Abstract
Over the past decade, permeable reactive barrier (PRB) technology has progressed through the conceptual, experimental and innovative stages to its current status as accepted standard practice for groundwater remediation. As represented in the schematic of Figure 16.1, a PRB can be defined as an in situ treatment zone positioned such that it passively captures a contaminant plume and removes or degrades the contaminants, discharging uncontaminated water. The recent development of PRB technology has been stimulated largely by the use of granular iron “walls” for treatment of groundwater containing chlorinated organic contaminants. While this continues to be the most common application of PRBs and is the subject of this chapter, it should be noted that many other reactive materials have been proposed and tested for removal of a wide range of groundwater contaminants (ITRC, 2005).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
16.1 Introduction and Background
Over the past decade, permeable reactive barrier (PRB) technology has progressed through the conceptual, experimental and innovative stages to its current status as accepted standard practice for groundwater remediation. As represented in the schematic of Figure 16.1, a PRB can be defined as an in situ treatment zone positioned such that it passively captures a contaminant plume and removes or degrades the contaminants, discharging uncontaminated water. The recent development of PRB technology has been stimulated largely by the use of granular iron “walls” for treatment of groundwater containing chlorinated organic contaminants. While this continues to be the most common application of PRBs and is the subject of this chapter, it should be noted that many other reactive materials have been proposed and tested for removal of a wide range of groundwater contaminants (ITRC, 2005).
Historically, the development of the granular iron PRB technology rests on the recognition of two advances: first, that metallic iron degrades chlorinated organic compounds, and second, that the reactions can proceed in situ under ambient groundwater conditions. Metals have been used as catalysts in the commercial production of chlorinated organic compounds since early in the 20th Century, and there is a further body of literature concerning the corrosion of metal containers used for storage and shipping. Because this body of literature concerns pure solvents rather than dilute aqueous solutions and the processes frequently occur at elevated temperatures and pressures, it was overlooked by the environmental community. Of greater relevance, Sweeny and Fischer (1972) showed zero-valent metals to be effective in degrading pesticides and other chlorinated organic compounds in aqueous solution. This work was also overlooked, possibly because it appeared only in the patent literature and also because it preceded, by several years, awareness of chlorinated solvents in groundwater as a major environmental problem.
In the mid-1980s, a graduate student at the University of Waterloo was studying the potential for sampling bias caused by sorption of chlorinated organic contaminants to well casings and other materials commonly used in groundwater sampling. While it was clear that contaminants were lost from solution as a consequence of diffusion into polymer materials, losses were also observed when solutions were in contact with a variety of metals, and the pattern of loss was not consistent with a diffusion process. No literature was found that was directly relevant; however, reductive dechlorination was proposed as the most likely cause (Reynolds et al., 1990). This was confirmed by subsequent tests that showed that several transition metals had the capacity to degrade a wide range of chlorinated aliphatic compounds (Gillham and O’Hannesin, 1994).
Through similar work concerning the degradation of carbon tetrachloride (CT) by iron, Matheson and Tratnyek (1994) supported the general observations of Gillham and O’Hannesin (1994) and further suggested three possible mechanisms of degradation, with direct electron transfer at the solid surface as the most probable. Stimulated in part by these early publications, the use of metals to degrade organic contaminants developed rapidly as a major area of environmental research, with over 1,100 citations currently listed on the web site maintained by Dr. Tratnyek (http://cgr.ese.ogi.edu/ironrefs/). Furthermore, as a consequence of its availability and relatively low cost, most of the research has focused on recycled granular iron as the reducing agent.
During the early investigations, substantial rates of reaction were observed under ambient temperature and pressure, leading to the proposal that the technology could be implemented in situ (Gillham and O’Hannesin, 1992; Gillham and Burris, 1992). This was supported by the initial field demonstration at Canadian Forces Base (CFB) Borden, Ontario, as reported in O’Hannesin (1993) and O’Hannesin and Gillham (1998). Prior to this initial field test, most studies had been conducted in the laboratory on a small scale and using very pure iron materials. The field test and the experiments conducted to design the test used cuttings and grindings from a local foundry, with no pre-treatment, demonstrating that the quality of the iron and the presence of oxides that are ubiquitous on iron surfaces were not a significant limitation to the technology. The five-year duration of the test, as reported in O’Hannesin and Gillham (1998), was also encouraging with respect to the long-term performance of granular iron PRBs.
The first installation of a granular iron PRB at an industrial site was completed early in 1995 (Warner et al., 2005). This was an important step in the development of the technology, not only because it was the first commercial application, but also because in developing the project, it was necessary to locate a large quantity of granular iron. In this particular application the material was obtained from Master Builders Inc. of Cleveland, Ohio. Other suppliers including Connelly GPM Inc. of Chicago, Illinois and Peerless Metal Powders and Abrasives Inc. of Detroit, Michigan were subsequently identified. All three products are derived from cuttings, grindings and other waste iron materials that are collected from manufacturing facilities, passed through a rotary kiln to remove cutting oils and other surface contaminants, and ground and screened to size. Traditionally, the resulting products are used as a wear-resistant additive to concrete, as catalysts and abrasives, and for a host of other applications. A variety of other iron materials have since been identified. The availability of large quantities of the reactant, at reasonable cost, was a major factor in the early commercial development of the technology. The initial installation continues to support the development of the technology in that the initial cost analysis was based on the assumption that the iron would need to be replaced every five years. As reported in Warner et al. (2005), there has been no appreciable decline in performance ten years after installation, and operation and maintenance costs continue to be low.
Recognizing the potential importance of the technology, a Permeable Reactive Barrier(s) Working Group was established in 1995 under the Remediation Technology Development Forum of the U.S. Environmental Protection Agency (USEPA). This organization played a major role in advancing the technology through independent evaluation and transfer of information. Through demonstration projects and the development of guidance documents, other agencies, such as the Department of Defense (DoD), the Department of Energy and the Interstate Technology & Regulatory Council also contributed significantly to technology development.
There is now a substantial body of literature concerning PRBs, ranging from very fundamental research to highly applied field installations, covering a wide range of groundwater contaminants. Consistent with the focus of this monograph, discussion will be limited primarily to chlorinated solvents, and though some aspects of the basic processes will be introduced, the primary focus will be on the applied aspects of the technology.
16.2 Reaction
16.2.1 Nature of the Reaction/Processes
The addition of water containing chlorinated organic compounds to iron results in two redox reactions, reduction of water accompanied by oxidation of the iron (Equation 16.1) and reduction of the chlorinated organic (R-Cl) accompanied by oxidation of the iron (Equation 16.2).
The carbon of chlorinated organic compounds is in an oxidized state, while the metallic form of iron is the most highly reduced; thus, thermodynamically, Equation 16.2 is very favorable for many contaminants. Furthermore, the metal has an abundant supply of mobile electrons. As a consequence of the reducing properties of Fe(0), redox potential (Eh) values are commonly in the range of −200 to −500 millivolts (mV). Furthermore, the release of OH− and the formation of H2, in Equation 16.1, as well as the consumption of H+ in Equation 16.2, leads to pH values that are commonly in the range of 9 to 10. At the elevated pH values, Fe(II) precipitates as Fe(OH)2, which is subsequently transformed to magnetite (Fe3O4). This has two important consequences: dissolved iron concentrations (resulting from both Equations 16.1 and 16.2), exiting the iron zone are generally very low (<2 milligrams per liter [mg/L]), and the iron surfaces become coated with a magnetite surface film of increasing thickness over time. The high pH values can further alter the natural geochemistry of the groundwater, resulting in the formation of secondary minerals, such as calcium carbonate and iron carbonate.
The reactions occur on the iron surface and thus rates of degradation depend on the available surface area density (surface area of iron per volume of solution) and on the condition of the surfaces. It should be noted that the reactions seldom occur on clean metallic surfaces. Commercial recycled iron materials, having passed through a rotary kiln, are covered with a thick high-temperature oxide double layer. The nature of the layer is variable, depending upon the conditions in the kiln, but generally includes an inner layer of magnetite and an outer layer of higher valent oxides such as hematite and maghemite (Ritter et al., 2002). Contaminants are adsorbed onto the outer surface of the oxide film and the reduction reaction occurs on the outer surface, requiring that the oxide film be conducting. While magnetite is an electron conductor, the high valance oxides are not. Fortunately however, under reducing conditions in an aqueous environment, the outer non-conducting oxides dissolve or are autoreduced to magnetite (Ritter et al., 2002, and references therein). As a semiconductor, it would be reasonable to expect rates of reaction to decline with increasing thickness of the magnetite films. This will be addressed further in Section 16.4.
16.2.2 Pathways
Various reaction mechanisms and pathways have been proposed for degradation of chlorinated aliphatic compounds. Two of the common mechanisms include hydrogenolysis and reductive β-elimination. In hydrogenolysis, a chlorine atom is replaced by a hydrogen atom, accompanied by the addition of two electrons (from the iron). In reductive β-elimination, two chlorine atoms are released, accompanied by the formation of an additional carbon-carbon bond. For chlorinated methanes, with a single carbon atom, the primary mechanism appears to be hydrogenolysis. A fraction of CT is sequentially reduced through a one-electron transfer pathway to form trichloromethane (chloroform [CF]) followed by dichloromethane (DCM), while the remainder is reduced through a two-electron transfer pathway to form methane, formate or carbon monoxide (CO) (McCormick and Adriaens, 2004).
In the case of chlorinated ethenes, the most common industrial solvents and those identified most commonly at hazardous waste sites, hydrogenolysis and β-elimination appear to proceed simultaneously. As shown in Figure 16.2, perchloroethene (PCE) can degrade sequentially by hydrogenolysis through trichloroethene (TCE), cis-dichloroethene (cis-DCE), vinyl chloride (VC) and finally ethene; alternatively, PCE and TCE can degrade through β-elimination to dichloroacetylene and chloroacetylene, respectively. Both of the chlorinated acetylenes are highly unstable and degrade rapidly, primarily through reductive dechlorination to acetylene (Arnold and Roberts, 2000). A third mechanism, hydrogenation, involves the addition of two hydrogen atoms across two carbon atoms with the removal of a C-C bond. An example is the reduction of acetylene to ethene as in Figure 16.2.
Proposed pathways for dechlorination of chlorinated ethenes. Pathways in thin lines have shown to be negligible when starting with PCE or TCE. Modified from Arnold and Roberts, 2000.
From both laboratory and field studies, for degradation of PCE and TCE, the chlorinated intermediates seldom exceed 5–10% of the initial compound. Indeed, Arnold and Roberts (2000) and Li and Farrell (2000) showed that >90% of TCE degrades through β-elimination. This is highly advantageous with respect to application of the technology in that relatively small amounts of the slower-degrading intermediates (dichloroethene [DCE] and VC) are produced. It also should be noted that both laboratory and field tests indicate that of the DCE isomers, cis-DCE is by far the most prevalent. The rate of degradation tends to decline with decreasing chlorine content of the compound; thus, for example, VC tends to degrade at lower rates than PCE. Thus, from practical considerations, VC could become the controlling compound in the design of a PRB, even if VC was not initially present in the contaminated water.
16.2.3 Kinetics of Degradation
Figure 16.3(a) shows TCE concentration versus distance along a column containing granular iron. The exponential decline in concentration suggests first-order kinetics and indeed, most laboratory tests, using both batch and column procedures, indicate the process to be first-order with respect to the concentration of the contaminant (pseudo first-order). That is,
Example of steady concentration profiles in a column of granular iron in which TCE is present in the influent solution. Different lines represent different initial concentrations: (a) concentration versus distance along the column, (b) Log concentration versus residence time, including the least-square fit of the first-order kinetic model (adapted from Orth and Gillham, 1996).
giving
where C is the concentration in solution at a particular time (t), Co is the initial concentration and k is the first-order rate constant. Figure 16.3(b) shows a least-squares best fit of Equation 16.4 to a semilog plot of the data in Figure 16.3(a). The excellent fit confirms consistency with the first-order equation.
