Abstract
The field of vaccinology underwent massive advances over the past decades with the introduction of virus-like particles (VLPs), a supra-molecular nanoparticle vaccine platform that resembles viral structures without the ability to replicate in hosts. This innovative approach has been remarkably effective, as evidenced by its profound immunogenicity and safety. These highly desirable intrinsic properties enabled their further development as vaccines against a multitude of diseases. To date, several VLP-based vaccines have already been commercialized and many more are undergoing clinical evaluation prior to FDA approval. However, efficacious vaccines against a plethora of pathogens are still lacking, which imposes a tremendous socioeconomic burden and continues to threaten public health throughout the globe. This is especially the case for several respiratory pathogens and protozoan parasites. In this review, we briefly describe the fundamentals of VLP vaccines and the unique properties that enable these to be such valuable vaccine candidates and summarize current advances in VLP-based vaccines targeting respiratory and parasitic diseases of global importance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Massive advances to the biomedical science have been made in the twenty-first century, resulting in the emergence of novel molecular technologies such as the construction of virus-like particles (VLPs). Vaccinology in particular harnessed the full power of these innovative approaches and application of these concepts invigorated vaccine development and research against infectious pathogens. Importantly, the virus-like particle (VLP) vaccines are of keen interest for vaccine design strategy due to several intrinsic properties that render VLPs more effective than the traditional vaccines. Traditionally, vaccine design strategies were predominantly based on using inactivated or live-attenuated pathogens but several drawbacks to these production methods have fueled and accelerated the development of alternative strategies. Attenuated vaccines are derived from weakened pathogens, and though these vaccines are capable of inducing long-term immunity, the possibility of the pathogen reverting to its virulent wild-type form remains a challenge to be addressed. Applying heat or formaldehyde to inactivate pathogens for use as vaccine components is much safer than the attenuated vaccines, but undesirable side effects including exuberant inflammation have necessitated the need for a safer vaccine (Gause et al. 2017). Moreover, vaccines developed using these methods were only effective against pathogens with relatively simple pathogenesis, whereas such strategies were deemed less effective against pathogens involving complex life cycles or host–pathogen interactions (Jennings and Bachmann 2008). Subunit vaccines expressing carbohydrate and protein antigens are much safer than these traditional vaccines, but their safety aspect comes at the cost of diminished immunogenicity (Moyle and Toth 2013). The VLPs are an exemplification of efficacious vaccines possessing favorable parameters of both subunit and traditional vaccines, notably the high immunogenicity of traditional vaccines and the safety aspect of subunit vaccines as represented by their replication-deficient nature.
The first VLP vaccines were constructed in the 1980s against hepatitis B virus (HBV). Only years later, the first VLP-based vaccine was approved for clinical use and many others followed. Over the past decades, with enormous improvements to genetic engineering technologies, VLP vaccine design strategies have made giant strides to achieve several developmental milestones (Plotkin 2014; Lua et al. 2014). Currently, a diverse array of VLP formulation methods have been developed and computational tools are actively being employed to enhance vaccine immunogenicity. VLP-based vaccines are being extensively studied as prophylactic tools against many microbial organisms. These include VLP-based vaccines against hepatitis A virus (HAV), hepatitis B virus (HBV), and human papillomavirus (HPV), all of which are commercially available while many other are undergoing clinical evaluation (Kushnir et al. 2012). Despite these endeavors, vaccines against etiological agents responsible for some of the most important diseases are still lacking. Viruses and parasites are especially of concern for causing a high rate of mortality throughout the globe. Influenza and respiratory syncytial virus (RSV) infections have been viral-borne respiratory diseases of importance and VLP-based vaccines are currently lacking for these pathogens or vaccine in general in the latter case. A vaccine against neglected but important tropical diseases such as malaria and toxoplasmosis is also direly needed, but decades of developmental effort have remained unfruitful. In this chapter, we will describe the VLP technology, and review the current progress in several major infectious diseases lacking an efficacious VLP vaccine, and highlight global attempts to develop one from both pre-clinical and clinical standpoints.
2 Virus-Like Particles (VLPs) and Properties Enabling Immunity Induction
VLPs are self-assembled highly immunogenic particles that mimic the morphology of the natural virus particle while lacking the genetic components required for replication. These particles are organized into repetitive elements, usually comprising a foreign antigen and a base platform. This repetitive aspect is of particular importance as mounting an efficient antibody response to foreign antigen epitope requires the foreign epitope to be expressed repetitively on a particle surface (Fehr et al. 1998; Bachmann et al. 1993). Importantly, the highly repetitive and densely presented antigens serve as a pathogen-associated structural pattern (PASP) that facilitates direct cross-linking with the B cell receptor without T cell assistance (Mohsen et al. 2017a; Brune and Howarth 2018).
Another factor that contributes to their application in vaccines is their small size. Ranging anywhere between 20 and 200 nm, these particles can be easily trafficked and they rapidly accumulate in the lymph nodes (Manolova et al. 2008). Interestingly, upon immunizing mice with bacteria-derived VLPs, the VLPs were found to have accumulated in the popliteal lymph nodes of the immunized mice in less than 10 min (Mohsen et al. 2017b). In the case of porcine parvovirus VLPs, it took less than 2 h for half of the entire murine splenic dendritic cells to be presented with the antigen from VLPs (Morón et al. 2002). In addition to their rapid drainage into the lymph nodes for enhancing B cell response, VLP vaccines also induce cytotoxic T cell response via major histocompatibility complex cross-presentation (Brune and Howarth 2018; Gomes et al. 2017). Moreover, particulate vaccines such as VLPs generally possess charged surfaces with intrinsic properties enabling receptor interaction, which allows enhanced interaction with APCs such as DCs than solubilized proteins (Bachmann and Jennings 2010).
2.1 VLP Production System
The VLP production and expression systems and the pitfalls associated with each system have been extensively described elsewhere (Kushnir et al. 2012; Zeltins 2013; Charlton Hume and Lua 2017; Donaldson et al. 2015). In brief, VLPs can be produced in a variety of prokaryotic and eukaryotic cells which includes bacterial, yeast, mammalian, insect, or plant cells. Each of these production strategies has its own limitations and all of these production methods require the presence of viruses for proper icosahedral structured VLP production. These viruses include the hepatitis B virus (HBV), bacteriophages MS2, Qβ, P22, the plant viruses cowpea chlorotic mottle virus (CCMV) and cowpea mosaic virus (CPMV), and the baculovirus system in insects.
Escherichia coli, along with other members in the family Enterobacteriacea, is highly sought after for VLP production. Infection of these bacteria using the bacteriophages MS2, Qβ, and the Salmonella typhimurium P22 is required to drive the VLP production process (Rohovie et al. 2017). Although bacteria-derived recombinant proteins are widely utilized for their low production cost and high yields, their use for VLP production is not recommended which can be attributed to the absence of mammalian-like post-translational modifications (PTM), and formation of inclusion bodies. Bacteria-derived VLPs are also unable to form proper disulfide bonds, have issues with protein stability and solubility, and the presence of endotoxins renders their use unacceptable for clinical trials (Zeltins 2013). The yeast VLP expression system is a bit more complex than bacteria, which shares many of the features of bacteria-derived VLPs, with the presence of notable differences in PTM especially glycosylation (Kushnir et al. 2012). However, the overall yield is much lower than bacteria-derived VLPs.
Numerous transgenic plants have been reported to date as potential source of VLP production (Bragard et al. 2000; Huang et al. 2006; Mason et al. 1996). Plant cells can produce VLPs at a higher yield at a low cost, but as with above, antigen glycosylation and other PTM processes are issues that need to be overcome (Lomonossoff and D’Aoust 2016). There are also stability issues such as antigens being degraded during in vivo delivery, which limits its applicability (Roldão et al. 2010). Plant-based system using Nicotiana sp. has been of concern due to the toxic alkaloid build-up. However, this issue has been overcome by crossing two transgenic Nicotiana spp. (Ling et al. 2012). VLPs produced using mammalian cells may be the optimal choice, as folding and PTM of complex proteins may not be an issue. Nevertheless, the mammalian cell approach is the most expensive method and requires stringent quality control, as they are prone to passenger virus infections (Plotkin et al. 2017). Other factors hindering its use are slow growth and low yield (Donaldson et al. 2015).
The baculovirus is a DNA virus that naturally infects insect cells. Although they can be transduced using mammalian cells, baculoviruses are incapable of replicating in mammalian cells and cytotoxicity is virtually none. Because these are non-pathogenic to humans, these have been widely utilized in the field of biomedical science (Luo et al. 2013). Baculovirus expression system using insect cells is quite frequently used and is a powerful tools for producing VLP vaccines (Fig. 1). Insect cells enable high production yields in relatively short-time frames and are much safer and easier to handle than mammalian cells, since several known human pathogens are absent. Commonly used cell lines are the insect cells belonging to the Lepidoptera family, notably Sf9 or Sf21 cells from Spodoptera frugiperda and High Five™ cells from Trichoplusia ni (Liu et al. 2013; Puente-Massaguer et al. 2020). Though this production method still remains as a favorable method of choice, there are limitation to this production method. First and foremost, co-production of enveloped baculovirus particles and other contaminants has been reported to impair vaccine efficacy. Consequently, these have to be removed through chemical inactivation or via downstream processing procedures that further affects the immunogenicity of VLPs (Rueda et al. 2000; Hervas-Stubbs et al. 2007). As with plant cells, insect cell-derived VLPs using baculovirus also have PTM-associated issues most notably the ones involving N-glycans. Although transgenic insect cell lines are currently being researched to overcome this problem, this still remains a work in progress and further developments are required (Brune and Howarth 2018).
