecular techniques, there are still important gaps in information regarding eumycetoma, mainly related to pathogenesis and management. The goal of this chapter is to review the epidemiologica and clinical aspects of eumycetoma, also known as eumycotic mycetoma.
Organisms and Epidemiology
More than 20 hyaline and pigmented moulds can cause eumycetomas (Table 1). Madurella mycetomatis is the predominant pathogen worldwide, followed by Pseudallescheria boydii/Scedesporium apiospermum, Leptosphaeria senegalensis, and Madurella grisea [7]. These four fungi account for approximately 95% of eumycetoma cases. S. apiospermum has been considered the anamorph (the asexual state) of P. boydii; but new molecular studies indicate they are two different species, and that P. boydii is a complex that includes at least eight phylogenetic species [8,9]. However, most of the existing literature does not differentiate between the species, and their individual involvement in human infections has not been determined. Hereafter, we will use the name P. boydii complex when referring to P. boydii or to S. apiospermum.
Although eumycetoma has been reported worldwide, most of the cases come from tropical and subtropical regions around the Tropic of Cancer, between 15° south and 30° north [10], with sporadic cases occurring in temperate zones. Over 60% of all cases are reported from India, Sudan, and Senegal [11–18]. Endemic regions are characteristically arid with a moderate rainy season (4–6 months), a rainfall of 50–1,000 mm per year, and daytime temperatures from 30°C to 37°C, with small variations between day and night [19].
Temperature, rainfall, type of soil, and prevalent vegetation influence the prevalence of specific eumycetoma agents in a particular region [20], with rainfall being the most influential factor. Black grain fungi cause eumycetomas in arid regions, whereas white grain fungi cause eumycetomas in regions with higher rainfall and without a significant dry season [21]. M. mycetomatis prevails in hot and dry areas with low rainfall and can be found in temperate zones, but it is rare in the equatorial zone [11]. P. boydii complex prevails in areas with hyperprecipitation (∼2,000 mm per year) [10,22] and has been reported sporadically in the northern temperate zone among sewage workers [23].
Most eumycetoma agents such as M. mycetomatis, M. grisea, P. boydii complex, and Neotestudina rosatii have been isolated from soil samples [24–27], and M. mycetomatis and P. boydii complex have also been isolated from termite mounds. L. senegalensis and L. tompkinsii are recovered from 50% of acacia dry thorns in the Senegal River region, but not from green thorns [25,27,28], suggesting that thorns may play a role as mechanical vectors. Recently, the use of molecular techniques has facilitated the study of natural reservoirs of M. mycetomatis. Polymerase chain reaction (PCR) detection followed by restriction fragment length polymorphism (RFLP) analysis have demonstrated the presence of the organism in 23% of soil samples from endemic areas of Sudan and have successfully linked environmental and clinical isolates [29].
Reports of eumycetoma affecting animals are unusual. Equine and canine cases due to M. mycetomatis have been reported [30,31]. Cases due to Curvularia lunata and Cladophialophora bantiana in dogs and an unspecified organism in buffalo have been reported [32–34].
Pathogenesis
Disease usually develops as a result of minor trauma that inoculates contaminated material, usually soil, into the skin or subcutaneous tissue. A history of any trauma at the site of eumycetoma is uncommon, ranging from 0% to 34% of cases, with the higher figures reported from endemic areas of Sudan and India [35,36]. This observation suggests that either these fungi do not need deep inoculation, or that disease occurs after a prolonged incubation period [37].
After inoculation, a poorly defined host response precludes the development of free fungal filaments in the infected tissue, and instead leads to the development of the characteristic grain. Neutrophil-mediated tissue reaction leads to partial grain disintegration, but most of the grain remains and perpetuates a chronic inflammatory response. Macrophages and multinucleated giant cells clear dead neutrophils and grain fragments, and an epithelioid granuloma develops [38]. Results of immunologic studies performed among patients affected by mycetomas are scarce and conflicting. Mahgoub and co-workers found a moderately decreased cell-mediated immune response [39], while Bendl et al. were not able to demonstrate any immunologic alterations in 15 patients [40].
The role of genetic predisposition to develop mycetoma has not been established. Although many residents of endemic areas have antibodies to M. mycetomatis, very few develop eumycetoma. It has been postulated that those who develop eumycetoma have inadequate neutrophil function resulting from polymorphisms in the functional expression of those genes that direct neutrophil function [41].
The role of melanin that is present in variable amounts in grains from certain organisms, such as M. mycetomatis, is not completely understood. Melanin has been linked to virulence and pathogenicity, and it is considered the most important component of the grain cement. Melanin strengthens the grain and protects fungal cells from antibodies, hydrolytic enzymes, strong oxidants, and azole antifungal agents [42,43].
Clinical Aspects
Clinical Manifestations
Eumycetoma principally affects otherwise normal men living and working in rural areas. The male-to-female ratio ranges between 3:1 and 5:1; the age at the time of diagnosis ranges from 3 to 77 years (mean 32.6 years). The average duration of symptoms ranges between 7.7 and 9.8 years, ranging from 1 month to 25 years [44–50]. Most patients with eumycetoma are not classically immunocompromised, although diabetes is a frequent comorbidity. Indeed, 9 of 26 mycetoma patients diagnosed over a 9-year period in the UK had diabetes as an underlying disease [10]. Eumycetoma has been reported in patients receiving chronic immunosuppressive therapy for renal and heart transplantation, leukemia, and idiopathic CD4 lymphopenia [51–56]. Eumycetoma has not been reported in an HIV-infected patient.