The rate constant (k) is a measure of the reaction rate and can be calculated directly from Equation 16.4. Alternatively, the half life (t½), the time required for one-half of the compound to disappear (C/Co = 0.5), can be determined from Equation 16.4 to give
The literature clearly shows that the measured rate constant (or half life) can be influenced by numerous factors:
-
Available surface area of the iron,
-
Type of iron,
-
Inorganic composition of the groundwater,
-
Temperature, and
-
Concentration/competition effects.
Available Surface Area—Since the reduction reaction is a surface process, the reaction rate will depend upon the available surface area of iron. Indeed, rate constants measured in batch tests, where there is generally a small amount of iron relative to the volume of solution, are much lower than measured in column tests. Similarly, if a particular application calls for a mixture of iron and sand rather than 100% iron, the rate constant will be lower in the iron-sand mixture. Thus, in order to compare results from different methods of measurement, it is common to normalize the measured rate constant to the surface area of iron per unit volume of solution. That is, following Johnson et al. (1996),
where kSA is the normalized rate constant (L water/square meter [m2]/kobs), kobs is the measured rate constant (hour−1 [hr−1]) and ρa is the surface area density of the iron (m2 iron/L water).
While the correlation reflected in Equation 16.6 provides a useful basis for comparison, it is far from perfect. The specific surface area of the iron (m2/gram [g]) is most frequently measured by the nitrogen-BET (Brunauer-Emmett-Teller) (Brunauer et al., 1938). There is speculation that the measured surface area may be sensitive to the surface oxide coatings and therefore may not provide a true measure of the reactive surface area. Undoubtedly, further variation is caused by differences in the iron surface characteristics and in the procedures used in different laboratories for measuring kobs. In addition, surface area changes over time.
From a practical perspective, Equation 16.6 also suggests that the highest reaction rates (kobs) will be obtained from materials having the greatest specific surface area, which would imply improved performance from materials of finer grain size. While this is generally the case, materials of finer grain size also have lower permeability and thus optimization of reactivity and grain size needs to be considered within the context of the particular application.
Type of Iron Material—The effectiveness in degrading organic compounds has been evaluated for many types of iron materials ranging from acid-washed, high-purity iron to the oxide-coated commercial materials, as well as slag, spent foundry sand and other waste products from the iron and steel industries. From these tests, it can be concluded that if metallic iron is present, the degradation reaction is likely to proceed; in the absence of metallic iron, degradation will proceed very slowly, if at all. It is not surprising that of the metallic iron materials that have been tested, there is considerable variation in kSA values, but considering the range in materials, it is perhaps surprising that there is not a greater range in reactivity. For example, using acid-washed electrolytic iron, Su and Puls (1999) obtained a kSA value for TCE of 0.308 ± 0.018 × 10−5 L/hr m2 while Jeen et al. (2006), using commercial Connelly material, obtained a value of 1.52 × 10−4 L/hr m2. Similarly, considering TCE and the most commonly used commercial materials, kSA values for Connelly are generally within the range of 2.0 × 10−5 L/hr m2 to 3.4 × 10−4 L/hr m2, while values for Peerless are normally in the range of 4.0 × 10−5 L/hr m2 to 1.3 × 10−4 L/hr m2. This suggests that the purity of the iron and the oxide coatings generally present on commercial materials do not have a significantly detrimental effect on reactivity. It follows that in selecting the commercial material to be used in a particular project, price is likely to be as big a factor as the reactivity of the material.
Inorganic Composition of the Groundwater—The inorganic composition of the influent groundwater can have a profound influence on the reactivity of commercial iron materials. Most of these effects concern long-term performance and will be discussed further in Section 16.4. However, there are common constituents of groundwater that influence the initial reactivity; bicarbonate is a notable example. In studies of the influence of anions on iron reactivity, several studies such as those reported in D’Andrea et al. (2005), Klausen et al. (2003) and Agrawal et al. (2002) have shown bicarbonate to significantly increase reactivity. The increased reaction rates appear to be a consequence of an increase in the corrosion rate of the iron due to its buffering property. Since most groundwater contains significant amounts of HCO− 3, initial reaction rates in the field are likely to be higher than one would anticipate based on laboratory tests performed using deionized water. Devlin and Allin (2005) found that Cl− and SO\(_4^ - \) had relatively little effect on reactivity. More generally, for design purposes, laboratory treatability tests should be conducted using groundwater from the particular site or water with a composition very similar to that present at the site.
Temperature—While reaction rates are most commonly measured at room temperature (about 22 degrees Celsius [°C]), groundwater temperatures, depending on location, can range from 5° to 15°C or more. Various studies, including Johnson et al. (1996), Su and Puls (1999), Deng et al. (1999), and O’Hannesin et al. (2004), have shown the temperature dependence of the degradation process to be consistent with the Arhenius equation. That is,
where kT2 and kT1 are the rate constants at two temperatures (T1 and T2 in degrees Kelvin [°K]), Ea (kilojoules per mole [kJ/mol]) is the activation energy and R is the universal gas constant. Thus, knowing k at one temperature (T1) and the activation energy, k can be calculated for any other temperature (T2) or more generally, a correction factor (kT2/kT1) can be calculated. The values of Ea depend upon the organic contaminant, character of the iron material and undoubtedly other factors; however, based on literature values Tratnyek et al. (2003) suggest a range from 15 to 55 kJ/mol. Based on measured values, Su and Puls (1998) indicate a range from 30 to 80 kJ/mol. As an example, using degradation rates measured at different temperatures in column tests, O’Hannesin et al. (2004) calculated an activation energy for TCE of 62 kJ/mol. Using 25°C as the reference temperature and expressing the reaction rate in terms of half life, Figure 16.4 shows the correction factor over the range from 5° to 25°C. At 10°C, for example, the reaction rate is almost four times lower (half life four times greater) than at 25°C. Thus, at 10°C, the residence time in a PRB would need to be four times greater than might be expected based only on a laboratory-measured value of the rate constant. Clearly, this is a factor that needs to be considered in PRB design.
Temperature correction factor for TCE with commercial iron material. The symbols are measured data and the line is a best fit of the Arrhenius equation with an activation energy (Ea) of 62 kJ/mol. The half life at a particular temperature is obtained by multiplying the half life at 25°C by the appropriate correction factor on the vertical axis. From O’Hannesin et al., 2004.
Concentration/Competition Effects—Evidence has been presented to indicate that at high contaminant concentrations, degradation does not follow pseudo first-order kinetics; this has been attributed to saturation of reactive sites. Further, apparent non-first-order behavior has been attributed to competition between reactants for reactive sites or competitive sorption processes. A brief review of this literature and the associated kinetic models is presented in Tratnyek et al. (2003). Under the conditions presented in Orth and Gillham (1996), as shown in Figure 16.3 part b, the first-order model gave a good fit to the data over an initial concentration range of 1 to 60 mg/L TCE. Thus, there is reason to believe that at the concentrations commonly found in most contaminant plumes, first-order kinetics should apply. Furthermore, considering that most commercial iron materials are covered with a thick layer of oxides (primarily magnetite), the reactive site model becomes somewhat tenuous. For the majority of experimental results and the concentrations commonly found in contaminant plumes, the first-order model appears to be adequate for practical applications.
16.2.4 Typical Reaction Rates
After 15 years of investigation, reaction rates for degradation by Fe(0) now have been measured for many compounds; some compounds, such as CT and TCE, have been measured numerous times using a variety of methods. Preparation of a representative and meaningful table of these results has therefore become a significant challenge. Therefore, and consistent with the purpose of this chapter, the discussion will be limited to the common chlorinated solvents and will draw heavily upon summary tables produced by others.
Table 16.1 shows rate constants for several chlorinated alkanes. The literature values summarize the range of values that have been reported. Though normalized values (kSA) are shown, there is still a considerable range. This variation is undoubtedly a consequence of the different measurement methods used (batch vs. column for example), different iron materials and possibly different solution matrices. For comparative purposes, a second column of half lives is provided in Table 16.1 from a database from treatability tests using groundwater from contaminated sites and commercial iron materials. The kobs values under the “Commercial Studies” heading were all obtained from column tests using 100% commercial granular iron. A typical value for ρa (Equation 16.6) for a granular iron column is about 7 m2/L. To provide a basis for comparison with the literature values, the kobs values were converted to kSA using this value. As indicated in Table 16.1, the kSA values calculated from the treatability tests generally fall within the range reported in the literature. Since the same procedures were used in the same laboratory, the range in values from the commercial database is believed to reflect, to some degree, the variation in the inorganic composition of the groundwater. For convenience, the range in half lives, calculated from the kobs values, is also shown.
16.3 Iron Prb Design
16.3.1 Interpretation and Application of Rate Data
A primary consideration in PRB design is the residence time required to reach objective concentrations. The objective concentration is the regulated or negotiated maximum concentration of each contaminant exiting the PRB and is commonly the Maximum Contaminant Level (MCL) as established by the USEPA. In determining the required residence time, in addition to the influent contaminant levels, the simultaneous production and degradation of intermediate products of degradation must be considered. The process is further complicated by the fact that in some cases, such as the chlorinated ethenes, there are multiple pathways. Because chlorinated ethenes are the most common industrial solvents identified at contaminated sites, they will form the basis for the following discussion.
Either through laboratory column tests, using groundwater from the particular site, or through the use of data from a site with similar geochemical conditions, degradation rate constants for the organic compounds of concern are estimated. This includes the group of compounds that may be present in the water initially, as well as potential degradation products. Because of the simultaneous production and degradation of some compounds, laboratory data is generally interpreted through the use of a multi-component kinetic model. For example, Figure 16.5 shows the possible pathways for degradation of the chlorinated ethenes.
Consistent with experimental results and Figure 16.2, there is no evidence that the pathways labeled at <1% exist and thus these will not be considered further. Each remaining pathway is characterized by a rate constant (k) and the mole fraction of the compound that follows that particular path (f). First-order rate equations can then be written for each compound; that is,
Using commercial software, the set of equations can be fit to the results of column tests, giving estimates of all k and f values. After applying temperature correction factors to the k values, the model then can be run to give concentrations of all compounds as a function of time. From this, the residence time required to meet the objective concentration can be determined for each contaminant.
In practice, after testing a wide range of site waters, the molar conversions (f values), particularly for the major pathways, fall within a narrow range and thus are provided as input to the model (percentage values on the pathways of Figure 16.5).
Figure 16.6 shows the results of a simulation for a particular site water. In this case, initial concentrations were 10,000 micrograms per liter (µg/L) TCE and 100 µg/L cis-DCE. The f values were those shown in Figure 16.5 and the rate constants were determined by fitting the set of first-order rate equations to the results of column treatability tests. The objective concentrations were taken to be the USEPA MCLs (5 µg/L for TCE, 70 µg/L for cis-DCE and 2 µg/L for VC). The cis-DCE concentration increased from the initial value of 100 µg/L to about 110 µg/L, before declining to below the MCL, while VC increased from zero to about 10 µg/L before declining. In this case, even though VC was not initially present in the site water, because of the lower k value, the necessary residence time (about 46 hours) was controlled by the production and subsequent degradation of VC.
Estimation of residence time, using the model of Figure 16.5, for a hypothetical contaminant plume (provided by EnviroMetal Technologies, Inc.).
16.3.2 Treatment Zone Design
Having determined the contact time required to meet the objective concentration, the next question of critical importance concerns the thickness of the PRB that is required in order to provide the necessary residence time. Transport of contaminants through a PRB involves advection, dispersion, reaction and sorption. Because the reactive material is installed as homogeneously as possible and because the flow-through thickness is relatively small (generally <1 m), dispersion is a weak process, and at steady-state concentration distribution, sorption is also a minor process. Thus, for practical purposes, the residence time within the PRB can be calculated assuming plug flow. In this case, the required thickness in the direction of flow (l) is simply given by
where VPRB is the groundwater velocity within the PRB and tR is the residence time required to meet the discharge concentration limits.
