Abstract
Background
Primary hyperoxaluria type 3 (PH3) is a recently described cause of childhood renal calculi. It results from mutations in the HOGA1 gene and most cases have been diagnosed after clinical ascertainment, exclusion of other genetic hyperoxalurias and mutation testing. Metabolite testing has not been widely applied but holds promise for the rapid screening and diagnosis of patients who are not specifically suspected to have PH3.
Case-Diagnosis/Treatment
Two cases presented with renal calculi. Urine metabolite testing by tandem mass spectrometry was performed as part of the routine diagnostic work-up for this condition. Both had significantly increased levels of the PH3 urine marker 4-hydroxyglutamate and related metabolites. The diagnosis of PH3 was confirmed by the finding of bi-allelic damaging HOGA1 mutations.
Conclusions
Urine screening by tandem mass spectrometry is a rapid, high-throughput test that can detect PH3 cases that may otherwise not be diagnosed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary hyperoxaluria (PH3, OMIM 613616) is caused by loss-of-function mutations HOGA1, resulting in deficiency of the mitochondrial enzyme 4-hydroxy-2-oxoglutarate aldolase which is active in the hydroxyproline catabolic pathway [1, 2]. This enzyme deficiency results in the accumulation of 4-hydroxy-2-oxoglutarate (HOG) within mitochondria. It is proposed that HOG is then transported to the cytosol where it is converted to oxalate by the concerted actions of an aldolase and lactate dehydrogenase [3]. An alternatively proposed mechanism for the production of oxalate is the inhibition of glyoxylate reductase by accumulating HOG [4]. Patients with PH3 typically present in the first few years of life with nephrolithiasis [5]. Renal function in PH3 appears to be relatively intact compared to primary hyperoxaluria type 1 (OMIM 259900) and asymptomatic PH3 individuals have been described [1, 5]. Only a few patients have been reported with compromised renal function [6, 7]. An in silico incidence of 1:136,900 has been estimated for PH3 from the mutant allele frequency [6]. However, exome sequencing (Exac database) detected many additional rare potentially damaging mutations which were not detected in homozygous or compound heterozygous conditions, potentially increasing twice the mutant allele frequency.
Most cases of PH3 have been diagnosed via Sanger sequencing of HOGA1 when there is high index of suspicion, e.g. significant oxaluria or oxalate stones, exclusion of other causes of hyperoxaluria or multiple affected siblings. Mutation testing is harder to justify in patients who may not fulfil the above criteria and is not suited to testing large numbers of such patients. Lately, next-generation sequencing (NGS) has been suggested as a scalable test for all three types of PH. However, this method is still rather expensive and time consuming [8]. Given the relatively large numbers of patients with a low index of suspicion of a genetic oxaluria (e.g. nephrolithiasis or mildly increased oxalate excretion) but who nevertheless require these conditions to be excluded, it would be helpful if there was a cheaper screening technique. We recently showed that 4-hydroxyglutamate (4OHGlu), the immediate precursor of HOG, is a useful PH3 diagnostic biomarker and developed a high-throughput screening method for this metabolite [9]. The current report describes the prospective application of this screening technique which led to the prompt diagnosis of two new cases of PH3 with low indices of suspicion.
Patients and methods
Patients
Patient 1
This male initially presented during infancy in the UK with hyperoxaluria and renal stones requiring lithotripsy. No diagnosis was made at the time but primary hyperoxaluria types 1 and 2 were excluded on the basis of normal excretion of glycolic and glyceric acids. He migrated to Australia and was referred for investigation of long-standing recurrent bilateral renal calculi at the age of 15 years. The calculi were observed on ultrasound and appeared larger than on a previous scan 9 months earlier. A new calculus was also identified in the lower pole of the left kidney. The calculi were noted to be more significant during infancy. The boy was otherwise well with no dysuria, haematuria or urinary tract infections. Between the age of 12 and 15 years, he was noted to have a mild oxaluria (Table 1). There was also an intermittent mild to moderate hypercalcuria of 0.38 to 1.77 mmol/mmol creatinine (reference range 0.04–0.70).