Male predominance among eumycetoma patients can be explained by higher rates of exposure to etiologic agents related to occupational cutaneous injury, such as might occur in farming. This is similar to the acquisition of other subcutaneous mycoses, such as sporotrichosis and chromoblastomycosis. Male predominance could also be a consequence of higher susceptibility to this disease, an explanation based on an inhibitory effect of progesterone on the growth of M. mycetomatis and Pyrenochaeta romeroi in the laboratory [57].
The incubation period of eumycetoma is not well established, as most patients seek care after long periods of disease and without recall of the inoculation event. Clinical characteristics and evolution of eumycetoma lesions are independent of the etiologic fungus; the clinical course depends on the anatomic location, duration of lesions, and medical intervention. Lesions begin as small, firm, painless, indurated subcutaneous nodules or plaques that gradually increase in size. The clinical course is somewhat slower for eumycetoma than for actinomycetoma. Initially, the lesion is well demarcated and may be encapsulated, especially when M. mycetomatis is the etiologic agent. The disease usually runs a chronic course from several years to decades, with lesions spreading slowly to adjacent structures by contiguous spread, and virtually never by hematogenous dissemination.
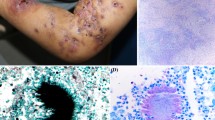
The tumor develops as a result of the enlargement of existing nodules and formation of new nodules. Generally it is firm and round but may be soft and lobulated. Enlarged nodules open to the skin through sinus tracts, discharging sanguineous, seropurulent or purulent exudate that contains grains (Figs. 1, 2, and 3). A history of sinus tracts discharging grains is present in up to 60% of the cases [14].
Sinus tracts develop relatively early in the course of disease; at least one-third of patients develop sinuses between 3 and 6 months, and almost all patients have sinus tracts within 1 year of the development of skin lesions [58]. Established sinuses heal and recur as new sinuses continue to develop. Sinus tracts are very characteristic of both eumycetoma and actinomycetoma and help support the clinical diagnosis, but also occur in other diseases and are not specific.
Destruction of adjacent structures can be dramatic and is especially characteristic late in the course of the disease. Destructive lesions are relatively painless. Pain, fever, and other systemic symptoms are not characteristic of eumycetoma, and when present, suggest a secondary bacterial infection. Bacterial cellulitis should be ruled out when pain is present, especially when edema and increasing discharge are evident. Massive fibrosis occurs after healing of involved tissue, contributing to the tumor-like appearance and woody texture of the affected area.
Eumycetoma lesions are located most frequently in areas with a high frequency of repeated trauma, especially the lower limbs. Feet, legs, and hands account for approximately 90% of black grain eumycetomas and 95% of M. mycetomatis eumycetomas [35,59]. Involvement of the foot is more common in eumycetomas than in actinomycetomas, occurring in 75–85% of cases [35,59]; legs and hands are involved in 7% and 6% of eumycetoma cases, respectively [60].
Extrapedal eumycetomas appear when repeated trauma occurs to other parts of the body. For example, lesions can occur on the abdominal wall in patients who do not wear a shirt and who carry organic products, such as vegetables or straw [61,62]. Rare anatomic sites described for eumycetoma include intraspinal [63], scalp [35], neck [13], mandible [64], eyelid [65], cheek [50], perineum [10], testicle [35], buttock [66,67], and thigh [10].
Multiple eumycetomas involving more than one anatomic site are rare. Most of these “double eumycetomas” described in the literature represent two lesions in the same anatomic region [68]. One report from Argentina of double eumycetoma described a patient with lesions on the foot and wrist, both lesions caused by M. grisea [69]. Eumycetoma caused by more than one fungus is also a rare clinical occurrence, but the occurrence of M. mycetomatis and M. grisea in a foot lesion has been reported [70].
Lymphatic spread is uncommon with eumycetomas, occurring in fewer than 3% of the cases [71]. It appears to be more frequent in actinomycetomas, possibly because the grains are smaller in this condition [72]. Among the agents of eumycetomas, M. mycetomatis has the lowest frequency of lymph node involvement, possibly because of the extensive fibrotic reaction that often accompanies this agent. Only 3 of 578 (0.5%) patients in one series experienced this complication [73].
In both eumycetoma and actinomycetoma, bone involvement occurs by contiguous spread, with changes occurring first in cortical bone [74]. Bone involvement occurs in up to 76% of cases and is more extensive with longer duration of disease [35,46]. In addition, bone lesions are more frequent and occur sooner when they are located in areas with thin subcutaneous tissue, such as the feet, hands, and skull.
Radiologic Findings
The most frequent radiologic findings in patients with eumycetomas are soft tissue swelling and osteolytic changes (Fig. 4). Loss of the cortical border and external erosion of the bone is the earliest osteolytic manifestation [7,75]. Later, the medullary canal and epiphysis are affected, resulting in bone destruction followed by bone remodeling [76]. Bone lesions can be manifested radiographically as demineralization, periosteal reaction, osteolysis, endosteal bone cavitation, sclerosis, and frank osteomyelitis [46]. Osteolytic lesions associated with eumycetoma are usually large and few in number, often with well-defined margins. Grossly, these lesions are filled with necrotic material and grains [20,76,77]. No particular pattern of bone involvement is associated with a specific eumycetoma agent. Moreover, it is not possible to differentiate between eumycetoma and actinomycetoma using radiologic studies [77,78].
Computerized tomography (CT) and MRI typically demonstrate bone lesions earlier than x-rays. CT should be used to evaluate pedal mycetomas, whereas MRI is preferred for extrapedal lesions. In addition, CT has greater sensitivity to detect early bone involvement, while MRI easily detects late manifestations as a coarse trabecular pattern, bone destruction, marrow infiltration, and sequestra [74]. MRI is also helpful for determining the extent of soft tissue involvement and for monitoring the response to treatment [74,78]. A “dot-in-circle sign” demonstrated by MRI is considered as a specific sign of both eumycetoma and actinomycetoma. It represents inflammatory granulomata containing grains and surrounded by a fibrous matrix [79].