Structure and production schematic of insect cell-based VLP vaccines. a A structural representation of a typical virus-like particle vaccine, which illustrates the matrix protein surrounded by the phospholipid bilayer and the repeated expression of antigens on the surface. b A VLP vaccine production schematic using the baculovirus expression system. Briefly, the antigen gene of interest needs to be cloned into the pFastBac™ vector and subsequently transformed into the DH10Bac competent cell. The helper plasmid and the baculovirus shuttle vector allows transposition to occur within the DH10Bac cells, resulting in a recombinant bacmid DNA. This bacmid DNA is then transfected into the Spodoptera frugiperda (Sf9) insect cell for recombinant baculovirus production. Finally, co-transfecting the recombinant baculovirus expressing the matrix protein with the recombinant baculovirus containing the antigen of interest into the insect cell results in the production of VLPs that can be used as vaccines post-purification
One of the latest methods for modular VLP vaccine design is based on the Tag/Catcher system introduced by Zakeri et al. (2012). The fundamental concept underlying this technology is the formation of an intramolecular isopeptide bond, which is frequently found in the collagen-binding adhesin (Cna) domains of the cell surface proteins belonging to numerous Gram-positive bacteria (Kang and Baker 2011). These isopeptide bonds were also observed in the CnaB2 domain of fibronectin-binding protein (FbaB) in Streptococcus pyogenes, where the lysine and the aspartate residues spontaneously formed a covalent bond of phenomenal strength that were resilient to diverse environmental conditions (Zakeri et al. 2012). Based on this finding, genetic fusion of the antigen of interest to the SpyTag and attaching this component to the SpyCatcher-expressing core protein would enable easy construction of VLPs.
2.2 Advantages and Disadvantages of VLPs Compared to Other Vaccine Platforms
There are multiple aspects that render VLP-based vaccines more effective than traditional vaccines. Since VLPs are completely devoid of pathogenic genomic material, replication is impossible and are therefore safe for use (Chackerian 2007). Furthermore, as discussed earlier, their structural resemblance to viruses and the geometric arrangement of highly repetitive antigens enables potent immune response induction (Fehr et al. 1998; Bachmann et al. 1993). Compared to other vaccines, protective efficacies are much greater in VLP vaccines than traditional vaccines. VLPs can be rapidly produced using various expression systems with robust, scalable production systems that are highly cost-efficient (Zhang et al. 2014). Currently, commercially available VLP-based vaccines are generally produced using the baculoviruses expression system or yeast expression system. One advantage of using the insect baculovirus system is that they are unable to replicate in mammalian cells, which further supports the notion of the VLPs being safe for use (Hervas-Stubbs et al. 2007). With regards to immunogenicity, VLPs generally do not require adjuvant usage as these are highly immunogenic by themselves. However, by supplementing these vaccines with adjuvants, the vaccine efficacy can be further enhanced.
There are several down-sides to the VLP-based vaccines. First and foremost, their cost of production is rather expensive compared to other vaccine platforms. Secondly, other factors such as downstream purification processes are required, as the presence of host cellular impurities can be detrimental during clinical trials (Effio and Hubbuch 2015). Besides cost, the purification steps also add a time penalty to vaccine production. There is also potential of side effects associated with VLP use. Although rare, HBV VLPs produced using yeast were reported to have elicited anaphylaxis in several patients (DiMiceli et al. 2006).
3 Influenza Virus
The influenza virus belongs to the Orthomyxoviridae family, which consists of the genera Alphainfluenzavirus, Betainfluenzavirus, Gammainfluenzavirus, and Deltainfluenzavirus along with few others. Its annual impact places a tremendous economic burden, with direct medical costs reported to be $10.4 billion in the USA alone (Molinari et al. 2007). Avian influenza is also of importance as its outbreaks can lead to the culling of millions of poultry that devastates the poultry industry and animal health. Circulating avian influenza virus has resulted in the culling of 400 million birds and the economic loss incurred was estimated to be around $20 billion USD (Short et al. 2015). With the continuous threat from annual influenza, an effective influenza vaccine that can be rapidly manufactured has become a priority. In 2013, the seasonal influenza vaccine FluBlok was approved for clinical use. This recombinant vaccine, unlike the previous vaccines manufactured from embryonated eggs, was produced using the insect cell Sf9 with high immunogenicity (Liu et al. 2013). With several advantages of the insect cell-based vaccine system, other studies are following suit and investigators are starting to shy away from the traditional egg-based vaccines which have been the standard for over 50 years (Cox and Hollister 2009). To date, multitudes of influenza VLP vaccine studies have been conducted and the overall findings were generally consistent, with vaccines inducing potent antibody responses and protection.
3.1 Influenza Vaccine Components
There are several genes of antigenicity that are frequently utilized as vaccine components. Among the genes encoded by the influenza virus, the most well-characterized proteins are the hemagglutinin (HA) and neuraminidase (NA). Apart from these, the virus also encodes several other structural and non-structural proteins such as the nucleoprotein (NP), matrix protein (M1), ion channel protein (M2), non-structural proteins (NS1, NS2), and several polymerase complexes (PB1, PB2, PA) (Jang and Seong 2020). Nevertheless, the highly mutating nature of influenza surface glycoproteins has become nothing more than a nuisance for vaccine development. Currently, the main targets for the commercialized influenza virus vaccines are the globular head domains of the HA. This has become problematic and research focusing on other areas that are more conserved has been emerging. Several examples of these conserved regions are the stalk domain of HA, the ectodomain of the ion channel (M2e), domains of NA, and even internal structural proteins such as M1 and NP (Krammer et al. 2018).
3.2 Seasonal Influenza VLP Vaccines
Although strategies for VLP construction differ between research groups, the overall result seems to be more or less similar. Nitrocellulose membrane-based filtration was used to concentrate the insect-derived VLPs expressing the HA and NA of the highly pathogenic avian influenza (HPAI) A/ Korea/ Mallard/W452/2014) H5N8. This filtration process did not affect the immunogenicity of the vaccines, as immunized mice mounted strong virus-specific antibody responses (Park and Song 2017). Plant cell-based VLPs are also being investigated as a potential influenza vaccine candidate. The plant Nicotiana benthamiana has been widely used to generate stable and highly immunogenic VLPs. Their immunization in mice even elicited protection in old mice that were experiencing multiple co-morbidities, indicating its potential for conferring protection in the elderly (Lindsay et al. 2018; Hodgins et al. 2019). A cocktail VLP vaccine constituted of H1, H3, H5, or H7 HAs conferred broad protection against several subtypes of influenza A virus, which include the 1918 H1, 1957 H2, as well as the avian H5, H6, H7, H10, and H11 subtypes (Schwartzman et al. 2015). NA VLP generated using the A/PR/8/34 (H1N1) successfully protected mice against both homologous and heterosubtypic lethal challenge infection (Quan et al. 2012).
Another strategy is utilizing computationally optimized broadly reactive antigen (COBRA). Using this method, the codon-optimized HA VLPs were produced in mammalian cells which were used to immunize mice and ferrets. Vaccinated animals were completely protected from lethal challenge infection with the clade 2.2 H5N1 virus A/Mongolia/Whooper Swan/244/2005 (Giles and Ross 2011). The key difference here is that ferrets were involved rather than simply testing the VLP vaccine efficacy in mice. This is crucial since the murine immune system is not an accurate depiction of human immunity. Through these results, the potential for VLPs as a seasonal influenza vaccine is quite encouraging.
3.3 Pandemic Influenza VLP Vaccine
Influenza A virus pandemics have occurred several times within the last century. Since its first report in 1918 with the H1N1 influenza virus, three other pandemics occurred in the years 1957, 1968, and 2009 involving the H2N2, H3N2, and H1N1pdm, each respectively (Viboud et al. 2020). These pandemics, which occurred in the twentieth and twenty-first century incurred millions of deaths (Ryu and Cowling 2020). Currently, developing pandemic influenza vaccines is of concern since the efficacy of seasonal influenza vaccines against novel pandemic influenza A virus is extremely low or negligible at best. This development process is further hampered through the time required for development upon correct matching of circulating pandemic vaccine strain, which can take up to six months (Krammer and Palese 2015; Krietsch Boerner 2020).
Several VLP vaccines against pandemic 2009 H1N1 have been constructed using the baculovirus expression vector system, and the conferred immunity enabled protection in both mice and ferret models (Quan et al. 2010; Song et al. 2011). HA VLPs containing the transmembrane domain of H3N2 exhibited protection against H7N9 challenge, a strain that has been perceived to have numerous outbreaks in countries such as China (Qin et al. 2018). VLPs targeting avian influenza have also been generated and they were proven to be efficacious in both murine and ferret models (Song et al. 2010). Replacing the influenza M1 protein with another protein from a different virus could be a potential vaccine design strategy. Using the gag protein isolated from bovine immunodeficiency virus (Bgag), VLPs expressing HAs of H5N1, H7N9, H9N2, and H10N8 have been constructed (Tretyakova et al. 2016). With multiple HA subtypes being expressed, this vaccine could potentially serve as a first line of defense upon avian influenza pandemic after efficacy confirmation in animal models.
3.4 Universal Influenza VLP Vaccine
Designing a universal influenza vaccine that broadly protects against various subtypes is highly desired, but the process has remained challenging simply due to the nature of the virus. RNA viruses such as the influenza virus use its own RNA polymerase for replication, which lacks fidelity and is quite error-prone. Consequently, this frequent build-up of mutations results in an antigenic drift that renders the host immune system ineffective since antibodies are unable to recognize the viral antigenic site (Zambon 1999). This is particularly important as exemplified during the 2014/2015 influenza season. At the time, the most prevalent strain circulating around the globe for H3N2 was A/Texas/50/2012, but by the end of the year, antigenic drift caused a transition from this strain to A/Switzerland/9,715,293/2013 being the most prevalent strain. Consequently, this resulted in suboptimal vaccine efficacy estimated to be 18% for the 2014/2015 season (Berlanda Scorza et al. 2016).
Numerous attempts have been made to produce a universal influenza vaccine conferring long-lasting cross-protection against various subtypes using the more conserved antigens aforementioned. Bivalent heterologous vaccine regimen involving immunization with both DNA vaccine and VLPs conferred broad protection against a variety of influenza subtypes. Vaccination using this method ensured the production of antibodies that bound to HAs from both group 1 and group 2 (Jiang et al. 2017).
Apart from this combinatorial vaccination approach, the VLP vaccines containing the heterologous tandem repeats of the M2 extracellular domain (M2e) were found to be highly cross-protective (Kim and Song 2013; Kim et al. 2014, 2017). Supplementing several vaccines, whether it be live-attenuated or pandemic split vaccines, with the M2e heterologous tandem repeat VLPs enhanced the efficacy of vaccines (Lee et al. 2019a). The efficacies of these M2e VLPs were further assessed in ferrets and chickens, which better reflect human and avian influenza pathogenesis, and the results appeared promising (Music et al. 2016; Song et al. 2016). The development of influenza VLP vaccines and its protective efficacies have been extensively reviewed elsewhere (Quan et al. 2020).
