Abstarct
Cardiac efficiency is defined in analogy to that of a hydraulic pump. The external power is calculated from pressure times flow (Chap. 15) and the ‘input power’ is calculated from cardiac oxygen consumption (Chap. 16). The ratio of external, or produced power, and input power is defined as efficiency. Therefore, both external power and input power need to be expressed in the same units. When glucose or free fatty acids are consumed oxygen consumption can be expressed in Joules and oxygen consumption per time in Watts, through the so-called caloric equivalent. For carbohydrate and fat metabolism it holds that 1 ml O2 ≈ 20 J and 1 ml O2/min ≈ 0.33 W. A review on cardiac energetics has been published by Suga [1].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.

Description
Power and Efficiency
Cardiac efficiency is defined in analogy to that of a hydraulic pump. The external power is calculated from pressure times flow (Chap. 15) and the ‘input power’ is calculated from cardiac oxygen consumption (Chap. 16). The ratio of external, or produced power, and input power is defined as efficiency. Therefore, both external power and input power need to be expressed in the same units. When glucose or free fatty acids are consumed oxygen consumption can be expressed in Joules and oxygen consumption per time in Watts, through the so-called caloric equivalent. For carbohydrate and fat metabolism it holds that 1 ml O2 ≈ 20 J and 1 ml O2/min ≈ 0.33 W. A review on cardiac energetics has been published by Suga [1].
Maximum Cardiac Efficiency and Maximum Power in the Intact Animal
The pressure and flow generated by the heart and the arterial load can be studied while keeping the Heart Rate, diastolic filling and contractility unaltered [2]. Power can be calculated from the pressure and flow. The Figure in the box, on the left, shows that, when power is plotted as a function of Cardiac Output it exhibits an optimal value. This can be understood with the cardiac pump function graph in mind, (top panel of box Figure, Chap. 14). For a high load (isovolumic contraction) pressure generated is high but flow is zero. Power, the product of pressure and flow, is therefore negligible. Inversely, for a very low load the heart generates a large flow but negligible pressure so that power is negligible as well. Thus, power, at some intermediate value of Cardiac Output, must be maximal. This power maximum was found, in the intact cat [3], to coincide with the working point, i.e., when a physiologic arterial load is present (see Fig. 17.1). It has also been reported that the left ventricle works at maximal efficiency [4].
The heart pumps at optimal power output. Power output of the heart studied in the intact animal for different arterial loads. Other determinants of pressure and flow, heart rate, diastolic filling and contractility, are kept constant. When the physiological arterial load is present, shown as the working point, power transfer is maximal. Adapted from ref. [3], used by permission
Local Work and Power
It is of great interest if local power and efficiency could be derived. However, this is difficult since local work, and local oxygen consumption are hard to measure accurately. Local work can be derived by local shortening times local stress. The first, local shortening can be obtained from (surface) markers attached to the muscle or by MRI-tagging (magnetically induced markers in the myocardium). Local forces are only possible to derive indirectly from pressure and anatomy (sophisticated forms of Laplace’s law, Chap. 9). This technique was used by Prinzen et al., to study effect of pacing site on local work [5]. When local oxygen consumption is also determined [6] local efficiency can be derived. However, local stresses derived from pressure are difficult to verify.
Heat Production and Transport
The heart is about 20–25% efficient. This implies that about 75% of the oxygen consumed is converted into heat. The heat is removed by diffusion to lumen and thorax, and convection by coronary flow, in about equal amounts (Fig. 17.2). In the mid-wall of the myocardium the temperature is a few tenths of a degree Celsius higher than in subepicardial and subendocardial layers allowing for diffusion of heat [7].
Assessment of Ventriculo-Arterial Coupling
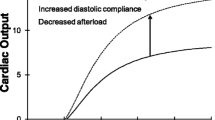
Optimum power and efficiency are assumed measures of good ventriculo-arterial coupling. Whether the heart functions on optimum power or efficiency can be derived from hemodynamic principles. The two parameters assumed to be the determining ones are the slope of the left ventricular End-Systolic Pressure–Volume Relation, E es or E max , and effective arterial elastance, E a . The effective arterial elastance is defined as E a = P es /SV, i.e., end-systolic (ventricular or aortic) pressure over Stroke Volume, (right side of the Figure in the box). The ratio E a /E es is considered a ventriculo-arterial coupling parameter and when E a /E es ≈ 1 external work is maximized, while for E a /E es ≈ 0.5 cardiac efficiency is maximal [8].