There is uncertainty in tR associated with the degree to which laboratory kobs values are applicable to field conditions, the partitioning of the degradation process between the different possible pathways (the “f” values) and the accuracy of the temperature correction factor. Furthermore, tR values can change over time as a consequence of changing influent concentrations and gradual reductions in the reactivity of the iron (see Section 16.4). While these uncertainties should not be overlooked, in most situations the greatest uncertainty is associated with VPRB.
The velocity within the PRB is related to the velocity in the aquifer (VAQ) through the ratio of porosities according to
where n AQ and n PRB are the porosities of the aquifer and PRB respectively. For unconsolidated aquifer materials, porosity values typically range from about 0.2 to 0.4, while the porosity of granular iron PRBs are generally in the range of about 0.45 to 0.5. Thus, in most situations, the velocity in the PRB will be lower than in the aquifer. Accurate determination of the aquifer velocity remains a significant challenge however.
In most PRB applications, estimates of groundwater velocity are based on the Darcy equation according to
where qAQ is the groundwater flux (Darcy flux), KAQ is hydraulic conductivity of the aquifer material and i is the hydraulic gradient. Determining KAQ presents considerable difficulty with respect to both accuracy and scale. While a detailed analysis is beyond the scope of this discussion, few hydrogeologists would claim a certainty greater than a factor of two for measured hydraulic conductivity values. Furthermore, the common methods of measurement such as pump-test analyses and, to a lesser degree, single-well response tests give integrated values that may not be relevant at the scale or location of the PRB. This is particularly the case for aquifers that are highly heterogeneous. For example, relatively thin layers of high hydraulic conductivity that are not identified or represented in the hydraulic conductivity values could have a significant influence on the ultimate performance of a PRB. While there is no satisfactory means to resolve this problem, several single-well tests along the anticipated alignment of the PRB, combined with detailed stratigraphic investigations, provide the best means to reduce the uncertainty in K values. Uncertainty in hydraulic gradient (i) also can be significant, particularly in regions of low gradient, and temporal variations in gradient also can occur seasonally or in response to the pumping schedule of local production wells.
The required residence time is sensitive to the influent concentration and thus it is common to vary the thickness of the PRB according to the concentration distribution within the contaminant plume. Similarly, the required thickness of the PRB is directly proportional to the velocity. Thus, it is common to vary the thickness according to major stratigraphic features having contrasting values of hydraulic conductivity.
Because the cost of iron is generally a significant component of total construction costs (frequently in the range of 30–50%) there is clearly an incentive to keep the wall as thin as possible. On the other hand, because of uncertainty in the required residence time, and particularly in the groundwater velocity distribution, an appropriate factor of safety is generally included in the design, with the safety factor requiring professional judgment based on the level of confidence in the various parameters.
Though the deterministic approach to design, as discussed above, is used most commonly, an alternative is a probabilistic approach, as discussed in Vidumsky and Landis (2001), Hocking et al. (2001) and Christians et al. (2006). In this case, the range and distribution of transport and reaction parameters are provided, and through a Monte Carlo simulation process, a probability distribution of PRB thickness is provided as the output. One can then select the thickness that will provide the acceptable probability of success. Conceptually, this approach is appealing in that it reduces the need for arbitrary safety factors in the design and also provides a means to examine the influence of uncertainty in the various parameters on the ultimate design. However, it does not incorporate heterogeneity explicitly, and knowledge of parameter uncertainty is still required in order to arrive at defensible results.
16.3.3 Possible PRB Configurations
Various configurations of PRBs have been implemented, including continuous walls, funnel-and-gate systems, and in situ reactive vessels and injected treatment zones. As represented in Figure 16.1, in a continuous wall configuration, the treatment material is distributed as a wall or panel across the entire width and depth of the contaminant plume. If the grain size of the iron is selected such that the hydraulic conductivity is greater than that of the aquifer, the wall will not significantly alter the natural groundwater flow path. Thus, there is no need for the “wall” to be keyed into a low permeable zone or to extend for a significant distance beyond the lateral limits of the plume. To date, the continuous wall PRB has been the most commonly used configuration.
A funnel-and-gate configuration uses low permeability materials (funnel) to direct groundwater towards one or more permeable treatment zones (gates). Directing or funneling the groundwater towards a treatment gate will increase the groundwater flow velocity in the gate relative to background by an amount that is approximately equal to the ratio of the width of the funnel and gate to the width of the gate. Because the purpose of the impermeable funnel sections is to divert the natural flow of water, it is necessary that the lateral funnel sections extend beyond the lateral extent of the plume in order to ensure complete capture of the plume. The total length of a funnel-and-gate system may be on the order of 1.2 to 2.5 times the plume width, depending on the number of gates, and the funnel-to-gate ratio. Because the hydraulic head on the upgradient side of the funnel and gate increases, there is the possibility that the contaminants will be pushed to greater depth and thus flow under the funnel-and-gate sections. To avoid this, it is preferable that the funnel and gate be keyed into an underlying low permeability layer. Alternatively, if no impermeable layer is present, it may be necessary to extend the funnel-and-gate system well below the initial depth of the plume. Starr and Cherry (1994) present numerical simulations of flow fields associated with various funnel-and-gate configurations.
Closely related to the funnel and gate design are in situ reaction vessels, which use funnels or collection trenches to capture the plume and pass the groundwater, through differences in hydraulic head, through a buried vessel containing the treatment material. These vessels also can be used to control and treat groundwater exiting from otherwise completely contained source areas. The use of in situ vessels is usually determined by specific site conditions; examples are described in Primrose et al. (2004). A particularly novel approach is the GeoSiphonTM, described in Phifer et al. (1999).
Because of the higher velocity through the treatment zone in funnel-and-gate configurations and treatment vessels, the flow-through thickness is generally greater than in a continuous wall configuration. Indeed, in principle, the same amount of iron is required to treat the same discharge of contaminated water independent of the configuration of the treatment zone. It should also be noted that the higher velocity in funnel-and-gate systems or vessels, and thus the higher flow rate per unit surface area, could result in accelerated passivation of the treatment zone relative to a continuous wall (see Section 16.4). On the other hand, having the treatment material confined to a gate or vessel makes it more convenient and less costly than in the case of a continuous wall should the reactive material require replacement at some time in the future.
16.3.4 Construction Methods
Several methods are available for construction of granular iron PRBs. The most suitable method will depend on several site-specific factors, such as the depth of installation, the nature of the geologic materials, surface/subsurface obstructions (e.g., buildings and utilities) and the required flow-through thickness. Where the minimum practical excavation width is wider than the required flow-through thickness of 100% granular iron, sand is frequently mixed with the granular iron as a bulking agent, thus reducing the amount and cost of iron. The sand is chosen to have hydraulic properties similar to those of the granular iron and if possible, to have mineralogical properties similar to the aquifer material. Below is a summary of methods used to install granular iron PRBs, the range in depth for which the method is applicable and the iron flow-through thickness that can be achieved.
16.3.4.1 Excavation Methods
Unsupported Excavation—This is the simplest and least expensive installation method and can be implemented using conventional backhoe equipment. The geologic material must have sufficient strength such that the trench remains open for a sufficient period of time to permit backfilling with granular iron or an iron/sand mixture. The method is generally limited to relatively shallow depths, and the minimum width of the trench is determined by the width of the excavator bucket, usually no narrower than about 1 foot (ft) (30 centimeters [cm]). The method has been used to install PRBs to depths of 20 ft (6 m) below ground surface (bgs) in till and highly fractured shale materials.
Continuous Trenching—Continuous trenching machines allow simultaneous excavation and backfilling without an open trench. Excavation is performed by a cutting chain immediately in front of a trench-box (boot) that extends the thickness and depth of the treatment zone. Both the cutting chain and boot are attached to the trenching machine. As the trencher advances, granular iron or an iron/sand mixture is added to the hopper and is expelled from the back of the boot, creating a continuous treatment zone. Trenchers are available for installing treatment zones from 1.5 ft (0.5 m) to 3 ft (0.9 m) in width and to depths of about 35 ft (11 m). Several passes of the trencher can be made if greater widths are required. A bench on which to operate the trencher can be excavated to near the water table to increase the depth that can be reached.
Continuous trenchers have high mobilization costs and require a period for assembly and therefore may not be cost effective for small projects. However, for large projects where the geological conditions are favorable, they provide a highly cost-effective and rapid means of installation.
Supported Excavation—Where the geologic materials do not have sufficient strength for a trench to remain open, which is normally the case, some form of mechanical trench support can be used. Temporary support methods such as trench boxes or hydraulic shoring have been used at several sites. The permeability of the soil must be sufficiently low such that dewatering is not required in order for these methods to be effective. Prefabricated trench boxes similar to those commonly used during installation of utilities and sewers can be used to maintain trench integrity during excavation and backfilling operations. The trench box is slid along the trench with excavation proceeding just ahead and backfilling occurring in the back half of the trench box. Typical trench boxes are 4 ft (1.2 m) wide, though custom trench boxes can be fabricated to smaller widths. An alternative is to use a system of temporary shoring. Generally, hydraulic shores are used to provide additional stability to the trench until it is backfilled. The temporary shores are placed in the excavation immediately after the trench is excavated to provide temporary support during the excavation and backfilling operations. Advantages of the shored excavation method are that the trench width can be as narrow as 2 ft (0.6 m) and two workers can place the hydraulic shores without the use of heavy equipment.
The trench box and hydraulic shoring methods are generally limited to depths no greater than about 20 ft (6 m).
Sheet Piling Cofferdam—Using sheet piling cofferdams is another method for providing mechanical support for open trenches. Sheet piling is driven around the perimeter of the treatment zone, and the soil within the sheet piling enclosure is excavated. Typically, internal bracing is required at greater depths. The sheet pile maintains the dimensions of the treatment zone during excavation and backfilling. After backfilling is complete, the sheet piling is removed and groundwater is allowed to flow through the treatment zone. The method is limited to minimum widths of about 3 ft (1 m) and maximum depths of about 40 ft (13 m).
A significant advantage of all of the mechanically supported trench methods is that they provide a high degree of control in placing the reactive materials and the boundaries of the treatment zones are well defined. They are, however, limited to relatively shallow depths.
Biopolymer Trenching—Installation of a treatment zone of granular iron using a biodegradable polymer (biopolymer) slurry is similar to constructing a conventional impermeable slurry wall. As the trench is excavated, a biopolymer slurry is added, and through density and viscosity effects, stabilizes the exposed trench walls. The biopolymer used is typically guar gum. Excavation continues through the biopolymer without the need for dewatering or mechanical shoring. Granular iron (or an iron/sand mixture) is placed through the biopolymer using a tremie pipe. Recirculation wells are spaced along the length of the trench to flush out the polymer slurry and to add enzyme solution to assist in breaking down residual polymer. Because of the high solubility of the biopolymer, any remaining material will be flushed out by the flow of groundwater through the treatment zone. Depths of up to 70 ft (21 m) bgs and a minimum width of 2 ft (0.6 m) or greater can be achieved using specialized backhoe equipment. Substantially greater depths could be achieved through the use of clam-shell excavators. The biopolymer is highly biodegradable and thus residual polymer can result in high levels of biological activity following installation. To minimize possible effects on performance, efforts should be made to flush as much polymer material as possible from the PRB during construction.
16.3.4.2 Injection Methods
Vertical Hydraulic Fracturing—Thin vertical treatment zones, up to 9 inches (23 cm) thick, can be installed using vertical hydrofracturing. This method uses a specialized tool that is inserted into a borehole to the required depth and oriented to control the direction of the fracture. The vertical interval of the fracture is isolated in the borehole by packers. The fluid used for fracturing consists of a biopolymer slurry similar to that used in biopolymer trenching; however, the polymer is cross-linked, resulting in a fluid of very high viscosity. The granular iron, of grain size considerably smaller than that normally used in trenching methods, is suspended in the highly viscous fluid. The fracturing fluid is pumped under low pressure (25 pounds per square inch [psi]) into the formation, which causes the subsurface material to separate, creating an iron treatment zone a few inches in thickness with a controlled vertical dimension. Several fractures propagated from boreholes located along the line of installation coalesce to create a continuous PRB. Though the maximum thickness of a single panel is about 9 in (20 cm), parallel panels can be installed to increase the total flow-through thickness of the iron zone. Using this method, PRBs have been constructed to depths of 120 ft (40 m) and technically, greater depths can be achieved. Further details concerning this method can be found in Hocking et al. (2004).