3.5 VLP Vaccines for Clinical Trials
To date, numerous clinical trials have been conducted using VLPs and most of the clinical results seem to point toward the conclusion that VLPs are highly immunogenic and safe for use in humans. An alum-adjuvanted plant-derived VLP vaccine expressing the HA protein of H5N1 (A/Indonesia/5/05) successfully induced cross-protection in ferrets and humans. Notably, in the clinical phase I trial, the VLPs were found to be safe and highly immunogenic as indicated by hemagglutination inhibiton (HI) and microneutralization responses, thereby paving the way for further evaluation. With regard to side effects, most of the symptoms such as pain at the immunization site were mild to self-limiting at best (Landry et al. 2010). In an FDA-approved clinical trial phase I/II study, immunization with the clade 2.1 H5N1 VLP expressing HA, NA, and M1 induced the production of antibodies, which conferred cross-protection against the other clade 2 subtypes (Khurana et al. 2011). VLP vaccines against other avian influenza strains, notably H7N9, have been on-going. Adjuvanted-VLP vaccines expressing H7 antigen promoted enhanced production of neutralizing antibodies compared to unadjuvanted VLPs, even at a lower dose (Chung et al. 2015).
With the start of the 2009 H1N1 influenza outbreak occurring in Mexico, there was a dire need for a vaccine but a vaccine became available in the USA, five months after the start of the outbreak. Phase II clinical trial results for the VLP vaccine against the 2009 H1N1 (A/California/04/2009) revealed that high HI titers were induced in the vast majority of the participants and were overall well-tolerated (López-Macías et al. 2011). Similar findings were observed from clinical trials conducted in Singapore using E. coli-derived VLP vaccine against H1N1 (A/California/07/2009). This bacteria-derived VLP vaccine successfully elicited influenza-specific CD4+ and CD8+ T cell responses following vaccination, along with a plethora of cytokines (Low et al. 2014; Skibinski et al. 2018). Though the results of this study appear promising, clinical evaluation involving human challenge infection with the influenza virus is required to confirm its protective efficacy. Recently, the immunogenicity and safety aspect of a quadrivalent VLP vaccine has been confirmed in the phase II clinical trial. The quadrivalent VLPs expressing the HA proteins of H1N1 (A/California/07/2009), H3N2 (A/Victoria/361/11), the Victoria lineage B/Brisbane/60/08, and the Yamagata lineage B/Massachusetts/02/2012 were produced in plants. Immunizing the participants with 30 μg of this VLPs ensured strong induction of both humoral and cellular immune responses and will be subjected to phase III clinical trial in the near future (Pillet et al. 2019).
Immunizing participants with the insect-derived 2009 H1N1 VLPs ensured durable antibody response that persisted up to 25 months post-immunization, and re-vaccinating these participants with the trivalent inactivated influenza vaccine enhanced the protective antibody levels (Valero-Pacheco et al. 2016). Several clinical trials assessing the efficacies of both H1 (A/California/07/2009) and H5 VLPs (A/Indonesia/5/05) have reported similar results. Immunization with H5 VLPs mounted durable antibody and cellular immune responses, while subjects immunized with H1-expressing VLP produced in plants induced stronger CD4+ T cell responses than patients receiving placebo or the trivalent inactivated vaccine (Landry et al. 2014). A clinical evaluation assessing the humoral immune response to glycosylated H1 and H5 VLPs revealed that their immunization did not exacerbate pre-existing allergic reactions nor incurred allergies or other hypersensitivity symptoms (Ward et al. 2014).
4 Respiratory Syncytial Virus (RSV)
RSV was first isolated in 1956 but designing a vaccine for this virus has remained a monumental task. In 1966, clinical trial using formalin-inactivated RSV (FI-RSV) vaccine sensitized the patients and eventually led to the death of two children (Efstathiou et al. 2020). Since this medical failure nearly half a century ago, there was a hesitancy for conducting RSV vaccine-related studies. However, with the resurgence of novel vaccine design platforms enabling improvements to previously utilized vaccination strategies, the effort to design an RSV vaccine has been re-ignited. To date, RSV infection still remains as a major risk factor for lower respiratory tract infection (LRTI)-related hospitalizations for infants. Statistically, RSV accounts for approximately 28% of all acute LRTI cases and 22% of all LRTI-related mortality in children (Shi et al. 2017). In the year 2017, the global cost estimate for RSV-associated LRTI management in children under five years of age was determined to be approximately $5.2 billion USD (Eichinger et al. 2020; Zhang et al. 2020). For these reasons, developing a successful RSV vaccine has become a global necessity.
4.1 RSV VLP Vaccine Antigen Components
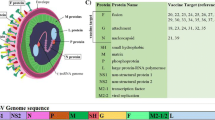
RSV is a negative-sense RNA virus consisting of ten genes that encode a total of 11 different proteins. The transmembrane proteins that are expressed on the viral surface are the fusion glycoprotein (F), attachment glycoprotein (G), and the small hydrophobic protein (SH). Other internal proteins encoded by the virus include the nucleoprotein (N), RNA polymerase (L), phosphoprotein (P), matrix protein (M), the transcription factors (M2-1, M2-2), and non-structural proteins (NS1, NS2) Efstathiou et al. 2020; Collins et al. 2013). Of the three proteins expressed on the surface, F and G proteins are more extensively studied than the SH protein, as both of these are capable of inducing effective antibody response (Ha et al. 2020; Cane 2001). The SH protein has also been tested as an antigen candidate, but their immunogenicity upon natural infection with the RSV was deemed weak as indicated by the low immunoglobulin levels in both humans and murine models (Akerlind-Stopner et al. 1993; Schepens et al. 2014).
Although the F protein is more conserved in comparison to the G protein, both are capable of inducing effective antibody responses. Structural analysis results have revealed that the RSV F protein can exist in either pre-fusion or a post-fusion conformation. Of the two conformations, majority of the research was focused on the pre-fusion (Pre-F) form since most of the neutralizing activity was directed here rather than the post-fusion form. One antigenic site of interest in the Pre-F protein is the “site Ø,” which was shown to elicit the strongest neutralizing antibody response against RSV (Ngwuta et al. 2015; Frey et al. 2020). Evidently, the antibody response induced through site Ø was as much as 100 times more potent than those induced upon treatment with the commercialized monoclonal antibody palivizumab (McLellan et al. 2013). Despite these insightful findings, it was revealed that site Ø was the least conserved site on the F protein in the A and B subtypes, while the regions outside of site Ø retained 96% similarity across subtypes (McLellan et al. 2013).
4.2 Experimental VLP-Based Vaccines for RSV
Preliminary RSV VLP vaccines tested in animal models appear to be promising. Thus far, various VLPs have been produced presenting F, G, or the two combined using numerous platforms. Chimeric VLPs were among the first VLP vaccines developed against RSV. Chimeric VLPs constructed using the newcastle disease virus (NDV) and RSV F or G proteins have demonstrated interesting results. The ectodomain of RSV G protein fused to the NDV HN protein stimulated immune response comparable to those induced by UV-inactivated RSV vaccine without exerting much side effects. Similar findings were also observed from chimeric VLP constructed using the RSV F protein fused to the NDV F protein, with the absence of pulmonary pathologies (Murawski et al. 2010; McGinnes et al. 2011). Recent investigation using this chimeric VLP elucidated that mutations stabilizing the structure of RSV F protein in its Pre-F conformation induce greater titer of antibody responses to cope with subsequent RSV infection (McGinnes Cullen et al. 2015). Immunizing mice that were previously exposed to RSV with this chimeric VLP vaccine resulted in a drastic increase in protective antibody titers (McGinnes Cullen et al. 2019). Most recently, these chimeric VLPs were used to assess the anti-RSV immunity and protective efficacy via maternal immunization in cotton rats. Immunized dams gave birth to pups that were also protected from RSV challenge, indicated by lessened pulmonary inflammation (Blanco et al. 2018).
Immunizing the mice twice with the either RSV F or G protein expressed on influenza matrix protein 1 (M1) conferred significant protection against RSV A2 challenge infection, with enhanced viral clearance being observed from RSV G VLP (Quan et al. 2011). In both mice and cotton rats, combining both RSV F and G protein elicited enhanced protection without undesirable side effects, whereas RSV G protein immunization alone exacerbated the pulmonary inflammatory damage incurred by RSV challenge infection (Lee et al. 2014; Hwang et al. 2017a). Several VLPs displaying tandem repeats of the RSV G protein suppressed pulmonary eosinophilia and viral titers in mice (Kim et al. 2018). VLPs constructed using this method have been applied with other vaccines and this heterologous immunization strategy appeared to be promising. Co-immunizing mice using these VLPs along with an RSV F DNA vaccine conferred durable antibody responses and protection while mitigating the inflammatory cell influx responsible for lung pathology (Hwang et al. 2014; Ko et al. 2015). In line with this notion, identical findings were also observed from cotton rats immunized using this strategy (Hwang et al. 2016). Interestingly, priming mice with RSV F VLPs then boosting with FI-RSV vaccine prevented the development of vaccine-enhanced respiratory diseases (Hwang et al. 2017b). Both active and passive immunization with the insect cell-derived recombinant RSV F VLPs conferred broad protection against RSV A and B subtypes in cotton rats (Raghunandan et al. 2014).
A comparative study assessing the efficacy of RSV F VLP to those of FI-RSV and live-attenuated RSV vaccines revealed interesting findings. In contrast to the latter two, RSV F VLP vaccine immunization elicited RSV neutralizing antibody response, CD8α+ and CD103+ dendritic cells while minimizing the pulmonary damage incurred by RSV challenge infection (Kim et al. 2015). Similarly, while a similar degree of neutralizing antibody responses was observed from both RSV F VLPs and F soluble protein, immunization with the former prevented vaccine-enhanced respiratory diseases while the latter was associated with lung histopathology upon RSV challenge (Lee et al. 2017a). The efficacies of a VLP vaccine generated using the matrix protein of human metapneumovirus (hMPV) matrix protein (M) expressing Pre-F, Post-F, or both were assessed in BALB/c mice. Contrary to the effects observed from FI-RSV vaccine immunization, VLP vaccines suppressed pulmonary inflammation. Interestingly, VLP vaccine displaying both Pre-F and Post-F immunogens induced the highest level of neutralizing antibody response, as well as the Th1 response required for viral clearance (Cimica et al. 2016).
