Abstract
Primary hyperoxaluria type 3 (PH3) is a recently identified inborn error of 4-hydroxyproline metabolism causing kidney stone disease. Diagnosis to date has relied on mutation detection. The excretion of 4-hydroxyglutamate (4OHGlu) was investigated in controls and a cohort of nine patients with PH3 and their parents using flow injection tandem mass spectrometry. 4OHGlu was stable in acidified urine samples and was not influenced by diet. Its measurement was readily incorporated into an existing multi-analyte panel for comprehensive screening for inborn errors of metabolism. There was a steady decline with age in 4OHGlu levels, expressed as μmol/mmol of creatinine, in controls. Levels in patients with PH3 ranged from 6.5 to 98 μmol/mmol of creatinine and were all significantly increased when compared to age-matched controls (<4.2). Levels in eight parents (obligatory carriers of the corresponding mutation) were moderately, but significantly increased, ranging from 0.6 to 2.5 (age-matched controls <1.4, p = 0.03). Urine 4OHGlu screening was used to prospectively diagnose PH3 in an 18-month-old boy with calcium oxalate kidney stone disease associated with hyperoxaluria. 4OHGlu was also increased in a stored newborn screening dried blood spot sample from this child (37 μmol/L, controls <2.53). 4OHGlu testing provides a robust and high-throughput biochemical screen for PH3.
Competing interests: None declared
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Newborn Screening
- Supplementary Material Table
- Kidney Stone Disease
- Electrospray Tandem Mass Spectrometry
- HOGA1 Gene
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The two main determinants of kidney stone disease among the paediatric age group are genetic factors and anatomic anomalies. Children, as compared to adults, are more likely to have an underlying metabolic derangement which results in a higher rate of stone recurrence. Urinary metabolic profiling is therefore recommended in all children with nephrolithiasis, as timely treatment may prevent stone formation (Habbig et al. 2011). Primary hyperoxaluria type 3 (PH3, OMIM 613616) is a recently identified childhood oxalate kidney stone disease caused by mutations in the HOGA1 gene, formerly DHDPSL (Belostotsky et al. 2010). A recent study gave an in silico estimate of ~1:165,000 for the incidence of PH3 in the US population (Hopp et al. 2013). HOGA1 encodes for 4-hydroxy-2-oxoglutarate aldolase (HOGA1), a mitochondrial enzyme in the 4-hydroxyproline (4OHPro) catabolic pathway which converts 4-hydroxy-2-oxoglutarate (HOG) to glyoxylate (Fig. 1). HOGA1 mutations cause decreased HOG aldolase activity (Riedel et al. 2012), and it was recently shown that patients with PH3 accumulate HOG, 2,4-dihydroxyglutarate (the reduced form of HOG) and 4-hydroxyglutamate (4OHGlu, the metabolic precursor of HOG) in their urine (Belostotsky et al. 2012). It was difficult to envisage how oxalate accumulates when glyoxylate, its immediate precursor, is downstream of the metabolic block. We recently obtained evidence (Belostotsky et al. 2012) of a “re-routing” mechanism in which accumulating HOG exits mitochondria and is converted to glyoxylate in the cytoplasm by a yet-to-be-identified aldolase enzyme, distinct from HOGA1 (Fig. 1).
Metabolic pathway for 4-hydroxyproline showing the enzymatic block in primary hyperoxaluria type 3 and the proposed metabolic re-routing mechanism. Enzymes are as follows: 1 aspartate aminotransferase, 2 4-hydroxy-2-oxo-glutarate aldolase, 3 unidentified aldolase, 4 lactate dehydrogenase. Principal PH3 urine metabolites that exit the cell are underlined
Until now, PH3 diagnosis has relied on mutation detection by sequencing of the HOGA1 gene in cases with a high index of suspicion, e.g. persistent hyperoxaluria and exclusion of other genetic (i.e. primary hyperoxaluria types 1 and 2) and acquired causes of hyperoxaluria. The finding of abnormal PH3 metabolites opens the possibility of more comprehensive biochemical screening for this disorder in subjects with a lower index of suspicion. This communication describes the performance of 4OHGlu as a biomarker for PH3, the development of a high-throughput tandem mass spectrometry urine screening method for symptomatic patients and the potential feasibility of newborn screening for PH3.