To determine these two parameters, several simplifications have been used. The E a can be approximated as follows. End-systolic pressure is close to mean arterial pressure (Fig. 17.3). With Cardiac Output, CO, being SV ⋅HR, and Heart Period T, in seconds, the inverse of HR, CO = SV/T, we find that P es /SV ≈ P mean /CO.T = R p /T. Thus the effective arterial elastance, E a , is primarily a measure of vascular or peripheral resistance, R p , and hardly reflects the compliant properties of the large conduit arteries. Therefore the term ‘elastance’ is misleading. Note that E a depends on vascular resistance, which is a purely arterial variable, and on heart period, T, which is a purely cardiac variable. Therefore, E a is a coupling parameter by itself. The E a can be derived from noninvasive measurements: mean pressure (by sphygmomanometer) and Cardiac Output (US or MRI), and Heart Rate.
The maximum or End-Systolic elastance, E es is calculated from E es = P es /(Ves − V d ). End-Systolic volume can be measured noninvasively, but V d is hard to estimate. To derive this intercept volume, at least one other point on the ESPVR should be obtained. This would require changes in diastolic filling that are often not feasible in very sick patients and in epidemiological studies. One method to determine the End-Systolic Pressure–Volume Relation is the one suggested by Sunagawa et al. [8], where an isovolumic left ventricular pressure is predicted from the pressure of an ejecting beat (Single Beat Method). However, none of the so-called single beat methods to determine the ESPVR has been shown to give accurate estimates [9]. Nevertheless, the Single Beat Method is, at present the best approach in practice.
In a number of studies it has simply been assumed that V d = 0 [10, 11]. This assumption leads to a very interesting simplification of the analysis. With the V d = 0, E es = P es /V es = P es /(V ed − SV). The ratio E a /E es then becomes equal to:
with EF, the Ejection Fraction. We see that the Pes disappears altogether only leaving the determination of Ejection Fraction. This implies that work is maximal when E a /E es = 1, thus when EF = 0.5. Similarly, cardiac efficiency is maximal when E a /E es = 0.5 or when EF = 0.67.
Thus the assumption of a negligible V d simplifies matters. However, negligible V d values are difficult to verify, mostly not correct, and are certainly leading to large errors in case of a dilated heart (Chaps. 13 and 15).
The effective arterial elastance (E a ), has the units of elastance but is not an elastance; it is closely related to total peripheral resistance. It is approximately R p /T and thus is not related to total arterial compliance. Several authors [12] have interpreted E a as a measure of total arterial compliance, an interpretation that leads to confusion and is clearly wrong.
Theory of Optimal Heart Size
Why does the left ventricle pump at maximum power, while a feedback control for power is not known to exist? A simple answer to this question can be given based on the following reasoning [13]. Consider the pump function graph (Fig. 17.4). The working point, i.e., the point where maximum power is found, is for a flow which is about 58% maximal flow, Q max . Mean ventricular pressure and Cardiac Output together determine the working point. Pressure is similar in mammals and Cardiac Output is determined by body size (Chap. 30). Several pump function graphs can be drawn through this working point. We begin by assuming that muscle stress is a given quantity, and that the ventricle is a sphere. On the one hand, a larger intercept of the pump function graph with the flow axis, i.e., a larger Q max , implies a larger ventricular lumen requiring a thicker wall (Law of Laplace, Chap. 9), to maintain muscle, or wall stress. On the other hand, with a larger Q max a smaller P max results so that the wall may be less thick. In this way it is possible to calculate ventricular volume for the different pump function graphs through the working point, each with its own Q max . Plotting ventricular volume as a function of Q max , results in the graph given in Fig. 17.4. The minimum volume is found when the working point is at about 60% of Q max , and this is the same value as where maximum power and efficiency are found. The minimum in heart volume thus corresponds to a working point where power and efficiency are about maximal. In other words, the size of the heart is minimized, and, for this minimal heart volume the heart pumps at maximal power.
Total ventricular volume, i.e., wall plus lumen volume, can be calculated assuming a spherical shape, right, and a fixed, maximal, wall stress, σ m , for isovolumic contractions. Thus P max relates to wall volume (Chap. 9), and larger P max implies larger wall volume to keep wall stress the same. Increased Cardiac Output relates to ventricular lumen and wall volume. Many Pump Function Graphs through the working point are possible, left, but the Pump Function Graph where the working point is about 60% of Q max , i.e., where maximal power and efficiency are found, corresponds with the smallest total (wall plus lumen) ventricular volume. Adapted from ref. [13], used by permission
Physiological and Clinical Relevance
Cardiac oxygen consumption and efficiency are still difficult to obtain in the patient. Modern techniques, such as Positron Emission Tomography (PET) and Magnetic Resonance Spectroscopy (MRS) may change this. Assessment of glucose metabolism with 18F-fluorodeoxyglucose measures glucose uptake into myocardial cells, but not its conversion by glycolysis. Myocardial oxidative metabolism can be measured by 11C-labeled acetate PET. For lipid metabolism, tracer examples are 123I-beta-methyl-p-iodophenyl pentadecanoic acid and 15-(O-123I-phenyl)-pentadecanoic acid. These tracers can be detected by planar scintigraphy and single-photon emission computed tomography (SPECT), which are more economical and more widely available than PET. With current MRS techniques, 31P-labeled magnetic resonance spectroscopy, Phosphate/Creatine and/or pH can be obtained in humans but this is not common yet. The hemodynamic determinants for oxygen consumption, as discussed in Chaps. 16 and 17, are only valid in single hearts during acute interventions and cannot be used in comparing different patients.
