Abstract
Alzheimer’s disease (AD) and other dementia disorders are characterized by a decline in cognitive abilities that interferes with daily functioning. Cognitive symptoms consist of progressive decline in memory, executive function, judgment, language, comprehension, and visuospatial ability, and cognitive profiles differ across dementia disorders (e.g., AD, frontotemporal dementia, vascular dementia, etc.). Dementia disorders are also associated with a range of neuropsychiatric symptoms and physical symptoms. Functionally, dementia disorders negatively impact the ability to maintain employment and engage in social activity, and conversely employment history and social participation have been shown to influence risk for dementia. Additionally, declining ability to complete daily activities such as grooming and meal preparation is a hallmark feature of dementia that is associated with negative outcomes. Methods of assessing everyday functioning include self- and informant-report as well as performance-based measures of everyday tasks that provide more objective assessment facilitated by standardized coding schemes. Everyday action research has revealed relations between cognitive/neuropsychiatric symptoms and functional decline in dementia and has highlighted the relevance of early functional changes to illness progression. Further, heterogeneity across dementia disorders in the extent and type of functional deficit has led to the development of a neurocognitive model that may help clarify the conceptualization and treatment of specific functional deficits. There are few empirical treatments to improve everyday function, but interventions targeting functional deficits and environmental adaptations have shown promise. Finally, racial/ethnic differences in dementia rates underscore the importance of a lifespan approach to examining socioeconomic, educational, and other lifestyle factors that interact with neurobiological components of risk and resilience and impact cognitive test performance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Dementia
- Cognition
- Everyday function
- Activities of daily living (ADL)
- Instrumental activities of daily living (IADL)
- Neuropsychiatry
- Cultural factors
Pearl, a 70-year-old homemaker, is scheduled for her annual physical with her primary care physician. Despite a confirmation call from the physician’s office the day before, Pearl missed the appointment. When the office called to reschedule, she was embarrassed and apologized for the error.
Henry, a 72-year-old truck driver, was on a new delivery route when he missed his highway exit. Having been a truck driver for 40 years, he was surprised when he felt lost and disoriented in a familiar city. When his employer called to inquire about the delivery, Henry complained that he must have received inaccurate directions for the trip.
Introduction
Alzheimer’s disease (AD ) and other dementia disorders are characterized by a decline in multiple cognitive abilities that interfere with work, daily functioning, or usual social activities (McKhann et al., 2011). Dementias may be classified based on their presumed etiology or neuropathology. AD is characterized by the buildup of beta-amyloid plaques and neurofibrillary tangles consisting of the protein tau, initially affecting the hippocampus and entorhinal cortex (Braak & Braak, 1991; see Chap. 1). Different forms of neuropathology characterize other dementia disorders. Dementia with Lewy bodies (DLB) involves cell loss and the presence of Lewy bodies, or abnormal protein aggregates that develop inside nerve cells throughout the limbic system and neocortex (Weintraub, Wicklund, & Salmon, 2012). Frontotemporal dementia ( FTD ) consists primarily of intraneuronal molecular abnormalities throughout frontal and temporal brain regions (Mackenzie et al., 2010). FTDs are also sub-classified according to the patient’s cognitive/behavioral presentation (i.e., progressive nonfluent aphasia, semantic dementia, behavioral variant; Neary et al., 1998). Vascular dementia results from cerebrovascular disease involving multiple or specifically localized infarctions, ischemic events, or hemorrhage (Wetzel & Kramer, 2008). Although additional dementia subtypes have been described, AD is the leading dementia diagnosis in older adults (Weintraub et al., 2012) and features prominently in the scientific literature; therefore, AD will be the primary focus of this review.
AD and other dementias typically arise in older adulthood and follow a deteriorating course (Finkel, Costa e Silva, Cohen, Miller, & Sartorius, 1996). Dementia may begin with the subtle forgetfulness and confusion illustrated in the case examples of Pearl and Henry, described above. In dementia, these symptoms worsen over time and can lead to loss of independence (Krenz, Larson, Buchner, & Canfield, 1988) and severe caregiver burden (Ory, Hoffman, Yee, Tennstedt, & Schulz, 1999). According to the World Alzheimer Report , there are currently 36 million people living with dementia worldwide (World Alzheimer Report, 2009), and this estimate is expected to grow to 42 million by the year 2020 (Ferri et al., 2005). There are numerous important challenges currently facing dementia researchers and clinicians (e.g., early detection, pharmacologic interventions, etc.), but this chapter will focus largely on the impact of dementia on everyday functioning, including employment, social functioning, and activities of daily living. We will begin with a brief overview of the cognitive, neuropsychiatric, and physical symptoms of dementia that are associated with everyday functioning. The chapter closes with a brief discussion of cultural issues that are highly relevant to understanding dementia symptoms and functional outcomes.
Symptomatology
Two years after her initial symptoms, Pearl’s friends tell her that she often repeats herself in conversation. Pearl is embarrassed by her memory difficulties, and she withdraws from social situations. Her physician tells her that she is in good general health, but she refers her to a neurologist and a neuropsychologist for further evaluation. The neuropsychological testing shows significant impairment in learning a word list and remembering figures that were presented during the examination. An MRI of the brain, which was ordered by the neurologist, shows generalized atrophy (i.e., shrinking).
Two years following his initial symptoms, Henry, who had retired from his job, was at a routine cardiology appointment when his wife complained to the cardiologist that Henry appeared more irritable and confused. At a recent family gathering, he made a few inappropriate comments that upset several people at the event. The cardiologist suggested that Henry see a neurologist and neuropsychologist. Cognitive testing showed intact learning of word lists and figures. However, Henry had trouble on tests requiring him to do two things at once or requiring him to alternate his attention from one activity to another. An MRI of his brain showed extensive white matter disease and multiple tiny strokes throughout his brain. Henry and his wife were unaware that he had ever had a stroke.
Cognitive Symptoms
Cognitive symptoms of dementia consist of progressive decline in various domains, including memory, executive function, judgment, language, comprehension, and visuospatial difficulties (Thune-Boyle, Iliffe, Cerga-Pashoja, Lowery, & Warner, 2012; Welsh-Bohmer & Warren, 2006). Episodic memory impairment is variable across dementia subtypes, but it is one of the hallmark features of AD (Geldmacher & Whitehouse, 1997). Episodic memory changes appear early in the disease and are generally attributed to difficulty consolidating or encoding new information (Hart, Kwentus, Taylor, & Harkins, 1987). Older memories, which were encoded prior to the onset of the dementia, are often well preserved. Thus, individuals with episodic memory impairment, such as Pearl in the case example above, may retain memories of events and people met prior to developing dementia, but may be unable to remember those occurring since the onset of illness (i.e., anterograde amnesia). Furthermore, because the episodic memory deficit disrupts new learning, and the memory was never encoded into the brain, the AD patient may receive little benefit from reminder cues in eliciting recall (Looi & Sachdev, 1999). Other forms of dementia spare the hippocampal formation (see Chap. 1); in these disorders information encoding may be preserved, but memory recall may be disrupted due to interference or poor retrieval of previously learned information.
Executive functioning refers to multiple cognitive processes that allow for the goal-directed control of attention, thinking, and behavior. Working memory refers to the ability to mentally maintain and manipulate information and is often associated with executive functioning. Impaired executive functions and working memory are observed following brain damage in many regions but they are typically associated with damage to the prefrontal cortex (PFC) and its related circuitry. They often manifest as disorganized behavior or difficulty planning and organizing one’s activities and can interfere with recall of information stored in memory. These impairments related to executive functions and working memory also often present early in the course of AD , with some suggesting that they follow the initial symptoms of episodic memory decline (Perry & Hodges, 1999) due to the accumulation of neurofibrillary tangles in the PFC (Weintraub et al., 2012). In other dementias, such as FTD -behavioral variant, executive function deficits are the first cognitive symptoms to appear and are the primary feature of the dementia (Neary et al., 1998). This pattern also is observed in individuals with dementia associated with chronic vascular disease, who typically present with deficits in executive functioning and relative sparing of episodic memory encoding (Libon, Price, Garrett, & Giovannetti, 2004). The case example of Henry, described above, illustrates relative sparing of memory but the presence of executive function deficits associated with cerebrovascular disease.
Later symptoms of cognitive dysfunction in AD include language difficulties, with initial expressive deficits resulting in anomia (inability generating the names of words) and empty speech (Bayles, Boone, Tomoeda, Slauson, & Kaszniak, 1989) and receptive difficulties that affect oral comprehension manifesting later in the course of language decline (Welsh-Bohmer & Warren, 2006). Individuals may thus experience the “tip-of-the-tongue” phenomenon and eventually exhibit conversational difficulties due to impairment in both understanding others as well as generating the names of words. Language and spatial deficits may be the initial and core cognitive symptom for some less common forms of dementia, including FTD -progressive nonfluent aphasia or semantic dementia (Neary et al., 1998).
Individuals with AD also experience visuospatial difficulties that result in problems with visual attention, or inability to attend to a portion of the visual field, visual memory and learning, reading, and perception of objects and faces (Benke, 1993; Jackson & Owsley, 2003). Object and face perception deficits may result in an inability to recognize items and people previously highly familiar to the individual. These deficits have been associated with damage to parieto-occiptal brain regions that prevents efficient use of visual information in guiding self-movement and maintaining spatial orientation (Tetewsky & Duffy, 1999). Individuals with these deficits may become more clumsy because of problems reaching to and grasping objects; they also may be more likely to experience difficulty finding their way back to a meeting spot and may become lost even in a familiar location.
The progression of cognitive decline, beginning with episodic memory and leading to executive function and language deficits, is the pattern often observed in people with suspected AD . However, there is great heterogeneity in the affected brain regions and neuropathology across individuals with dementia disorders (Weintraub & Mesulam, 2009). Therefore, it is not surprising that the extent of specific cognitive impairment is highly variable across dementia subtypes (Weintraub & Mesulam, 2009). A comprehensive neuropsychological evaluation is useful for characterizing the cognitive presentation of the disorder and educating patients and caregivers on the specific pattern of cognitive deficits and strengths.