Materials and Methods
Nine patients with PH3 from four unrelated families of Ashkenazi-Jewish descent were enrolled in this study. The diagnosis was confirmed by mutation screening of HOGA1. Three families have been previously described (Belostotsky et al. 2010). The affected individuals from the fourth family are compound heterozygotes for the following mutations: c.107C>T and c.944_946delAGG. Of note, four out of nine patients have never formed a kidney stone despite having persistent hyperoxaluria. Their newborn screening dried blood spots were not available for analysis.
Random urine samples were collected from patients and their parents. Controls were random urine samples submitted for routine screening for inborn errors of metabolism including those causing kidney stone disease. Ages ranged from 4 days to 86 years. The influence of diet was checked by performing an oral loading test of 20 g of gelatine, containing ~14% 4-hydroxyproline (Eastoe 1955), on a healthy male volunteer and collecting random urine samples. Urines were stored at −30°C. Urine creatinine was measured by a kinetic Jaffe reaction using a Cobas Mira analyser. 4OHGlu, 4OHPro and acetyl chloride were purchased from Sigma Aldrich; n-butanol, acetonitrile and methanol were from Merck, while 2H3-glutamate and 2H3-4-hydroxyproline were from CDN Isotopes.
Urine 4OHGlu and 4OHPro were measured using a modification of a previously described multi-analyte method for comprehensive screening for inborn errors of metabolism using electrospray tandem mass spectrometry (Pitt et al. 2002). Briefly, aliquots of urine containing 20 nmoles of creatinine were mixed with 20 μL of internal standard solution (containing 100 μmol/L of 2H3-glutamate, 2H3-4-hydroxyproline and other internal standards). Amino acids and other metabolites were then converted to n-butyl esters and analysed by flow injection analysis electrospray tandem mass spectrometry in positive ion multiple reaction monitoring mode as previously described using a Waters Acquity TQD system. The relevant mass transitions are given in Supplementary Material Table 1 and were added to an existing panel of ~115 metabolites.
The assay was calibrated with three standards (12.5, 25 and 50 μmol/L) and a blank, all prepared in a liquid matrix that approximately matched salt and urea concentrations in urine samples. Urine samples from different control individuals were spiked with 4OHGlu and 4OHPro for determining the assay recovery. To assess storage stability, aliquots of a spiked urine sample were stored at various temperatures with and without acidification to pH 2 using hydrochloric acid. Urine blotters were prepared applying samples to Whatman 903 paper, dried and stored at room temperature.
Neonatal dried blood spots were eluted with methanol containing the internal standards, dried and then derivatised as described above. Mass spectrometry transitions for 4OHGlu and its internal standard (see Supplementary Material Table 1) were added to a standard newborn screening amino acid/acyl-carnitine panel. De-identified dried blood spots from controls were analysed with approval from the Royal Children’s Hospital Human Research Ethics Committee.
Results
Assay Performance
The analytical performance for urine 4OHGlu and 4OHPro is summarised in Supplementary Material Fig. 1 and Table 2. Linearities were >0.984, imprecision <11.7% (normal and high levels) and recovery 83–112% for both analytes. A typical calibration curve is shown in Supplementary Fig. 1. As expected, the performance of 4OHPro was superior because a stable isotope internal standard was used.
A spiked urine sample was analysed after 28 days of storage under various conditions and expressed as a percentage of the same sample stored at −80 °C. Samples stored at −30 °C, acidified and stored at room temperature, and on blotters, gave values of 101%, 92% and 99%, respectively.
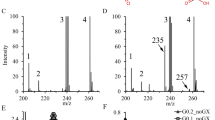
Intake of 4OHPro, in the form of gelatine, caused an increase in urine 4OHPro, peaking at 1.7 μmol/mmol of creatinine, with a smaller concomitant increase in 4OHGlu levels, peaking at 0.6 μmol/mmol of creatinine (Fig. 2), well within the reference range.