Jetting—Installation of an iron PRB by jetting is a variation of the jet-grouting procedure that is well developed for construction of impermeable barriers. The same equipment is used to inject granular iron to form an iron PRB. High pressures, of about 5,000 to 6,000 psi, are used to erode the soil and deposit fine-grained iron in the natural aquifer material. The jetting tool is advanced into the formation to the desired depth and the iron, suspended in biodegradable slurry, is injected from the nozzles as the tool is withdrawn. If the tool is rotated, a columnar iron zone is created. The diameter of injection depends on several factors, but diameters of 2 to 7 ft (0.6 to 2.1 m) can be achieved. If the tool is not rotated, and has only two opposing nozzles, a thin diaphragm treatment wall can be created. Diaphragm walls may be 2 to 3 inches (5 to 8 cm) of 100% iron near the point of injection but may be several inches of an iron-aquifer material mixture further away.
Direct Push Injection—This method typically involves hydraulic fracturing, in which a slurry solution is injected at a pressure that exceeds the combined lithostatic pressure and cohesive strength of the formation. Rods are pushed into the subsurface to the required depth, and then retracted, exposing an injection nozzle. The granular iron is suspended in a biopolymer slurry and pumped into the formation at a rate that exceeds the ability of the formation to accept the fluid. As a result, the pressure increases until fractures form along horizontal planes of weakness. The fluid invades the fractures, depositing a layer of iron. Enzymes are added to the biopolymer slurry during injection to accelerate degradation of the polymer.
Pneumatic Fracturing and Injection—Pneumatic fracturing is the injection of gas, usually nitrogen, at high pressure and flow rate in order to create fractures or fissures in the unconsolidated or rock matrix. Fractures or fissures occur when the pressure of injected gas exceeds the natural in situ stresses and the flow rate exceeds the natural permeability of the soils. In cohesive geologic materials, pneumatic fracturing enhances the permeability by creating fracture networks, while in rock, the effect is generally dilation and extension of existing discontinuities, thereby improving the interconnection between existing fractures. In materials with low cohesive strength, the iron can be pneumatically injected without first fracturing the formation. More commonly, however, the zone of interest is first fractured, then using the same equipment, fine-grained iron is added to the nitrogen stream and is subsequently deposited in the fracture network. Further information is provided in McCall et al. (2004).
16.3.4.3 Other Methods
Augered Boreholes—Treatment zones of iron can be constructed by an array of augered boreholes. When the required depth is achieved, the iron can be placed either through the auger stem as the augers are removed or directly into an open borehole.
Caissons—Caisson installation involves driving a large circular steel caisson into the ground and augering out the native material. The caisson is then backfilled with iron and removed. Although caissons as large as 15 ft (4.6 m) in diameter can be installed, smaller diameters are more common. Overlapping or tangential caisson-emplaced treatment zones can be used to create a larger permeable treatment zone.
Soil Mixing—Soil-mixing equipment generally uses one or more large-diameter augers to thoroughly mix iron into the soil. The iron is initially mixed with biodegradable slurry and pumped to the mixing augers while they are advanced slowly through the soil. Over time, the biodegradable slurry breaks down, allowing the groundwater to flow through the treatment zone. Alternatively, the iron can be placed in a smaller diameter borehole and mixed into the adjacent aquifer material using the soil-mixing equipment.
Mandrel (or H-Beam)—A mandrel is a hollow steel box or H-beam that is driven into the ground to create a thin continuous treatment zone. A disposable shoe at the leading edge of the mandrel prevents aquifer material from entering the cavity as the mandrel is advanced. Once the mandrel reaches the maximum depth of the treatment zone the shoe is driven from the end of the H-beam and iron is placed inside the mandrel. As the mandrel is removed, the iron fills the void that is created. The process is then repeated with the mandrel being driven adjacent to the iron zone previously installed. The mandrel can be modified for a particular site, but its open-end dimensions range from 3 to 5 in (8 to 13 cm) by 20 to 30 in (51 to 76 cm).
16.3.5 Installations in Fractured Rock
Innovative PRB designs and construction methods are being developed for groundwater remediation in fractured bedrock. If the bedrock is sufficiently weathered and can be economically excavated, possibly with the aid of a rock hammer, a PRB can be constructed using trenching methods similar to those described for unconsolidated media. As an example, a PRB was installed to a depth of 14 feet (4.2 m) in a highly weathered shale using a conventional excavator with no mechanical support of the trench walls (personal communication, EnviroMetal Technologies Inc.).
Rock trenchers are capable of excavating a continuous trench in bedrock, such as limestone. This equipment has been used for several years to install utilities in areas where bedrock is relatively shallow. Excavation is performed by a cutting chain that extends the width and depth of the finished treatment zone. A bench on which to operate the trencher can be excavated to near the water table to increase the total depth attainable with the trencher. A continuous rock trencher has been used to install a PRB in limestone bedrock to a depth of 18 feet (5.5 m).
Waterjet technology has been used extensively in the mining industry, particularly for cutting and quarrying blocks of granite and other rock types. High-pressure waterjet units can be used to cut a thin (3 to 6 in [7.5 to 15 cm]) vertical slot in the bedrock along the length of the proposed alignment, allowing a PRB to be constructed (Elmore et al., 2003).
Installation of an array of closely-spaced boreholes backfilled with granular iron also could be used to intercept fractures and provide treatment. The array of boreholes would need to be designed to capture the groundwater plume through a combination of intercepting the fractures and creating connecting pathways between fractures. Treatment would occur as the groundwater flowed through the granular iron in the boreholes.
Refractive flow and treatment (RFT) configurations rely on creating zones of relatively high hydraulic conductivity contrast to divert groundwater flow to a treatment zone. Precision blasting can be an effective means of producing highly conducting and elongated rubble zones (Dick and Edwards, 1997). Pneumatic and hydraulic fracturing also have been used as a means of emplacing iron along pre-existing planes of weakness in bedrock formations.
16.4 Long-term Performance
16.4.1 Definition and Potential Factors
The major appeal of granular iron PRBs is a reduced cost for remediation. As the capital cost of a PRB is generally comparable to that of a pump-and-treat system, the main economic advantage accrues from greatly reduced operation and maintenance costs. To realize these benefits, the PRB must perform effectively, without significant maintenance or replacement of the iron, for long periods of time. The question then is how long is long? Because of particular circumstances at the site, the first commercial installation of a PRB was shown to be economically viable even if the iron had to be replaced every five years. In that particular case, as noted previously, the PRB continues to perform adequately after 10 years of operation, without replacement of the iron (Warner et al., 2005). Taking yet another approach, net present value calculations suggest that replacement of iron in a PRB every 10 to 15 years adds very little to the total cost. Thus, for the purpose of this discussion, long-term performance will be defined as adequate treatment for a period of 10 to 15 years.
The record of field performance is encouraging, although only a small number of installations have reached the 10-year criterion and none have yet reached the 15-year criterion. It is therefore important to consider the factors that may limit long-term performance and the evidence that is available, primarily from laboratory tests, concerning their potential effects over time. Generally included among these factors are persistence of the iron, competing oxidants, precipitate formation (implications for both hydraulic and reactivity changes) and microbial effects.
16.4.2 Persistence of Metallic Iron
As indicated in Equation 16.1 and 16.2, metallic iron is not only consumed through oxidation by the chlorinated organic compounds but also by reaction with water. The reaction with water proceeds whether or not chlorinated compounds are present and except in the presence of very high contaminant concentrations, corrosion of iron will be dominated by reactions with water.
Using hydrogen evolution as a measure of the anaerobic corrosion rate of iron in water, Reardon (2005) measured values for various iron materials that ranged between 0.1 and 0.6 millimoles (mmol)/kilograms (kg) Fe/day, with a value of 0.2 mmol/kg Fe/day for a material commonly used in PRB construction (Reardon, 2005). The iron corrosion rate is strongly dependent on grain size (specific surface area), as well as on temperature and the type of iron. Furthermore, the corrosion rate depends upon the anions in solution. The literature is not entirely consistent in that Reardon (1995) showed enhancement in the order HCO\(_3^ - \) > SO\(_4^{2 - } \) > Cl\(^ - \), while Devlin and Allin (2005) showed SO\(_4^{2 - } \) and Cl\(^ - \) had little effect on iron reactivity. Deng et al. (1998) observed enhancement in the order PO\(_4^{3 - } \) > BO\(_3^{3 - } \) > H2SiO4. Reardon (1995) also observed a decline in hydrogen production (decline in corrosion rate) over time.
Using a corrosion rate of 0.3 mmol/kg Fe/day, it can readily be shown that the metallic iron in a PRB should persist for periods of up to 100 years or more. While there is considerable uncertainty in this estimate, one can reasonably expect that the iron will persist for several decades and well in excess of the 10 to 15 years used in our definition of long-term performance.
Over the past several years, there has been considerable interest in the injection of nano-scale iron for groundwater remediation. Because of the much smaller grain size and thus a vastly greater specific surface area, nano-iron corrodes at a much higher rate, and using normalized specific surface area, it can be shown that nano-iron is likely to be consumed in a matter of weeks to a few months (Liu and Lowry, 2006). Thus, it would appear that injection of nano-iron would be more appropriate in situations where high mass removal is required over short periods of time, as in source-zone treatment, rather than in the construction of PRBs for plume control and treatment.
16.4.3 Competing Oxidants
In addition to water and chlorinated solvents, other oxidants, such as dissolved oxygen, nitrate, chromate and permanganate, can be present in groundwater. Dissolved oxygen reacts quickly with iron, forming high valency iron oxides, such as hematite and maghemite. As noted previously, these are passivating oxides and therefore have the potential to adversely affect PRB performance. In laboratory column tests, Mackenzie et al. (1999) noted the formation of iron oxides near the influent end of the column, and attributed a significant decline in permeability to oxide accumulation.
Oxygen, though potentially present at concentrations of about 8 mg/L, depending upon temperature, is frequently absent in contaminant plumes and is seldom present at concentrations in excess of 1–2 mg/L. In the initial test of the PRB concept at Borden (O’Hannesin and Gillham, 1998) the dissolved oxygen concentration in the groundwater entering the PRB was about 1–3 mg/L. After five years of operation, there was only slight evidence of the formation of higher valence iron oxides, and this was restricted to the first 2–3 cm of the influent surface, with no evidence of a decline in hydraulic conductivity. While the evidence from the numerous cores from other commercial installations is generally less precise (e.g., Vogan et al., 1998), it is also the case that no evidence of declining performance as a consequence of oxygen invasion has been reported. Nevertheless, acknowledging that little high-quality field data is available, in contaminant plumes with high concentrations of dissolved oxygen (greater than perhaps 3 or 4 mg/L), a reduction in hydraulic conductivity and reactivity as a consequence of iron oxide formation should be anticipated.
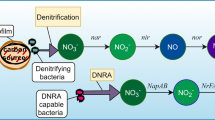
Nitrate is frequently a constituent of shallow groundwater at concentrations of a few mg/L to tens of mg/L NO\(_3^ - \) - N, with most of the nitrate being attributed to anthropogenic sources, such as agricultural fertilizers or disposal of septic effluent. In addition, waste streams from manufacturing processes can contain NO\(_3^ - \) - N at several tens of mg/L and in some cases, these concentrations may occur as cocontaminants with chlorinated solvents. Numerous laboratory studies have shown NO\(_3^ - \) to be rapidly reduced to NH\(_4^ + \) by iron, accompanied by oxidation of the iron, resulting in the formation of passivating higher valency iron oxides (Ritter et al., 2003; Lu, 2005).