Immunizing cotton rats with mammalian cell-derived VLPs expressing the RSV F and G proteins elicited potent neutralizing antibody responses that enhanced viral clearance in both lower and upper respiratory tracts (Walpita et al. 2015). RSV VLPs expressing the various forms of F and G proteins have been produced using different platforms: through one VLP expressing M + P proteins and the other expressing M + M2-1 proteins (Ha et al. 2020). The F and G proteins, regardless of how they were presented as truncated form, full length, or peptide form, induced RSV F and G protein-specific antibody responses and lessened the lung viral titer upon challenge infection in mice (Ha et al. 2020). In cotton rats, pre-fusion F VLPs conferred significant protection against RSV in the offspring of immunized dams (Cullen et al. 2020). As with the influenza virus VLP vaccines, the protective efficacies of these aforementioned RSV VLP vaccines have been thoroughly reviewed elsewhere (Quan et al. 2020).
4.3 RSV Vaccines for Clinical Trials
Clinical VLP-based RSV vaccine studies are extremely limited. The most advanced vaccine is the VLP vaccine manufactured from Novavax using the Sf9/rBV technology (Mazur et al. 2018). This VLP vaccine, which expresses the F protein of RSV, has recently completed the phase III clinical evaluation which involved the immunization of 4,636 healthy pregnant women. The pregnant women were intramuscularly immunized with a single dose of RSV F protein VLP vaccine and the infants were followed up to a year for LRTI and safety assessment. Overall, although the vaccines were safe and incurred minimal adverse events, the vaccine failed to meet its pre-specified success criterion (Madhi et al. 2020). Despite these results, the vaccines are promising and further improvements will be required prior to FDA approval. Apart from this vaccine, the most recent RSV VLP vaccine that will be undergoing phase I clinical trial is the synthetic VLP vaccine V-306 [ClinicalTrials.gov ID: NCT04519073].
5 Toxoplasmosis
The causative organism of toxoplasmosis is the protozoan parasite Toxoplasma gondii which belongs to the phylum Apicomplexa. It has been reported that virtually all animals, including but not limited to mammals and avians, are capable of being infected by this parasite (Black and Boothroyd 2000). To date, due to their ubiquitous nature, more than 1 billion individuals throughout the globe have been estimated to be infected with T. gondii. Furthermore, T. gondii infection is one of the most common parasite-associated food-borne illness which requires hospitalization (Pappas et al. 2009; Montazeri et al. 2020). Though majority of the infected individuals remain asymptomatic with mild flu-like symptoms, their presence can have devastating consequences which can be fatal at times. In particular, acquired immune deficiency syndrome patients and pregnant women are especially at risk of encephalitis and congenital toxoplasmosis. Their infection in livestock can result in abortion or stillbirth, thus inflicting substantial damage to the agricultural industry (Wang et al. 2019). To lessen the socioeconomic burden incurred by T. gondii, vaccines are urgently needed. Thus far, despite decades of research, clinical toxoplasmosis vaccine remains unavailable while a commercial live-attenuated vaccine is available for ovines (Dubey 2009).
Currently, a variety of strategies have been implemented to develop T. gondii vaccines for clinical use. Traditional vaccines based on inactivated parasites have failed to demonstrate efficacy in multitudes of the models tested to date, while live-attenuated vaccines still face safety concerns that renders them unacceptable for use in humans as attenuated strains can revert back to virulent wild type. An extensively large number of protein families belonging to T. gondii have been tested as potential vaccine candidates (Rezaei et al. 2019). The major antigens used in these recombinant subunit vaccines include members of the dense granule antigens (GRA), microneme proteins (MIC), rhoptry proteins (ROP), and the surface antigens (SAG) (Li and Zhou 2018). Vaccines expressing these antigens were capable of eliciting sufficient antibody responses but failed to confer full protection against pathogens, and as such, further development is required. DNA vaccines have also proven to be effective, but the immune response induced in animals has been elicited to varying degree. Particularly, immunization with DNA vaccine in large animals such as ovines have demonstrated subpar efficacy (Li and Zhou 2018). In comparison to the aforementioned strategies, VLP-based T. gondii vaccines have proven to be highly efficacious, often conferring full protection against lethal challenge infection. Further efficacy testing in higher-order eukaryotes is still needed, but the future of T. gondii VLP vaccines appears promising.
5.1 Toxoplasma Gondii Antigens and VLP Vaccines
Numerous T. gondii vaccine studies have been conducted using DNA, recombinant protein subunit, or live-attenuated vaccines. Contrary to this, VLP-based T. gondii vaccine studies are extremely limited in number and all of them are experimental vaccines that have not been clinically assessed. Although different vaccine platforms were used, these earlier vaccine studies have delineated the antigenicity of numerous parasitic proteins that may serve as potential vaccine candidate antigens. Among the numerous antigen candidates reported to date, studies were predominantly focused on proteins involved in the parasite’s motility, replication, host cell attachment, or invasion. Examples of these include the T. gondii GRA, MIC, ROP, SAG, and many others such as the apical membrane antigen (AMA) (Wang et al. 2019).
One of the earliest VLP-based T. gondii vaccines was developed using the inner membrane complex expressed on the spherical influenza virus M1 protein (Lee et al. 2016a, b). Using an identical format, several other vaccines expressing the rhoptry proteins were constructed and their efficacies were assessed in mice (Lee et al. 2017b). Other antigens that are involved in the host cell invasion by the T. gondii were expressed using VLPs and their efficacies were assessed (Kim et al. 2020). The efficacies of these VLPs were further enhanced by expressing multiple antigens (Lee et al. 2018a, b; Kang et al. 2020,2019). Chimeric VLP vaccines only conferred partial protection in mice against the virulent T. gondii RH strain (Guo et al. 2019). Overall, all of the aforementioned VLP vaccines were tested in mice with varying degrees of efficacies (Table 1). Therefore, further evaluation and improvements to the T. gondii VLP vaccines are required prior to clinical trials.
6 Malaria
Malaria still remains as one of the deadliest diseases affecting humans. In the year 2019, the number of malaria incidences was 229 million cases worldwide, and the number of deaths was estimated to be 409,000, with 67% of the deaths occurring in children under five years of age (World Health Organization 2020). Decades of effort have enabled a considerable reduction in global malaria disease burden and the mortality associated with it between the years 2000 and 2017 (Weiss et al. 2019). However, recent meta-analysis data has revealed that the overall rates of congenital and neonatal malaria in the endemic regions were approximately 40% and 12%, respectively (Danwang et al. 2020). The high prevalence associated with the disease requires additional efforts and investment to overcome. From a socioeconomic standpoint, malaria is of utmost importance as the incidence of malaria was inversely correlated with economic growth. Notably, industrial growth was much slower in malaria-endemic areas despite having the same labor intensity (Sarma et al. 2019). Even in areas where malaria is not endemic, the economic impact of malaria cannot be neglected. From the years 2000 to 2014, malaria-associated total hospital costs and total charges were roughly $176 million and $555 million in the USA, respectively (Khuu et al. 2019). With these issues underscoring the importance of malaria, developing an effective vaccine to prevent malaria onset is crucial.
6.1 Malaria Vaccine Components
Malaria life cycle is quite complex, and as such, designing an effective vaccine remains an arduous task. Malaria vaccines can be subdivided into three distinct groups that target specific antigens: transmission-blocking vaccines (TBV), pre-erythrocytic vaccine, and blood-stage vaccine (Duffy and Patrick Gorres 2000). In the TBV, the antigens currently being investigated are the Pfs25, Pfs230, Pfs48/45, and Pvs230. Pre-erythrocytic vaccines are generally designed based on the circumsporozoite protein (CSP), but there are vaccines targeting the sporozoites such as the P. falciparum sporozoites (PfSPZ). Finally, the blood-stage antigens are the most numerous of the three, which includes but not limited to apical membrane antigen 1 (AMA1), the merozoite surface proteins (MSP), erythrocyte binding antigen 175 (EBA-175), P. falciparum reticulocyte-binding protein homolog 5 (PfRh5), P. falciparum schizont egress antigen 1 (PfSEA1), P. falciparum glutamic-acid-rich protein (PfGARP), and many others.
6.2 Experimental Malaria VLP Vaccines Using Animal Models
Numerous VLP-based malaria vaccines have been generated to date targeting various stages of the disease (Table 2). A chimeric VLP expressing the antigens of P. falciparum fused to the surface protein of the duck hepatitis B virus has been proposed as a potential TBV (Wetzel et al. 2019). Prime immunization using P. falciparum P47 antigen-expressing VLP vaccine conjugated to an Acinetobacter bacteriophage followed by boost immunization with the Pfs47 monomer elicited up to 98% transmission reducing activity (TRA) in mice (Yenkoidiok-Douti et al. 2019).
Another antigen from P. falciparum was used to generate a VLP vaccine expressed on top of the HBV surface antigen and its immunization in mice demonstrated high level of antibody response and TRA (Marini et al. 2019). A chimeric VLP vaccine expressing P. falciparum Pfs25 fused to Alfalfa mosaic virus coat protein completely blocked parasitic transmission over the span of six months (Jones et al. 2013). Immunizing mice with malarial antigens such as the Pfs25 or complex lysine and cysteine-rich inter-domain region (CIDR) expressed using SpyCatcher:SpyTag plug-and-display technology induced a strong antibody response with only a single immunization (Brune et al. 2016). Multimerizing the Pfs25 and Pfs28 protein with the SpyCatcher-IMX313-SnoopCatcher enhanced the murine antibody response to both antigens nearly 100-fold with only a single immunization (Brune et al. 2017). Several VLP display technologies have been assessed using the Pfs25 vaccines. Compared to Pfs25 protein genetically fused to the IMX313 heptamer, antibody inductions were vastly enhanced when the Pfs25 proteins were expressed using the SpyCatcher-AP205 VLPs or chemically conjugated to Qβ phage. The sheer amounts of antibodies induced were found to be the highest from Qβ-VLPs, whereas the highest quality of Pfs25-specific antibodies was elicited from SpyCatcher-AP205 VLP immunization (Leneghan et al. 2017).