4OHGlu Levels in Controls and PH3 Families
Urine 4OHGlu in μmol/mmol of creatinine for controls, PH3 patients and their parents are shown in Fig. 3. There was a steady decline in 4OHGlu with age in controls (<11.1 for <0.5 years of age; <4.2 for 0.5–1 years; <2.9 for 1–5 years; <1.8 for 5–10 years; <1.4 for >10 years). Levels were grossly increased in PH3 patients (all >0.5 years), ranging from 6.5 to 98 and also showing an apparent decline with age. The levels in eight PH3 obligate heterozygous parents ranged from 0.6 to 2.5, showing a moderate but significant (p = 0.03) increase when compared to controls >10 years. There was an approximate correlation between 4OHGlu and 4OHPro levels in both controls and PH3 patients, and consideration of both of these metabolites appeared to give greater discrimination between controls and PH3 (see Supplementary Material Fig. 2).
4OHGlu levels were normal for age in subjects with kidney stone diseases such as cystinuria (n = 22), xanthine oxidase deficiency (n = 2), hyperoxaluria type 1 (n = 4) and hereditary orotic aciduria (n = 1). One patient with hyperoxaluria type 2 had a slight increase at 3.5 μmol/mmol of creatinine (controls <1.4).
We subsequently screened a larger population of children with kidney stone disease for PH3. Patient A was diagnosed after paediatric nephrologists throughout Australia and New Zealand were contacted and offered biochemical testing for suspected cases of PH3. He was an 18-month-old boy with multiple calcium oxalate kidney stones from the age of 13 months. Urine oxalate levels ranged from 179 to 379 μmol/mmol of creatinine (reference range: 29–174) with normal glycolate and glycerate. Consistently increased urine 4OHGlu levels were detected by the screening method (Fig. 3), and urine HOG and 2,4-dihydroxyglutarate were also increased (data not shown). The diagnosis of PH3 was confirmed by the finding of a novel deletion (c.803_805delTGC causing an in-frame deletion: p.Lys268del) and a known splice site variant (c.700+5G>T) in the HOGA1 gene.
The newborn screening dried blood spot card, stored for 2 years, was retrieved and analysed with parental consent. This showed an increase in 4OHGlu (37 μmol/L; storage-matched controls <2.1, n = 50, fresh controls <2.5, n = 50). Each of the storage-matched and fresh control groups included ten premature babies (30–35 week gestation) to check for possible influences of prematurity. There were no significant differences in 4OHGlu levels between premature and normal gestation babies.
Discussion
Since the initial report of HOGA1 mutations causing PH3 (Belostotsky et al. 2010), several cohorts of patients with persistent hyperoxaluria of unknown aetiology (also referred to as non-type I/type II PH) have been retrospectively studied, and HOGA1 mutations have been identified in ~45% of these patients (Beck et al. 2013; Monico et al. 2011; Williams et al. 2012). These findings have significant implications for the way in which patients with hyperoxaluria are investigated and also suggest possible treatments for PH3, now that the gene and enzyme involved have been identified. 4OHPro is almost exclusively derived from collagen (Fig. 1), from either endogenous turnover or dietary sources, and it may be possible to modulate 4OHPro metabolism in PH3, either through restriction of dietary collagen or by pharmaceutical means, to minimise stone formation. Reliable biomarkers for PH3 would be very useful for further research in these areas as well as for the initial diagnosis of PH3 which has relied on HOGA1 mutation detection to date.
Our previous study (Belostotsky et al. 2012) found large increases of these metabolites in the urine of patients with PH3, and these findings were later replicated for HOG (Riedel et al. 2012). The analytical methods used in those studies are poorly suited for routine diagnostic work and screening large numbers of samples. 4OHGlu could potentially be measured by standard methods of amino acids analysis which are widely available in biochemical genetics laboratories. Preliminary investigations showed that the peak of 4OHGlu eluted close to normal amino acid urine constituents in both ion exchange (Biochrom 30 system) and reverse phase (Waters Masstrak/Acquity system) chromatography, precluding reliable measurement. We therefore added 4OHGlu to a panel of metabolites measured by electrospray tandem mass spectrometry as part of comprehensive screening for inborn errors of metabolism. A stable isotope version of 4OHGlu was unavailable commercially and we were unable to synthesise one. We chose 2H3-glutamate as a “surrogate” internal standard for 4OHGlu. Despite this limitation, analytical performance was acceptable for a screening method (see Supplementary Material Table 2) with between batch CVs <12% and recoveries ranging from 83% to 112%.