Column tests show progressive passivation from the influent end, with the zone of nitrate reduction and TCE reduction progressing further into the column over time, with near complete passivation as the eventual consequence. Unfortunately, there is insufficient information to indicate if there is a threshold below which NO\(_3^ - \) is not a problem; furthermore, there is no data available to indicate if the effect of nitrate in field situations is similar to observations in the laboratory. Though incomplete, the available information suggests that if nitrate mass flux is significant, special precautions should be taken. These could include running treatability tests for a sufficient period of time to ensure that any negative effects of nitrate can be evaluated.
In some cases, potential problems may be averted by constructing the PRB with added thickness to accommodate a degree of passivation by nitrate. Alternatively, and particularly in cases of very high nitrate concentration, a nitrate removal zone in advance of the iron PRB could be considered. This zone would include an organic carbon source to promote denitrification. Encouraging, but preliminary, research has shown that iron surfaces passivated by nitrate may be rejuvenated by passing water containing no nitrate over these surfaces (Lu, 2005).
Chromate frequently occurs in groundwater as a cocontaminant with chlorinated solvents, particularly in contaminant plumes associated with metal-plating facilities. There is considerable literature available concerning the removal of chromium from contaminated water using Fe(0) (Blowes et al., 1997; Pratt et al., 1997; Astrup et al., 2000). Generally, Cr(VI) is reduced to Cr(III), which precipitates as chromium hydroxides or mixed Fe(III)−Cr(III) oxides. As a consequence of the chromium precipitates, or possibly as a consequence of the formation of higher valence iron oxides, treatment of chromate has a gradual passivating effect on the iron. However, the literature concerning the effect of chromate removal on treatment of chlorinated solvents is considerably less extensive. In laboratory column tests, Yang (2006) showed that TCE did not appear to have a significant effect on the removal rate of chromate; however, the removal of chromate had a significant passivating effect on the degradation of TCE; this was attributed to the formation of chromium precipitates, as well as the formation of tri-valent iron oxides on the iron surfaces as observed using Raman spectroscopy.
An iron PRB was installed at Elizabeth City, North Carolina, for removal of both chromium and TCE. A comprehensive discussion of this project, including treatability tests, design, installation, performance monitoring and mathematical simulation, is provided in Wilkin and Puls (2003). Concentrations of chromate and TCE entering the PRB were as high as 4.7 and 4.2 mg/L, respectively (Wilkin and Puls, 2003). Performance monitoring indicated complete removal of the chromate near the influent face of the PRB and first-order decay of the TCE concentrations, with a minor discharge of cis-DCE at one location where the influent TCE concentration was particularly high. As reported in Wilkin and Puls (2003), the performance remained satisfactory after five years of operation. Thus, while it is clear that the precipitation of chromium can have an adverse effect on reactivity towards TCE, it appears that at moderate concentrations, there should be no difficulties in designing a PRB such that it meets the criterion for long-term performance.
In situ chemical oxidation is an emerging technology for treatment of chlorinated-solvent source zones. Of the potential oxidants, considerable attention has been directed towards permanganate, added either as NaMnO4 or KMnO4 (Gonullu and Farquhar, 1989; Thomson et al., 2000; Siegrist et al., 2001). With the expectation that removal of the source zone will not be complete, it has been proposed that PRB technology could be implemented as a polishing step downgradient of such a treatment area. Depending on the distance between MnO\(_4^ - \) application and the PRB, in some cases MnO\(_4^ - \) may not be entirely consumed and therefore may enter the PRB. Okwi et al. (2005) reported the simultaneous addition of TCE and MnO\(_4^ - \) to laboratory columns containing granular iron. At the high MnO\(_4^ - \) concentrations used (5,000 mg/L), the columns were quickly passivated with respect to both TCE and MnO\(_4^ - \) removal. In contact with iron, the Mn(VII) of MnO\(_4^ - \) is quickly reduced to Mn(II), which subsequently precipitates as various oxides/hydroxides of Mn. Furthermore, because MnO\(_4^ - \) is a strong oxidant, the passivating higher valent oxides of iron were also identified. Though somewhat speculative, the authors considered the iron oxides to be the primary passivating agents, with the Mn precipitates possibly playing a secondary role. Because of the particularly high MnO\(_4^ - \) concentrations used, the results cannot be readily transferred to the field, but are nevertheless of interest in that they clearly indicate that high concentrations of strong oxidants can rapidly degrade the performance of iron PRBs.
16.4.4 Formation of Secondary Minerals
Because of the low Eh and high pH characteristics of iron PRBs, secondary minerals are likely to form in virtually all groundwater environments. These have the potential to form coatings on the iron and thus reduce the reactivity; if present in sufficient quantities, they could also reduce the pore space, leading to a decline in hydraulic conductivity. Should the permeability decline sufficiently, then much of the plume could be diverted around the PRB, rendering it ineffective.
A wide range of secondary minerals have been identified in laboratory columns and in field PRBs; however, in most cases, there is insufficient information to predict the effect that these may have on long-term performance. Furthermore, it is generally not practical to perform laboratory treatability tests for a sufficient period of time to give useful information concerning the effects of secondary minerals on long-term performance, and only recently have suitable predictive models been under development.
The major mineral phases that have been identified in iron PRBs include various iron oxides, hydroxides and oxyhydroxides, intermediate products (green rusts), carbonates (aragonite, calcite and siderite) and iron sulfides (Blowes et al., 1997; Gu et al., 1999; Phillips et al., 2000; Roh et al., 2000; Phillips et al., 2003). In most cases, the primary source of dissolved iron that reacts with anions to form iron-bearing mineral phases is from oxidation of the iron metal. Bicarbonate (HCO\(_3^ - \)) is present in virtually all groundwaters at concentrations ranging from a few to several hundred mg/L. Furthermore, in the PRBs that have been examined, carbonates commonly appear to be the predominant secondary minerals present as aragonite, calcite (CaCO3) or siderite (FeCO3). While often not observed in laboratory studies, sulfate reduction is ubiquitous in iron PRBs in the field. However, with the exception of a few isolated cases with extremely high natural sulfate (in excess of 100s of mg/L), sulfides do not appear to be a major mineral phase influencing PRB performance. Thus the following discussion will be limited primarily to secondary minerals of carbonate.
In cases where core samples have been collected, the greatest accumulation of precipitates appears very near the upgradient face of the PRB. Vogan et al. (1999) reported calcium carbonate accumulations in a funnel-and-gate system after two years of operation. Though there was no observable change in performance, core samples revealed about 6 weight (wt) % CaCO3 in the sample collected from the upgradient interface, with a rapid decline with distance (1 wt % at a distance of 15 cm from the interface). The 6 wt % carbonate was calculated to be equivalent to a loss of initial porosity of almost 5% per year. From changes in inorganic chemical compositions, McMahon et al. (1999) estimated that the total porosity of a PRB was reduced by about 0.35% per year as a consequence of calcite and siderite precipitation. This estimate assumed that the precipitates formed uniformly throughout the PRB; though solid-phase analyses were not reported, visual inspection indicated a greater abundance of precipitates near the influent face.
In general, based on both laboratory and field evidence, and because the primary source of dissolved iron is corrosion by water, iron (oxy) hydroxides will form throughout a PRB. However, because these hydroxides ultimately transform to magnetite, which is electron-conducting, they do not substantially reduce the reactivity of the iron and the rate of formation does not cause a significant decline in permeability.
Projecting the empirical field evidence, it would be reasonable to expect continued precipitate formation near the influent surface of a PRB, ultimately resulting in reduced permeability and bypass of contaminated groundwater around the PRB (or the need for some form of maintenance). However, controlled experimental data indicates that precipitation may have a greater effect on the reactivity of the iron rather than on the hydraulic performance of the PRB. In long-term column tests with dissolved CaCO3 in the influent solution, Zhang and Gillham (2005) observed the formation of aragonite near the influent end of the columns early in the test. With the passage of time, however, the pH near the influent end declined, suggesting reduced iron reactivity; there was no further precipitation in this region. Indeed, Ca2+ and HCO\(_3^ - \) were carried further into the column and precipitated where the iron remained reactive, and the pH values were elevated. Thus, rather than precipitating at the influent to the degree that the iron was substantially impermeable, aragonite was deposited as a moving front that progressed through the column. The results suggested that at about 3 wt % CaCO3, the iron was essentially non-reactive; at this percentage, the permeability was reduced by about half an order of magnitude. Though passivation of the iron is generally considered to be undesirable, it can be advantageous in that it prevents the continued formation of precipitates to the point that the PRB becomes impermeable. Assuming a groundwater velocity of 5 cm/day with a CaCO3 concentration of 100 mg/L and using highly simplified plug-flow assumptions, Zhang and Gillham (2005) suggested that the passivating front would progress through a PRB at a rate of about 1 cm/year; this factor could be readily accommodated in the initial design of a PRB.
Various efforts have been made to develop mathematical models for predicting PRB performance. The initial attempts used geochemical equilibrium models (Schumaker, 1995; Gaveskar et al., 1998; Morrison et al., 2001) to represent the chemical changes occurring within a PRB; more recently, complex reactive transport models have been developed (Mayer et al., 2001; Yabusaki et al., 2001; Li et al., 2005). Though the reactive transport models represent a significant advance over the equilibrium models, the reactivity of the iron is assumed to be constant in all cases. If the observations of Zhang and Gillham (2005) are generally applicable, then the current models are significantly limited in terms of their ability to predict long-term performance.
Jeen et al. (2006; 2007) conducted column tests similar to those of Zhang and Gillham (2005) and obtained results showing similar patterns of precipitate formation. In addition, an empirical relationship between the iron reactivity and the amount of precipitate present was derived. The relationship was then incorporated into the multi-component reactive transport model MIN3P presented in Mayer et al. (2001). The model was highly successful in reproducing the various concentration profiles observed in the columns, as well as the distribution of precipitates. Furthermore, assuming conditions similar to those in the laboratory, a groundwater velocity of 10 cm/day with influent concentrations of 100 mg/L CaCO3 and 10 mg/L TCE, modeling results indicated that a PRB of 50 cm thickness would perform effectively for periods in excess of 40 years. The model presented in Jeen et al. (2007) appears to accommodate the major factors that influence long-term performance of PRBs. However, because of its complexity and the large number of interacting parameters, its practicality and accuracy for predicting long-term performance and its usefulness as a design tool remain uncertain.
Because of the extremely wide range of geochemical conditions that can be encountered, it is difficult to arrive at generally applicable conclusions regarding long-term performance. Clearly, if high concentrations of oxidants are present, passivation of the iron must be anticipated and accommodated in the design; this could include increasing the thickness of the PRB or designing special features for removal of the oxidant. In some cases, the PRB concept may not be appropriate. It is also apparent from the empirical field evidence and experimental observations that secondary minerals will form in virtually all PRBs; their influence on performance is strongly dependent on the groundwater velocity and the concentration of precipitating species in the groundwater. The evidence suggests that precipitates are not likely to have a significantly detrimental effect on permeability but will cause a reduction in reactivity of the iron. In most groundwater, this can probably be accommodated through a modest addition to the thickness of the PRB. It appears that there are few situations where the 10–15 year criterion for long-term performance cannot be achieved with relative ease; in many environments, performance periods of 20–40 years or more are likely to be achievable.