Complications
The most common complication is secondary bacterial infection, which occurs in up to 66% of patients with black grain eumycetomas [80]. Massive bone destruction induced by eumycetomas can produce pathologic fractures [81]. Other complications are related to the site of disease, e.g., deformity of the foot in tumoral pedal eumycetoma. Rare complications include bronchopleural-cutaneous fistulae [82] and palatal deformity and dysfunction [83].
Differential Diagnosis
The differential diagnosis of eumycetoma lesions at any stage of their evolution should always include actinomycetoma due to aerobic filamentous actinomycetes and botryomycosis, due to gram-positive and gram-negative bacteria.
Small eumycetoma lesions may be confused with folliculitis, soft tissue tumors. or cystic lesions [84], while exophytic verrucous eumycetoma lesions of the foot can mimic verrucous tuberculosis, blastomycosis, chromoblastomycosis, and sporotrichosis. More extensive tumoral pedal lesions without sinus tracts should be differentiated from elephantiasis of the foot, as well as benign and malignant tumors. When bone involvement is present, the differential diagnosis includes bacterial osteomyelitis, osseous tuberculosis, osteosarcoma, and other malignant bone tumors. Extrapedal lesions should be differentiated from dermatophytic pseudomycetoma when the scalp is affected. In addition, cutaneous tuberculosis, endemic fungal diseases, such as blastomycosis and coccidioidomycosis, and cutaneous nocardiosis should be excluded.
Diagnosis
When draining sinus tracts are present, these provide the optimum material for microscopic examination and culture. Grains in discharged fluid are visible to the naked eye and can be collected from dressings covering a draining sinus tract. If discharged grains are not available, a deep skin biopsy taken from a small abscess or around a sinus tract is necessary for both culture and histopathologic studies. Fine-needle aspiration also can be useful for the diagnosis of eumycetoma [85]. Specimens should be submitted for macroscopic and microscopic examination and cultured appropriately.
Evaluation of Grains
Grain color, size, shape, and consistency should be noted because these characteristics help to guide identification of the causative fungus. For example, M. mycetomatis grains are large, black, and hard; L. senegalensis grains are large, black, and firm to hard; M. grisea and P. romeroi grains are small, black, and soft to firm; and P. boydii complex and Aspergillus nidulans grains are large, white, and soft. After macroscopic examination, grains should be placed in a drop of 10–20% KOH on a slide, compressed between two slides, and examined under microscopy. This direct examination will differentiate the grains of eumycetomas from the grains of actinomycetomas. Eumycetoma grains contain intertwined, broad hyphae (2–5 μm), and may contain large swollen cells (15 μm or more) at the periphery (Fig. 5).
Culture
Culture is essential for an etiologic diagnosis; however, performing cultures with eumycetoma specimens is laborious and complicated by a high rate of bacterial contamination. Prior to culture, grains should be washed several times with sterile saline solution to reduce bacterial and mould contamination, then crushed using sterile technique and plated on Sabouraud’s dextrose agar containing chloramphenicol. Medium containing cycloheximide should be avoided because it inhibits the growth of some eumycetoma agents, such as some Fusarium spp. and Aspergillus spp. Specimens should be incubated at both room temperature and at 37°C for 6–8 weeks.
Species identification is based on both macroscopic and microscopic examination of colonies. Other tests may be helpful. For example, patterns of sugar assimilation and optimal growth temperature differentiate M. mycetomatis from M. grisea. The former can utilize glucose, galactose, lactose, and maltose, but not sucrose, and grows well at 37°C. By comparison, M. grisea can utilize glucose, galactose, maltose, and sucrose, but not lactose, and grows well at 30°C [55]. More recently, molecular techniques, such as the random amplification of polymorphic DNA (RAPD), RFLP, and DNA sequencing are being used for identification of various fungal species. These techniques are particularly useful when routine fungal isolation has failed [86–88].
Histopathology
The basic histopathologic picture of eumycetoma is chronic nonspecific granulomatous inflammation, with a central focus of acute inflammatory reaction surrounding one or more grains. Grains can be difficult to visualize in tissue sections, making it necessary to examine numerous sections of the biopsy. A zone formed by histiocytes surrounds the central and abscessed focus; this is surrounded by an outer zone consisting of new capillaries, isolated histiocytes, plasma cells, mast cells, and eosinophils. Lymphocytes characteristically are found infiltrating the fibrous tissue of the outer zone [19,36]. The fungal hyphae, which constitute the main element of the grain, are more easily observed with the use of periodic acid–Schiff (PAS) or methenamine silver stains.
As shown in Table 2, histopathologic characteristics on hematoxylin-eosin stain of black grain eumycetomas can be quite distinctive, and may allow for a presumptive diagnosis [89,90]. For example, M. mycetomatis grains, vesicular type, show a dense brown cement-like substance with hyphae and large chlamydospores in the periphery (Fig. 6). By contrast, Exophiala jeanselmei grains do not have cement-like substance. On the other hand, pale grain eumycetomas have similar histopathologic findings, making their differentiation uncertain [89]. The use of immunofluorescent antibodies facilitates the identification of the etiologic agent in tissue sections. A specific fluorescent antibody conjugate for identification of P. boydii is available in some areas [91,92], and monoclonal antibodies against Aspergillus galactomannan have been used to identify Aspergillus species without cross-reactivity with other fungi [93,94].
