Abstract
Probiotics are susceptible to factors such as stomach acid, enzymes, and bile salts. Also, when incorporated into food matrices, intrinsic or processing factors like low pH, high water activity, or high cooking temperatures can negatively affect the viability of microorganisms. Encapsulation technology can ensure the safe delivery of probiotics to the gut and better survival during processing and storage. Several techniques are used to protect probiotics, for example, emulsion, extrusion, spray-drying, freeze-drying, liposome, electrospinning, and others. Here, we describe in detail the main methods of encapsulation of probiotics, including emulsion, extrusion, and spray-drying techniques.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key words
1 Introduction
The consumption of probiotic products has increased exponentially due to the range of benefits these microorganisms can offer to human health. However, it is still a challenge to ensure the viability of probiotics to the consumer, as they have a noticeable loss of viability after passing through the digestive tract. In addition, when incorporated into commercial products, intrinsic or processing factors such as low pH, high water activity, or high cooking temperatures can negatively affect the viability of microorganisms [1].
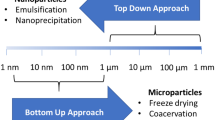
Microencapsulation emerges as an alternative to circumvent these limitations. This technique is based on trapping probiotics within an encapsulating matrix, ensuring safe delivery to the intestine at appropriate therapeutic levels to provide human health benefits [2]. Several microencapsulation techniques can be used to encapsulate probiotics (Table 1). However, emulsion, extrusion, and spray-drying techniques occupy a prominent place, considering their low cost, simplicity of handling, and the possibility of producing large-scale microcapsules. Thus, throughout this chapter, we will address only these most used techniques.
The encapsulating matrix can be formed using different wall materials, also known as carriers. Sodium alginate has been widely used due to its low cost, biocompatibility, food grade, and targeted delivery of probiotics (soluble in basic medium, for example, in the intestine) [3]. Wall materials such as chitosan, gelatin, milk proteins, pectin, carrageenan, prebiotics, and different types of starch have also occupied a prominent place for the microencapsulation of probiotic strains. The criteria for choosing a suitable encapsulating agent are mainly based on its physicochemical properties (molecular mass, solubility, glass transition temperatures, crystallinity, film formation, and emulsifying properties). A good wall material must also be easy to handle during the encapsulation process. In addition, it cannot react or injure the probiotic strain during the encapsulation and storage process and, finally, it must meet the solubility properties of the microcapsule by releasing the probiotics at the site of action [1]. To describe the methodology of this chapter, we will consider alginate (ALG) and whey proteins (WPI) as encapsulating agents and the strain of Lacticaseibacillus rhamnosus GG as active material. Alginate was chosen because it is necessary to use a hydrocolloid for the crosslinking process in the emulsion and extrusion methods. In addition, it is considered GRAS (Generally Recognized as Safe) and low cost. However, other wall materials have been widely used [4,5,6].
2 Material
For the production of microcapsules, the following materials are needed:
-
Freeze-dried probiotic cells;
-
De Man Rogosa and Sharpe (MRS) broth;
-
Glycerol.
-
Bacteriological oven;
-
Centrifuge;
-
Saline solution;
-
Soybean oil;
-
Alginate (ALG);
-
Whey proteins (WPI);
-
Calcium carbonate;
-
Acid organic;
-
Span 80;
-
Calcium chloride;
-
Spray-drier.
3 Methods
3.1 Preparation of Probiotic Suspension
To obtain the stock solution, freeze-dried probiotic cells can be rehydrated in sterile skim milk (25 g L−1) or with De Man Rogosa and Sharpe (MRS) broth added with glycerol (20 g L−1) and stored in sterile Falcon vials at −20 ± 2 °C [18] (see Note 1). Then, the stock solution is added to sterile MRS broth and incubated (37 ± 1 °C for 48 h) to reach the stationary phase (see Note 2). After the incubation time, the probiotic cells are harvested by centrifugation (1000 × g) for 10 min at a temperature of 25 ± 1 °C and washed twice with saline solution (0.9 g 100 mL−1). Cell pellets should be kept at 4 ± 1 °C until encapsulation procedure.