4OHGlu was stable in urine with recoveries >90%, when samples were stored frozen or on urine blotters. Acidification with hydrochloric acid also prevented the breakdown of 4OHGlu in liquid urine at room temperature, with a recovery of 92% after 28 days storage. For routine diagnostic work, we recommend acidification of samples (to prevent decomposition of 4OHGlu, oxalate and other metabolites) and transport in the frozen state or at ambient temperatures. In contrast, HOG is unstable at ambient temperatures (Dekker and Maitra 1975) making it less reliable as a diagnostic biomarker. We also found urine 4OHGlu to be relatively unaffected by dietary influences (Fig. 2).
There was a decline in urine 4OHGlu levels in the first few years of life (Fig. 3). This is consistent with increased collagen turnover (Mora et al. 1998) and 4OHPro metabolism in young children. Indeed, 4OHPro excretion steadily declines with age (Shih 2003), and there was a rough correlation between 4OHGlu and 4OHPro (Supplementary Material Fig. 2). 4-OHGlu levels in ten PH3 patients were much higher than age-matched controls and ranged from 6.5 to 98 μmol/mmol of creatinine. A decline in 4OHGlu levels with age was also apparent in the PH3 patients, presumably the result of a similar mechanism to controls. In our previous study, we did not detect a significant difference between controls and obligate carriers (parents) of PH3 patients (Belostotsky et al. 2012). However, this, more extensive, study showed that some carriers do indeed have modest, but significant, increases in 4OHGlu levels compared to age-matched controls. Heterozygous HOGA1 mutations may be a risk factor for stone formation (Monico et al. 2011), and increased levels of 4OHGlu in carriers may be useful in stratifying this risk.
4OHGlu testing was easily added to a multi-analyte test for comprehensive screening for inborn errors of metabolism (Pitt et al. 2002), and we now test all urines submitted to our laboratory for 4OHGlu. This resulted in rapid biochemical diagnosis of PH3 in patient A. 4OHGlu was specific for PH3 with normal values being obtained for most subjects with other genetic forms of kidney stone diseases. One patient with primary hyperoxaluria type 2 had a slight increase in 4OHGlu levels that did not overlap with the PH3 range which may reflect accumulation of metabolites in the 4-hydroxyproline pathway. When samples are analysed in parallel in negative ion mode, biomarkers for these other kidney stone diseases are readily detected, e.g. hyperoxaluria type 1 (glycolate), hyperoxaluria type 2 (glycerate), xanthine oxidase deficiency (xanthine), hereditary orotic aciduria (orotate) and cystinuria (cystine and arginine).
Retrospective analysis of the stored newborn screening dried blood spot from patient A showed a ~25-fold increase in 4OHGlu relative to controls. This feature may be useful in situations where a rapid diagnosis is requested, e.g. diagnosis of subsequent siblings. PH3 does not meet the traditional criteria for newborn screening test, but 4OHGlu testing would only involve a minor modification of the current newborn tandem mass spectrometry screening test with minimal additional laboratory costs. Newborn screening laboratories may wish to consider the feasibility of newborn screening for PH3 in order to prevent the “diagnostic odyssey” that many PH3 patients experience. Further studies would be required to determine the metrics of this screening, in particular the false positive rate.