Serology
There is no reliable serologic test available for diagnosis of eumycetoma. Lack of standardized preparation of antigens has hampered development of such a test. In addition, many etiologic agents of eumycetoma require independent testing with several antigens or the use of a polyvalent antigen preparation. Immunodiffusion (ID) and counterimmunoelectrophoresis (CIE) have been the most widely used tests for detecting antibodies in eumycetoma patients, but both have shown inconsistent results [95–97]. Enzyme-linked immunosorbent assay (ELISA) is more sensitive and reproducible than ID and CIE [98]; its limitation is that asymptomatic patients from endemic areas may also show elevated antibody titers by ELISA.
Serologic assays may play a role in the follow-up of patients on antifungal treatment after the specific etiology is established.
Treatment
Antifungal Agents
No evidence-based treatment recommendations are available for eumycetoma, as no large randomized clinical trials have been conducted. Moreover, the few clinical reports of the use of antifungal agents in the treatment of eumycetomas often involve a limited number of patients, do not differentiate by presence of bone involvement, and fail to establish a definitive response status due to the limited follow-up period. Even though new triazole drugs have been shown to be effective in treating eumycetomas, current options remain limited because of insufficient clinical data.
The in vitro activity of various antifungal agents against the organisms causing eumycetoma does not reliably predict clinical response. For example, amphotericin B has good in vitro activity against M. mycetomatis, M. grisea, and E. jeanselmei, but in vivo responses are poor, and clinical data do not support an important role for amphotericin B in the treatment of eumycetomas [99,100]. Liposomal amphotericin B has been used to treat eumycetomas caused by M. grisea and Fusarium species, producing temporary remission followed by clinical relapses 6 months after therapy was stopped [101]. Based on these limited anecdotal data, most experts believe that there is little role for amphotericin B in the treatment of eumycetoma.
Black grain eumycetoma agents are sensitive in vitro to the older azoles, with itraconazole demonstrating the most activity, followed by ketoconazole and miconazole [102]. Among the new triazoles, posaconazole is highly active in vitro against Aspergillus species and P. boydii complex [103,104], and voriconazole is active in vitro against M. mycetomatis, M. grisea, and E. jeanselmei. Voriconazole also has in vitro fungicidal activity against Aspergillus species and is more active than itraconazole against P. boydii complex isolates [105–107].
In general, itraconazole and ketoconazole appear to perform better against black grain than white grain eumycetomas [108]. Fluconazole 400 mg per day is not effective for eumycetoma caused by M. mycetomatis, M. grisea, or P. boydii complex [109].
For eumycetomas due to M. mycetomatis or M. grisea, itraconazole 100 mg twice daily is the regimen of choice. Preliminary results from the evaluation of posaconazole as salvage therapy of patients with various fungal infections resistant or refractory to standard treatment, including a small number of eumycetomas due to a variety of agents, are encouraging. Based on these data, posaconazole administered 200 mg orally four times daily or 400 mg twice daily is an acceptable alternative treatment for eumycetomas caused by M. mycetomatis or M. grisea [110]. Ketoconazole is also an effective agent for eumycetoma due to M. mycetomatis with reported rates of success greater than 70% when 400 mg or more daily is given for more than 6 months [99,101,108,111]. Ketoconazole at doses of 400 mg or more daily should be the first treatment option in areas in which the cost of itraconazole is prohibitive. Periodic evaluations of liver enzymes are mandatory, especially when ketoconazole is administered for prolonged periods.
Management of P. boydii complex infections is challenging because this fungus has intrinsic resistance to some antifungal agents, including fluconazole and amphotericin B. Ketoconazole alone or combined with surgery has been tried with varied outcomes [112–114]. Voriconazole demonstrates good in vitro fungicidal activity against P. boydii complex [106], and has been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) as salvage therapy for refractory scedosporiosis. Voriconazole has been successfully used to treat patients with severe P. boydii complex infections, including CNS and disseminated disease [115–119]. There are very limited data on the use of voriconazole specifically for the treatment of eumycetoma caused by P. boydii complex. However, based on the results noted above, voriconazole, 200 mg twice daily, could be considered a preferred regimen in areas in which the cost is not prohibitive [120,121]. Similarly, itraconazole alone or combined with surgery has been effective in some cases [120,122] and is considered an alternative therapy.
The optimal therapeutic regimen for Acremonium eumycetoma is unknown due to the scarcity of reports concerning therapy of this condition. Ketoconazole is not an effective treatment [114]; some eumycetomas caused by Acremonium species have been treated successfully with itraconazole. One patient with eumycetoma caused by A. falciforme responded satisfactorily to itraconazole 200 mg daily for 10 weeks [37], and a patient with A. kiliense eumycetoma, who had failed 3 years of ketoconazole 400 mg daily, rapidly improved when treated with itraconazole 300 mg daily [99]. Similarly, treatment of eumycetomas caused by Aspergillus species, Arthrographis kalrae, or L. senegalensis has not been established. Based on anecdotal reports and in vitro results, itraconazole 100 mg twice daily is considered the treatment of choice, and voriconazole is a promising alternative [105–107,123].
An unsatisfactory response to antifungal therapy correlates with the duration and extent of disease, susceptibility of the causative organism, and drug concentrations in the affected tissues. The latter is influenced by the pharmacokinetics of the agent used, the amount of fibrosis, and the local blood supply [124]. The absence of ischemic changes and necrosis in mycetoma lesions indicates that blood supply probably does not contribute significantly to the failure of medical treatment. However, it is likely that antifungal agents cannot reach adequate concentrations in grains surrounded by fibrotic and abscessed tissue.
Surgery
Early surgery can be curative for small and well-defined eumycetoma lesions, and is used to remove the greater bulk of the lesion when used as an adjunct to antifungal treatment. Antifungal therapy reduces the size of lesions when administered prior to surgery, and reduces recurrences when used following surgical debridement [99,111]. Additional indications for surgery in the management of eumycetoma are less well defined. Use of surgery to drain sinuses and remove grains or as a measure to reduce pain and swelling caused by inflammation is generally discouraged. Most experts advocate delaying surgery until patients have completed several months of antifungal chemotherapy [99]. Radical surgical procedures should generally be avoided [76].