3.2 Encapsulation of Probiotics by Emulsion
The emulsion technique consists of mixing two immiscible phases, called the dispersed or discontinuous phase, and the oily or continuous phase [1]. In this method, ALG gelation can be performed internally or externally (Fig. 1). In internal gelation, the alginate is previously solubilized with calcium carbonate, and then an aliquot of organic acid is added to the mixture after emulsification to promote gelation. As the organic acid enters the aqueous phase, it interacts with calcium carbonate, releasing calcium ions and carbonic acid. The calcium ions react with the alginate through complexation with the carboxylic groups of the polymer, forming the “egg box model” structure [19]. On the other hand, in external gelation, the complexation reaction of the carboxylic groups of ALG occurs through contact with a solution of calcium chloride.
-
1.
Preparing the dispersed phase: Mix 5% (w/v) of WPI in 100 mL of sterile distilled water under stirring at 400 rpm. Then, gently add 1% (w/v) of ALG (see Note 3), and leave under stirring until the alginate is entirely homogenized.
-
2.
Addition of cell biomass: Aseptically, an aliquot (~ 9 log CFU mL−1) of the probiotic biomass should be added to the dispersed phase and then homogenized at 400 rpm for 5 min. It is recommended to add a biomass content that reaches a viable cell count of around 9 to 10 log CFU g−1 (see Note 4).
-
3.
Preparing the continuous phase: Add 300 mL of soybean oil to a beaker. Add 3% (v/v) of an emulsifying agent (Span 80) in the same container and leave it under stirring at 400 rpm until complete homogenization (see Note 5).
-
4.
Mixing the two phases: In a beaker, mix the dispersed and continuous phases and leave under stirring at 400 rpm for 20 min or until the complete formation of the emulsion (see Note 6).
-
5.
ALG cross-linking process: While stirring, add an aliquot of a 1.5% (w/v) calcium chloride solution to form the gelled microcapsules (see Note 7). Then, turn off the agitation and add 200 mL of sterile distilled water to attract the microcapsules to the aqueous phase.
-
6.
Collecting the microcapsules: Discard the emulsion supernatant, collect the microcapsules by vacuum filtration, and keep them at 4 °C until drying.
-
7.
Drying of microcapsules: Gelled microcapsules can be dried in a spray-dryer, freeze-dryer, or fluidized bed dryer (see Note 8). After drying, the microcapsules can be packed in airtight packaging and kept at room temperature until use (see Note 9).
3.3 Encapsulation of Probiotics by Extrusion
The extrusion technique (Fig. 1) involves mixing the cellular biomass of the probiotic with the polymeric solution (ALG and WPI) and then forming droplets by passing the solution through a nozzle or atomizing nozzle [20].
-
1.
Preparing the dispersed phase: Mix 5% (w/v) f WPI in 100 mL of sterile distilled water under stirring at 400 rpm. Then, gently add 1% (w/v) of ALG (see Note 3), and leave under stirring until the alginate is entirely homogenized.
-
2.
Addition of cell biomass: Aseptically, an aliquot (~ 9 log CFU mL−1) of the probiotic biomass should be added to the dispersed phase and then homogenized at 400 rpm for 5 min. It is recommended to add an aliquot with a viable cell count of around 8 to 9 log CFU g−1 (see Note 4). It is worth emphasizing that the dispersed phase containing the hydrocolloid must be prepared just before use.
-
3.