Neuropsychiatric Symptoms
Individuals with dementia disorders also exhibit a range of neuropsychiatric symptoms. These symptoms include anxiety, apathy, depressed mood, hallucinations, and delusions, as well as behavioral manifestations such as aggression, restlessness, sleep disturbance, agitation, wandering, culturally inappropriate behaviors, sexual disinhibition, hoarding, cursing, and shadowing (Desai, Schwartz, & Grossberg, 2012; Finkel et al., 1996; Lyketsos et al., 2000). Although neuropsychiatric symptoms are not included in the diagnostic criteria for dementia, they are important with regards to the developmental trajectory of dementia, as risk of progression from mild cognitive impairment (MCI ) to dementia has been associated with higher levels of neuropsychiatric symptoms. Specifically sleep disturbance, apathy, and anxiety have been shown to predict increased rates of progression to dementia (Somme, Fernandez-Martinez, Molano, & Zarranz, 2013). Further, increased presence of hallucinations, anxiety, and apathy have been found to be associated with greater global functional impairment (Wadsworth et al., 2012), highlighting the impact of these features on dementia severity. Interestingly, the course of specific emotional symptomatology has been shown to vary, with depression and anxiety decreasing and apathy increasing over the course of dementia progression (Wetzels, Zuidema, de Jonghe, Verhey, & Koopmans, 2010). The case example of Pearl described above illustrates how initial distress and anxiety concerning symptoms may lead to subsequent withdrawal and apathy. Others, like Henry, may exhibit diminished insight, increased irritability, and socially inappropriate behaviors. These distinct emotional trajectories have important implications with regards to the developmental course of dementia disorders as well as relevant treatment approaches at different stages of illness.
Another important consideration in assessing the course of dementia disorders with regards to emotional symptoms is whether these mood factors constitute the cause of cognitive and functional decline rather than a byproduct of disease. Depression and other psychiatric symptoms can, themselves, lead to cognitive disorders that share a similar presentation to dementia or can co-occur with dementia and increase the extent of impairment (Welsh-Bohmer & Warren, 2006). Further, depressive symptoms in older age are common, with rates around 20 % for individuals over the age of 65 (Blazer, Hughes, & George, 1987). It is therefore critical to understand and distinguish between emotional and cognitive factors involved in declining abilities, as their progression and response to treatment may be highly influenced by their etiology.
Psychotic symptoms are prevalent but variable across the dementia disorders, with hallucinations most common and observed earliest in the course of DLB (Borroni, Agosti, & Padovani, 2008; Johnson, Watts, Chapin, Anderson, & Burns, 2011), as opposed to the lower prevalence of these symptoms in some FTD subtypes (Rabinovici & Miller, 2010) and their later onset in AD (Lyketsos et al., 2000). When present in dementia disorders, psychotic symptoms tend to consist of relatively simple, non-bizarre delusions such as delusions of stealing, reference, infidelity, grandiosity, and persecutory delusions rather than hallucinations or implausible delusions, and they most commonly arise in the moderate-to-severe stages of the disorder (Bassiony & Lyketsos, 2003; Lyketsos et al., 2000). Conversely, older adults with initial psychotic symptoms have been shown to be at increased risk for progression to dementia (Kohler et al., 2012), further reinforcing the interactive process of underlying features of dementia and accompanying symptoms.
Physical Symptoms
Dementia is also associated with an array of physical symptoms that can pose health risks beyond the cognitive and psychiatric limitations of the disease. Some of these features may result from disorders that confer risk for dementia, including stroke or other cardiovascular risk factors, whereas others develop as dementia progresses, such as difficulty swallowing, which can lead to risk for pneumonia (Phelan, Borson, Grothaus, Balch, & Larson, 2012). Dementia has been shown to significantly predict increased risk for hospitalization, with admission rates for circulatory, geritourinary, infectious, neurological, and respiratory disorders occurring more often in older adults with dementia than those without (Phelan et al., 2012). A major physical feature that has been associated with cognitive decline is mobility impairment (Buchman, Boyle, Leurgans, Barnes, & Bennett, 2011). Although motor, sensory, and coordination deficits typically occur in late-stage AD (McKhann et al., 1984), it has been shown that balance impairments potentially related to early dysfunction of the vestibular and hippocampal systems arise as early as diagnosis of MCI , a precursor to dementia (Leandri et al., 2009).
Given mobility impairments, it is not surprising that a highly prevalent physical correlate of dementia disorders is increased risk of falling (Allan, Ballard, Rowan, & Kenny, 2009; van Doorn et al., 2003). Several risk factors for the incidence of falls include leg weakness, gait and balance impairments, functional impairment, visual impairment, hypotension, cognitive impairment, and medication use (Rubenstein, Josephson, & Robbins, 1994). These are common among the older population in general and may increase fall risk in individuals with and without dementia. However, several physical risk factors specific to falls in dementia have been identified and may aid in the distinction between healthy and pathological aging, including previous history of falls or recurrent falls, use of cardiac medications, autonomic symptoms, orthostatic hypotension, and limited physical activity (Allan et al., 2009), as well as deficits in executive functioning (Mirelman et al., 2012).
Symptomatology and Everyday Functioning
Predictably, this wide array of symptoms often leads to substantial functional impairment. In fact, functional impairment is a core diagnostic criterion of AD and dementia; this criterion is useful for distinguishing AD/dementia from normal cognitive aging and MCI , in which everyday functioning is unimpaired or only minimally disrupted. The extent and presentation of functional impairment in dementia is variable across individuals depending on the course of illness (Gure, Kabeto, Plassman, Piette, & Langa, 2010), functional expectations, and both protective factors and risk factors. Given that dementia is a disorder of older age, there is a lifelong opportunity for gradual development and interaction of these factors. This poses difficulty in distinguishing among factors that confer risk, those that delay illness, and those that emerge as correlates of dementia. However, increased understanding of relations between the complex factors involved in illness progression and functional deficits is important, as the resulting functional impairment can impede performance in domains critical to independent functioning in adulthood (Krenz et al., 1988). In the sections that follow, we will review the literature on everyday functioning in dementia with emphasis on employment, social functioning, and activities of daily living. The literature on functional outcomes in dementia is largest for activities of daily living, and this topic is the focus of our research laboratory; therefore, the review of activities of daily living will be more detailed and extensive.
Employment
The ability to secure and maintain employment is a societal expectation of adults that requires cognitive, physical, and functional competence, all of which may be compromised in dementia. It is therefore important to consider the impact of these symptoms on individuals with dementia as well as MCI , which typically precedes dementia and thus has a higher likelihood of onset prior to the age of retirement. However, relative to the literature on neurologic disorders that affect younger adults, there has been very little research devoted to employment in dementia. Much of the research on employment has instead focused on caregivers and the extent to which caregiving demands reduce workforce participation and financial resources (van Vliet, de Vugt, Bakker, Koopmans, & Verhey, 2010). This focus on caregivers is likely due to the fact that many older adults who develop dementia disorders such as AD may have already retired from their professions or are close to retirement age. In the near future, however, as longevity and age of retirement steadily increase, we suspect that more research will be devoted to understanding employment outcomes in dementia.
Although research on employment in dementia is minimal, findings from previous studies have warranted exploration of the impact of certain features of dementia disorders on the ability to maintain employment. This literature suggests that employment may be influenced by a multitude of factors, including cognitive, behavioral, and psychiatric symptoms of dementia previously described. Cognitive symptoms in other illnesses (e.g., prospective memory impairment in HIV-infected individuals) have been shown to independently predict unemployment (Woods, Weber, Weisz, Twamley, & Grant, 2011). Thus, there is reason to expect that memory impairment and other cognitive symptoms of dementia may confer risk for difficulty maintaining employment in this population as well. Further, studies on employment in FTD have shown socially undesirable behaviors and psychiatric symptoms (e.g., depression, anxiety) to be associated with departure from employment or job loss (Morhardt, 2011; Mychack, Kramer, Boone, & Miller, 2001).
Interestingly, studies have shown that previous high work complexity can serve as a protective or delaying factor in the development and onset of dementia (Andel et al., 2005; Fratiglioni & Wang, 2007; Seidler et al., 2004; Smyth et al., 2004). It has not only been found that complexity of paid work predicted levels of intellectual functioning, but that this effect was greater for older (late 50s to early 80s) than younger (early 40s to early 50s) workers (Schooler, Mulatu, & Oates, 1999). A separate study showed that individuals with a history of consistent unemployment have been found to be at greater risk of developing dementia (Li et al., 1991), suggesting that a failure to be involved in work-related experiences may be detrimental to outcomes in later life. These studies support the notion that employment, particularly complex work activities, may delay or preclude the cognitive decline associated with MCI and dementia. The reasons employment may be protective against dementia are not entirely clear. Some have suggested that employment may increase one’s cognitive reserve (Stern, 2002, 2012). Cognitive reserve is a concept that has been described as a store of cognitive “energy” or “power” that may be developed during one’s lifetime through participation in diverse cognitive activities and challenges, such as education, employment, and leisure activities. Cognitive reserve is thought to be protective against the effects of brain damage or disease. For example, individuals with the same degree of brain damage may differ in their degree of cognitive impairment as a function of their cognitive reserve; the person with greater cognitive reserve will show fewer cognitive difficulties.
It is important to consider the potential for circularity in arguments for cognitive reserve. For example, the association between increased work complexity and preserved cognitive functioning in older adults may be explained by higher premorbid cognitive ability, specific associations between low education levels and vascular damage, or over-diagnosing dementia in individuals with low levels of education (Fratiglioni & Wang, 2007). An alternative explanation is that work may be enjoyable and prevent depressive symptoms and social isolation; however, Cohen-Mansfield and colleagues (2012) found that exposing individuals with dementia to task/work stimuli did not increase their experience of pleasure. The precise mechanism by which employment, particularly work that is complex in nature, enhances the aging process is unclear, and may involve some interaction of engagement in mental, physical, and interpersonal activities. Nonetheless, maintaining steady and challenging employment throughout the lifespan appears to benefit individuals as they age.
Conversely, there may be situations where high work complexity may be very stressful and consequently confer risk for dementia and other negative health outcomes. For most people, however, research indicates that work-related stress is unrelated to risk for dementia (Crowe, Andel, Pedersen, & Gatz, 2007), suggesting that this form of stress may not exacerbate cognitive deficits associated with dementia. Given the association between work complexity and decreased risk for dementia, it is alternatively possible that work-related stress does, in fact, confer some risk for decline, but that the protective impact of work complexity outweighs the impact of this risk. The notion that highly complex or stimulating activities may effectively counter harmful consequences of stress presents important implications regarding prevention and intervention practices for both younger and older adults.