Outcomes
An accepted time of follow-up to define cure for eumycetoma has not been firmly established. Many experts require 2 years of relapse-free survival, while others consider a patient cured only after 3 years have passed without evidence of relapse [40]. Assessment of stability, improvement, cure, or relapse is based on clinical and mycologic parameters, including the amount of discharge, degree of swelling, evolution of fistulae, radiographic findings, the results of histopathologic studies, and culture results.
References
Godfrey J. Disease of the foot not hitherto described. Lancet. 1846;1:593–4.
Carter HV. On a new and striking form of fungus disease, principally affecting the foot, and prevailing endemically in many parts of India. Trans Med Phys Soc Bombay. 1860;6:104–42.
Brumpt E. Les mycétomes. Arch Parasitol. 1906;10:489–564.
Pinoy E. Actinomycoses and mycetomas. Bull Inst Pasteur. 1913;11:929–38.
Chalmers AJ, Christopherson JB. A Sudanese actinomycosis. Ann Trop Med Parasitol. 1916;10:223–82.
Chalmers AJ, Archibald RG. A Sudanese maduromycosis. Ann Trop Med Parasitol. 1916;10:169–222.
McGinnis MR, Fader RC. Mycetoma: A contemporary concept. Infect Dis Clin N Am. 1988;2:939–54.
Gilgado F, Cano J, Gene J, Sutton DA, Guarro J. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J Clin Microbiol. 2008;46:766–71.
Harun A, Perdomo H, Gilgado F, Chen SC, Cano J, Guarro J, et al. Genotyping of Scedosporium species: a review of molecular approaches. Med Mycol. 2009;47:406–14.
Hay RJ, Mahgoub ES, Leon G, Al-Sogair S, Welsh O. Mycetoma. J Med Vet Mycol. 1992;30 suppl 1:41–9.
Mariat F. On the geographic distribution and incidence of mycetoma agents. Bull Soc Pathol Exot Filiales. 1963;56:35–45.
Klokke AH, Swamidasan G, Anguli R, Verghese A. The causal agents of mycetoma in South India. Trans R Soc Trop Med Hyg. 1968;62:509–16.
Gumaa SA, Mahgoub ES, El Sid MA. Mycetoma of the head and neck. Am J Trop Med Hyg. 1986;35:594–600.
Hazra B, Bandyopadhyay S, Saha SK, Banerjee DP, Dutta G. A study of mycetoma in eastern India. J Commun Dis. 1988;30:7–11.
Venugopal PV, Venugopal TV. Treatment of eumycetoma with ketoconazole. Australas J Dermatol. 1993;34:27–9.
Maiti PK, Ray A, Bandyopadhyay S. Epidemiological aspects of mycetoma from a retrospective study of 264 cases in West Bengal. Trop Med Int Health. 2002;7:788–92.
Dieng MT, Sy MH, Diop BM, Niang SO, Ndiaye B. Mycetoma: 130 cases. Ann Dermatol Vénéréol. 2003;130:16–9.
Bakshi R, Mathur DR. Incidence and changing pattern of mycetoma in western Rajasthan. Indian J Pathol Microbiol. 2008;51:154–5.
Lavalle P. Mycetoma. In: Canizares O, Harman RRM, editors. Clinical Tropical Dermatology. 2nd ed. Boston: Blackwell Scientific Publication; 1992. p. 41–60.
Boiron P, Locci R, Goodfellow M, et al. Nocardia, nocardiosis and mycetoma. Med Mycol. 1998;36 Suppl 1:26–37.
Buot G, Lavalle P, Mariat F, Suchil P. Étude épidémiologique des mycétomes au Mexique. Bull Soc Pathol Exot. 1987;80:329–39.
Mahgoub ES. Mycetoma. In: Mahgoub ES, editor. Tropical mycoses. Beerse, Belgium: Janssen Research Council; 1989. p. 57–74.
Cooke WB, Kahler PW. Isolation of potentially pathogenic fungi from polluted water and sewage. Public Health Rep. 1955;70:689–94.
Borelli D. Madurella mycetomi y Madurella grisea. Arch Venez Med Trop Parasit Med. 1962;4:195–211.
Segretain G, Mariat F. Recherche sur la presence d’agents de mycètomes dans le sol et sur les épineux du Sénégal et de la Mauritanie. Bull Soc Pathol Exot. 1968;61:194–201.
Thirumalachar MJ, Padhye AA. Isolation of Madurella mycetomi from soil in India. Hindustan Antibiot Bull. 1968;10:314–8.
Segretain G, Mariat F. Recherche sur l´écologie des agents de mycètomes fungiques au Sénégal. 5th Congress International Society Human and Animal Mycology, Paris, France. pp. 153–154, 1971.
Segretain G. Epidémiologie des mycétomes. Ann Soc Belge Méd Trop. 1972;52:277–86.
Ahmed A, Adelmann D, Fahal A, Verbrugh H, van Belkum A, de Hoog S. Environmental occurrence of Madurella mycetomatis, the major agent of human eumycetoma in Sudan. J Clin Microbiol. 2002;40:1031–6.
Van Amstel SR, Ross M, Van Den Berg SS. Maduromycosis (Madurella mycetomatis) in a horse. J South Afr Vet Assoc. 1984;55:81–3.
Lambrechts N, Collett MG, Henton M. Black grain eumycetoma (Madurella mycetomatis) in the abdominal cavity of a dog. J Med Vet Mycol. 1991;29:211–4.
Elad D, Orgal U, Yakobson B, et al. Eumycetoma caused by Curvularia lunata in a dog. Mycopathologia. 1991;116:113–8.