Forming the gelled microcapsules: Once the feed solution (FS) (polymer solution + probiotic) is prepared, the FS is dripped into a 1.5% (w/v) calcium chloride (see Note 7) gelling solution under stirring at 200 rpm. The dripping of the FS into the gelling solution is carried out using an atomizing nozzle. In this case, the FS is pumped by a peristaltic pump, and the droplets are quickly transformed into solid particles through the complexation of ALG with calcium ions. Another simplified form can be used, for example, using a syringe to perform the drip (see Note 10). After the dripping step, it is interesting to leave the microcapsules to rest (~20 to 30 min) in the CaCl2 solution to solidify the microcapsules completely. The formation of large particles and the low production rate are the main disadvantages of this technique for use in the food industry. However, to overcome this, the extrusion process can be combined with ultrasound, jet cutting, electrostatic field, and rotating disk (Fig. 1).
-
4.
Collecting the gelled microcapsules: Collect the microcapsules by vacuum filtration and keep them at 4 °C until drying.
-
5.
Drying of microcapsules: Gelled microcapsules can be dried in a spray-dryer, freeze-dryer, or fluidized bed dryer (see Note 8). After drying, the microcapsules can be packed in airtight packaging and kept at room temperature until use (see Note 9).
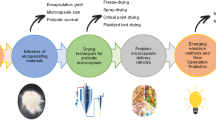
3.4 Encapsulation of Probiotics by Spray-Drying
The spray-drying encapsulation technique (Fig. 2) is well established for large-scale industrial applications and is considered an economically viable technique. In this technique, the suspension containing the wall materials and probiotics is atomized in a drying chamber with concurrent hot air, which instantly removes water from the atomized solution [21]. Microcapsules are removed from the drying chamber by a negative pressure cyclone system and can be collected on the drying chamber bottom or in the collection flask (see Note 11).
-
1.
Preparing the feed solution: Mix 5% (w/v) of WPI in 100 mL of sterile distilled water under stirring at 400 rpm. Then, gently add 1 (w/v) of ALG (see Note 3), and leave under stirring until the alginate is entirely homogenized (see Note 12).
-
2.
Addition of cell biomass: Aseptically, an aliquot of the probiotic biomass should be added to the dispersed phase and then homogenized at 400 rpm for 5 min. It is recommended to add an aliquot with a viable cell count of around 8 to 9 log CFU g−1 (see Note 4).
-
3.
Encapsulation process: Turn on the spray-dryer equipment and operate it with the concurrent flow (see Note 13) with an inlet temperature of 150 °C and an outlet temperature of 50 °C (see Note 14). Program the drying airflow of 35 m3 h−1, and compressor air pressure of 0.7 MPa [23].
-
4.
Then, turn on the peristaltic pump to pump the FS and program supply flow to 20 mL min−1. It was found that slow drying kinetics leads to significant inactivation of the dehydration of Lactiplantibacillus plantarum, while a rapid drying rate could instantly stabilize the cells and thereby prevent this inactivation [24]. In addition, a high drying rate during the first stage of drying, when facilitated by hydraulic membrane permeability, may limit bacterial adaptation because of exposure for too short a time to the gradual withdrawal of moisture. It is recommended that the FS be kept under magnetic stirring at room temperature during the encapsulation process (see Note 15).
-
5.
Before FS entry, sterile distilled water at room temperature must be pumping until stabilization of the inlet temperature.
-
6.
Collecting the dry microcapsules: After complete evaporation of the water, collect the microcapsules from the collector located at the bottom of the equipment, store them in hermetic packages and keep them at room temperature until use (see Note 9).
The analysis of microcapsules is an important step in the microencapsulation process. Microcapsules must be characterized before use to observe their physical, chemical, and biological properties. Table 2 shows the characteristics of the probiotic microcapsules obtained by the emulsion, extrusion, and spray-drying techniques and the main characterization analyses.
4 Notes
-
1.
You can use other cryoprotectants. MRS for LAB only, if strains from other species (E. coli, Bacillus, Saccharomyces), other broths should be used.
-
2.
Cells in the stationary phase are more resistant and have a higher encapsulation yield than cells in the log phase [33].
-
3.