P. falciparum CSP expressed on the envelope protein of the chikungunya virus induced a strong immune response upon challenge infection with the malaria sporozoites (Urakami et al. 2017). Although a challenge infection was not performed, strongly enhanced antibody responses were also observed from immunization with a VLP vaccine expressing the full-length CSP of P. vivax (Almeida et al. 2014; Andersson et al. 2017). Anchoring the VAR2CSA ID1-DBL2X-ID2a domain to the TM-CT of influenza A virus HA enhanced the antibody responses as well as parasite inhibition (Andersson et al. 2017). The Pfs48/45 VLP vaccines supplemented with adjuvants were confirmed to inhibit P. falciparum transmission (Singh et al. 2017,2019). Sander and his research group developed several VLP vaccines that induced long-lasting antibody responses in mice, which was maintained at high levels for months (Janitzek et al. 2016; Thrane et al. 2016). VLP vaccine designed using the Asp-Ala-Asp-Pro (NANP) repeat epitopes of the P. falciparum CSP has been proposed as a potential optimization strategy since the number of NANP was correlated with the degree of complement fixation to CSP (Kingston et al. 2019). Conjugating the P. falciparum CIDRα1 domain of erythrocyte membrane protein 1 induced antibody responses, though more research needs to be conducted for its future application (Harmsen et al. 2020).
P. vivax is another species capable of causing human malaria. Vaccines designed using the P. vivax cell-traversal protein for ookinetes and sporozoites (CelTOS) have demonstrated interesting results. Priming with adenoviral vaccine followed by VLP or recombinant protein boost immunization conferred modest protection in mice (Alves et al. 2017). VLP vaccine expressing the CSP of P. vivax conferred 100% sterile protection against transgenic P. berghei challenge and is currently under further development for clinical evaluation (Salman et al. 2017). Similar results were observed from VLPs expressing the T and B cell epitopes of P. falciparum and P. vivax CSP epitopes (Whitacre et al. 2015). Synergistic effect of combinatorial vaccinations using CSP-expressing Rv21 VLP and viral vectored antigens expressing CSP and thrombospondin-related adhesive protein (TRAP) improved the protective efficacy of the vaccine compared to the vaccine used alone, which could also be increased through adjuvant use (Atcheson et al. 2018). A chimeric VLP vaccine expressing the P. yoelii CSP T cell or B cell epitopes induced CD8+ T cell responses and antibody responses (Pattinson et al. 2019).
In stark contrast to P. vivax or P. falciparum, studies investigating the efficacy of rodent malaria VLP vaccines are extremely scarce. Several VLPs expressing the AMA-1, MSP-8, and MSP-9 of P. berghei elicited potent protection and prolonged the survival times, but failed to confer 100% protection in mice (Lee et al. 2019b, 2020a, b).
A VLP vaccine expressing the P. vivax thrombospondin-related adhesive protein (TRAP) adjuvanted with microcrystalline tyrosine (MCT) conferred partial protection against rodent malaria P. berghei (Cabral-Miranda et al. 2017). Recently, a P. falciparum thrombospondin-related adhesive protein (TRAP)-expressing VLP vaccine has been generated using the cucumber mosaic virus that has been chemically fused to the tetanus toxin T cell epitope. This VLP vaccine, when adjuvanted with the dioleoylphosphatidylserine (DOPS), induced stronger humoral and cellular immune responses than those induced by alum-adjuvanted VLPs upon challenge infection with transgenic P. berghei (Cabral-Miranda et al. 2018).
6.3 Clinical Malaria VLP Vaccines in Trial
Several clinical trials have been conducted using various VLP-based malaria vaccines. A Pfs25 VLP vaccine produced from the plant Nicotiana benthamiana has recently underwent phase 1 study. Although substantial Pfs25-specific antibody responses were generated, the TRA was determined to be suboptimal (Chichester et al. 2018). A VLP vaccine expressing the PfRH5 constructed using the Drosophila melanogaster Schneider 2 (S2) cell line was confirmed to be highly immunogenic in mice and induced functional growth inhibitory antibodies against P. falciparum in vitro. As such, this RH5.1 VLP vaccine adjuvanted with the AS01B has been approved for clinical trial phase I/IIa in the United Kingdom (Jin et al. 2018). Phase III clinical trial results for the malaria vaccine RTS,S/AS01 have reported that the vaccines initially protected the patients against malaria, but its protective efficacy waned over time (Olotu et al. 2016). With the disappointing efficacy demonstrated by RTS,S, developing an alternative vaccine using different strategies has become a necessity. Recently, at the University of Oxford’s Jenner Institute, a RTS,S-like vaccine was designed which was labeled as R21 that were more immunogenic than its predecessor RTS,S. This vaccine was reported to be undergoing several clinical trials in the United Kingdom and West Africa (Collins et al. 2017).
7 Conclusion
Developing efficacious vaccines for diseases is extremely difficult and more efforts are needed to eradicate some of the infectious diseases of global importance. Advances in biotechnology have enabled construction of VLP vaccines and the efficacies of these vaccines appear to be promising. With the recent emergence of the novel coronavirus pandemic affecting the entire world, rapid production of vaccines with outstanding efficacy such as VLPs has become a global necessity.
References
Akerlind-Stopner B, Hu A, Mufson MA, Utter G, Norrby E (1993) Antibody responses of children to the C-terminal peptide of the SH protein of respiratory syncytial virus and the immunological characterization of this protein. J Med Virol 40(2):112–120
Almeida AP, Dias MO, Vieira Cde A, Chávez-Olórtegui C, Gazzineli RT, Rodrigues MM, Fujiwara RT, Bruna-Romero O (2014) Long-lasting humoral and cellular immune responses elicited by immunization with recombinant chimeras of the plasmodium vivax circumsporozoite protein. Vaccine 32(19):2181–2187
Alves E, Salman AM, Leoratti F, Lopez-Camacho C, Viveros-Sandoval ME, Lall A, El-Turabi A, Bachmann MF, Hill AV, Janse CJ, Khan SM et al (2017) Evaluation of plasmodium vivax cell-traversal protein for ookinetes and sporozoites as a preerythrocytic P. Vivax vaccine. Clin Vaccine Immunol 24(4):e00501–16
Andersson AC, Resende M, Salanti A, Nielsen MA, Holst PJ (2017) Novel adenovirus encoded virus-like particles displaying the placental malaria associated var2csa antigen. Vaccine 35(8):1140–1147
Atcheson E, Bauza K, Salman AM, Alves E, Blight J, Viveros-Sandoval ME, Janse CJ, Khan SM, Hill AVS, Reyes-Sandoval A (2018) Tailoring a plasmodium vivax vaccine to enhance efficacy through a combination of a csp virus-like particle and trap viral vectors. Infect Immun 24(4):e00114–18
Bachmann MF, Jennings GT (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10(11):787–796
Bachmann MF, Rohrer UH, Kündig TM, Bürki K, Hengartner H, Zinkernagel RM (1993) The influence of antigen organization on b cell responsiveness. Science 262(5138):1448–1451
Berlanda Scorza F, Tsvetnitsky V, Donnelly JJ (2016) Universal influenza vaccines: shifting to better vaccines. Vaccine 34(26):2926–2933
Black MW, Boothroyd JC (2000) Lytic cycle of toxoplasma gondii. Microbiol Mol Biol Rev 64(3):607–623
Blanco JCG, Pletneva LM, McGinnes-Cullen L, Otoa RO, Patel MC, Fernando LR, Boukhvalova MS, Morrison TG (2018) Efficacy of a respiratory syncytial virus vaccine candidate in a maternal immunization model. Nat Commun 9(1):1904
Bragard C, Duncan GH, Wesley SV, Naidu RA, Mayo MA (2000) Virus-like particles assemble in plants and bacteria expressing the coat protein gene of Indian peanut clump virus. J Gen Virol 81(Pt 1):267–272
Brune KD, Howarth M (2018) New routes and opportunities for modular construction of particulate vaccines: stick, click, and glue. Front Immunol 9:1432
Brune KD, Leneghan DB, Brian IJ, Ishizuka AS, Bachmann MF, Draper SJ, Biswas S, Howarth M (2016) Plug-and-display: decoration of virus-like particles via isopeptide bonds for modular immunization. Sci Rep 6:19234
Brune KD, Buldun CM, Li Y, Taylor IJ, Brod F, Biswas S, Howarth M (2017) Dual plug-and-display synthetic assembly using orthogonal reactive proteins for twin antigen immunization. Bioconjug Chem 28(5):1544–1551
Cabral-Miranda G, Heath MD, Mohsen MO, Gomes AC, Engeroff P, Flaxman A, Leoratti FMS, El-Turabi A, Reyes-Sandoval A, Skinner MA, Kramer MF et al (2015) Virus-like particle (VLP) plus microcrystalline tyrosine (mct) adjuvants enhance vaccine efficacy improving t and b cell immunogenicity and protection against plasmodium berghei/vivax. Vaccines (Basel) 5(2)
Cabral-Miranda G, Salman AM, Mohsen MO, Storni FL, Roesti ES, Skinner MA, Heath MD, Kramer MF, Khan SM, Janse CJ, Hill AVS et al (2018) Dops adjuvant confers enhanced protection against malaria for VLP-trap based vaccines. Diseases 6(4):107
Cane PA (2001) Molecular epidemiology of respiratory syncytial virus. Rev Med Virol 11(2):103–116
Chackerian B (2007) Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines 6(3):381–390
Charlton Hume HK, Lua LHL (2017) Platform technologies for modern vaccine manufacturing. Vaccine 35(35 Pt A):4480–4485
Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, Lee CK, Ockenhouse CF, Morin MJ, Streatfield SJ, Yusibov V (2018) Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: a phase 1 dose-escalation study in healthy adults. Vaccine 36(39):5865–5871