16.4.5 Microbiological Effects
Precipitation of iron oxides in the screens of pumping wells is a well-known phenomenon that prompted initial concern regarding biofouling of iron PRBs. However, under the strongly reducing and high pH conditions of most PRBs, biofouling has not been documented and, indeed, the environment is quite hostile for most microbes. In most cases where samples have been collected for microbial analyses, populations have been no greater within the PRB than in the natural groundwater (Matheson and Tratnyek, 1994; Vogan et al., 1998). There are exceptions however; in a groundwater containing 120 mg/L of nitrate, Gu et al. (2001) found biomass in an iron PRB of one to three orders of magnitude higher than in the groundwater, with an abundance of sulfate reducers and denitrifiers. Indeed, where there is a decline in sulfate concentration across a PRB, the presence of sulfate-reducing bacteria should be anticipated. High levels of biological activity were also reported in one gate of a funnel-and-gate system installed in Denver, Colorado (Wilkin and Puls, 2003); subsequent investigation (Hart et al., 2004) showed that smearing (decreasing hydraulic conductivity) of the trench walls had caused the gate to be “sealed off” from groundwater flow, creating an essentially stagnant area within the gate. Thus, with the exception of a small number of special conditions, biofouling is not likely to be of concern with respect to the performance of iron PRBs.
There is speculation and preliminary evidence that PRBs may enhance downgradient biodegradation (Scherer et al., 2000). In particular, the dissolved hydrogen exiting the PRB may stimulate downgradient biological activity, which could promote the degradation of compounds not normally degraded by iron, such as DCM and 1,2 dichloroethane (Vidumsky, 2003; Stening et al., 2008). Other contributions to this topic include Wildman and Alvarez (2001), Weathers et al. (1997), and Sfeir et al. (2000).
16.5 Case Studies
The following case studies have been selected to illustrate the deterministic approach typically used to design iron PRBs.
16.5.1 DoD Facility, New York
In 1998, the U.S. Army Corps of Engineers (USACE) installed a PRB at a DoD facility in eastern New York (Wood and Heckelman, 2002). Shallow groundwater at the site occurs in unconsolidated deposits comprising a 4 ft (1.2 m) thick fill unit and a 2 to 6 ft (0.6 to 1.8 m) thick lacustrine unit, overlying weathered bedrock (USEPA, 2001; Anderson, 2001). To examine the feasibility of PRB application at the facility, groundwater from the site was pumped through a 1.7 ft long column of 100% Connelly GPM iron at a constant flow rate. Samples collected along the length of the column were analyzed for the primary contaminants present (PCE, TCE, cis-DCE and VC) allowing concentration versus distance profiles to be obtained (Figure 16.7). These data were converted to concentration versus time profiles using the column flow rate (1 ft/day) and fitted with a first-order model to obtain the half lives shown in Figure 16.7. The half lives, increased by a factor of two when corrected for groundwater temperature, were then input to a first-order simulation model together with anticipated field-scale influent concentrations. It was determined (Figure 16.8) that 2.5-days would be the residence time required to reach USEPA MCLs.
Concentration versus distance profiles obtained in column test using groundwater from U.S. DoD facility, New York, and half lives obtained from first-order model fit to these data (modified from EnviroMetal Technologies Inc., 1998).
Simulation of field-scale residence time requirement based on column test results, U.S. DoD facility, New York (from Goldstein et al., 2000).
Concurrent with the bench-scale design activity, a groundwater modeling study was conducted to assess a variety of PRB configurations (Gaule et al., 2000). Configurations incorporating funnel sections or reactive walls non-perpendicular to flow created underflow and reduced capture zone efficiency. A configuration with two staggered PRBs aligned perpendicular to flow was most cost-effective. At the design velocity of 0.15 ft/day (4.5 cm/day), the models indicated that a wall of 0.82 ft (25 cm) of 100% iron would provide the required residence time.
The shallow depth of this installation meant that construction could be economically completed with a trench dug with a backhoe and maintained with temporary hydraulic shoring (Anderson, 2001). To accommodate the 2.5 ft (75 cm) width of the excavator bucket, iron was mixed at a 1:1 ratio with local sand, which created an additional safety factor in the design. An upgradient 180-ft PRB and offset downgradient 90-ft PRB were keyed into the weathered bedrock.
Construction costs, including the cost of iron, were about $300,000 U.S., and cost savings of over $3,000,000 U.S. over a 30-year life cycle were estimated by the USACE (Gaule et al., 2001). Three years of monitoring data indicated that volatile organic compound (VOC) concentrations remained below regulatory standards in groundwater exiting the PRBs.
16.5.2 Former Industrial Facility, Massachusetts
The depth of a chlorinated solvent plume migrating towards municipal supply wells from a former industrial facility in Needham, Massachusetts, necessitated the use of biopolymer trenching methods to complete the PRB installation (Richards, 2002). The PRB was designed to intercept an overburdened TCE plume, about 550 ft (165 m) wide and 57 ft (17 m) deep, to the local bedrock.
Two sources of granular iron were evaluated for use in this application, one from Connelly GPM, Inc., Chicago, Illinois, and the other from Peerless Metal Powders & Abrasives, Inc., Detroit, Michigan. The grain size of both iron sources ranged from 0.25 to 2.0 millimeters (mm) (−8 to +50 U.S. standard mesh size). Laboratory column tests were completed as described in the above case study, but in this case, each column was packed with a mixture of 68% by volume granular iron and 32% by volume sand, in order to simulate the sand/iron mixture anticipated to be implemented in the field. Biodegradable slurry was mixed into the sand/iron mixture to simulate the construction technique. Groundwater was collected from the site, and laboratory grade TCE was added to the groundwater to increase the concentration to about 500 µg/L of TCE. A flow velocity of 78 cm/day was selected to simulate actual field conditions. All column experiments were performed at room temperature.
The TCE concentration versus residence time data from the columns (Figure 16.9), fitted using a first-order kinetic model, resulted in a TCE half life of 1.0 hour for the Peerless iron and 1.1 hours for the Connelly iron (Duchene and Jacobson, 2002). The degradation rates were increased by a factor of three to account for the temperature difference between the laboratory test and the expected in situ temperature.
Concentration versus time profiles obtained in column test using groundwater from a former industrial facility in Massachusetts, and half lives obtained from first-order model fit to these data (modified from Duchene and Jacobson, 2002).
In situ permeability tests and a range of hydraulic gradients were evaluated to determine suitable groundwater flow velocities for design. The design influent TCE concentrations were determined from historic water quality data. First-order simulations (Figure 16.10) indicated that a residence time of 13 hours was required for the PRB placed in the core of the plume to degrade the TCE to below the USEPA MCL of 5 µg/L.
Simulation of field-scale residence time requirement based on column test results from a former industrial facility in Massachusetts (modified from EnviroMetal Technologies., 2000).
The flow-through thickness of iron required was obtained by multiplying the residence time by the groundwater flow velocity. As the TCE concentration and groundwater flow velocity varied throughout the plume, the plume was divided into two areas for design, Zone A and Zone B, were assigned according to the differences in expected TCE mass flux. The design thickness in the central portion of the plume with higher TCE flux was 1.7 ft (50 cm) of 100% iron (Zone A); the remainder of the PRB had a design thickness of 0.5 ft (15 cm) of 100% iron (Zone B). Sand was used as a bulking agent to enable a 2-ft wide trench to be excavated and backfilled. A cross-section of the installation is shown in Figure 16.11, modified from Duchene and Jacobson, 2002.
Cross-section of PRB showing the two iron zones and monitoring well network; Zone A = 1.7 ft iron plus sand, Zone B = 0.5 ft iron plus sand (modified from Duchene and Jacobson, 2002).
Construction of the PRB was completed below a two-lane residential street. Biodegradable slurry was used as liquid shoring and was added as the trench excavation proceeded to provide stability for the trench walls. Soda ash and a biostat are frequently added to the slurry to slow microbial breakdown. Adding the biostat raised regulatory concerns about the potential to contaminate groundwater, so the biostat was not used (Huber et al., 2001). The contractor modified the construction process to account for the fact that there was no additional protection against premature microbial breakdown of the mixture.
The iron/sand mixture was prepared in an off-site pugmill and delivered to the site in trucks. The iron/sand mixture was placed through the slurry by the tremie method, which displaces the biodegradable slurry. After placement of the backfill was completed, residual slurry in the PRB was broken down through circulation of an enzymatic breaker fluid and natural biological degradation, allowing groundwater to flow through the PRB.
Monitoring of PRB performance was accomplished using monitoring wells arranged in nine transects of two wells that intersect the PRB in the direction of groundwater flow. Groundwater samples are analyzed for pH, Eh, dissolved oxygen and VOCs. The contractor performed the initial monitoring to provide evidence that the biopolymer had not affected the iron reactivity. To the authors’ knowledge, routine groundwater monitoring of VOCs has shown that the PRB has performed essentially as designed for several years following installation.
16.6 Stage of Technology Development
16.6.1 Number of Sites and Performance
Iron PRB technology for chlorinated solvents was commercialized by the University of Waterloo, Waterloo, Ontario, through EnviroMetal Technologies Inc. (ETI), founded in 1992. When introduced in the early 1990s, the PRB concept was a significant departure from conventional treatment technologies and was therefore greeted with considerable skepticism. Through the efforts of the research community, the scientific basis of the technology has evolved, and through numerous pilot-scale demonstrations by “arms-length” agencies such as USEPA and DoD, considerable confidence in the practical viability of the concept has been developed. ETI currently lists over 200 applications of the technology, approximately 40 of which are demonstration projects, with over 150 full-scale commercial applications. The largest number of applications is in the United States, with several in Europe and 15 in Japan. Thus, it is reasonable to classify iron PRBs as “an accepted standard practice.”
Of the sites listed by ETI, most continue to perform in a manner consistent with the initial design. Of the approximately 10 sites that have not met expectations, insufficient design information concerning the characteristics of the plume (size, groundwater velocity, and/or concentration distribution) has been the major cause of compromised performance. In these cases, the problem was generally resolved by increasing the length of the PRB or the thickness at particular locations. A small number of cases of insufficient performance can be attributed to construction artifacts, such as smearing of the trench wall, that have impeded flow through the PRB. The need for careful and thorough site characterization prior to the design and installation of a PRB cannot be overemphasized. To this point, a PRB has not failed as a consequence of lower-than-expected performance of the iron material.
Although a substantial number of PRBs have been installed and the record of performance is enviable, relative to the many thousands of sites for which the technology could be applicable, the number of installations remains small. This could be due in part to low incentives for cleanup and in part to the high initial capital cost. This situation may change as the record of performance at existing installations lengthens, leading to more demonstrable evidence of the economic benefits.
16.6.2 Future Developments
Even though the iron PRB technology is firmly established, numerous opportunities to improve the technology exist. Some of these are addressed very briefly below.
Installation Methods—While numerous methods for installing PRBs are available, continued improvement and development, particularly with a view to reducing cost, would be beneficial. This is particularly the case for installations at depths of greater than 80–100 ft (24–30 m) and for bedrock plume applications.
Prediction of Performance—Though the empirical evidence and experimental results are encouraging, or even convincing, with respect to long-term performance, predicting a reasonable expectation for the period of performance in a wide range of geochemical conditions continues to be an elusive goal. The model presented in Jeen et al. (2007) may prove to be a useful step in this direction. As the period of record for several installations now approaches (or exceeds) ten years, these sites could provide useful data for testing and improving predictive models.
Sequential Treatment—Many contaminant plumes include several contaminants, not all of which can be adequately treated by iron; in some cases, while iron treats the parent compound, it may not treat degradation products, which may also be toxic. Sequential treatment systems that include different reactive materials to treat the different contaminants may be a useful and practical approach in these situations. Work is proceeding in this area (Fiorenza et al., 2000, for example), and significant developments can be expected over the next few years.
PRB–Monitored Natural Attenuation—Iron PRB technology has been combined with monitored natural attenuation (MNA) at only a few sites. Because of the first-order kinetics associated with granular iron, large amounts of mass are removed very quickly at high concentrations, while at low concentration, rates of mass removal become very low. Thus, in aquifers where natural attenuation is an active process, a relatively thin, and thus less costly, PRB could be used to remove much of the mass, relying on downgradient natural attenuation as a polishing process to meet MCLs.