Patient 2
Patient 2 presented at 7 months of age with initial symptoms of dysuria. He was initially thought to have a urinary tract infection but urine culture was negative. A renal ultrasound was performed which showed bilateral renal calculi including a large left calculus obstructing the pelvic-ureteric junction. Urine oxalate was increased in two samples collected at 6 and 6.5 months of age (Table 1). Given the rarity of renal calculi in infancy and the likelihood of this being secondary to a metabolic disorder, patient 2 proceeded to have a full investigation including a urine metabolic screen. He required a left percutaneous nephrolithotomy to remove the left calculus. There was insufficient material for analysis due to the friable and fragmented nature of the stone.
Methods
Urine 4OHGlu was measured by flow injection tandem mass spectrometry in the same laboratory as previously described [9]. Standard urine organic acid screening by gas chromatography-mass spectrometry was also used to measure HOG and 2,4-dihydroxyglutaric in the same laboratory as a confirmatory test. HOGA1 sequencing was performed as previously described [1]. Urine oxalate was measured using an enzymatic method (Trinity Biotech’s Oxalate Kit) in two laboratories. Values were expressed as micromoles per millimoles creatinine and mmol/1.73 m2/day due to reporting differences between the two laboratories used.
Results
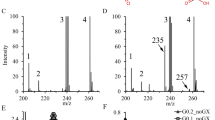
Results are summarised in Table 1. Urine oxalate was increased in multiple samples from both patients although it was only slightly increased in patient 2. Urine screening showed large increases in 4OHGlu, consistent with PH3 in both patients. Follow-up urine organic acid analysis also demonstrated significant increases in the PH3 metabolites 2,4-dihydroxyglutaric and HOG. Urine biomarkers for other genetic nephrolithiasis disorders (cystine, glycolic, glyceric, orotic and xanthine) were normal.
Sequencing of the HOGA1 showed that patient 1 was compound heterozygous for the most frequent PH3 splicing mutation IVS700+5G>T [1, 6] and a novel c.251T>C (Leu84Pro) missense mutation predicted to be damaging by Mutation Taster and PolyPhen-2.
Patient 2 is a compound heterozygote for the p.Glu315del mutation frequently described in PH3 [1, 6] and a novel c.860G>A, p. Gly287Glu missense mutation predicted to be damaging by Mutation Taster and PolyPhen-2. Interestingly, the large family which was the first to be identified as having HOGA1 mutations causing PH3 (family 1 in [1]) had compound heterozygous mutations in the same positions but with another substitution at position 287 (p.Gly287Val).
Discussion
In addition to oxalate, three additional metabolites accumulate in PH3 and appear to be specific for this condition. HOG, the immediate substrate of the deficient enzyme accumulates in urine as well as 2,4-dihydroxyglutaric (the reduced form of HOG) and 4OHGlu, the precursor to HOG in the hydroxyproline catabolic pathway. For the biochemical diagnosis of PH3, measurement of urine HOG by ion chromatography-mass spectrometry [4, 9] and the simultaneous measurement of HOG and 2,4-dihydroxyglutaric acid by gas chromatography-mass spectrometry [10] have been shown to be useful. However, these are specialised, low-throughput tests that are only likely to be applied to the differential diagnosis of patients with significant documented hyperoxaluria. Furthermore, HOG is unstable in urine which may explain the partial overlap of HOG levels between PH3 patients and controls [9]. Also, reliable measurement of urine oxalate is fraught with technical difficulties and which may result in equivocal results. Moreover, patients with PH3 can occasionally have normal or only slightly increased oxalate excretion [11], as exemplified by patient 2, with the potential that PH3 may be overlooked.
We recently showed that urine 4OHGlu is a reliable and stable marker for PH3 that can simply be added to an existing metabolite screening panel for inborn errors of metabolism [3, 9]. It can therefore be applied as a general screening test to diagnose PH3 in situations where oxaluria may subtle or not apparent for the reasons outlined above. Our initial studies indicated 100% specificity and sensitivity when age-appropriate reference ranges are applied [9]. Further studies are needed to determine if this performance will apply for larger-scale, prospective screening and mutation testing of HOGA1 is recommended to confirm all screen positive patients. Metabolite screening also offers the advantage of faster turn-around time and lower cost compared to genetic testing as well as providing a more definitive diagnosis when the patient carries HOGA1 variants of unknown significance. Another advantage of this approach is that this single test can diagnose most genetic causes of renal calculi including PH1, PH2, PH3, cystinuria, xanthinuria and hereditary orotic aciduria as the relevant individual biomarkers can be readily included in the same tandem mass spectrometry analysis [12]. This approach allowed us to rapidly diagnose PH3 in these two cases. The tandem mass spectrometry technology used to measure 4OHGlu is now widely available in many metabolic screening laboratories and we advocate the inclusion of 4OHGlu in metabolite panels used to screen all patients with nephrolithiasis of uncertain aetiology.