Chung KY, Coyle EM, Jani D, King LR, Bhardwaj R, Fries L, Smith G, Glenn G, Golding H, Khurana S (2015) Iscomatrix™ adjuvant promotes epitope spreading and antibody affinity maturation of influenza a H7N9 virus like particle vaccine that correlate with virus neutralization in humans. Vaccine 33(32):3953–3962
Cimica V, Boigard H, Bhatia B, Fallon JT, Alimova A, Gottlieb P, Galarza JM (2016) Novel respiratory syncytial virus-like particle vaccine composed of the postfusion and prefusion conformations of the f glycoprotein. Clin Vaccine Immunol 23(6):451–459
Collins PL, Fearns R, Graham BS (2013) Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol 372:3–38
Collins KA, Snaith R, Cottingham MG, Gilbert SC, Hill AVS (2017) Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep 7:46621
Cox MM, Hollister JR (2009) Flublok, a next generation influenza vaccine manufactured in insect cells. Biologicals 37(3):182–189
Cullen LM, Boukhvalova MS, Blanco JCG, Morrison TG (2020) Comparisons of antibody populations in different pre-fusion f VLP-immunized cotton rat dams and their offspring. Vaccines (Basel) 8(1):133
Danwang C, Bigna JJ, Nzalie RNT, Robert A (2020) Epidemiology of clinical congenital and neonatal malaria in endemic settings: A systematic review and meta-analysis. Malar J 19(1):312
DiMiceli L, Pool V, Kelso JM, Shadomy SV, Iskander J (2006) Vaccination of yeast sensitive individuals: review of safety data in the US vaccine adverse event reporting system (vaers). Vaccine 24(6):703–707
Donaldson B, Al-Barwani F, Young V, Scullion S, Ward V, Young S (2015) Virus-like particles, a versatile subunit vaccine platform. In: Foged C, Rades T, Perrie Y, Hook S (eds) Subunit vaccine delivery. New York, NY, Springer, New York, pp 159–180
Dubey JP (2009) Toxoplasmosis in sheep–the last 20 years. Vet Parasitol 163(1–2):1–14
Duffy PE, Patrick Gorres J (2000) Malaria vaccines since 2000: Progress, priorities, products. NPJ Vaccines 5:48
Effio CL, Hubbuch J (2015) Next generation vaccines and vectors: designing downstream processes for recombinant protein-based virus-like particles. Biotechnol J 10(5):715–727
Efstathiou C, Abidi SH, Harker J, Stevenson NJ (2020) Revisiting respiratory syncytial virus’s interaction with host immunity, towards novel therapeutics. Cell Mol Life Sci 1–14
Eichinger KM, Kosanovich JL, Gidwani SV, Zomback A, Lipp MA, Perkins TN, Oury TD, Petrovsky N, Marshall CP, Yondola MA, Empey KM (2020) Prefusion rsv f immunization elicits th2-mediated lung pathology in mice when formulated with a th2 (but not a th1/th2-balanced) adjuvant despite complete viral protection. Front Immunol 11:1673
Fehr T, Skrastina D, Pumpens P, Zinkernagel RM (1998) T cell-independent type i antibody response against b cell epitopes expressed repetitively on recombinant virus particles. Proc Natl Acad Sci U S A 95(16):9477–9481
Frey SJ, Varner C, Arsiwala A, Currier MG, Moore ML, Kane RS (2020) The design of vaccines based on the shielding of antigenic site Ú of a respiratory syncytial virus fusion protein immunogen. Adv Healthc Mater e2000714
Gause KT, Wheatley AK, Cui J, Yan Y, Kent SJ, Caruso F (2017) Immunological principles guiding the rational design of particles for vaccine delivery. ACS Nano 11(1):54–68
Giles BM, Ross TM (2011) A computationally optimized broadly reactive antigen (cobra) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 29(16):3043–3054
Gomes AC, Mohsen M, Bachmann MF (2017) Harnessing nanoparticles for immunomodulation and vaccines. Vaccines (Basel) 5(1):6
Guo J, Zhou A, Sun X, Sha W, Ai K, Pan G, Zhou C, Zhou H, Cong H, He S (2009) Immunogenicity of a virus-like-particle vaccine containing multiple antigenic epitopes of toxoplasma gondii against acute and chronic toxoplasmosis in mice. Front Immunol 10:592
Ha B, Yang JE, Chen X, Jadhao SJ, Wright ER, Anderson LJ (2020) Two rsv platforms for g, f, or g+f proteins vlps. Viruses 12(9):906
Harmsen C, Turner L, Thrane S, Sander AF, Theander TG, Lavstsen T (2020) Immunization with virus-like particles conjugated to cidrα1 domain of plasmodium falciparum erythrocyte membrane protein 1 induces inhibitory antibodies. Malar J 19(1):132
Hervas-Stubbs S, Rueda P, Lopez L, Leclerc C (2007) Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J Immunol 178(4):2361–2369
Hodgins B, Pillet S, Landry N, Ward BJ (2019) A plant-derived vlp influenza vaccine elicits a balanced immune response even in very old mice with co-morbidities. PLoS ONE 14(1):e0210009
Huang Z, Santi L, LePore K, Kilbourne J, Arntzen CJ, Mason HS (2006) Rapid, high-level production of hepatitis b core antigen in plant leaf and its immunogenicity in mice. Vaccine 24(14):2506–2513
Hwang HS, Kwon YM, Lee JS, Yoo SE, Lee YN, Ko EJ, Kim MC, Cho MK, Lee YT, Jung YJ, Lee JY et al (2014) Co-immunization with virus-like particle and DNA vaccines induces protection against respiratory syncytial virus infection and bronchiolitis. Antiviral Res 110:115–123
Hwang HS, Lee YT, Kim KH, Park S, Kwon YM, Lee Y, Ko EJ, Jung YJ, Lee JS, Kim YJ, Lee YN et al (2016) Combined virus-like particle and fusion protein-encoding DNA vaccination of cotton rats induces protection against respiratory syncytial virus without causing vaccine-enhanced disease. Virology 494:215–224
Hwang HS, Kim KH, Lee Y, Lee YT, Ko EJ, Park S, Lee JS, Lee BC, Kwon YM, Moore ML, Kang SM (2017a) Virus-like particle vaccines containing f or f and g proteins confer protection against respiratory syncytial virus without pulmonary inflammation in cotton rats. Hum Vaccin Immunother 13(5):1031–1039
Hwang HS, Lee YT, Kim KH, Ko EJ, Lee Y, Kwon YM, Kang SM (2017b) Virus-like particle vaccine primes immune responses preventing inactivated-virus vaccine-enhanced disease against respiratory syncytial virus. Virology 511:142–151
Jang YH, Seong BL (2020) Call for a paradigm shift in the design of universal influenza vaccines by harnessing multiple correlates of protection. Expert Opin Drug Discov 15(12):1441–1455
Janitzek CM, Matondo S, Thrane S, Nielsen MA, Kavishe R, Mwakalinga SB, Theander TG, Salanti A, Sander AF (2016) Bacterial superglue generates a full-length circumsporozoite protein virus-like particle vaccine capable of inducing high and durable antibody responses. Malar J 15(1):545
Jennings GT, Bachmann MF (2008) The coming of age of virus-like particle vaccines. Biol Chem 389(5):521–536
Jiang W, Wang S, Chen H, Ren H, Huang X, Wang G, Chen Z, Chen L, Chen Z, Zhou P (2017) A bivalent heterologous DNA virus-like-particle prime-boost vaccine elicits broad protection against both group 1 and 2 influenza a viruses. J Virol 91(9):e02052–16
Jin J, Tarrant RD, Bolam EJ, Angell-Manning P, Soegaard M, Pattinson DJ, Dulal P, Silk SE, Marshall JM, Dabbs RA, Nugent FL et al (2018) Production, quality control, stability, and potency of cGMP-produced plasmodium falciparum RH5.1 protein vaccine expressed in drosophila s2 cells. NPJ Vaccines 3:32
Jones RM, Chichester JA, Mett V, Jaje J, Tottey S, Manceva S, Casta LJ, Gibbs SK, Musiychuk K, Shamloul M, Norikane J et al (2013) A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against plasmodium falciparum in immunized mice. PLoS ONE 8(11):e79538
Kang HJ, Baker EN (2011) Intramolecular isopeptide bonds: protein crosslinks built for stress? Trends Biochem Sci 36(4):229–237
Kang HJ, Lee SH, Kim MJ, Chu KB, Lee DH, Chopra M, Choi HJ, Park H, Jin H, Quan FS (2019) Influenza virus-like particles presenting both toxoplasma gondii rop4 and rop13 enhance protection against T. Gondii infection. Pharmaceutics 11(7):342
Kang HJ, Chu KB, Lee SH, Kim MJ, Park H, Jin H, Moon EK, Quan FS (2020) Toxoplasma gondii virus-like particle vaccination alleviates inflammatory response in the brain upon T. gondii infection. Parasite Immunol 42(6):e12716
Khurana S, Wu J, Verma N, Verma S, Raghunandan R, Manischewitz J, King LR, Kpamegan E, Pincus S, Smith G, Glenn G et al (2011) H5n1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. J Virol 85(21):10945–10954
Khuu D, Eberhard ML, Bristow BN, Javanbakht M, Ash LR, Shafir SC, Sorvillo FJ (2019) Economic impact of malaria-related hospitalizations in the united states, 2000–2014. J Infect Public Health 12(3):424–433
Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM (2013) Virus-like particles containing multiple m2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther 21(2):485–492
Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, Song JM, Song BM, Lee YJ, Choi JG, Kang HM et al (2014) Supplementation of influenza split vaccines with conserved m2 ectodomains overcomes strain specificity and provides long-term cross protection. Mol Ther 22(7):1364–1374
Kim KH, Lee YT, Hwang HS, Kwon YM, Kim MC, Ko EJ, Lee JS, Lee Y, Kang SM (2015) Virus-like particle vaccine containing the f protein of respiratory syncytial virus confers protection without pulmonary disease by modulating specific subsets of dendritic cells and effector t cells. J Virol 89(22):11692–11705
Kim YJ, Lee YT, Kim MC, Lee YN, Kim KH, Ko EJ, Song JM, Kang SM (2017) Cross-protective efficacy of influenza virus m2e containing virus-like particles is superior to hemagglutinin vaccines and variable depending on the genetic backgrounds of mice. Front Immunol 8:1730
Kim AR, Lee DH, Lee SH, Rubino I, Choi HJ, Quan FS (2018) Protection induced by virus-like particle vaccine containing tandem repeat gene of respiratory syncytial virus g protein. PLoS ONE 13(1):e0191277