Alginate should be added gently to not form lumps. You can place it on a foil film and spray it on the solution. Another way to avoid the formation of lumps is to homogenize them in warm water (40–50 °C).
-
4.
Adding an aliquot of L. rhamnosus GG with a low viable cell count may compromise delivery to the gut at levels suitable for promoting human health.
-
5.
Any oil can be used. The emulsifying agent is chosen according to the lipophilic hydrophilic balance (LHB); generally, the most used are Tween 80 and Span 80.
-
6.
Using slow agitation rates (400–500 rpm) is recommended. High agitation rates can damage the probiotics‘cell wall.
-
7.
Other types of salt can be used for ALG crosslinking, such as calcium citrate. However, it is desirable to use low concentrations. High salt concentrations have a detergent effect, which dissolves bacterial membranes and even causes cell death.
-
8.
Another drying method can be used. However, those are more commonly used. The drying of microcapsules is important both from a microbiological and technological point of view, as it increases the lifespan of microorganisms. In addition, drying the microcapsules makes it possible to incorporate probiotics into low-moisture food matrices.
-
9.
These microcapsules can be used in the products described in other chapters of this edition to improve survivability in processing, storage, and TGI.
-
10.
The formation of large particles and the slow production rate are the main disadvantages of this technique for use in the food industry.
-
11.
For probiotic microcapsules, the ideal in bench spray-dryers or pilots is to collect only the product from the collector due to the greater control of the exit temperature of the process.
-
12.
During the process, encapsulated microorganisms can undergo several stresses, including heat stress and dehydration. Encapsulating agents such as gelatin, gum arabic, and cellulose acetate phthalate has been reported as protective agents capable of forming a physical barrier resistant to hot air [21]. In addition, disaccharides are encouraged as they can preserve the structure of probiotic cell proteins and membranes through a connection at sites that previously interacted with water [34].
-
13.
Spray flow can be applied in three ways (concurrent, countercurrent, or mixed flow). However, the choice of spray flow will depend on the direction in which air and liquid (e.g., feed solution) enter the drying chamber. In the first case (concurrent), the product is in contact with the colder air, preferable for drying thermosensitive materials, such as probiotics.
-
14.
The lower the Toutlet, the higher the post-drying viability. Toutlet is therefore considered to be the principal drying parameter that affects the viability of spray-dried LAB, and any lack of monitoring and control of the latter may be markedly detrimental [21].
-
15.
Agitation prevents materials in solution from settling.
References
Camelo-Silva C, Verruck S, Ambrosi A, Di Luccio M (2022) Innovation and trends in probiotic microencapsulation by emulsification techniques. Food Eng Rev 14:462–490. https://doi.org/10.1007/s12393-022-09315-1
Hill C, Guarner F, Reid G et al (2014) Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Marcial-Coba MS, Knøchel S, Nielsen DS (2019) Low-moisture food matrices as probiotic carriers. FEMS Microbiol Lett 366:1–11. https://doi.org/10.1093/femsle/fnz006
Niño-Vásquez IA, Muñiz-Márquez D, Ascacio-Valdés JA et al (2022) Co-microencapsulation: a promising multi-approach technique for enhancement of functional properties. Bioengineered 13:5168–5189. https://doi.org/10.1080/21655979.2022.2037363
de la Cruz P-CA, Ortega D, García-Triana A et al (2020) A brief review of edible coating materials for the microencapsulation of probiotics. Coatings 10:1–36. https://doi.org/10.3390/coatings10030197