Guillot J, Garcia-Hermoso D, Degorce F, et al. Eumycetoma caused by Cladophialophora bantiana in a dog. J Clin Microbiol. 2004;42:4901–3.
Ramachandran PK. Mycetoma caused by an unknown fungus in Indian buffaloes (Bos bubalis). Ceylon Vet J. 1968;16:77–80.
Abbott P. Mycetoma in the Sudan. Trans R Soc Trop Med Hyg. 1956;50:11–24.
Yu AM, Zhao S, Nie LY. Mycetomas in northern Yemen: identification of causative organisms and epidemiologic considerations. Am J Trop Med Hyg. 1993;48:812–7.
Lee MW, Kim JC, Choi JS, Kim KH, Greer DL. Mycetoma caused by Acremonium falciforme: successful treatment with itraconazole. J Am Acad Dermatol. 1995;32:897–900.
Fahal AH, el Toum EA, el Hassan AM, Mahgoub ES, Gumaa SA. The host tissue reaction to Madurella mycetomatis: new classification. J Med Vet Mycol. 1995;33:15–7.
Mahgoub ES, Gumma SA, El Hassan AM. Immunological status of mycetoma patients. Bull Soc Pathol Exot Filiales. 1977;70:48–54.
Bendl BJ, Mackey D, Al-Saati F, Sheth KV, Ofole SN, Bailey TM. Mycetoma in Saudi Arabia. J Trop Med Hyg. 1987;90:51–9.
van de Sande WW, Fahal A, Verbrugh H, van Belkum A. Polymorphisms in genes involved in innate immunity predispose toward mycetoma susceptibility. J Immunol. 2007;179:3065.
Findlay GH, Vismer HF. Black grain mycetoma. A study of the chemistry, formation and significance of the tissue grain in Madurella mycetomi infection. Br J Dermatol. 1974;91:297–303.
van de Sande WW, de Kat J, Coppens J, et al. Melanin biosynthesis in Madurella mycetomatis and its effect on susceptibility to itraconazole and ketoconazole. Microbes Infect. 2007;9:1114–23.
Green Jr WO, Adams TE. Mycetoma in the United States: a review and report of seven additional cases. Am J Clin Pathol. 1964;42:75–91.
Develoux M, Audoin J, Treguer J, Vetter JM, Warter A, Cenac A. Mycetoma in the Republic of Niger: clinical features and epidemiology. Am J Trop Med Hyg. 1985;38:386–90.
Castro LG, Belda Junior W, Salebian A, Cuce LC. Mycetoma: a retrospective study of 41 cases seen in Sao Paulo, Brazil, from 1978 to 1989. Mycoses. 1993;36:89–95.
Queiroz-Telles F, McGinnis MR, Salkin I, Graybill JR. Subcutaneous mycoses. Infect Dis Clin North Am. 2003;17:59–85.
Daoud M, Ezzine Sebai N, Badri T, Mokhtar I, Fazza B, Kamoun MR. Mycetoma: retrospective study of 13 cases in Tunisia. Acta Dermatoven APA. 2005;14:153–6.
N’diaye B, Dieng MT, Perez A, Stockmeyer M, Bakshi R. Clinical efficacy and safety of oral terbinafine in fungal mycetoma. Int J Dermatol. 2006;45:154–7.
Mirza SH, Gardezi AH, Khan Y, Wiqar MA. Subcutaneous facial mycosis in a child due to Madurella mycetomatis. J Pak Med Assoc. 2007;57:466–8.
Van Etta LL, Peterson LR, Gerding DN. Acremonium falciforme (Cephalosporium falciforme) mycetoma in a renal transplant patient. Arch Dermatol. 1983;119:707–8.
Meis JF, Schouten RA, Verweij PE, Dolmans W, Wetzels JF. Atypical presentation of Madurella mycetomatis mycetoma in a renal transplant patient. Transpl Infect Dis. 2000;2:96–8.
O’Riordan E, Denton J, Taylor PM, Kerr J, Short CD. Madura foot in the U.K.: fungal osteomyelitis after renal transplantation. Transplantation. 2002;73:151–3.
Geyer AS, Fox LP, Husain S, et al. Acremonium mycetoma in a heart transplant recipient. J Am Acad Dermatol. 2006;55:1095–100.
Satta R, Sanna S, Cottoni F. Madurella infection in an immunocompromised host. Int J Dermatol. 2000;39:939–41.
Neumeister B, Zollner TM, Krieger D, Sterry W, Marre R. Mycetoma due to Exophiala jeanselmei and Mycobacterium chelonae in a 73-year-old man with idiopathic CD4+ T lymphocytopenia. Mycoses. 1995;38:271–6.
Mendez-Tovar LJ, de Bièvre C, Lopez-Martinez R. Effets des hormones sexuelles humaines sur le development in vitro des agents d´eumycetomes. J Mycol Med. 1991;1:141–3.
Lynch JB. Mycetoma in the Sudan. Ann R Coll Surg. 1964;35:319–40.
Destombes P, Mariat L, Rosati G, Segretain G. Les mycétomes en Somalie – conclusions d´une enquête menée de 1959 à 1964. Acta Trop. 1977;34:335–73.
Bustamante B, Campos PE. Eumycetoma. In: Kauffman CA, editor. Atlas of fungal infections. 2nd ed. Philadelphia: Current Medicine; 2007. p. 203.
Lopez-Martinez R, Mendez-Tovar LJ, Lavalle P, Welsh O, Saul A, Macotela Ruiz E. Epidemiology of mycetoma in Mexico: study of 2105 cases. Gac Méd Méx. 1992;128:477–81.