References
Beck BB, Baasner A, Buescher A et al (2013) Novel findings in patients with primary hyperoxaluria type III and implications for advanced molecular testing strategies. Eur J Hum Genet 21(2):162–172
Belostotsky R, Pitt JJ, Frishberg Y (2012) Primary hyperoxaluria type III-a model for studying perturbations in glyoxylate metabolism. J Mol Med (Berl) 90(12):1497–1504
Belostotsky R, Seboun E, Idelson GH et al (2010) Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87(3):392–399
Dekker EE, Maitra U (1975) DL-2-keto-4-hydroxyglutarate-1. Methods Enzymol 41:115–118
Eastoe JE (1955) The amino acid composition of mammalian collagen and gelatin. Biochem J 61(4):589–600
Habbig S, Beck BB, Hoppe B (2011) Nephrocalcinosis and urolithiasis in children. Kidney Int 80(12):1278–1291
Hopp K, Cogal AG, Hakonarson H, Milliner DS, Harris PC (2013) Estimated incidence of primary hyperoxaluria using population allele frequencies of disease variants [Abstract]. J Am Soc Nephrol 24:529A
Monico CG, Rossetti S, Belostotsky R et al (2011) Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol 6(9):2289–2295
Mora S, Prinster C, Proverbio MC et al (1998) Urinary markers of bone turnover in healthy children and adolescents: age-related changes and effect of puberty. Calcif Tissue Int 63(5):369–374
Pitt J, Belostotsky R, Frishberg Y (2012) The metabolic basis of primary hyperoxaluria type 3 [Abstract]. J Inherit Metab Dis 35(Suppl 1):S24
Pitt JJ, Eggington M, Kahler SG (2002) Comprehensive screening of urine samples for inborn errors of metabolism by electrospray tandem mass spectrometry. Clin Chem 48(11):1970–1980
Riedel TJ, Knight J, Murray MS, Milliner DS, Holmes RP, Lowther WT (2012) 4-Hydroxy-2-oxoglutarate aldolase inactivity in primary hyperoxaluria type 3 and glyoxylate reductase inhibition. Biochim Biophys Acta 1822(10):1544–1552
Shih VE (2003) Amino acid analysis. In: Blau N, Duran M, Blaskovics ME, Gibson KM (eds) Physician’s guide to the laboratory diagnosis of metabolic diseases, 2nd edn. Springer, Berlin, pp 11–26
Williams EL, Bockenhauer D, van’t Hoff WG et al (2012) The enzyme 4-hydroxy-2-oxoglutarate aldolase is deficient in primary hyperoxaluria type 3. Nephrol Dial Transplant 27(8):3191–3195
Acknowledgements
We thank Dr. Barry Lewis and Mr. Lawrence Greed (Princess Margaret Hospital for Children, Perth) for supplying oxalate data and retrieving the newborn screening card from patient A and Ms. Avantika Mishra, Ms. Mary Eggington, Mr. Kai Mun Hong and Ms. Maggie Tan for performing analyses. A preliminary report of sections of this work was previously been presented at the SSIEM Annual 2012 Symposium, Birmingham (Pitt et al. 2012). Sections of this work were supported by the Victorian Government’s Operational Infrastructure Support Program.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
Communicated by: Rodney Pollitt, PhD
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Compliance with Ethics Guidelines
Compliance with Ethics Guidelines
Author Contributions
Ruth Belostotsky: performed mutation analysis, provided samples from the Israeli cohort, revised the manuscript
Frank Willis: clinically managed, organised samples and consent for patient A, revised the manuscript
Nicholas Tzanakos: performed dried blood spot analysis, revised the manuscript
Yaacov Frishberg: advised on study design, managed and provided data for the Israeli cohort, contributed to the manuscript
James Pitt: conceived, designed and supervised the study, wrote the manuscript, acts as guarantor
Funding Disclosure: None relevant to this research
Conflicts of Interest
James Pitt, Ruth Belostotsky, Frank Willis, Nicholas Tzanakos and Yaacov Frishberg declare that they have no conflict of interest.
Ethical Approval
Control neonatal dried blood spots were analysed with the approval of the Royal Children’s Hospital Human Research Ethics Committee. This study was also approved by the Helsinki Committee of the Shaare Zedek Medical Center, Jerusalem, Israel.
Consent: Parental consent was obtained for testing the neonatal blood spot from patient A.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Animal Rights: Not applicable, no animal experiments were performed
Rights and permissions
Copyright information
© 2014 SSIEM and Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Pitt, J.J., Willis, F., Tzanakos, N., Belostotsky, R., Frishberg, Y. (2014). 4-Hydroxyglutamate Is a Biomarker for Primary Hyperoxaluria Type 3. In: Zschocke, J., Gibson, K., Brown, G., Morava, E., Peters, V. (eds) JIMD Reports, Volume 15. JIMD Reports, vol 15. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8904_2013_291
Download citation
DOI: https://doi.org/10.1007/8904_2013_291
Received:
Revised:
Accepted:
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43750-6
Online ISBN: 978-3-662-43751-3
eBook Packages: MedicineMedicine (R0)