Corrêa-Filho LC, Moldão-Martins M, Alves VD (2019) Advances in the application of microcapsules as carriers of functional compounds for food products. Appl Sci 9:571. https://doi.org/10.3390/app9030571
Bakhtiyari M, Hamidi-Esfahani Z, Barzegar M (2022) Optimization of co-encapsulation of L. plantarum cells and Silybum marianum seed extract and evaluation of protective effect of extract on cells survival in simulated gastrointestinal fluids. LWT 165:113733. https://doi.org/10.1016/J.LWT.2022.113733
Rodrigues FJ, Cedran MF, Pereira GA et al (2022) Effective encapsulation of reuterin-producing Limosilactobacillus reuteri in alginate beads prepared with different mucilages/gums. Biotechnol Reports 34:e00737. https://doi.org/10.1016/J.BTRE.2022.E00737
Verruck S, de Carvalho MW, de Liz GR et al (2017) Survival of Bifidobacterium BB-12 microencapsulated with full-fat goat’s milk and prebiotics when exposed to simulated gastrointestinal conditions and thermal treatments. Small Rumin Res 153:48–56. https://doi.org/10.1016/j.smallrumres.2017.05.008
Meireles Mafaldo Í, de Medeiros VPB, da Costa WKA et al (2022) Survival during long-term storage, membrane integrity, and ultrastructural aspects of lactobacillus acidophilus 05 and Lacticaseibacillus casei 01 freeze-dried with freshwater microalgae biomasses. Food Res Int 159:111620. https://doi.org/10.1016/J.FOODRES.2022.111620
Thantsha MS, Labuschagne PW, Mamvura CI (2014) Supercritical CO 2 interpolymer complex encapsulation improves heat stability of probiotic bifidobacteria. World J Microbiol Biotechnol 30:479–486. https://doi.org/10.1007/s11274-013-1465-3
Hosseini SF, Ansari B, Gharsallaoui A (2022) Polyelectrolytes-stabilized liposomes for efficient encapsulation of lactobacillus rhamnosus and improvement of its survivability under adverse conditions. Food Chem 372:131358. https://doi.org/10.1016/j.foodchem.2021.131358
Xu C, Ma J, Wang W et al (2022) Preparation of pectin-based nanofibers encapsulating lactobacillus rhamnosus 1.0320 by electrospinning. Food Hydrocoll 124:107216. https://doi.org/10.1016/J.FOODHYD.2021.107216
Martinez CJ, Kim JW, Ye C et al (2012) A microfluidic approach to encapsulate living cells in uniform alginate hydrogel microparticles. Macromol Biosci 12:946–951. https://doi.org/10.1002/mabi.201100351
Yao M, Lu Y, Zhang T et al (2021) Improved functionality of Ligilactobacillus salivarius Li01 in alleviating colonic inflammation by layer-by-layer microencapsulation. Biofilmes e Microbiomas 58:1–10. https://doi.org/10.1038/s41522-021-00228-1
Mirzamani SS, Bassiri AR, Tavakolipour H et al (2021) Survival of fluidized bed encapsulated lactobacillus acidophilus under simulated gastro-intestinal conditions and heat treatment during bread baking. J Food Meas Charact 15:5477–5484. https://doi.org/10.1007/s11694-021-01108-0
Kuo CC, Clark S, Qin H, Shi X (2022) Development of a shelf-stable, gel-based delivery system for probiotics by encapsulation, 3D printing, and freeze-drying. LWT 157:113075. https://doi.org/10.1016/J.LWT.2022.113075
Camelo-Silva C, da Barros EL, Verruck S et al (2022) How ice cream manufactured with concentrated milk serves as a protective probiotic carrier? An in vitro gastrointestinal assay. Food Sci Technol 42:e28621. https://doi.org/10.1590/fst.28621
Martín MJ, Lara-Villoslada F, Ruiz MA, Morales ME (2015) Microencapsulation of bacteria: a review of different technologies and their impact on the probiotic effects. Innov Food Sci Emerg Technol 27:15–25. https://doi.org/10.1016/j.ifset.2014.09.010
Sultana M, Chan ES, Pushpamalar J, Choo WS (2022) Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci Technol 123:69–86. https://doi.org/10.1016/J.TIFS.2022.03.006