Social Functioning and Participation in Society
The diagnostic criteria for dementia stipulate a significant decline in social or occupational functioning (McKhann et al., 2011). In contrast to task/work stimuli, social stimuli have been shown to increase pleasure or positive affect in individuals with dementia, potentially due to increased feelings of social isolation that accompany dementia disorders (Cohen-Mansfield, Marx, Thein, & Dakheel-Ali, 2011; Cohen-Mansfield et al., 2012). However, apathy, a previously discussed neuropsychiatric symptom of dementia disorders, is associated with low social engagement (Landes, Sperry, Strauss, & Geldmacher, 2001), suggesting that a decrease in emotionally driven social initiative may underlie social isolation associated with illness. It has also been suggested that individuals living at home with dementia may experience reduced social participation due to difficulties using the telephone (Nygard & Starkhammar, 2003) rather than reduced proactive social behavior. Although changes in social functioning and participation in society are common across dementia disorders, it appears likely that the mechanisms driving these changes vary greatly among different etiologies of disease, and potentially within these etiological subgroups as well.
Similarly to work complexity, a high level of social activity may serve as a protective factor in the progression of dementia disorders. It has been found that active participation in society predicts significantly later onset of dementia (Paillard-Borg, Fratiglioni, Xu, Winblad, & Wang, 2012), and degree of social participation has been shown to negatively correlate with risk for developing dementia (Paillard-Borg, Fratiglioni, Winblad, & Wang, 2009). Maintaining a high number of leisure activities in older age may provide stimulation in physical, mental, and social domains, yielding a cumulative advantage in the aging process (Karp et al., 2006). This combined effect is important given the lack of identification of a single modifiable lifestyle factor consistently related to decreased risk of AD (Daviglus et al., 2010) and inconsistent findings regarding the types of leisure activities that are beneficial to older adults (Wang, Xu, & Pei, 2012). Relatedly, a latent class analysis revealed a lifestyle pattern including abstinence from smoking, eating a healthy diet, exercising, and engaging in social activities that was associated with lower AD risk (Norton et al., 2012). Participation in society may thus be a multifaceted aspect of one’s lifestyle that promotes healthy cognitive aging through multiple pathways.
Although the specific mechanisms by which social participation confers an advantage in the aging process are unclear, some research has suggested that, similar to employment findings, complexity of leisure activities in both middle and older age influences intellectual functioning (Schooler & Mulatu, 2001). Further, this relation was shown to be reciprocal, such that highly complex leisure activities supported, and were supported by, higher levels of intellectual functioning. These findings are not surprising given research showing that mental stimulation can serve to increase synaptogenesis in adulthood, resulting in enhanced brain function (Churchill et al., 2002). In line with this evidence, physical exercise, which may also increase with social participation, has positive effects on vasculature that can also counter risk factors in aging (Churchill et al., 2002). It is becoming increasingly clear that neurobiological and environmental mechanisms are highly interactive and that these interactions can have important effects on trajectories that determine outcomes in older age much earlier than the typical onset of dementia.
Activities of Daily Living
One day, Pearl invited her daughter to her house for lunch. When her daughter arrived, Pearl had prepared only drinks. She had not prepared any food items. When her daughter questioned her about it, Pearl apologized and admitted that she must have forgotten about the sandwiches that she had intended to prepare. Concerned about her mother’s level of functioning, Pearl’s daughter invited Pearl to move into her home.
Henry was preparing dinner while his wife was out for the day. When his wife retuned, she discovered several pots in the refrigerator and flour and baking soda on the set table in place of the salt and pepper. Henry, who was typically meticulous when he cooked, had made a mess in the kitchen. Since that day, Henry’s wife took over the responsibility for dinners, but Henry still managed to prepare light meals when all of the objects were set out for him.
Consistent with declining social and occupational functioning, difficulty performing everyday activities, such as grooming and meal preparation, is a hallmark feature of dementia. A large literature shows that difficulties with everyday tasks are associated with a wide spectrum of negative outcomes, including decreased quality of life, frustration, depression (Espiritu et al., 2001; Hargrave, Reed, & Mungus, 2000), and institutionalization (Knopman et al., 1999; Smith, Kokmen, & O’Brien, 2000) as well as caregiver burden (DeBettignies, Mahurin, & Pirozzolo, 1990) and higher costs of care (Albert et al., 1999). In contrast to employment and other domains functioning, much of the outcome literature in dementia has focused on activities of daily living.
Assessment of Activities of Daily Living
The literature on everyday activities has used a range of methods. Most studies have relied on questionnaires that are completed by caregivers and designed to gauge a patient’s need for assistance on a variety of everyday tasks (Lawton & Brody, 1969). Newer questionnaires have been designed to collect more detailed information regarding the breakdown of everyday tasks across various domains (e.g., memory, visuospatial tasks, etc.; Farias et al., 2006, 2008; Glosser et al., 2002; Jefferson et al., 2008). Some studies have used performance-based methods to address the drawbacks associated with questionnaire data, including the potential for unreliable reports (Arguelles, Loewenstein, Eisdorfer, & Arguelles, 2001; Zanetti et al., 1995; Zanetti, Geroldi, Frisoni, Bianchetti, & Trabucchi, 1999), variability in patients’ daily routines, and the difficulty in meaningfully characterizing difficulties. Performance-based methods require patients to complete everyday tasks in the laboratory so that task accomplishment and errors may be scored and compared to normative data. These measures have the potential to be more objective than questionnaires, but they face the challenge of generalizability to real-world functioning. It is therefore important to develop coding schemes that may be used to relate performance on these measures to other constructs, including cognitive measures and caregiver- or self-report.
The methods used to code errors on performance-based tasks may differ across studies, but most researchers have generally used coding schemes that include omissions (failure to perform a task step), various types of commissions (inaccurate execution of a task step; e.g., sequence, perseveration, etc.), and action additions (performance of an extra, off-task step). Table 3.1 provides a list of error types, definitions, and examples of an error taxonomy that was developed by Schwartz and colleagues [(Buxbaum, Schwartz, & Montgomery, 1998; Schwartz, Reed, Montgomery, Palmer, & Mayer, 1991; Schwartz et al., 1995, 1998, 1999); see also Forde & Humphreys, 2002; Humphreys & Forde, 1998; Park et al., 2012; Ramsden, Kinsella, Ong, & Storey, 2008 for variants of this coding scheme]. Work from our group has shown that different error categories are associated with different cognitive difficulties, with higher rates of omission associated with episodic memory impairment and higher rates of commission associated with executive dysfunction (Giovannetti et al., 2008). Not all performance-based tasks incorporate this level of error analysis, possibly because it requires videotaped assessments and a great deal of time. Despite the resources and time required, detailed error coding offers the opportunity to characterize both overall level of impairment (i.e., total errors) as well as specific functional problems (i.e., distributions of error types).
Past studies have shown only modest correlations between caregiver/self-report questionnaires and direct observation of task performance (DeBettignies et al., 1990; Giovannetti et al., 2008; Giovannetti, Libon, & Hart, 2002; Kuriansky, Gurland, Fleiss, & Cowan, 1976). A study from our lab that used the detailed error analysis described above showed that caregiver ratings were associated only with omission errors on performance-based tasks in which participants completed goal-directed, everyday activities (e.g., make toast and coffee) in the lab (Giovannetti et al., 2008). This may suggest that caregivers do not notice other forms of error (e.g., commissions, action additions) or that omission errors are most disruptive to patients’ independent functioning. More research is needed to understand the complex relations among various forms of everyday action assessment. Until these relations are understood, it is important to consider data from multiple sources and emphasize points of convergence across methods.
One consistent finding that has emerged from multiple methods is that everyday functioning is associated with cognitive impairment, such that individuals with greater cognitive impairment typically show greater functional disability (Barberger-Gateau & Fabrigoule, 1997; Lavery et al., 2005; Royall, Palmer, Chiodo, & Polk, 2004; Schmeidler, Mohs, & Aryan, 1998). Early in the course of the illness, individuals show difficulties with complex daily tasks, such as financial accounting and medication management (i.e., instrumental activities of daily living). These tasks likely pose difficulties early in the course of the illness because they involve many steps that must be performed over a lengthy period of time and are less routinized than more basic activities. Basic activities of daily living, such as grooming and eating, tend to remain relatively preserved until later in the course of the illness as the individual experiences moderate to severe cognitive deficits (Barberger-Gateau & Fabrigoule, 1997). Problems in everyday activities, even complex tasks, were initially considered a distinguishing feature of dementia and uncharacteristic of MCI , but newer diagnostic criteria for MCI have been expanded to allow for “mild problems” on “complex” tasks as long as “independence” is maintained “with minimal aids or assistance” (Albert et al., 2011). Thus, the presence and level of functional impairment have enormous implications for the diagnosis of dementia and cognitive disorders in older adults.
Among individuals with MCI , frequent assessment of everyday functioning is important for tracking progression of symptoms. Investigators have shown that changes in functional abilities in people with MCI are highly predictive of conversion to dementia (Sikkes et al., 2011). In fact, a sharp decline in functional/cognitive ability in people with MCI more accurately predicted conversion to dementia than CSF markers and brain volumes (Gomar, Bobes-Bascaran, Conejero-Goldberg, Davies, & Goldberg, 2011). Thus, treatments designed specifically to improve everyday activities might not only serve to improve a wide ranges of outcomes in people with dementia but may be protective against conversion to dementia in people with MCI . Although there is insufficient evidence to recommend any specific interventions for improving everyday activities at this time, several studies on interventions have been published and are reviewed below.
Neuropsychiatric/Cognitive Symptoms and Activities of Daily Living
In addition to global cognitive status, neuropsychiatric symptoms are strongly associated with the ability to perform daily activities in dementia. Investigators have shown a relation between depression and daily functional status in dementia disorders (Ormel et al., 1998; Sarkisian et al., 2000), but some have suggested that this relation may be best explained by apathy, suggesting the importance of motivation loss in functional decline (Boyle et al., 2003; Tekin, Fairbanks, O’Connor, Rosenberg, & Cummings, 2001). As mentioned earlier, depressive and cognitive symptoms are tightly linked, making it difficult to determine whether it is possible or relevant to tease apart the relative influence of either factor on functional outcome. However, a large study of over 5,000 older adults showed that both depression and cognitive deficits contributed to functional decline in the early stages of a dementia disorder; later in the course of the disorder, only cognitive difficulties contributed to further functional decline (Mehta, Yaffe, & Covinsky, 2002). This study suggests that the influence of depression or other neuropsychiatric factors on functioning may change during the course of the illness. Relatively few studies have considered the interaction among depression, other neuropsychiatric symptoms, cognitive decline, and daily functioning, and even fewer have explored these relations using longitudinal study designs. Therefore, it is difficult to know the specific role of depression or other neuropsychiatric symptoms on functional abilities throughout the course of the illness.