Elhardello OA, Adam ES, Adam I. Abdominal wall mycetoma presented as obstructed incisional hernia of cesarean section in Eastern Sudan. Infect Dis Obstet Gynecol. 2007, on-line publication (Article ID 74643, doi:10.1155/2007/74643).
Arbab MA, el Hag IA, Abdul Gadir AF, Siddik H el-R. Intraspinal mycetoma: report of two cases. Am J Trop Med Hyg. 1997;56:27–9.
Gumma SA, Satir AA, Shehata AH, Mahgoub ES. Tumor of the mandible caused by Madurella mycetomii. Am J Trop Med Hyg. 1975;24:471–4.
Aldrige J, Kirk R. Mycetoma of the eyelid. Br J Ophthalmol. 1940;24:211–2.
Soni N, Gupta A, Shekhawat NS. Mycetoma - an unusual site. Surgery. 2000;127:709–10.
Ly F, Develoux M, Deme A, Dangou JM, Kane A, Ndiaye B, et al. Tumoral mycetoma of the buttock. Ann Dermatol Vénéréol. 2000;127:67–9.
Ravisse P, Huerre M, De Bièvre C, et al. Les mycétomes en Mauritanie: Étude histologique de 150 cas. J Mycol Med. 1992;2:154–9.
Negroni R, Lopez-Daneri G, Arechavala A, Bianchi MH, Robles AM. Clinical and microbiological study of mycetomas at the Muniz hospital of Buenos Aires between 1989 and 2004. Rev Argent Microbiol. 2006;38:13–8.
Niño FL. Coexistencia de Madurella mycetomi y de M. grisea en una misma observacion de maduromicosis podal negra. Mycopathologia. 1962;16:323–32.
Mahgoub ES. Mycetoma. Semin Dermatol. 1985;4:230–9.
Camain R. Processus d´extension et de limitation des mycétomes africains. Bull Soc Pathol Exot. 1968;61:517–23.
El Hassan AM, Mahgoub ES. Lymph node involvement in mycetoma. Trans R Soc Trop Med Hyg. 1972;66:165–9.
Sharif HS, Clark DC, Aabed MY, et al. Mycetoma: comparison of MR imaging with CT. Radiology. 1991;178:865–70.
Tomimori-Yamashita J, Ogawa MM, Hirata SH, Fischman O, Michalany NS, Yamashita HK, et al. Mycetoma caused by Fusarium solani with osteolytic lesions on the hand: case report. Mycopathologia. 2001;153:11–4.
McGinnis MR. Mycetoma. Dermatol Clin. 1996;14:97–104.
Abd El-Bagi ME, Fahal AH. Mycetoma revisited: Incidence of various radiographic signs. Saudi Med J. 2009;30:529–33.
Czechowski J, Nork M, Haas D, Lestringant G, Ekelund L. MR and other imaging methods in the investigation of mycetomas. Acta Radiol. 2001;42:24–6.
Sarris I, Berendt AR, Athanasous N, Ostlere SJ. MRI of mycetoma of the foot: two cases demonstrating the dot-in-circle sign. Skeletal Radiol. 2003;32:179–83.
Ahmed AO, Abugroun ES. Unexpected high prevalence of secondary bacterial infection in patients with mycetoma. J Clin Microbiol. 1998;36:850–1.
Fahal AH, Sheik HE, El Hassan AM. Pathological fractures in mycetoma. Trans R Soc Trop Med Hyg. 1996;90:675–67.
Fahal AH, Sharfi AR, Sheik HE, El Hassan AM, Mahgoub ES. Internal fistula formation: An unusual complication of mycetoma. Trans R Soc Trop Med Hyg. 1996;90:550–2.
Fahal AH, Yagi HI, El Hassan AM. Mycetoma-induced palatal deficiency and pharyngeal plexus dysfunction. Trans R Soc Trop Med Hyg. 1996;90:676–7.
Lopez-Cepeda LD, Mora-Ruiz S, Padilla-Desgarennes M del C, Ramos-Garibay JA. Small eumycetic mycetoma due to black grain. Arch Dermatol. 2005;141:783–4.
Gabhane SK, Gangane N, Anshu. Cytodiagnosis of eumycotic mycetoma: a case report. Acta Cytol. 2008;52:354–6.
Ahmed A, van de Sande W, Verbrugh H, et al. Madurella mycetomatis strains from mycetoma lesions in Sudanese patients are clonal. J Clin Microbiol. 2003;41:4537–41.
Hemashettar BM, Siddaramappa B, Munjunathaswamy BS, et al. Phaeoacremonium krajdenii, a cause of white grain eumycetoma. J Clin Microbiol. 2006;44:4619–22.
Desnos-Ollivier M, Bretagne S, Dromer F, et al. Molecular identification of black-grain mycetoma agents. J Clin Microbiol. 2006;44:3517–23.
Hay RJ, Mackenzie DWR. The histopathological features of pale grain eumycetoma. Trans R Soc Trop Med Hyg. 1982;76:839–44.
Hay RJ. Mycetoma (Maduromycosis). In: Strickland GT, editor. Hunter`s Tropical Medicine and Emerging Infectious Diseases. 8th ed. Philadelphia: W.B. Saunders; 2000. p. 537–41.
Chandler FW, Kaplan W, Ajello L. A Colour Atlas and Textbook of the Histopathology of Mycotic Diseases. London: Wolfe Medical Publication; 1980. p. 76–83. and 222–239.
Jackson JA, Kaplan W, Kaufman L, Standard P. Development of fluorescent-antibody reagents for demonstration of Pseudallescheria boydii in tissues. J Clin Microbiol. 1983;18:668–73.
Fenelon LE, Hamilton AJ, Figueroa JI, et al. Production of specific monoclonal antibodies to Aspergillus species and their use in immunohistochemical identification of aspergillosis. J Clin Microbiol. 1999;37:1221–3.