Iron-Clay Combinations for Source-Zone Treatment—PRBs were developed for plume control; however, granular iron has been applied using various emplacement methods for source-zone treatment (ITRC, 2005). In particular, a granular iron/clay slurry can be mixed into a dense nonaqueous phase liquid (DNAPL) source zone using mixing augers or by some other suitable means. The clay acts as a lubricant during injection and further reduces the permeability in the source zone, reducing the potential for discharge of dissolved contaminants. Following mixing, the DNAPL will be distributed as small droplets. As the iron degrades contaminants in the dissolved phase, the droplets will continue to dissolve, eventually consuming the DNAPLs. Because of the mixing process, this technology should be relatively immune to the effects of geologic heterogeneity, a significant limitation to several of the other proposed methods for source zone treatment. The viability of this concept has been demonstrated in numerous laboratory studies and in field applications (Wadley et al., 2005; Shackleford et al., 2005; Olson, 2005).
Bimetals—There is considerable evidence (Sweeney and Fischer, 1972, Odziemkowski et al., 1998) that plating a very small amount of a more noble metal, such as palladium or nickel, onto the surface of iron can increase the degradation rates of many chlorinated compounds by a factor of ten or more. Use of such plating could reduce the amount of reactive material required in a PRB by a factor of ten, with possible cost reductions and a greater range of options for installation, particularly at greater depths. Unfortunately, the noble metal acts as a catalyst and as in most catalyzed reactions, the catalyst becomes poisoned over a period of time (Lin et al., 2004; Odziemkowski et al., 1998), requiring reactivation or replacement at intervals of a few weeks to a few months. Thus, there appears to be little potential for using these materials in PRBs, though if cost-effective methods for reactivation can be developed, they may have application in aboveground treatment systems.
Nano-iron—Interest in iron having particle sizes in the nanometer (nm) range (generally 10–100 nm) was stimulated by the early work of Wang and Zhang (1997). Because of the small particle size and correspondingly high specific surface area, nano-iron is substantially more reactive than the iron materials normally used in PRB applications; the reactivity can be increased even further by plating a more noble metal onto the iron. In addition to the higher reactivity, it has been proposed that because of the small particle size, nano-iron could be injected and transported well beyond the point of injection by natural groundwater flow. Because of the high reactivity, even with water, it is unlikely that nanoparticles will persist in the subsurface for periods of greater than a few weeks to months (Liu and Lowry, 2006), and because of their high cost, repeated injections may not be practical. In addition, no record was found of tests where the nanoparticles have been shown to migrate significant distances from the injection point (e.g., Tratnyek and Johnson, 2006; Liu and Lowry, 2006). Based on the current state of the technology, the use of nano-iron is probably most appropriate in situations where high rates of mass removal are required over short periods of time, as may be the case in source zone treatment.
References
Agrawal A, Ferguson WJ, Gardner BO, Christ JA, Bandstra JZ, Tratnyek PG. 2002. Effects of carbonate species on the kinetics of dechlorination of 1,1,1–trichloroethane by zero-valent iron. Environ Sci Technol 36:4326–4333.
Anderson G. 2001. Dual Reactive Barrier Walls for the Remediation of CHC Contamination, Watervliet Arsenal, NY. Presented at the Remediation Technology Development Forum (RTDF) In-Situ Permeable Reactive Barriers Applications and Deployment Short Course, New York, NY, USA, May.
Arnold WA, Roberts AL. 2000. Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environ Sci Technol 34:1794–1805.
Astrup T, Stipp SLS, Christensen TH. 2000. Immobilization of chromate from coal fly ash leachate using an attenuating barrier containing zero-valent iron. Environ Sci Technol 34:4163–4168.
Blowes DW, Ptacek CJ, Jambor JL. 1997. In-situ remediation of Cr(VI)-contaminated groundwater using permeable reactive walls: Laboratory studies. Environ Sci Technol 31:3348–3357.
Brunauer S, Emmett PH, Teller E. 1938. Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319.
Christians GL, Ash RE, Wilson DJ. 2006. PRB Wall Design Optimization Monte Carlo Probabilistic Approach Saves $1,500,000. Proceedings, Fifth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 22–25, Paper C-07.
D’Andrea PD, Lai KCK, Kjeldsen P, Lo IMC. 2005. Effect of groundwater inorganics on the reductive dechlorination of TCE by zero-valent iron. Water Air Soil Pollut 162:401–420.
Deng B, Hu S, Burris DR. 1998. Effect of Iron Corrosion Inhibitors Trichloroethylene Reductions. Proceedings, First International Conference Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, May 18–21. Battelle Press, Columbus, OH, USA, C1–5:341–346.
Deng B, Burris DR, Campbell TJ. 1999. Reduction of vinyl chloride in metallic iron-water systems. Environ Sci Technol 33:2651–2656.
Devlin JF, Allin KO. 2005. Major anion effects on the kinetics and reactivity of granular iron in glass-encased magnet batch reactor experiments. Environ Sci Technol 39:1868–1874.
Dick V, Edwards D. 1997. Bedrock Refractive-Flow Cells: A Passive Treatment Analog to Funnel-and-Gate. Proceedings, International Containment Technology Conference and Exhibition, St. Petersburg, FL, USA, February 9–12, pp 459–466.
Duchene M, Jacobson R. 2002. Use of Granular Iron for Remediation of a Drinking Water Aquifer Contaminated with TCE. Proceedings, 75th Annual Water Environment Federation (AWEF) Annual Meeting, Chicago, IL, USA, September 28–October 2, pp 82–91.
Elmore C, Summers D, Vogan J, Duchene M. 2003. A Novel Waterjetting Method for PRB Emplacement in Bedrock Environments. Poster Presentation, RTDF Permeable Reactive Barriers (PRBs) Action Team Meeting, Niagara Falls, NY, USA, October 15–16.
EnviroMetal Technologies, Inc. 1998. Bench Scale Treatability Study of the EnviroMetal Process for In-Situ Treatment of Groundwater at the Siberia Area, Watervliet Arsenal, Watervliet, NY, USA. Study Performed for the U.S. Army Corps of Engineers, Baltimore District, Baltimore, MD, USA. March.
EnviroMetal Technologies Inc. 2000. Bench Scale Treatability Studies in Support of a Permeable Reactive Barrier Design for the Microwave Development Laboratories Inc. Site, Needham, Massachusetts. December.
Fiorenza S, Oubre CL, Ward CH, eds. 2000. Sequenced Reactive Barriers for Groundwater Remediation, Lewis Publishers, Boca Raton, FL, USA. 730 p.
Gaule C, Land D, Goldstein K. 2000. Reactive Barrier Wall for the Remediation of Chlorinated Hydrocarbon Contamination, Design and Installation of an Innovative Technology, Watervliet Arsenal, NY. Presented at the U.S. Army Corps of Engineers (USACE) Defense Environmental Restoration Program Conference.
Gaule C, Goldstein K, Heckelman C. 2001. Dual Reactive Walls for the Remediation of CHC Contamination, Watervliet Arsenal, New York: Design and Installation of an Innovative Technology. International Containment and Remediation Technology Conference, Boca Raton, FL, USA, June 10–13, Paper 156.
Gaveskar AR, Gupta N, Sass BM, Janosy RJ, O’Sullivan D. 1998. Permeable Barriers for Groundwater Remediation: Design, Construction, and Monitoring. Battelle Press, Columbus, OH, USA. 176 p.
Gillham RW, Burris DR. 1992. In Situ Treatment Walls: Chemical Dehalogenation, Denitrification, and Bioaugmentation. Proceedings, Subsurface Restoration Conference Dallas, TX, USA, June 21–24, pp 66–68.
Gillham RW, O’Hannesin SF. 1992. Metal-Catalysed Abiotic Degradation of Halogenated Organic Compounds. Proceedings, International Association of Hydrogeologists (IAH) Conference: Modern Trends in Hydrogeology, Hamilton, Ontario, Canada, May 10–13, pp 94–103.
Gillham RW, O’Hannesin SF. 1994. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 32:958–967.
Goldstein KR, O’Hannesin SF, McDonald S, Gaule C, Anderson GA, Marsh R. 2000. Dual Permeable Reactive Barrier Walls Remediate Chlorinated Hydrocarbon Contamination. Proceedings, Second International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, May 22–25. Battelle Press, Columbus, OH, USA, Vol C-2, pp 273–279.
Gonullu T, Farquhar GJ. 1989. Oxidation to remove TCE from soil. Internal report. Department of Civil Engineering, University of Waterloo. www.civil.uwaterloo.ca/groundwater/oxlitrev.html. Accessed July 6, 2009.
Gu B, Phelps TJ, Liang L, Dickey MJ, Roh Y, Kinsall BL, Palumbo AV, Jacobs GK. 1999. Biogeochemical dynamics in zero-valent columns: Implications for permeable reactive barriers. Environ Sci Technol 33:2170–2177.
Gu B, Liang L, Zhou J, Phillips D. 2001. In-Situ Microbial Investigation of a Fe(0) Reactive Barrier. Proceedings, American Chemical Society National Meeting San Diego, CA, USA, April 1–5, 41:1173–1180.
Hart S, May M, Kleinschmidt J. 2004. Denver Federal Center IM#1: Hydraulic Fix of Gate 2. Abstract, Battelle Fourth International Conference on the Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 24–27.
Hocking G, Wells S, Ospina R. 2001. Probabilistic Design of Permeable Reactive Barriers. Presented at the 2001 International Containment and Remediation Technology Conference, Boca Raton, FL, USA, June 10–13, Paper 223.
Hocking G, Wells SL, Givens CN. 2004. A deep iron permeable reactive barrier constructed below 100-foot depth. Proceedings, Fourth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 24–27, 3A.09/1–3A.09/7.
Huber D, Manganaro D, Hurley P, Commerford J, Richards P. 2001. The use of the Biostat Troysan 142 in a Permeable Reactive Barrier and its potential effects on public health and the environment. Proceedings, Contaminated Soils, Sediments and Water Conference, Amherst, MA, USA, October 22–25.
ITRC (Interstate Technology & Regulatory Council). 2005. Federal Remediation Technologies Roundtable Meeting, Arlington, VA, USA, May 25.
Jeen S-W, Gillham RW, Blowes D. 2006. Effects of carbonate precipitates on long-term performance of granular iron for reductive dechlorination of TCE. Environ Sci Technol 40:6432–6437.
Jeen S-W, Mayer KU, Gillham RW, Blowes DW. 2007. Reactive transport modeling of trichloroethene treatment with declining reactivity of iron. Environ Sci Technol 41:1432–1438.
Johnson TL, Scherer MM, Tratnyek PG. 1996. Kinetics of halogenated organic compound degradation by iron metal. Environ Sci Technol 30:2634–2640.
Klausen J, Vikesland PJ, Kohn T, Burris DR, Ball WP, Roberts AL. 2003. Longevity of granular iron in groundwater treatment processes: Solution composition effects on reduction of organohalides and nitroaromatic compounds. Environ Sci Technol 37:1208–1218.
Li L, Benson CH, Lawson EM. 2005. Impact of mineral fouling on hydraulic behavior of permeable reactive barriers. Ground Water 43:582–596.
Li T, Farrell J. 2000. Reductive dechlorination of trichloroethene and carbon tetrachloride using iron and palladized-iron cathodes. Environ Sci Technol 34:173–179.
Lin CJ, Lo SL, Liou YH. 2004. Dechlorination of trichloroethylene in aqueous solution by noble metal-modified iron. J Hazard Mater B116:219–228.
Liu Y, Lowry GV. 2006. Effect of particle age (Fe0 content) and solution pH on NZVI reactivity: H2 evolution and TCE dechlorination. Environ Sci Technol 40:6085–6090.
Lu Q. 2005. Effect of Oxidant (Nitrate) on TCE Degradation by Granular Iron. MSc Thesis, Department of Earth Sciences, University of Waterloo, Ontario, Canada.
Mackenzie PD, Horny DP, Sivavec TM. 1999. Mineral precipitation and porosity losses in granular iron columns. J Hazard Mater 68:1–17.
Matheson LJ, Tratnyek PG. 1994. Processes affecting remediation of contaminated groundwater by dehalogenation with iron. Environ Sci Technol 28:2045–2052.