As described earlier in the review of symptomatology , there is great heterogeneity in the pattern of cognitive deficits experienced by people with dementia. Some patients may show marked deficits in episodic memory and relative sparing of executive processes, whereas others may present with the opposite pattern of findings. This heterogeneity underscores the importance of determining which specific cognitive symptoms are most strongly associated with decline in everyday activities. To date, however, research on this question has yielded inconsistent results. Many studies show only modest relations between measures of daily functioning and measures of specific cognitive abilities (Royall et al., 2007). Studies that show positive results often highlight the role of executive functions in everyday activities (Cahn-Weiner et al., 2007; Pereira, Yassuda, Oliveira, & Forlenza, 2008). A recent study showed that executive function significantly predicted informant rating of instrumental activities of daily living, even after controlling for overall level of cognitive impairment, episodic memory abilities, and apathy (Marshall et al., 2011). Other studies emphasize episodic memory in addition to executive functions (Farias, Mungas, Reed, Haan, & Jagust, 2004; Farias et al., 2009; Goldstein, McCue, Rogers, & Nussbaum, 1992; Jefferson et al., 2008). These mixed results may be in part due to methodological difficulties in assessing functional ability such as potential report bias, emphasizing the importance of multiple sources of data and further refinement of functional measures.
Heterogeneity in Performance of Activities of Daily Living Across Diagnostic Groups
Although studies exploring relations between specific tests and degree of functioning have been mixed (i.e., variable-centered studies), research comparing individuals with different dementia diagnoses has reported relatively consistent findings of differing degrees of functional deficit across diagnostic groups. Gure and colleagues (2010) reported that individuals with AD showed less functional impairment on caregiver ratings than those diagnosed with vascular dementia or dementia due to other etiologies. This difference was significant even after controlling for dementia severity and other demographic factors. Using a combination of both caregiver ratings and performance-based measures, Mioshi et al. (2007) showed individuals with FTD -behavioral variant had greater functional impairment than individuals with AD or other forms of FTD (i.e., progressive nonfluent aphasia, semantic dementia). Other studies have shown the relation between global cognitive impairment and functional difficulties to differ across subgroups, with FTD -behavioral variant showing the strongest relation and primary progressive aphasia showing the weakest relation (Bouwens, van Heugten, & Verhey, 2009).
Recent studies have suggested that individuals with different dementia diagnoses may also exhibit different types of functional deficit. Work from our group has shown that individuals with subcortical ischemic vascular dementia (VaD) showed a different pattern of impairment than people with AD on a performance-based test of everyday activities. Specifically, individuals with VaD performed more poorly on performance-based variables associated with executive functioning—task accomplishment (i.e., omission errors, such as failing to turn on the toaster when instructed to make a slice of toast) under conditions with distractor objects and errors reflecting inaccurate task performance (i.e., commission errors, such as applying jelly to a slice of bread and then putting the bread in the toaster). By contrast, people with AD performed worse on action variables associated with episodic memory—task accomplishment under conditions with multiple goals (e.g., make toast and coffee; Giovannetti, Schmidt, Sestito, Libon, & Gallo, 2006). Our group has shown similar differences between people with AD and people with dementia associated with Parkinson’s disease (PDD) , with AD participants showing a higher proportion of omission errors and PDD participants showing a higher proportion of commission errors on everyday tasks. Bangen et al. (2010) also recently reported significant differences between MCI subgroups on performance-based tests of functional abilities; participants with amnestic MCI demonstrated significantly worse performance on financial management, and participants with non-amnestic MCI performed significantly worse on health and safety tasks. These findings imply that the unique cognitive or neuropsychiatric symptoms associated with specific dementia or MCI syndromes may lead to qualitatively different patterns of functional impairment.
A Neurocognitive Model of Impairment in Activities of Daily Living
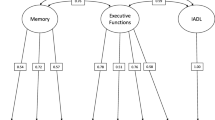
Along these lines, our research group has attempted to frame distinct functional impairment patterns within a neurocognitive model (hereafter Omission-Commission Model; Giovannetti et al., 2008; Giovannetti et al., 2012; Kessler, Giovannetti, & MacMullen, 2007; Seidel et al., 2011). Simply put, the model posits a link between specific neurocognitive deficits and specific functional deficits. As mentioned briefly earlier, failures in completing everyday task steps (omission errors) are associated with deficits in episodic memory and degraded task representations (semantic knowledge). By contrast, inaccuracies during the performance of everyday task steps (commission errors, such as incorrectly sequencing steps) are associated with executive control/working memory deficits (Giovannetti et al., 2008; Giovannetti et al., 2012; Giovannetti, Buxbaum, Biran, & Chatterjee, 2005). Several papers mentioned earlier that reported meaningfully different patterns of everyday action errors between groups of patients with different diagnoses provide support for this model (Giovannetti et al., 2006, 2012; Kessler et al., 2007). Further support for the Omission-Commission Model was reported in a study of 70 people with AD who completed a performance-based test of everyday functioning. The most frequent error categories—omission, sequence, perseveration, substitution, addition—were evaluated using a principal component analysis (PCA). Results yielded three distinct error components: omission, commission (sequence, perseveration, substitution), and addition (see Fig. 3.1). Furthermore, the three error components were associated with different neuropsychological processes. Commissions were related to measures of executive functioning and omissions were related to measures of overall cognitive impairment and episodic memory (Giovannetti et al., 2008).
Component loading plot from the rotated principal component analysis (PCA) of NAT error types from a sample of 70 participants with dementia. The x-axis shows loadings on component 1, which reflects commission errors. The variables that are circled (perseveration, sequence, and substitution errors) are closest to 1 on the x-axis indicating that they load most strongly on component 1-commissions. Consequently, these error variables are often grouped together to form a single commission error category. The omission error type loaded most strongly (i.e., closest to 1) on component 2-Omissions, which is represented on the y-axis. Addition errors fell between omission and commissions errors on the component plot. (Adapted from Giovannetti et al. 2008)
The third error category reported in the PCA study, addition errors, includes behaviors that are tangential to the overall task goal(s) (i.e., putting a pencil in an envelope along with a folded letter). This error category was unrelated to any of the neuropsychological measures included in the study of 70 dementia participants, and it is not frequently observed in participants with AD . However, addition errors are quite common in people with executive function difficulties (e.g., schizophrenia; Kessler et al., 2007), and it is possible that these errors reflect reduced cognitive control over performance (e.g., utilization behavior) or multiple deficits (see Seidel et al., 2011).
Error Monitoring and Detection
Another important facet of everyday functioning in dementia is the extent to which patients are aware of and detect their errors. Some have argued that improving error monitoring might be a more effective approach to improving daily functioning than attempting to eliminate or reduce errors (Reason, 1990). For the purpose of understanding everyday error monitoring, performance-based measures have been fruitful in deconstructing the timing and process by which an individual detects, interprets, and successfully rectifies errors on an everyday task (Bettcher & Giovannetti, 2009; Bettcher, Giovannetti, Klobusicky, et al., 2011; Bettcher, Giovannetti, Libon, et al., 2011; Bettcher, Giovannetti, Macmullen, & Libon, 2008; Giovannetti et al., 2002; Hart, Giovannetti, Montgomery, & Schwarts, 1998). Using these paradigms, individuals with dementia show clear decrements in detecting errors on everyday tasks when compared to older adults without dementia, even after controlling for differences in error rate (Bettcher & Giovannetti, 2009; Bettcher, Giovannetti, Klobusicky, et al., 2011; Bettcher, Giovannetti, Libon, et al., 2011; Bettcher et al., 2008; Giovannetti et al., 2002; Hart et al., 1998). Individuals with mild to moderate dementia detect approximately 20–30 % of errors compared to approximately 75 % of the errors detected by healthy older adults (Bettcher & Giovannetti, 2009; Bettcher, Giovannetti, Klobusicky, et al., 2011; Bettcher, Giovannetti, Libon, et al., 2011; Bettcher et al., 2008; Giovannetti et al., 2002).
Detailed analyses of everyday error monitoring have yielded more surprising findings, with results showing little relation between the number of errors one makes in a daily task and error detection or correction. The total number of everyday action errors was weakly and not significantly correlated with the proportion of errors detected in a study of people with various forms of dementia (r = −0.25, n = 54, p = 0.07; Giovannetti et al., 2002). Total errors and error detection/correction also have been associated with different cognitive processes (Bettcher, Giovannetti, Klobusicky, et al., 2011; Bettcher, Giovannetti, Libon, et al., 2011; Bettcher et al., 2008; Giovannetti et al., 2002), further supporting the distinction between overall error rates and detection/correction. Another notable finding is that even individuals with markedly low error detection are highly likely to correct errors that are detected. A majority (76 %) of detected errors are subsequently corrected by participants with dementia, suggesting that this population may experience relatively greater deficits in recognizing action errors than in acting to correct these errors once detected (Bettcher, Giovannetti, Klobusicky, et al., 2011; Bettcher, Giovannetti, Libon, et al., 2011; Bettcher et al., 2008; Giovannetti et al., 2002). These studies suggest that high rates of everyday action errors and deficient error detection may be the consequence of distinct neurocognitive deficits and that interventions focused on increasing error detection may improve error correction and in turn improve everyday functioning.
In summary, detailed analyses of performance-based assessments have shown that different error patterns may be observed across patients and populations, and that different error patterns may be reliably and meaningfully associated with distinct neuropsychological deficits (i.e., the Omission-Commission Model ). This model may serve to help clarify the conceptualization and treatment of specific everyday action deficits across a range of tasks and contexts.
Treatment of Impairments in Activities of Daily Living
Despite the relatively large literature on everyday action impairment reviewed above, relatively few studies have evaluated interventions designed to improve everyday functioning. One highly cited randomized control trial (Ball et al., 2002) examining the effect of 5–6 weeks of cognitive training interventions on everyday functioning in healthy older adults showed no effects on daily activities, although the training did improve performance on cognitive tasks. The study authors suggested that the null effects, or lack of significant improvement in daily activities following training, might have been due to the fact that their healthy sample actually experienced very few difficulties with daily activities (i.e., ceiling effects). However, null findings also were reported in a review of nine randomized control studies of cognitive training in dementia, where ceiling effects should not have interfered with results (Clare, Woods, Moniz Cook, Orrell, & Spector, 2008). Nevertheless, other methodological limitations, including the use of potentially insensitive functional outcome measures and small sample sizes, make it premature to draw firm conclusions regarding the efficacy of cognitive training interventions for people with dementia (Clare et al., 2008).