Choi JK, Mauger J, McGowan KL. Immunohistochemical detection of Aspergillus species in pediatric tissue samples. Am J Clin Pathol. 2004;121:18–25.
Murray IG, Mahgoub ES. Further studies on the diagnosis of mycetoma by double diffusion in agar. Sabouraudia. 1968;6:106–10.
Gumma SA, Mahgoub ES. Counterimmunoelectrophoresis in the diagnosis of mycetoma and its sensitivity as compared to immunodiffusion. Sabouraudia. 1975;13:309–15.
Hay RJ, Mackenzie DWR. Mycetoma (madura foot) in the United Kingdom - a survey of forty-four cases. Clin Exp Dermatol. 1983;8:553–62.
Wethered DB, Markey MA, Hay RJ, Mahgoub ES, Gumma SA. Humoral immune responses to mycetoma organisms: characterization of specific antibodies by the use of enzyme-linked immunosorbent assay and immunoblotting. Trans R Soc Trop Med Hyg. 1988;82:918–23.
Welsh O. Mycetoma: current concepts in treatment. Int J Dermatol. 1991;30:387–98.
Restrepo A. Treatment of tropical mycoses. J Am Acad Dermatol. 1994;31:S91–102.
Welsh O, Salinas MC, Rodriguez MA. Treatment of eumycetoma and actinomycetoma. Curr Topics Med Mycol. 1995;6:47–71.
Venugopal PV, Venugopal TV, Ramakrishna ES, Ilavarasi S. Antimycotic susceptibility testing of agents of black grain eumycetoma. J Med Vet Mycol. 1993;31:161–4.
Marco F, Pfaller MA, Messer SA, Jones RN. In vitro activity of a new triazole antifungal agent, Sch 56592, against clinical isolates of filamentous fungi. Mycopathologia. 1998;141:73–7.
Sabatelli F, Patel R, Mann PA, et al. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother. 2006;50:2009–15.
Clancy CJ, Nguyen MH. In vitro efficacy and fungicidal activity of voriconazole against Aspergillus and Fusarium species. Eur J Clin Microbiol Infect Dis. 1998;17:573–5.
McGinnis MR, Pasarell L. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. J Clin Microbiol. 1998;36:2353–5.
Cuenca-Estrella M, Ruiz-Díez B, Martínez-Suárez JV, Monzón A, Rodríguez-Tudela JL. Comparative in-vitro activity of voriconazole (UK-109, 496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother. 1999;43:149–51.
Poncio-Mendes R, Negroni R, Bonifaz A, Pappagianis D. New aspects of some endemic mycoses. Med Mycol. 2000;38 suppl 1:237–41.
Diaz M, Negroni R, Montero-Gei F, et al. A Pan-American 5-year study of fluconazole therapy for deep mycoses in the immunocompetent host. Clin Infect Dis. 1992;14 Suppl 1:S68–76.
Pitisuttithum P, Negroni R, Graybill JR, et al. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005;56:745–55.
Mahgoub ES, Gumma SA. Ketoconazole in the treatment of eumycetoma due to Madurella mycetomii. Trans R Soc Trop Med Hyg. 1984;78:376–9.
Symoens J, Moens M, Dom J, et al. An evaluation of two years of clinical experience with ketoconazole. Rev Infect Dis. 1980;2:674–87.
Drohuet E, Dupont B. Laboratory and clinical assessment of ketoconazole in deep-seated mycosis. Am J Med. 1983;74:30.
Hay RJ. Ketoconazole in the treatment of fungal infection. Clinical and laboratory studies. Am J Med. 1983;74:16–9.
Poza G, Montoya J, Redondo C, et al. Meningitis caused by Pseudallescheria boydii treated with voriconazole. Clin Infect Dis. 2000;30:981–2.
Muñoz P, Marín M, Tornero P, Martín-Rabadán P, Rodríguez-Creixems M, Bouza E. Successful outcome of Scedesporiun apiospermum disseminated infection treated with voriconazole in a patient receiving corticosteroid therapy. Clin Infect Dis. 2000;31:1499–501.
Nesky MA, McDougal EC, Peacock JE. Pseudallesheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallesheriasis. Clin Infect Dis. 2000;31:673–7.
Porte L, Khatibi S, Hajj LE, et al. Scedosporium apiospermum mycetoma with bone involvement successfully treated with voriconazole. Trans R Soc Trop Med Hyg. 2006;100:891–4.
Troke P, Aguirrebengoa K, Arteaga C, et al. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52:1743–50.
Lexier R, Walmsley SL. Successful treatment of Madura foot caused by Pseudallescheria boydii with Escherichia coli superinfection: a case report. Can J Surg. 1999;42:307–9.
Turner PG. Madura foot or plantar fibromatosis. J Bone Joint Surg Br. 1989;71:531.
Queiroz-Telles F, Queiroz-Telles JE. Treatment of paracoccidiodomycosis and Pseudallescheria boydii mycetoma with itraconazole: a preliminary report of two cases. Rev Iberica Micología. 1988;5 Suppl 1:72.
Degavre B, Joujoux JM, Dandurand M, Guillot B. First report of mycetoma caused by Arthrographis kalrae: successful treatment with itraconazole. J Am Acad Dermatol. 1997;37:318–20.
Fahal AH, el Hag IA, Gadir AF, el Lider AR, el Hassan AM, Baraka OZ, et al. Blood supply and vasculature of mycetoma. J Med Vet Mycol. 1997;35:101–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Bustamante, B., Campos, P.E. (2011). Eumycetoma. In: Kauffman, C., Pappas, P., Sobel, J., Dismukes, W. (eds) Essentials of Clinical Mycology. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6640-7_24
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6640-7_24
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6639-1
Online ISBN: 978-1-4419-6640-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)