Abbreviations
- PH3:
-
Primary hyperoxaluria type 3
- HOG:
-
4-Hydroxy-2-oxoglutarate
- 4OHGlu:
-
4-Hydroxyglutamic acid
References
Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y (2010) Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87:392–399
Monico CG, Rossetti S, Belostotsky R, Cogal AG, Herges RM, Seide BM, Olson JB, Bergstrahl EJ, Williams HJ, Haley WE, Frishberg Y, Milliner DS (2011) Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol 6:2289–2295
Belostotsky R, Pitt JJ, Frishberg Y (2012) Primary hyperoxaluria type III—a model for studying perturbations in glyoxylate metabolism. J Mol Med (Berl) 90:1497–1504
Riedel TJ, Knight J, Murray MS, Milliner DS, Holmes RP, Lowther WT (2012) 4-Hydroxy-2-oxoglutarate aldolase inactivity in primary hyperoxaluria type 3 and glyoxylate reductase inhibition. Biochim Biophys Acta 1822:1544–1552
Ben-Shalom E, Frishberg Y (2015) Primary hyperoxalurias: diagnosis and treatment. Pediatr Nephrol 30:1781–1791
Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, Lieske JC, Milliner DS, Harris PC, Rare Kidney Stone C (2015) Phenotype-genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J Am Soc Nephrol 26:2559–2570
Allard L, Cochat P, Leclerc AL, Cachat F, Fichtner C, De Souza VC, Garcia CD, Camoin-Schweitzer MC, Macher MA, Acquaviva-Bourdain C, Bacchetta J (2015) Renal function can be impaired in children with primary hyperoxaluria type 3. Pediatr Nephrol 30:1807–1813
Feldkötter M, Wei AZ, Langman CB, Ventzke A, Hoppe B (2014) Urinary hydroxy-oxo-glutarate (HOG) as diagnostic factor for primary hyperoxaluria type 3 [abstract SA-OR087]. Annual Scientific Meeting of the American Society of Nephrology. Journal of the American Society of Nephrology, Philadelphia, USA, p 100A
Pitt JJ, Willis F, Tzanakos N, Belostotsky R, Frishberg Y (2015) 4-hydroxyglutamate is a biomarker for primary hyperoxaluria type 3. JIMD reports 15:1–6
Clifford-Mobley O, Hewitt L, Rumsby G (2016) Simultaneous analysis of urinary metabolites for preliminary identification of primary hyperoxaluria. Ann Clin Biochem 53:485–494
Beck BB, Baasner A, Buescher A, Habbig S, Reintjes N, Kemper MJ, Sikora P, Mache C, Pohl M, Stahl M, Toenshoff B, Pape L, Fehrenbach H, Jacob DE, Grohe B, Wolf MT, Nurnberg G, Yigit G, Salido EC, Hoppe B (2013) Novel findings in patients with primary hyperoxaluria type III and implications for advanced molecular testing strategies. Eur J Hum Genet 21:162–172
Pitt JJ, Eggington M, Kahler SG (2002) Comprehensive screening of urine samples for inborn errors of metabolism by electrospray tandem mass spectrometry. Clin Chem 48:1970–1980
Acknowledgements
We thank Robert Dunlop, Ava Mishra, Kai Mun Hong, Mary Eggington and John Odontiadis for their expert assistance with metabolite screening.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Greed, L., Willis, F., Johnstone, L. et al. Metabolite diagnosis of primary hyperoxaluria type 3. Pediatr Nephrol 33, 1443–1446 (2018). https://doi.org/10.1007/s00467-018-3967-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-3967-6