In contrast to studies on cognitive training, positive results have been reported for interventions that specifically target functional outcomes in dementia. Extensive training and repetition of everyday activities improve performance on the trained tasks, although training effects do not generalize to untrained tasks (e.g., procedural memory stimulation; Avila et al., 2004; Farina et al., 2002; Josephsson et al., 1993; Zanetti et al., 2001, 2009). Positive benefits also have been reported following a brief (approximately 15 min) pictorial and video review of task objects and the sequence of task steps just prior to performance of everyday tasks (Bettcher, Giovannetti, Libon, et al., 2011). This intervention showed reduced error rates and greater error detection on a performance-based task when evaluated in the laboratory; the results emphasized the importance of targeting degraded knowledge of everyday objects and tasks in interventions for dementia patients.
A wide variety of environmental adaptations also have been suggested for dementia patients (e.g., cue cards, sparse workspace), and some have shown positive effects even without extensive training or repetition (Brennan, Giovannetti, Libon, Bettcher, & Duey, 2009; Giovannetti et al., 2007). Gitlin, Corcoran, Winter, Boyce, and Hauck (2001) explored the effect of individualized home-based environmental adaptations among 171 dementia participants following caregiver training and showed improved functioning and reduced caregiver burden as assessed via caregiver ratings. Our group conducted a laboratory-based study to test the efficacy of arranging objects in the workspace in the order that they should be used in the task and a visual cue to remind participants to monitor performance. Participants did not perform the task repeatedly and did not undergo extensive training with explanation of the adaptations. The results showed significantly reduced error rates on performance-based tests (Giovannetti et al., 2007), but the adaptations had no effect on error detection or correction (Bettcher, Giovannetti, Klobusicky, et al., 2011).
In sum, environmental adaptations may be implemented by the caregiver and may benefit patients even if they are introduced without extensive training or practice. It is quite possible that combining environmental adaptations with repeated practice and patient training will yield even larger positive effects. Additionally, new technologies will allow for adaptations to be seamlessly introduced into the home environment (e.g., smart home; Cook, 2012). Further research using large samples, randomized control designs, and meaningful outcome variables is crucial to determine the effectiveness of interventions and adaptations for daily functioning. An important goal of this work also must be to determine whether and how patients may be matched to interventions that specifically target their unique functional deficit, as this approach may increase the benefit and reduce the cost of interventions/adaptations.
Cultural Considerations
Several epidemiological studies have shown significant differences in the prevalence and incidence of dementia across racial/ethnic groups, with higher rates of cognitive impairment in African-American and Hispanic individuals than Caucasian individuals (Tang et al., 2001). However, it is important to note that this pattern is not equivocally reported in the literature. There are large studies that show no meaningful differences in cognitive impairment across racial/ethnic groups (Fillenbaum et al., 1998; Fitzpatrick et al., 2004), although to our knowledge there are no studies showing better cognitive performance or outcomes in African-American or Hispanic participants as compared to Caucasian participants.
Innumerable environmental factors may explain the discrepancies in cognitive performance across ethnic/racial groups, including segregation, migration patterns, socioeconomic position, discrimination, educational and occupational opportunities, diet, and many others (Glymour & Manly, 2008). In a review paper, Glymour and Manly (2008) propose that these factors may be best understood through a multidimensional lifespan perspective. This perspective considers the complex interaction of risk and protective factors across the entire life course. For example, as reviewed earlier in this chapter, both social participation and high work complexity, which may be strongly affected by racial/ethnic group, may help to buffer against risk factors for development of dementia. Conversely, failure to participate in these activities in young adulthood may serve to exacerbate or maintain these risk factors, promoting a trajectory of decline. Importantly, the effect of risk factors related to social and work behaviors likely interact and accumulate over many years, placing individuals of certain ethnic/racial groups at increased risk for dementia from a very early age. Early experiences across domains may influence outcomes in adolescence, adulthood, and older adulthood, with interactions growing more complex over time. In fact, dementia research has suggested that interactions not only among lifestyle factors but also between lifestyle and pathobiological features of Alzheimer’s disease (e.g., amyloid deposition) play a role in the clinical expression of the disorder (Lopez, Becker, & Kuller, 2012). Thus, the effects of these interactive processes can promote and deflect specific trajectories over the lifespan before emergence of the clinical features of illness.
In addition to cascading influences of experiences over time, there is also extensive evidence for multifinality—individuals with similar lifestyle patterns achieve very different outcomes. This makes isolating mechanisms of risk and resilience incredibly challenging. Among individuals who develop MCI , suggesting a shared transition from normal cognition to dementia, some go on to develop dementia, others remain stable in their mild impairment, and still others improve to a state of healthy aging (Winblad et al., 2004). Further, the association between dementia vulnerability and protective factors varies with age, as these factors have been shown to differentially influence risk for dementia in “younger older adults,” under the age of 80, and “older older adults,” over the age of 80 (Lopez et al., 2012). High variability in outcomes among seemingly similar risk groups and in the relative impact of risk factors further underscores the need for characterization of these dynamic processes in order to develop successful prevention and intervention strategies for individuals with dementia.
In addition to considering the development of risk and protective factors over the lifespan, it is important to consider whether the racial/ethnic differences in dementia reported in some studies reflect true disparities in cognitive abilities or simply differences on tests that are used to diagnose dementia. Cognitive tests are strongly influenced by education, which may bias tests to overestimate cognitive decline in African American and Hispanic individuals—groups with limited educational quality/opportunities/achievement (Manly, Jacobs, Touradji, Small, & Stern, 2002). In addition to education, many other factors associated with race and ethnicity may influence the meaning of low test scores across racial/ethnic groups (e.g., stereotype threat; Steele & Aronson, 1995). Investigators have suggested several solutions to reduce or eliminate test bias, including the use of racial/ethnic-specific norms and tests developed using Item-Response Theory (see Pedraza & Mungas, 2008). We suggest replacing traditional cognitive tests used to diagnose dementia and MCI with performance-based measures of everyday functioning, which have shown no significant relation to gender or education in many studies (Buxbaum et al., 1998; Giovannetti, Libon, Buxbaum, & Schwartz, 2002; Giovannetti et al., 2002, 2006; Schwartz et al., 1998, 1999; Schwartz, Buxbaum, Ferraro, Veramonti, & Segal, 2003; Schwartz, Segal, Veramonti, Ferraro, & Buxbaum, 2002; Sestito, Schmidt, Gallo, Giovannetti, & Libon, 2005).
Some investigators have argued that the differences between various racial and ethnic groups are greater than that which can be accounted for solely by test bias (see Glymour & Manly, 2008 for a review). In fact, test bias does not adequately explain the higher incidence of dementia in African-American compared to Caucasian individuals in longitudinal studies (Tang et al., 2001), as the influence of test bias would be expected to remain constant over the course of several years. Nevertheless, it is essential to understand the potential for any degree of racial/ethnic bias in measures used to diagnose dementia, as sensitive measures are crucial for early detection and adequately informed treatment recommendations.
Conclusions
As reviewed in this chapter, dementia is a degenerative disorder of older age that can lead to a wide range of impairments in cognitive, neuropsychiatric, and physical domains as well as everyday functional difficulties. Reduced participation in societal and work-related activities has been shown to constitute both an outcome and a risk factor for progression of the disorder, and difficulty performing everyday activities has been linked to a range of negative outcomes. A major goal of dementia research is to develop measures that accurately assess functional abilities, particularly given that interventions targeting functional deficits have been shown to be most effective in the literature to date. Performance-based measures have the potential to capture real-world functioning in the laboratory and to characterize functional deficits in dementia using error taxonomies that quantify task performance and can be related to measures of other constructs. These objective measures are also important in addressing differences in cognitive impairment reported across racial and ethnic groups. A lifespan approach to dementia can help characterize socioeconomic, educational, and other lifestyle factors that interact with neurobiological components of risk and resilience. Increased understanding of these interactions is critical for the development of effective prevention and intervention strategies to maximize independence and successful outcomes in older age.
References
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 270–279.
Albert, S. M., Michaels, K., Padilla, M., Pelton, G., Bell, K., Marder, K., et al. (1999). Functional significance of mild cognitive impairment in elderly patients without a dementia diagnosis. The American Journal of Geriatric Psychiatry, 7, 213–220.
Allan, L. M., Ballard, C. G., Rowan, E. N., & Kenny, R. A. (2009). Incidence and prediction of falls in dementia: A prospective study in older people. PLoS One, 4(5).
Andel, R., Crowe, M., Pedersen, N. L., Mortimer, J., Crimmings, E., Johansson, B., et al. (2005). Complexity of work and risk of Alzheimer’s disease: A population-based study of Swedish twins. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60(5), 251–258.
Arguelles, S., Loewenstein, D. A., Eisdorfer, C., & Arguelles, T. (2001). Caregivers’ judgments of the functional abilities of the Alzheimer’s disease patient: Impact of caregivers’ depression and perceived burden. Journal of Geriatric Psychiatry and Neurology, 14(2), 91–98.
Avila, R., Bottino, C. M. C., Carvalho, I. A. M., Santos, C. B., Seral, C., & Miotto, E. C. (2004). Neuropsychological rehabilitation of memory deficits and activities of daily living in patients with Alzheimer’s disease: A pilot study. Brazilian Journal of Medical and Biological Research, 37(11), 1721–1729.
Ball, K., Berch, D. B., Helmers, K. F., Jobe, J. B., Leveck, M. D., Marsiske, M., et al. (2002). Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA, 288(18), 2271–2281.
Bangen, K. J., Jak, A., Schiehser, D. M., Delano-Wood, L., Tuminello, E., Han, S. D., et al. (2010). Complex activities of daily living vary by mild cognitive impairment subtype. Journal of the International Neuropsychological Society, 16(4), 630–639.
Barberger-Gateau, P., & Fabrigoule, C. (1997). Disability and cognitive impairment in the elderly. Disability & Rehabilitation, 19(5), 175–193.
Bassiony, M. M., & Lyketsos, C. G. (2003). Delusions and hallucinations in Alzheimer’s disease: Review of the brain decade. Psychosomatics, 44(5), 388–401.
Bayles, K. A., Boone, D. R., Tomoeda, C. K., Slauson, T. J., & Kaszniak, A. W. (1989). Differentiating Alzheimer’s patients from the normal elderly and stroke patients with aphasia. The Journal of Speech and Hearing Disorders, 54(1), 74–87.