‘Output power’ requires the measurement of aortic or ventricular pressure and flow. Thus for the calculation of efficiency, which is the ratio of ‘Output power’ and ‘Input power’, many measurements are required and therefore efficiency is not calculated routinely. In Chap. 30 it is shown that in healthy mammals cardiac metabolism is proportional to body mass to the power −1/4, implying that metabolism per gram of heart tissue decreases with body mass.
Related Issues
Contractile efficiency. On the basis of the Pressure Volume Area concept (Chap. 16) the contractile efficiency has been defined as the inverse of the slope of the Pressure Volume Area – VO2 relation [1]. This definition of efficiency only accounts for the mechanical aspects of oxygen consumption and does not take into account the oxygen expenditure related to activation and basic metabolism. Therefore this contractile efficiency is about twice the actual cardiac efficiency.
Power in cardiogenic shock. Although power is a rather abstract measure, it has been shown that it is the strongest hemodynamic correlate of mortality in cardiogenic shock [14].
Economy. At the extremes of the pump function graph, the heart generates neither pressure nor flow. External power and thus efficiency is zero. In isolated heart studies (Langendorff preparations) where the heart contracts isovolumically or in isolated cardiac muscle studies, when the muscle contracts isometrically, contraction economy can be used instead. Economy of contraction is defined as oxygen consumption used for isovolumic or isometric contractions.
References
Suga H. Ventricular energetics. Physiol Rev 1990;70:247–277.
Elzinga G, Westerhof N. Pump function of the feline left heart: changes with heart rate and its bearing on the energy balance. Cardiovascular Res 1980;14:81–92.
Toorop GP, Van den Horn GJ, Elzinga G, Westerhof N. Matching between feline left ventricle and arterial load: optimal external power or efficiency. Am J Physiol 1988;254:H279–H285.
Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol 1986;250:R1021–R1027.
Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol 1999;33:1735–1742.
van Beek JH, van Mil HG, King RB, de Kanter FJ, Alders DJ, Bussemaker J. A (13)C NMR double-labeling method to quantitate local myocardial O(2) consumption using frozen tissue samples. Am J Physiol Heart Circ Physiol 1999;277:H1630–H1640.
ten Velden GH, Elzinga G, Westerhof N. Left ventricular energetics. Heat loss and temperature distribution of canine myocardium. Circ Res 1982;50:63–73.
Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in the isolated canine left ventricle. Circ Res 1985;56:586–595.
Kjorstad KE, Korvald C, Myrmel T. Pressure-volume-based single beat estimations cannot predict left ventricular contractility in vivo. Am J Physiol 2002;282:H1739–H1750.
Grosu A, BomGrosu A, Bombardini T, Senni M, Duino V, Gori M, Picano E. End-systolic pressure/volume relationship during dobutamine stress echo: a prognostically useful non-invasive index of left ventricular contractility. Eur Heart J 2005;26:2404–2412.
Saba PS, Roman MJ, Ganau A, Pini R, Jones EC, Pickering TG, Devereux RB. Relationship of effective arterial elastance to demographic and arterial characteristics in normotensive and hypertensive adults. J Hypertens 1995;13:971–977.
Senzaki H, Iwamoto Y, Ishido,H, Masutani S,Taketazu S, Kobayashi T, Katogi T, Kyo S. Ventricular–Vascular stiffening in patients with repaired coarctation of aorta. Circulation 2008;118:191–198.
Elzinga G, Westerhof N. Matching between ventricle and arterial load. An evolutionary process. Circ Res 1991;68:1495–1500.
Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004;44:340–348.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2010 Springer US
About this chapter
Cite this chapter
Westerhof, N., Stergiopulos, N., Noble, M.I.M. (2010). Cardiac Power and Ventriculo-Arterial Coupling. In: Snapshots of Hemodynamics. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-6363-5_17
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6363-5_17
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4419-6362-8
Online ISBN: 978-1-4419-6363-5
eBook Packages: MedicineMedicine (R0)