Huang S, Vignolles ML, Chen XD et al (2017) Spray drying of probiotics and other food-grade bacteria: a review. Trends Food Sci Technol 63:1–17. https://doi.org/10.1016/j.tifs.2017.02.007
de Liz GR, Verruck S, Canella MHM et al (2020) Stability of bifidobacteria entrapped in goat’s whey freeze concentrate and inulin as wall materials and powder properties. Food Res Int 127:108752. https://doi.org/10.1016/j.foodres.2019.108752
Verruck S, Santana F, de Olivera MC, Prudencio ES (2018) Thermal and water sorption properties of Bifidobacterium BB-12 microcapsules obtained from goat’s milk and prebiotics. LWT – Food Sci Technol 98:314–321. https://doi.org/10.1016/J.LWT.2018.08.060
Perdana J, Bereschenko L, Fox MB et al (2013) Dehydration and thermal inactivation of lactobacillus plantarum WCFS1: comparing single droplet drying to spray and freeze drying. Food Res Int 54:1351–1359. https://doi.org/10.1016/j.foodres.2013.09.043
Razavi S, Janfaza S, Tasnim N et al (2021) Microencapsulating polymers for probiotics delivery systems: preparation, characterization, and applications. Food Hydrocoll 120:106882. https://doi.org/10.1016/J.FOODHYD.2021.106882
Arepally D, Goswami TK (2019) Effect of inlet air temperature and gum Arabic concentration on encapsulation of probiotics by spray drying. LWT 99:583–593. https://doi.org/10.1016/j.lwt.2018.10.022
Bhusari SN, Muzaffar K, Kumar P (2014) Effect of carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol 266:354–364. https://doi.org/10.1016/J.POWTEC.2014.06.038
de Paula DA, EMF M, de Costa NA et al (2019) Use of gelatin and gum arabic for microencapsulation of probiotic cells from lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int J Biol Macromol 133:722–731. https://doi.org/10.1016/j.ijbiomac.2019.04.110
Silva MP, Tulini FL, Martins E et al (2018) Comparison of extrusion and co-extrusion encapsulation techniques to protect lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT – Food Sci Technol 89:392–399. https://doi.org/10.1016/j.lwt.2017.11.008
Beldarrain-Iznaga T, Villalobos-Carvajal R, Leiva-Vega J, Sevillano Armesto E (2020) Influence of multilayer mi;croencapsulation on the viability of lactobacillus casei using a combined double emulsion and ionic gelation approach. Food Bioprod Process 124:57–71. https://doi.org/10.1016/j.fbp.2020.08.009
Dantas A, Verruck S, Machado Canella MH et al (2021) Current knowledge about physical properties of innovative probiotic spray-dried powders produced with lactose-free milk and prebiotics. LWT 151:112175. https://doi.org/10.1016/J.LWT.2021.112175
Brodkorb A, Egger L, Alminger M et al (2019) INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc 14:991–1014. https://doi.org/10.1038/s41596-018-0119-1
Jananee J, Preeti S (2017) Molecular basis of stationary phase survival and applications. Front Microbiol 8:2000. https://doi.org/10.3389/fmicb.2017.02000
Perdana J, Fox MB, Siwei C et al (2014) Interactions between formulation and spray drying conditions related to survival of lactobacillus plantarum WCFS1. Food Res Int 56:9–17. https://doi.org/10.1016/j.foodres.2013.12.007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this chapter
Cite this chapter
Camelo-Silva, C., Figueredo, L.L., Zanetti, V.C., Ambrosi, A., Di Luccio, M., Verruck, S. (2023). Microencapsulation of Probiotics. In: Gomes da Cruz, A., Silva, M.C., Colombo Pimentel, T., Esmerino, E.A., Verruck, S. (eds) Probiotic Foods and Beverages . Methods and Protocols in Food Science . Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3187-4_14
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3187-4_14
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3186-7
Online ISBN: 978-1-0716-3187-4
eBook Packages: Springer Protocols