Benke, T. (1993). Two forms of apraxia in Alzheimer’s disease. Cortex, 29(4), 715–725.
Bettcher, B. M., & Giovannetti, T. (2009). From cognitive neuroscience to geriatric neuropsychology: What do current conceptualizations of the action error handling process mean for older adults? Neuropsychology Review, 19(19172399), 64–84.
Bettcher, B. M., Giovannetti, T., Klobusicky, E., Wambach, D., Eppig, J., & Libon, D. J. (2011). To err is human, to monitor divine: Environmental adaptations reduce everyday errors but do not improve monitoring. Journal of Clinical and Experimental Neuropsychology, 33(22133138), 1049–1058.
Bettcher, B. M., Giovannetti, T., Libon, D. J., Eppig, J., Wambach, D., & Klobusicky, E. (2011). Improving everyday error detection, one picture at a time: A performance-based study of everyday task training. Neuropsychology, 25(21639639), 771–783.
Bettcher, B. M., Giovannetti, T., Macmullen, L., & Libon, D. J. (2008). Error detection and correction patterns in dementia: A breakdown of error monitoring processes and their neuropsychological correlates. Journal of International Neuropsychological Society, 14(18282318), 199–208.
Blazer, D., Hughes, D. C., & George, L. K. (1987). The epidemiology of depression in an elderly community population. Gerontologist, 27(3), 281–287.
Borroni, B., Agosti, C., & Padovani, A. (2008). Behavioral and psychological symptoms in dementia with Lewy-bodies (DLB): Frequency and relationship with disease severity and motor impairment. Archives of Gerontology and Geriatrics, 46(1), 101–106.
Bouwens, S. F., van Heugten, C. M., & Verhey, F. R. (2009). Association between cognition and daily life functioning in dementia subtypes. International Journal of Geriatric Psychiatry, 24(7), 764–769.
Boyle, P. A., Malloy, P. F., Salloway, S., Cahn-Weiner, D. A., Cohen, R., & Cummings, J. L. (2003). Executive dysfunction and apathy predict functional impairment in Alzheimer disease. The American Journal of Geriatric Psychiatry, 11(2), 214–221.
Braak, H., & Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica, 82(4), 239–259.
Brennan, L., Giovannetti, T., Libon, D. J., Bettcher, B. M., & Duey, K. (2009). The impact of goal cues on everyday action performance in dementia. Neuropsychological Rehabilitation, 19(18923960), 562–582.
Buchman, A. S., Boyle, P. A., Leurgans, S. E., Barnes, L. L., & Bennett, D. A. (2011). Cognitive function is associated with the development of mobility impairments in community-dwelling elders. The American Journal of Geriatric Psychiatry, 19(6), 571–580.
Buxbaum, L. J., Schwartz, M. F., & Montgomery, M. (1998). Ideational apraxia and naturalistic action. Cognitive Neuropsychology, 15, 617–643.
Cahn-Weiner, D. A., Farias, S. T., Julian, L., Harvey, D. J., Kramer, J. H., Reed, B. R., et al. (2007). Cognitive and neuroimaging predictors of instrumental activities of daily living. Journal of International Neuropsychological Society, 13, 747–757.
Churchill, J. D., Galvez, R., Colcombe, S., Swain, R. A., Kramer, A. F., & Greenough, W. T. (2002). Exercise, experience and the aging brain. Neurobiology of Aging, 23(5), 941–955.
Clare, L., Woods, R. T., Moniz Cook, E. D., Orrell, M., & Spector, A. (2008). Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database of Systematic Reviews, 4.
Cohen-Mansfield, J., Marx, M. S., Freedman, L. S., Murad, H., Thein, K., & Dakheel-Ali, M. (2012). What affects pleasure in persons with advanced stage dementia? Journal of Psychiatric Research, 46(3), 402–406.
Cohen-Mansfield, J., Marx, M. S., Thein, K., & Dakheel-Ali, M. (2011). The impact of stimuli on affect in persons with dementia. Journal of Clinical Psychiatry, 72(4), 480–486.
Cook, D. J. (2012). How smart is your home? Science, 335(6076), 1579–1581.
Crowe, M., Andel, R., Pedersen, N. L., & Gatz, M. (2007). Do work-related stress and reactivity to stress predict dementia more than 30 years later? Alzheimer Disease & Associated Disorders, 21(3), 205–209.
Daviglus, M. L., Bell, C. C., Berrettini, W., Bowen, P. E., Connolly, E. S., Cox, N. J., et al. (2010). NIH state-of-the-science conference statement: Preventing Alzheimer’s disease and cognitive decline. NIH Consensus and State-of-the-Science Statements, 27(4).
DeBettignies, B. H., Mahurin, R. K., & Pirozzolo, F. J. (1990). Insight for impairment in independent living skills in Alzheimer’s disease and multi-infarct dementia. Journal of Clinical and Experimental Neuropsychology, 12, 355–363.
Desai, A. K., Schwartz, L., & Grossberg, G. T. (2012). Behavioral disturbance in dementia. Current Psychiatry Reports, 14(4), 298–309. doi:10.1007/s11920-012-0288-5.
Espiritu, D. A. V., Rashid, H., Mast, B. T., Fitzgerald, J., Steinberg, J., & Lichtenberg, P. A. (2001). Depression, cognitive impairment and function in Alzheimer’s disease. International Journal of Geriatric Psychiatry, 16(11), 1098–1103.
Farias, S. T., Cahn-Weiner, D. A., Harvey, D. J., Reed, B. R., Mungas, D., Kramer, J. H., et al. (2009). Longitudinal changes in memory and executive functioning are associated with longitudinal changes in instrumental activities of daily living in older adults. The Clinical Neuropsychologist, 23(3), 446–461.
Farias, S. T., Mungas, D., Reed, B., Haan, M. N., & Jagust, W. J. (2004). Everyday functioning in relation to cognitive functioning and neuroimaging in community-dwelling Hispanic and non-Hispanic older adults. Journal of the International Neuropsychological Society, 10(3), 342.
Farias, S. T., Mungas, D., Reed, B., Harvey, D. J., Cahn-Weiner, D., & DeCarli, C. (2006). MCI is associated with deficits in everyday functioning. Alzheimer Disease & Associated Disorders, 20, 217–223.
Farias, S. T., Mungas, D., Reed, B. R., Cahn-Weiner, D., Jagust, W., Baynes, K., et al. (2008). The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology, 22(4), 531–544.
Farina, E., Fioravanti, R., Chiavari, L., Imbornone, E., Alberoni, M., Pomati, S., et al. (2002). Comparing two programs of cognitive training in Alzheimer’s disease: A pilot study. Acta Neurologica Scandinavica, 105(5), 365–371.
Ferri, C. P., Prince, M., Brayne, C., Brodaty, H., Fratiglioni, L., Ganguli, M., et al. (2005). Global prevalence of dementia: A Delphi consensus study. Lancet, 366(9503), 2112–2117.
Fillenbaum, G. G., Heyman, A., Huber, M. S., Woodbury, M. A., Leiss, J., Schmader, K. E., et al. (1998). The prevalence and 3-year incidence of dementia in older Black and White community residents. Journal of Clinical Epidemiology, 51(7), 587–595.
Finkel, S. I., Costa e Silva, J., Cohen, G., Miller, S., & Sartorius, N. (1996). Behavioral and psychological signs and symptoms of dementia: A consensus statement on current knowledge and implications for research and treatment. International Psychogeriatrics, 8(Suppl 3), 497–500.
Fitzpatrick, A. L., Kuller, L. H., Ives, D. G., Lopez, O. L., Jagust, W., Breitner, J. C. S., et al. (2004). Incidence and prevalence of dementia in the Cardiovascular Health Study. Journal of American Geriatrics Society, 52(2), 195–204.
Forde, E. M. E., & Humphreys, G. W. (2002). Dissociations in routine behaviour across patients and everyday tasks. Neurocase, 8, 151–167.
Fratiglioni, L., & Wang, H.-X. (2007). Brain reserve hypothesis in dementia. Journal of Alzheimer's Disease, 12(1), 11–22.
Geldmacher, D. S., & Whitehouse, P. J. (1997). Differential diagnosis of Alzheimer’s disease. Neurology, 48(5 Suppl 6), 2–9.
Giovannetti, T., Bettcher, B. M., Brennan, L., Libon, D. J., Kessler, R. K., & Duey, K. (2008). Coffee with jelly or unbuttered toast: Commissions and omissions are dissociable aspects of everyday action impairment in Alzheimer’s disease. Neuropsychology, 22(2), 235–245.
Giovannetti, T., Bettcher, B. M., Libon, D. J., Brennan, L., Sestito, N., & Kessler, R. K. (2007). Environmental adaptations improve everyday action performance in Alzheimer’s disease: Empirical support from performance-based assessment. Neuropsychology, 21(17605578), 448–457.
Giovannetti, T., Britnell, P., Brennan, L., Siderowf, A., Grossman, M., Libon, D. J., et al. (2012). Everyday action impairment in Parkinson’s disease dementia. Journal of International Neuropsychological Society, 18(22621995), 787–798.
Giovannetti, T., Buxbaum, L. J., Biran, I., & Chatterjee, A. (2005). Reduced endogenous control in alien hand syndrome: Evidence from naturalistic action. Neuropsychologia, 43(15488908), 75–88.
Giovannetti, T., Libon, D. J., Buxbaum, L. J., & Schwartz, M. F. (2002). Naturalistic action impairments in dementia. Neuropsychologia, 40(11931925), 1220–1232.
Giovannetti, T., Libon, D. J., & Hart, T. (2002). Awareness of naturalistic action errors in dementia. Journal of International Neuropsychological Society, 8(12164673), 633–644.
Giovannetti, T., Schmidt, K. S., Sestito, N., Libon, D. J., & Gallo, J. L. (2006). Everyday action in dementia: Evidence for differentia deficits in Alzheimer’s disease versus subcortical vascular dementia. Journal of the International Neuropsychological Society, 12, 45–53.
Gitlin, L. N., Corcoran, M., Winter, L., Boyce, A., & Hauck, W. W. (2001). A randomized, controlled trial of a home environmental intervention effect on efficacy and upset in caregivers and on daily function of persons with dementia. The Gerontologist, 41(1), 4–14.
Glosser, G., Gallo, J., Duda, N., de Vries, J. J., Clark, C. M., & Grossman, M. (2002). Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 15(3), 198–206.