Mayer KU, Blowes DW, Frind EO. 2001. Reactive transport modeling of an in situ reactive barrier for the treatment of hexavalent chromium and trichloroethylene in groundwater. Water Resour Res 37:3091–3103.
McCall J, Swanson G, Cheng HC, Brooks P, Chan DB, Chen S, Menack J. 2004. Demonstration of ZVI Injection for In-Situ Remediation of Chlorinated Solvents at Hunter Shipyard. Proceedings, Fourth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA. Battelle Press, Columbus OH, USA, Paper 2E-09.
McCormick ML, Adriaens P. 2004. Carbon tetrachloride transformation on the surface of nanoscale biogenic magnetite particles. Environ Sci Technol 38:1045–1053.
McMahon PB, Dennehy KF, Sanstrom MW. 1999. Hydraulic and geochemical performance of a permeable reactive barrier containing zero-valent iron: Denver Federal Center. Ground Water 37:396–404.
Miehr R, Tratnyek PG, Bandstra JZ, Scherer MM, Alowitz M, Bylaska EJ. 2004. The diversity of contaminant reduction reactions by zero-valent iron: Role of the reductate. Environ Sci Technol 38:139–147.
Morrison SJ, Metzler DR, Carpenter CE. 2001. Uranium precipitation in a permeable reactive barrier by progressive irreversible dissolution of zero valent iron. Environ Sci Technol 35:385–390.
Odziemkowski MS, Gillham RW, Focht R. 1998. Electroless hydrogenation of trichloroethylene by FE-Ni(P) galvanic couples. Electrochem Soc Proc 90:91–103.
O’Hannesin SF. 1993. A field demonstration of a permeable reaction wall for the in situ abiotic degradation of halogenated aliphatic organic compounds. Unpublished MSc Thesis, Department of Earth Sciences, University of Waterloo, Ontario, Canada.
O’Hannesin SF, Gillham RW. 1998. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 36:164–170.
O(Hannesin SF, Przepiora A, Gillham RW. 2004. Effect of Temperature and Iron Content on Iron PRB Design. Proceedings, Fourth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 24–27, 3A.03/1-3A.03/5.
Okwi GJ, Thomson NR, Gillham RW. 2005. The impact of permanganate on the ability of granular iron to degrade trichloroethylene. Ground Water Monit Remediat 25:123–128.
Olson M. 2005. In-Situ Remediation via Admixing Zero-Valent Iron and Clay: Performance Prediction. MSc Thesis, Department of Civil Engineering, Colorado State University, Fort Collins, CO, USA.
Orth S, Gillham RW. 1996. Dechlorination of trichloroethene in aqueous solution using Fe. Environ Sci Technol 30:66–71.
Phifer MA, Sappington FC, Nichols RL, Ellis N, Cardoso-Neto JE. 1999. GeoSiphon/ Geoflow Treatment Systems. Waste Management 99 Symposia, Tuscon, AZ, USA, February 28–March 4, Session 29, Paper 2.
Phillips DH, Gu B, Watson DB, Roh Y, Liang L, Lee SY. 2000. Performance evaluation of a zerovalent iron reactive barrier: mineralogical characteristics. Environ Sci Technol 34:4169–4176.
Phillips DH, Watson DB, Roh Y, Gu B. 2003. Mineralogical characteristics and transformations during long-term operation of a zero valent iron reactive barrier. J Environ Qual 32:2033–2045.
Pratt AR, Blowes DW, Ptacek CJ. 1997. Products of chromate reduction on proposed subsurface remediation material. Environ Sci Technol 31:2492–2498.
Primrose A, Butler L, Wiemelt K, Castaneda N, Gavaskar AR, Chen ASC. 2004. Successful Field Implementation of Permeable Reactive Barriers at Rocky Flats. Proceedings, Fourth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 24–27, Paper 3A-28.
Reardon EJ. 1995 Anaerobic corrosion of granular iron: Measurement and interpretation of hydrogen evolution rates. Environ Sci Technol 29:2936–2945.
Reardon EJ. 2005. Zerovalent irons: Styles of corrosion and inorganic control on hydrogen pressure buildup. Environ Sci Technol 39:7311–7317.
Reynolds GW, Hoff JT, Gillham RW. 1990. Sampling bias caused by materials used to monitor halocarbons in groundwater. Environ Sci Technol 24:135–142.
Richards P. 2002. Construction of a permeable reactive barrier in a residential neighbourhood. Remediat J 12:65–79.
Ritter K, Odziemkowski MS, Gillham RW. 2002. An in-situ study of the role of surface films on granular iron in the permeable iron wall technology. J Contam Hydrol 55:87–111.
Ritter K, Odziemkowski MS, Simpraga R, Gillham RW, Irish DE. 2003. An in situ study of the effect of nitrate on the reduction of trichloroethylene by granular iron. J Contam Hydrol 6:121–136.
Roh Y, Lee SY, Elless MP. 2000. Characterization of corrosion products in the permeable reactive barriers. Environ Geol 40:184–194.
Scherer MM, Balko BA, Gallagher DA, Tratnyek PG. 1998. Correlation analysis of rate constants for dechlorination by zero-valent iron. Environ Sci Technol 32:3026–3033.
Scherer MM, Richter S, Valentine RL, Alvarez PJJ. 2000. Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Crit Rev Environ Sci Technol 30:363–411.
Schumaker TT. 1995. Identification of Precipitates Formed on Zero-Valent Iron in Anaerobic Aqueous Solutions. MSc Thesis, Department of Earth Sciences, University of Waterloo, Waterloo, Ontario, Canada. 148 p.
Sfeir HA, Randall A, Reinhart D, Chopra M, Clausen C. 2000. Biotic Attenuation and Zero-Valent Iron Permeable Barrier Technology. Proceedings, Second International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, May 22–25. Battelle Press, Columbus, OH, USA, C2–6:323–329.
Shackleford CD, Sale TC, Liberati MR. 2005. In-Situ Remediation of Chlorinated Solvents using Zero-Valent Iron and Kaolin Mixtures: A Case History. Geo-Frontiers 2005, American Society of Civil Engineers (ASCE), Reston, VA, USA.
Siegrist RL, Urynowicz MA, West OR, Crimi ML, Lowe KS. 2001. Principles and Practices of In Situ Chemical Oxidation Using Permanganate. Battelle Press, Columbus, OH, USA. 336 p.
Starr RC, Cherry JA. 1994. In situ remediation of contaminated groundwater: The funnel-and-gate system. Ground Water 32:465–76.
Stening J, Manefield M, Zemb O, Przepiora A, Vogan JL. 2008. Reductive Dechlorination of 1,2–Dichloroethane in a Reactive Iron Barrier. Proceedings, Sixth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Montery, CA, May 19–22. Batttelle Press, Columbus, OH, USA, Paper A-039.
Su C, Puls RW. 1998. Temperature effect on reductive dechlorination of trichloroethene by zero-valent metals. In Physical, Chemical, and Thermal Technologies: Remediation of Chlorinated and Recalcitrant Compounds: Proceedings, First International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 18–21, pp 317–322.
Su C, Puls RW. 1999. Kinetics of trichloroethene reduction by zero valent iron and tin: Pretreatment effect, apparent activation energy, and intermediate products. Environ Sci Technol 33:163–168
Sweeney KH, Fischer JR. 1972. Reductive Degradation of Halogenated Pesticides. U.S. Patent No. 3,640,821.
Thomson NR, Hood ED, MacKinnon LK. 2000. Source zone mass removal using permanganate: Expectations and potential limitations. Proceedings, Second International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, May 22–25. Battelle Press, Columbus, OH, USA, Volume C2–6, pp 9–16.
Tratnyek PG, Johnson RL. 2006. Nanotechnologies for environmental clean-up. Nanotoday 1:44–48.
Tratnyek PG, Scherer MM, Johnson TL, Matheson LJ. 2003. Permeable reactive barriers of iron and other zero-valent metals. Environmental Science and Pollution Control Series, 26 (Chemical Degradation Methods for Wastes and Pollutants):371–421.
USEPA (U.S. Environmental Protection Agency). 2001. Permeable Reactive Barrier Summaries Watervliet Arsenal, Watervliet, NY, USA. http://www.rtdf.org/public/permbarr/prbsumms/profile.cfm?mid=79. Accessed July 2, 2009.
Vidumsky JE. 2003. Impacts of a Zero-Valent Iron Permeable Reactive Barrier on Dowgradient Biodegradation Processes. Summary of the RTDF Permeable Reactive Barrier Action Team Meeting, October 2003, Niagara Falls, NY, USA. www.rtdf.org/public/permbarr/minutes/101603/index.htm. Accessed July 2, 2009.
Vidumsky JE, Landis RC. 2001. Probabilistic Design of a Combined Permeable Barrier and Natural Biodegradation Remedy. Proceedings, Sixth International In Situ and On-Site Bioremediation Symposium, San Diego, CA, USA, June 4–7, 8:185–191.
Vogan JL, Butler BJ, Odziemkowski MS, Friday G, Gillham RW. 1998. Inorganic and Biological Evaluation of Cores from Permeable Iron Reactive Barriers. Proceedings, First International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, May 18–21. Battelle Press, Columbus, OH, USA, Paper C1–6.
Vogan JL, Focht RM, Clark DK, Graham SL. 1999. Performance evaluation of a permeable reactive barrier for remediation of dissolved chlorinated solvents in groundwater. J Hazard Mater 68:97–108.
Wadley S, Gillham RW, Gui L, 2005. Remediation of DNAPL source zones using granular iron: Laboratory tests and field demonstration. Ground Water 43:9–18.
Wang C-B, Zhang W-X. 1997. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156.
Warner SD, Longino BL, Zhang M, Bennett P, Szerdy FS, Hamilton LA. 2005. The first commercial permeable reactive barrier composed of granular iron: Hydraulic and chemical performance at 10 years of operation. International Association of Hydrological Sciences (IAHS) Publication 298:32–42.
Weathers L, Parkin G, Alvarez PJJ. 1997. Utilization of cathodic hydrogen as election donor for chloroform metabolism by a mixed methanogenic culture. Environ Sci Technol 33:880–885.
Wildman MJ, Alvarez PJJ. 2001. RDX degradation using an integrated Fe(0)-microbial treatment approach. 1st World Water Congress, Part 2: Industrial Wastewater and Environmental Contaminants, 2000, Water Sci Technol 43:25–33.
Wilkin RT, Puls RW. 2003 Elizabeth City PRB Monitoring Results. 3.1.1 Contaminant Behavior. Capstone Report on the Application, Monitoring, and Performance of Permeable Reactive Barriers for Ground-Water Remediation: Volume–Performance Evaluations at Two Sites. EPA/600/R-03/045a. National Risk Management Research Laboratory, Office of Research and Development, USEPA, Cincinnati, OH, USA, August 9–13.
Wood S, Heckelman C. 2002. Performance of Dual Reactive Walls Monitored at Watervliet Arsenal, NY. EPA 542-N-02-002. USEPA Groundwater Currents 43, April.
Yabusaki S, Cantrell K, Sass B, Steefel C. 2001. Multicomponent reactive transport in an in situ zero-valent iron cell. Environ Sci Technol 35:1493–1503.
Yang Y. 2006. Reduction of TCE and Chromate by Granular Iron in the Presence of Dissolved CaCO3. MS Thesis, Department of Earth Sciences, University of Waterloo, Waterloo, Ontario, Canada.
Zhang Y, Gilham RW. 2005. Effects of gas generation and precipitates on performance of Fe° PRBs. Ground Water 43:113–121.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Gillham, R.W., Vogan, J., Gui, L., Duchene, M., Son, J. (2010). Iron Barrier Walls for Chlorinated Solvent Remediation. In: Stroo, H., Ward, C. (eds) In Situ Remediation of Chlorinated Solvent Plumes. SERDP/ESTCP Environmental Remediation Technology. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-1401-9_16
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1401-9_16
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-1400-2
Online ISBN: 978-1-4419-1401-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)