Glymour, M. M., & Manly, J. J. (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18(3), 223–254.
Goldstein, G., McCue, M., Rogers, J., & Nussbaum, P. D. (1992). Diagnostic differences in memory test based predictions of functional capacity in the elderly. Neuropsychological Rehabilitation, 2(4), 307–317.
Gomar, J. J., Bobes-Bascaran, M. T., Conejero-Goldberg, C., Davies, P., & Goldberg, T. E. (2011). Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer’s disease neuroimaging initiative. Archives of General Psychiatry, 68(9), 961–969.
Gure, T. R., Kabeto, M. U., Plassman, B. L., Piette, J. D., & Langa, K. M. (2010). Differences in functional impairment across subtypes of dementia. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 65(4), 434–441.
Hargrave, R., Reed, B., & Mungus, D. (2000). Depressive symptoms and functional ability in dementia. Journal of Geriatric Psychiatry and Neurology, 13, 72–77.
Hart, R. P., Kwentus, J. A., Taylor, J. R., & Harkins, S. W. (1987). Rate of forgetting in dementia and depression. Journal of Consulting and Clinical Psychology, 55(1), 101–105.
Hart, T., Giovannetti, T., Montgomery, M. W., & Schwarts, M. F. (1998). Awareness of errors in naturalistic action after traumatic brain injury. The Journal of Head Trauma Rehabilitation, 13(5), 16–28.
Humphreys, G. W., & Forde, E. M. E. (1998). Disordered action schema and action disorganisation syndrome. Cognitive Neuropsychology, 15(771-811).
Jackson, G. R., & Owsley, C. (2003). Visual dysfunction, neurodegenerative diseases, and aging. Neurologic Clinics, 21(3), 709–728.
Jefferson, A. J., Byerly, L. K., Vanderhill, S., Lambe, S., Wong, S., Ozonoff, A., et al. (2008). Characterization of activities of daily living in individuals with mild cognitive impairment. The American Journal of Geriatric Psychiatry, 16(5), 375–383.
Johnson, D. K., Watts, A. S., Chapin, B. A., Anderson, R., & Burns, J. M. (2011). Neuropsychiatric profiles in dementia. Alzheimer Disease & Associated Disorders, 25(4), 326–332.
Josephsson, S., Backman, L., Borell, L., Bernspang, B., Nygard, L., & Ronnberg, L. (1993). Supporting everyday activities in dementia: An intervention study. International Journal of Geriatric Psychiatry, 8(5), 395–400.
Karp, A., Paillard-Borg, S., Wang, H.-X., Silverstein, M., Winblad, B., & Fratiglioni, L. (2006). Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders, 21(2), 65–73.
Kessler, R. K., Giovannetti, T., & MacMullen, L. R. (2007). Everyday action in schizophrenia: Cognitive performance patterns and underlying mechanisms. Neuropsychology, 21(4), 439–447. doi:10.1037/0894-4105.21.4.439.
Knopman, D. S., Berg, J. D., Thomas, R., Grundman, M., Thal, L. J., & Sano, M. (1999). Nursing home placement is related to dementia progression: Experience from a clinical trial. Alzheimer’s Disease Cooperative Study. Neurology, 52, 714–718.
Kohler, S., Allardyce, J., Verhey, F. R., McKeith, I. G., Matthews, F., Brayne, C., et al. (2012). Cognitive decline and dementia risk in older adults with psychotic symptoms: A prospective cohort study. Am J Geriatr Psychiatry. doi: 10.1097/JGP.0b013e31826573af.
Krenz, C., Larson, E. B., Buchner, D. M., & Canfield, C. G. (1988). Characterizing patient dysfunction in Alzheimer’s-type dementia. Medical Care, 26(5), 453–461.
Kuriansky, J. B., Gurland, B. J., Fleiss, J. L., & Cowan, D. (1976). The assessment of self-care capacity in geriatric psychiatric patients by objective and subjective methods. Journal of Clinical Psychology, 32(1), 95–9102.
Landes, A. M., Sperry, S. D., Strauss, M. E., & Geldmacher, D. S. (2001). Apathy in Alzheimer’s disease. Journal of American Geriatrics Society, 49(12), 1700–1707.
Lavery, L. L., Starenchal, S. M., Flynn, W. B., Stoeff, M. A., Schaffner, R., & Newman, A. B. (2005). The clock drawing test is an independent predictor of incident use of 24-hour care in a retirement community. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 60(7), 928–932.
Lawton, M. P., & Brody, E. M. (1969). Assessment of older people: Sepf-maintaining and instrumental activities of daily living. Gerontologist, 9, 179–186.
Leandri, M., Cammisuli, S., Cammarata, S., Baratto, L., Campbell, J., Simonini, M., et al. (2009). Balance features in Alzheimer’s disease and amnestic mild cognitive impairment. Journal of Alzheimer’s Disease, 16(1), 113–120.
Li, G., Shen, Y. C., Chen, C. H., Zhau, Y. W., Li, S. R., & Lu, M. (1991). A three-year follow-up study of age-related dementia in an urban area of Beijing. Acta Psychiatrica Scandinavica, 83(2), 99–9104.
Libon, D. J., Price, C. C., Garrett, K. D., & Giovannetti, T. (2004). From Binswanger’s disease to leuokoaraiosis: What we have learned about subcortical vascular dementia. The Clinical Neuropsychologist, 18(1), 83–100.
Looi, J. C., & Sachdev, P. S. (1999). Differentiation of vascular dementia from AD on neuropsychological tests. Neurology, 53(4), 670–678.
Lopez, O. L., Becker, J. T., & Kuller, L. H. (2012). Patterns of compensation and vulnerability in normal subjects at risk of Alzheimer’s disease. Journal of Alzheimer’s Disease. doi:10.3233/JAD-2012-129015.
Lyketsos, C. G., Steinberg, M., Tschanz, J. T., Norton, M. C., Steffens, D. C., & Breitner, J. C. (2000). Mental and behavioral disturbances in dementia: Findings from the Cache County study on memory in aging. The American Journal of Psychiatry, 157(5), 708–714.
Mackenzie, I. R., Neumann, M., Bigio, E. H., Cairns, N. J., Alafuzoff, I., Kril, J., et al. (2010). Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathologica, 119(1), 1–4. doi:10.1007/s00401-009-0612-2.
Manly, J. J., Jacobs, D. M., Touradji, P., Small, S. A., & Stern, Y. (2002). Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of International Neuropsychological Society, 8, 341–348.
Marshall, G. A., Rentz, D. M., Frey, M. T., Locascio, J. J., Johnson, K. A., & Sperling, R. A. (2011). Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 300–308.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 34(7), 939–944.
McKhann, G., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 263–269.
Mehta, K. M., Yaffe, K., & Covinsky, K. E. (2002). Cognitive impairment, depressive symptoms, and functional decline in older people. Journal of American Geriatrics Society, 50(6), 1045–1050.
Mioshi, E., Kipps, C. M., Dawson, K., Mitchell, J., Graham, A., & Hodges, J. R. (2007). Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology, 68(24), 2077–2084.
Mirelman, A., Herman, T., Brozgol, M., Dorfman, M., Sprecher, E., Schweiger, A., et al. (2012). Executive function and falls in older adults: New findings from a five-year prospective study link fall risk to cognition. PLoS One, 7(6).
Morhardt, D. (2011). Accessing community-based and long-term care services: Challenges facing persons with frontotemporal dementia and their families. Journal of Molecular Neuroscience, 45(3), 737–741.
Mychack, P., Kramer, J. H., Boone, K. B., & Miller, B. L. (2001). The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology, 56(11 Suppl 4), S11–S15.
Neary, D., Snowden, J. S., Gustafson, L., Passant, U., Stuss, D., Black, S., et al. (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554.
Norton, M. C., Dew, J., Smith, H., Fauth, E., Piercy, K. W., Breitner, J. C. S., et al. (2012). Lifestyle behavior pattern is associated with different levels of risk for incident dementia and Alzheimer’s disease: The Cache County study. Journal of American Geriatrics Society, 60(3), 405–412.
Nygard, L., & Starkhammar, S. (2003). Telephone use among noninstitutionalized persons with dementia living alone: Mapping out difficulties and response strategies. Scandinavian Journal of Caring Sciences, 17(3), 239–249.
Ormel, J., Kempen, G. I., Deeg, D. J., Brilman, E. I., van Sonderen, E., & Relyveld, J. (1998). Functioning, well-being, and health perception in late middle-aged and older people: Comparing the effects of depressive symptoms and chronic medical conditions. Journal of American Geriatrics Society, 46, 39–48.
Ory, M. G., Hoffman, R. R., 3rd, Yee, J. L., Tennstedt, S., & Schulz, R. (1999). Prevalence and impact of caregiving: A detailed comparison between dementia and nondementia caregivers. Gerontologist, 39(2), 177–185.
Paillard-Borg, S., Fratiglioni, L., Winblad, B., & Wang, H.-X. (2009). Leisure activities in late life in relation to dementia risk: Principal component analysis. Dementia and Geriatric Cognitive Disorders, 28(2), 136–144.
Paillard-Borg, S., Fratiglioni, L., Xu, W., Winblad, B., & Wang, H.-X. (2012). An active lifestyle postpones dementia onset by more than one year in very old adults. Journal of Alzheimer’s Disease, 31(4), 835–842.
Park, N. W., Lombardi, S., Gold, D. A., Tarita-Nistor, L., Gravely, M., Roy, E. A., et al. (2012). Effects of familiarity and cognitive function on naturalistic action performance. Neuropsychology, 26(2), 224–237.
Pedraza, O., & Mungas, D. (2008). Measurement in cross-cultural neuropsychology. Neuropsychology Review, 18(3), 184–193.
Pereira, F. S., Yassuda, M. S., Oliveira, A. M., & Forlenza, O. V. (2008). Executive dysfunction correlates with impaired functional status in older adults with varying degrees of cognitive impairment. International Psychogeriatrics, 20, 1104–1115.
Perry, R. J., & Hodges, J. R. (1999). Attention and executive deficits in Alzheimer’s disease. A critical review. Brain, 122(Pt 3), 383–404.
Phelan, E. A., Borson, S., Grothaus, L., Balch, S., & Larson, E. B. (2012). Association of incident dementia with hospitalizations. JAMA, 307(2), 165–172.
Rabinovici, G. D., & Miller, B. L. (2010). Frontotemporal lobar degeneration: Epidemiology, pathophysiology, diagnosis and management. CNS Drugs, 24(5), 375–398.
Ramsden, C. M., Kinsella, G. J., Ong, B., & Storey, E. (2008). Performance of everyday actions in mild Alzheimer’s disease. Neuropsychology, 22(1), 17–26.
Reason, J. (1990). Human error. New York: Cambridge University Press.
Royall, D. R., Lauterbach, E. C., Kaufer, D., Malloy, P., Coburn, K. L., & Black, K. J. (2007). The cognitive correlates of functional status: A review from the Committee on Research of the American Neuropsychiatric Association. Journal of Neuropsychiatry and Clinical Neurosciences, 19(3), 249–265.
Royall, D. R., Palmer, R., Chiodo, L. K., & Polk, M. J. (2004). Declining executive control in normal aging predicts change in functional status: The Freedom House Study. Journal of the American Geriatric Society, 52, 346–352.
Rubenstein, L. Z., Josephson, K. R., & Robbins, A. S. (1994). Falls in the nursing home. Annals of Internal Medicine, 121(6), 442–451.
Sarkisian, C. A., Liu, H., Gutierrez, P. R., Seeley, D. G., Cummings, S. R., & Mangione, C. M. (2000). Modifiable risk factors predict functional decline among older women: A prospectively validated clinical prediction tool. The Study of Osteoporotic Fractures Research Group. Journal of American Geriatrics Society, 48, 170–178.
Schmeidler, J., Mohs, R. C., & Aryan, M. (1998). Relationship of disease severity to decline on specific cognitive and functional measures in Alzheimer’s disease. Alzheimer Disease & Associated Disorders, 3, 146–151.
Schooler, C., & Mulatu, M. S. (2001). The reciprocal effects of leisure time activities and intellectual functioning in older people: A longitudinal analysis. Psychology and Aging, 16(3), 466–482.
Schooler, C., Mulatu, M. S., & Oates, G. (1999). The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychology and Aging, 14(3), 483–506.
Schwartz, M. F., Buxbaum, L. J., Ferraro, M., Veramonti, T., & Segal, M. (2003). The naturalistic action test. Bury St. Edmunds, UK: Thames Valley Test Company.
Schwartz, M. F., Buxbaum, L. J., Montgomery, M. W., Fitzpatrick-DeSalme, E., Hart, T., Ferraro, M., et al. (1999). Naturalistic action production following right hemisphere stroke. Neuropsychologia, 37, 51–66.
Schwartz, M. F., Montgomery, M. W., Buxbaum, L. J., Lee, S. S., Carew, T. G., Coslett, H. B., et al. (1998). Naturalistic action impairment in closed head injury. Neuropsychology, 12, 13–28.
Schwartz, M. F., Montgomery, M. W., Fitzpatrick-DeSalme, E. J., Ochipa, P., Coslett, H. B., & Mayer, N. H. (1995). Analysis of a disorder of everyday action. Cognitive Neuropsychology, 12, 863–892.
Schwartz, M. F., Reed, E. S., Montgomery, M. W., Palmer, C., & Mayer, M. H. (1991). The quantitative description of action disorganization after brain damage: A case study. Cognitive Neuropsychology, 8, 381–414.
Schwartz, M. F., Segal, M. E., Veramonti, T., Ferraro, M., & Buxbaum, L. J. (2002). The Naturalistic Action Test: A standardised assessment for everyday-action impairment. Neuropsychological Rehabilitation, 12, 311–339.
Seidel, G., Giovannetti, T., Price, C. C., Towler, S. D., Tanner, J. J., Mitchell, S., et al. (2011). Neuroimaging predictors of IADLs and everyday action errors in dementia. Journal of the International Neuropsychological Society, 17-S1, 281.
Seidler, A., Nienhaus, A., Bernhardt, T., Kauppinen, T., Elo, A. L., & Frolich, L. (2004). Psychosocial work factors and dementia. Occupational and Environmental Medicine, 61(12), 962–971.
Sestito, N., Schmidt, K. S., Gallo, J. L., Giovannetti, T., & Libon, D. J. (2005). Using the Naturalistic Action Test (NAT) to assess everyday action in healthy older adults and patients with dementia. Journal of the International Neuropsychological Society, 11(Suppl 1), 90–91.
Sikkes, S. A. M., Visser, P. J., Knol, D. L., de Lange-de Klerk, E. S. M., Tsolaki, M., Frisoni, G. B., et al. (2011). Do instrumental activities of daily living predict dementia at 1- and 2-year follow-up? Findings from the Development of Screening guidelines and diagnostic Criteria for Predementia Alzheimer’s disease study. Journal of American Geriatrics Society, 59(12), 2273–2281.
Smith, G. E., Kokmen, E., & O’Brien, P. C. (2000). Risk factors for nursing home placement in a population-based dementia cohort. Journal of the American Geriatric Society, 48, 519–525.
Smyth, K. A., Fritsch, T., Cook, T. B., McClendon, M. J., Santillan, C. E., & Friedland, R. P. (2004). Worker functions and traits associated with occupations and the development of AD. Neurology, 63(3), 498–503.
Somme, J. H., Fernandez-Martinez, M., Molano, A., & Zarranz, J. J. (2013). Neuropsychiatric symptoms in amnestic mild cognitive impairment: Increased risk and faster progression to dementia. Curr Alzheimer Res, 10(1), 86–94.
Steele, C. M., & Aronson, J. (1995). Stereotype threat and the intellectual test performance of African Americans. Journal of Personal and Social Psychology, 69(5), 797–811.
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of International Neuropsychological Society, 8(3), 448–460.
Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology, 11(11), 1006–1012. doi:10.1016/S1474-4422(12)70191-6.
Tang, M. X., Cross, P., Andrews, H., Jacobs, D. M., Small, S., Bell, K., et al. (2001). Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 56(1), 49–56.
Tekin, S., Fairbanks, L. A., O'Connor, S., Rosenberg, S., & Cummings, J. L. (2001). Activities of daily living in Alzheimer’s disease: Neuropsychiatric, cognitive, and medical illness influences. The American Journal of Geriatric Psychiatry, 9(1), 81–86.
Tetewsky, S. J., & Duffy, C. J. (1999). Visual loss and getting lost in Alzheimer’s disease. Neurology, 52(5), 958–965.
Thune-Boyle, I. C., Iliffe, S., Cerga-Pashoja, A., Lowery, D., & Warner, J. (2012). The effect of exercise on behavioral and psychological symptoms of dementia: Towards a research agenda. International Psychogeriatrics, 24(7), 1046–1057.
van Doorn, C., Gruber-Baldini, A. L., Zimmerman, S., Hebel, J. R., Port, C. L., Baumgarten, M., et al. (2003). Dementia as a risk factor for falls and fall injuries among nursing home residents. Journal of American Geriatrics Society, 51(9), 1213–1218.
van Vliet, D., de Vugt, M. E., Bakker, C., Koopmans, R. T. C. M., & Verhey, F. R. J. (2010). Impact of early onset dementia on caregivers: A review. International Journal of Geriatric Psychiatry, 25(11), 1091–1100.
Wadsworth, L. P., Lorius, N., Donovan, N. J., Locascio, J. J., Rentz, D. M., Johnson, K. A., et al. (2012). Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dementia and Geriatric Cognitive Disorders, 34(2), 96–111. doi:10.1159/000342119.
Wang, H.-X., Xu, W., & Pei, J.-J. (2012). Leisure activities, cognition and dementia. Biochimica et Biophysica Acta, 1822(3), 482–491.
Weintraub, S., & Mesulam, M. (2009). With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain, 132(11), 2906–2908.
Weintraub, S., Wicklund, A. H., & Salmon, D. P. (2012). The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med, 2(4), a006171. doi:10.1101/cshperspect.a006171.
Welsh-Bohmer, K., & Warren, L. H. (2006). Neurodegenerative dementias. In D. Attix & K. Welsh-Bohmer (Eds.), Geriatric neuropsychology: Assessment and intervention. New York, NY: Guilford Press.
Wetzel, M. E., & Kramer, J. H. (2008). The neuropsychology of vascular dementia. Handbook of Clinical Neurology, 88, 567–583. doi:10.1016/S0072-9752(07)88030-4.
Wetzels, R. B., Zuidema, S. U., de Jonghe, J. F. M., Verhey, F. R. J., & Koopmans, R. T. C. M. (2010). Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. The American Journal of Geriatric Psychiatry, 18(12), 1054–1065.
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., et al. (2004). Mild cognitive impairment—beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256(3), 240–246.
Woods, S. P., Weber, E., Weisz, B. M., Twamley, E. W., & Grant, I. (2011). Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabilitation Psychology, 56(1), 77–84.
World Alzheimer Report. (2009). Alzheimer’s Disease International.
Zanetti, O., Binetti, G., Magni, E., Rozzini, L., Bianchetti, A., & Trabucchi, M. (2009). Procedural memory stimulation in Alzheimer’s disease: Impact of a training programme. Acta Neurologica Scandinavica, 95(3), 152–157.
Zanetti, O., Frisoni, G. B., Deleo, D., Dellobuono, M., Bianchetti, A., & Trabucchi, M. (1995). Reality orientation therapy in Alzheimer-disease—useful or not—a controlled-study. Alzheimer Disease & Associated Disorders, 9(3), 132–138.
Zanetti, O., Geroldi, C., Frisoni, G. B., Bianchetti, A., & Trabucchi, M. (1999). Contrasting results between caregiver’s report and direct assessment of activities of daily living in patients affected by mild and very mild dementia: The contribution of the caregiver’s personal characteristics. Journal of American Geriatrics Society, 47(2), 196–202.
Zanetti, O., Zanieri, G., Di Giovanni, G., De Vreese, L. P., Pezzini, A., Metitieri, T., et al. (2001). Effectiveness of procedural memory stimulation in mild Alzheimer’s disease patients: A controlled study. Neuropsychological Rehabilitation, 11(3–4), 263–272.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag New York
About this chapter
Cite this chapter
Rycroft, S.S., Giovannetti, T. (2017). Alzheimer’s Disease and Other Dementia Disorders. In: Chiaravalloti, N., Goverover, Y. (eds) Changes in the Brain. Springer, New York, NY. https://doi.org/10.1007/978-0-387-98188-8_3
Download citation
DOI: https://doi.org/10.1007/978-0-387-98188-8_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-0-387-98187-1
Online ISBN: 978-0-387-98188-8
eBook Packages: MedicineMedicine (R0)