Kim MJ, Lee SH, Kang HJ, Chu KB, Park H, Jin H, Moon EK, Kim SS, Quan FS (2020) Virus-like particle vaccine displaying toxoplasma gondii apical membrane antigen 1 induces protection against T. Gondii ME49 infection in mice. Microb Pathog 142:104090
Kingston NJ, Kurtovic L, Walsh R, Joe C, Lovrecz G, Locarnini S, Beeson JG, Netter HJ (2019) Hepatitis B virus-like particles expressing plasmodium falciparum epitopes induce complement-fixing antibodies against the circumsporozoite protein. Vaccine 37(12):1674–1684
Ko EJ, Kwon YM, Lee JS, Hwang HS, Yoo SE, Lee YN, Lee YT, Kim MC, Cho MK, Lee YR, Quan FS et al (2015) Virus-like nanoparticle and DNA vaccination confers protection against respiratory syncytial virus by modulating innate and adaptive immune cells. Nanomedicine 11(1):99–108
Krammer F, Palese P (2015) Advances in the development of influenza virus vaccines. Nat Rev Drug Discov 14(3):167–182
Kramer F, García-Sastre A, Palese P (2018) Is it possible to develop a “universal” influenza virus vaccine? Potential target antigens and critical aspects for a universal influenza vaccine. Cold Spring Harb Perspect Biol 10(7):a028845
Krietsch Boerner L (2020) The flu shot and the egg. ACS Cent Sci 6(2):89–92
Kushnir N, Streatfield SJ, Yusibov V (2012) Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 31(1):58–83
Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, Vézina LP (2010) Preclinical and clinical development of plant-made virus-like particle vaccine against avian h5n1 influenza. PLoS ONE 5(12):e15559
Landry N, Pillet S, Favre D, Poulin JF, Trépanier S, Yassine-Diab B, Ward BJ (2014) Influenza virus-like particle vaccines made in Nicotiana benthamiana elicit durable, poly-functional and cross-reactive t cell responses to influenza ha antigens. Clin Immunol 154(2):164–177
Lee S, Quan FS, Kwon Y, Sakamoto K, Kang SM, Compans RW, Moore ML (2014) Additive protection induced by mixed virus-like particles presenting respiratory syncytial virus fusion or attachment glycoproteins. Antiviral Res 111:129–135
Lee DH, Lee SH, Kim AR, Quan FS (2016a) Virus-like nanoparticle vaccine confers protection against toxoplasma gondii. PLoS ONE 11(8):e0161231
Lee DH, Kim AR, Lee SH, Quan FS (2016b) Cross-protection induced by toxoplasma gondii virus-like particle vaccine upon intraperitoneal route challenge. Acta Trop 164:77–83
Lee Y, Lee YT, Ko EJ, Kim KH, Hwang HS, Park S, Kwon YM, Kang SM (2017a) Soluble f proteins exacerbate pulmonary histopathology after vaccination upon respiratory syncytial virus challenge but not when presented on virus-like particles. Hum Vaccin Immunother 13(11):2594–2605
Lee SH, Kim AR, Lee DH, Rubino I, Choi HJ, Quan FS (2017b) Protection induced by virus-like particles containing toxoplasma gondii microneme protein 8 against highly virulent RH strain of toxoplasma gondii infection. PLoS ONE 12(4):e0175644
Lee SH, Kang HJ, Lee DH, Kang SM, Quan FS (2018a) Virus-like particle vaccines expressing toxoplasma gondii rhoptry protein 18 and microneme protein 8 provide enhanced protection. Vaccine 36(38):5692–5700
Lee SH, Kang HJ, Lee DH, Quan FS (2018b) Protective immunity induced by incorporating multiple antigenic proteins of toxoplasma gondii into influenza virus-like particles. Front Immunol 9:3073
Lee DH, Chu KB, Kang HJ, Lee SH, Chopra M, Choi HJ, Moon EK, Inn KS, Quan FS (2019a) Protection induced by malaria virus-like particles containing codon-optimized AMA-1 of plasmodium berghei. Malar J 18(1):394
Lee YT, Kim KH, Ko EJ, Kim MC, Lee YN, Hwang HS, Lee Y, Jung YJ, Kim YJ, Santos J, Perez DR et al (2019b) Enhancing the cross protective efficacy of live attenuated influenza virus vaccine by supplemented vaccination with m2 ectodomain virus-like particles. Virology 529:111–121
Lee SH, Chu KB, Kang HJ, Basak S, Kim MJ, Park H, Jin H, Moon EK, Quan FS (2020a) Virus-like particles expressing plasmodium berghei msp-8 induce protection against P. Berghei infection. Parasite Immunol 42(11):e12781
Lee SH, Kang HJ, Chu KB, Basak S, Lee DH, Moon EK, Quan FS (2020b) Protective immunity induced by virus-like particle containing merozoite surface protein 9 of plasmodium berghei. Vaccines (Basel) 8(3):428
Leneghan DB, Miura K, Taylor IJ, Li Y, Jin J, Brune KD, Bachmann MF, Howarth M, Long CA, Biswas S (2017) Nanoassembly routes stimulate conflicting antibody quantity and quality for transmission-blocking malaria vaccines. Sci Rep 7(1):3811
Li Y, Zhou H (2018) Moving towards improved vaccines for toxoplasma gondii. Expert Opin Biol Ther 18(3):273–280
Lindsay BJ, Bonar MM, Costas-Cancelas IN, Hunt K, Makarkov AI, Chierzi S, Krawczyk CM, Landry N, Ward BJ, Rouiller I (2018) Morphological characterization of a plant-made virus-like particle vaccine bearing influenza virus hemagglutinins by electron microscopy. Vaccine 36(16):2147–2154
Ling HY, Edwards AM, Gantier MP, Deboer KD, Neale AD, Hamill JD, Walmsley AM (2012) An interspecific Nicotiana hybrid as a useful and cost-effective platform for production of animal vaccines. PLoS ONE 7(4):e35688
Liu F, Wu X, Li L, Liu Z, Wang Z (2013) Use of baculovirus expression system for generation of virus-like particles: successes and challenges. Protein Expr Purif 90(2):104–116
Lomonossoff GP, D’Aoust MA (2016) Plant-produced biopharmaceuticals: a case of technical developments driving clinical deployment. Science 353(6305):1237–1240
López-Macías C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, Arriaga-Pizano L, Hickman SP, Allende M, Lenhard K, Pincus S et al (2011) Safety and immunogenicity of a virus-like particle pandemic influenza a (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine 29(44):7826–7834
Low JG, Lee LS, Ooi EE, Ethirajulu K, Yeo P, Matter A, Connolly JE, Skibinski DA, Saudan P, Bachmann M, Hanson BJ et al (2014) Safety and immunogenicity of a virus-like particle pandemic influenza a (H1N1) 2009 vaccine: results from a double-blinded, randomized phase i clinical trial in healthy Asian volunteers. Vaccine 32(39):5041–5048
Lua LH, Connors NK, Sainsbury F, Chuan YP, Wibowo N, Middelberg AP (2014) Bioengineering virus-like particles as vaccines. Biotechnol Bioeng 111(3):425–440
Luo WY, Lin SY, Lo KW, Lu CH, Hung CL, Chen CY, Chang CC, Hu YC (2013) Adaptive immune responses elicited by baculovirus and impacts on subsequent transgene expression in vivo. J Virol 87(9):4965–4973
Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simões EAF, Swamy GK, Agrawal S, Ahmed K, August A, Baqui AH et al (2020) Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 383(5):426–439
Malaria: World Health Organization, Geneva (2020). https://www.who.int/news-room/fact-sheets/detail/malaria
Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF (2008) Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol 38(5):1404–1413
Marini A, Zhou Y, Li Y, Taylor IJ, Leneghan DB, Jin J, Zaric M, Mekhaiel D, Long CA, Miura K, Biswas S (2019) A universal plug-and-display vaccine carrier based on HBsAg VLP to maximize effective antibody response. Front Immunol 10:2931
Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ (1996) Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci U S A 93(11):5335–5340
Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, Buchholz UJ, Openshaw PJ, McLellan JS, Englund JA, Mejias A et al (2018) The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 18(10):e295–e311
McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG (2011) Assembly and immunological properties of newcastle disease virus-like particles containing the respiratory syncytial virus f and g proteins. J Virol 85(1):366–377
McGinnes Cullen L, Schmidt MR, Kenward SA, Woodland RT, Morrison TG (2015) Murine immune responses to virus-like particle-associated pre- and postfusion forms of the respiratory syncytial virus f protein. J Virol 89(13):6835–6847
McGinnes Cullen L, Schmidt MR, Morrison TG (2019) Effect of previous respiratory syncytial virus infection on murine immune responses to f and g protein-containing virus-like particles. J Virol 93(9):e00087–19
McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A et al (2013) Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340(6136):1113–1117
Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF (2017a) Major findings and recent advances in virus-like particle (vlp)-based vaccines. Semin Immunol 34:123–132
Mohsen MO, Gomes AC, Cabral-Miranda G, Krueger CC, Leoratti FM, Stein JV, Bachmann MF (2017b) Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J Control Release 251:92–100
Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB (2007) The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 25(27):5086–5096
Montazeri M, Mikaeili Galeh T, Moosazadeh M, Sarvi S, Dodangeh S, Javidnia J, Sharif M, Daryani A (2020) The global serological prevalence of toxoplasma gondii in felids during the last five decades (1967–2017): a systematic review and meta-analysis. Parasit Vectors 13(1):82
Morón G, Rueda P, Casal I, Leclerc C (2002) Cd8alpha- cd11b+ dendritic cells present exogenous virus-like particles to cd8+ t cells and subsequently express cd8alpha and cd205 molecules. J Exp Med 195(10):1233–1245
Moyle PM, Toth I (2013) Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 8(3):360–376
Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG (2010) Newcastle disease virus-like particles containing respiratory syncytial virus g protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol 84(2):1110–1123
Music N, Reber AJ, Kim MC, York IA, Kang SM (2016) Supplementation of H1N1pdm09 split vaccine with heterologous tandem repeat m2e5x virus-like particles confers improved cross-protection in ferrets. Vaccine 34(4):466–473
Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang GY, Druz A et al (2015) Prefusion f-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 7(309):309ra162
Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, Kaslow DC, Njuguna P, Marsh K, Bejon P (2016) Seven-year efficacy of RTS, S/AS01 malaria vaccine among young African children. N Engl J Med 374(26):2519–2529
Pappas G, Roussos N, Falagas ME (2009) Toxoplasmosis snapshots: global status of toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 39(12):1385–1394
Park YC, Song JM (2017) Preparation and immunogenicity of influenza virus-like particles using nitrocellulose membrane filtration. Clin Exp Vaccine Res 6(1):61–66
Pattinson DJ, Apte SH, Wibowo N, Chuan YP, Rivera-Hernandez T, Groves PL, Lua LH, Middelberg APJ, Doolan DL (2019) Chimeric murine polyomavirus virus-like particles induce plasmodium antigen-specific cd8(+) t cell and antibody responses. Front Cell Infect Microbiol 9:215
Pillet S, Couillard J, Trépanier S, Poulin JF, Yassine-Diab B, Guy B, Ward BJ, Landry N (2019) Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-two randomized phase ii clinical trials in 18 to 49 and ≥50 years old adults. PLoS ONE 14(6):e0216533
Plotkin S (2014) History of vaccination. Proc Natl Acad Sci U S A 111(34):12283–12287
Plotkin S, Robinson JM, Cunningham G, Iqbal R, Larsen S (2017) The complexity and cost of vaccine manufacturing—an overview. Vaccine 35(33):4064–4071
Puente-Massaguer E, Gòdia F, Lecina M (2020) Development of a non-viral platform for rapid virus-like particle production in sf9 cells. J Biotechnol 322:43–53
Qin J, Zhang Y, Shen X, Gong L, Peng O, Liu Y, Xue C, Cao Y (2018) H7 virus-like particles assembled by hemagglutinin containing H3N2 transmembrane domain and m1 induce broad homologous and heterologous protection in mice. Vaccine 36(33):5030–5036
Quan FS, Vunnava A, Compans RW, Kang SM (2010) Virus-like particle vaccine protects against 2009 h1n1 pandemic influenza virus in mice. PLoS ONE 5(2):e9161
Quan FS, Kim Y, Lee S, Yi H, Kang SM, Bozja J, Moore ML, Compans RW (2011) Virus like particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis 204(7):987–995
Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, Kang SM (2012) Influenza M1 VLPS containing neuraminidase induce heterosubtypic cross-protection. Virology 430(2):127–135
Quan FS, Basak S, Chu KB, Kim SS, Kang SM (2020) Progress in the development of virus-like particle vaccines against respiratory viruses. Expert Rev Vaccines 19(1):11–24
Raghunandan R, Lu H, Zhou B, Xabier MG, Massare MJ, Flyer DC, Fries LF, Smith GE, Glenn GM (2014) An insect cell derived respiratory syncytial virus (RSV) f nanoparticle vaccine induces antigenic site ii antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine 32(48):6485–6492
Rezaei F, Sarvi S, Sharif M, Hejazi SH, Pagheh AS, Aghayan SA, Daryani A (2019) A systematic review of toxoplasma gondii antigens to find the best vaccine candidates for immunization. Microb Pathog 126:172–184
Rohovie MJ, Nagasawa M, Swartz JR (2017) Virus-like particles: next-generation nanoparticles for targeted therapeutic delivery. Bioeng Transl Med 2(1):43–57
Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM (2010) Virus-like particles in vaccine development. Expert Rev Vaccines 9(10):1149–1176
Rueda P, Fominaya J, Langeveld JP, Bruschke C, Vela C, Casal JI (2000) Effect of different baculovirus inactivation procedures on the integrity and immunogenicity of porcine parvovirus-like particles. Vaccine 19(7–8):726–734
Ryu S, Cowling BJ (2020) Human influenza epidemiology. Cold Spring Harb Perspect Med
Salman AM, Montoya-Díaz E, West H, Lall A, Atcheson E, Lopez-Camacho C, Ramesar J, Bauza K, Collins KA, Brod F, Reis F et al (2017) Rational development of a protective p. Vivax vaccine evaluated with transgenic rodent parasite challenge models. Sci Rep 7:46482
Sarma N, Patouillard E, Cibulskis RE, Arcand JL (2019) The economic burden of malaria: revisiting the evidence. Am J Trop Med Hyg 101(6):1405–1415
Schepens B, Sedeyn K, Vande Ginste L, De Baets S, Schotsaert M, Roose K, Houspie L, Van Ranst M, Gilbert B, van Rooijen N, Fiers W et al (2014) Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO Mol Med 6(11):1436–1454
Schwartzman LM, Cathcart AL, Pujanauski LM, Qi L, Kash JC, Taubenberger JK (2015) An intranasal virus-like particle vaccine broadly protects mice from multiple subtypes of influenza a virus. MBio 6(4):e01044
Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I et al (2017) Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 390(10098):946–958
Short KR, Richard M, Verhagen JH, van Riel D, Schrauwen EJ, van den Brand JM, Mänz B, Bodewes R, Herfst S (2015) One health, multiple challenges: the inter-species transmission of influenza a virus. One Health 1:1–13
Singh SK, Thrane S, Janitzek CM, Nielsen MA, Theander TG, Theisen M, Salanti A, Sander AF (2017) Improving the malaria transmission-blocking activity of a plasmodium falciparum 48/45 based vaccine antigen by SpyTag/SpyCatcher mediated virus-like display. Vaccine 35(30):3726–3732
Singh SK, Thrane S, Chourasia BK, Teelen K, Graumans W, Stoter R, van Gemert GJ, van de Vegte-Bolmer MG, Nielsen MA, Salanti A, Sander AF et al (2019) Pfs230 and pfs48/45 fusion proteins elicit strong transmission-blocking antibody responses against plasmodium falciparum. Front Immunol 10:1256
Skibinski DAG, Jones LA, Zhu YO, Xue LW, Au B, Lee B, Naim ANM, Lee A, Kaliaperumal N, Low JGH, Lee LS et al (2018) Induction of human t-cell and cytokine responses following vaccination with a novel influenza vaccine. Sci Rep 8(1):18007
Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, Chen LM, Hogan RJ, Donis RO, Compans RW, Kang SM (2010) Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 405(1):165–175
Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM (2011) Vaccination inducing broad and improved cross protection against multiple subtypes of influenza a virus. Proc Natl Acad Sci U S A 108(2):757–761
Song BM, Kang HM, Lee EK, Jung SC, Kim MC, Lee YN, Kang SM, Lee YJ (2016) Supplemented vaccination with tandem repeat m2e virus-like particles enhances protection against homologous and heterologous hpai h5 viruses in chickens. Vaccine 34(5):678–686
Thrane S, Janitzek CM, Matondo S, Resende M, Gustavsson T, de Jongh WA, Clemmensen S, Roeffen W, van de Vegte-Bolmer M, van Gemert GJ, Sauerwein R et al (2016) Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J Nanobiotechnology 14:30
Tretyakova I, Hidajat R, Hamilton G, Horn N, Nickols B, Prather RO, Tumpey TM, Pushko P (2016) Preparation of quadri-subtype influenza virus-like particles using bovine immunodeficiency virus gag protein. Virology 487:163–171
Urakami A, Sakurai A, Ishikawa M, Yap ML, Flores-Garcia Y, Haseda Y, Aoshi T, Zavala FP, Rossmann MG, Kuno S, Ueno R et al (2017) Development of a novel virus-like particle vaccine platform that mimics the immature form of alphavirus. Clin Vaccine Immunol 24(7):e00090–17
Valero-Pacheco N, Pérez-Toledo M, Villasís-Keever M, Núñez-Valencia A, Boscó-Gárate I, Lozano-Dubernard B, Lara-Puente H, Espitia C, Alpuche-Aranda C, Bonifaz LC, Arriaga-Pizano L et al (2016) Antibody persistence in adults two years after vaccination with an h1n1 2009 pandemic influenza virus-like particle vaccine. PLoS ONE 11(2):e0150146
Viboud C, Gostic K, Nelson MI, Price GE, Perofsky A, Sun K, Sequeira Trovão N, Cowling BJ, Epstein SL, Spiro DJ (2020) Beyond clinical trials: evolutionary and epidemiological considerations for development of a universal influenza vaccine. PLoS Pathog 16(9):e1008583
Walpita P, Johns LM, Tandon R, Moore ML (2015) Mammalian cell-derived respiratory syncytial virus-like particles protect the lower as well as the upper respiratory tract. PLoS ONE 10(7):e0130755
Wang JL, Zhang NZ, Li TT, He JJ, Elsheikha HM, Zhu XQ (2019) Advances in the development of anti-toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trends Parasitol 35(3):239–253
Ward BJ, Landry N, Trépanier S, Mercier G, Dargis M, Couture M, D’Aoust MA, Vézina LP (2014) Human antibody response to n-glycans present on plant-made influenza virus-like particle (vlp) vaccines. Vaccine 32(46):6098–6106
Weiss DJ, Lucas TCD, Nguyen M, Nandi AK, Bisanzio D, Battle KE, Cameron E, Twohig KA, Pfeffer DA, Rozier JA, Gibson HS et al (2019) Mapping the global prevalence, incidence, and mortality of plasmodium falciparum, 2000–17: a spatial and temporal modelling study. Lancet 394(10195):322–331
Wetzel D, Chan JA, Suckow M, Barbian A, Weniger M, Jenzelewski V, Reiling L, Richards JS, Anderson DA, Kouskousis B, Palmer C et al (2019) Display of malaria transmission-blocking antigens on chimeric duck hepatitis B virus-derived virus-like particles produced in Hansenula polymorpha. PLoS ONE 14(9):e0221394
Whitacre DC, Espinosa DA, Peters CJ, Jones JE, Tucker AE, Peterson DL, Zavala FP, Milich DR (2015) P. Falciparum and P. Vivax epitope-focused VLPS elicit sterile immunity to blood stage infections. PLoS One 10(5):e0124856
Yenkoidiok-Douti L, Williams AE, Canepa GE, Molina-Cruz A, Barillas-Mury C (2019) Engineering a virus-like particle as an antigenic platform for a pfs47-targeted malaria transmission-blocking vaccine. Sci Rep 9(1):16833
Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M (2012) Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci U S A 109(12):E690-697
Zambon MC (1999) Epidemiology and pathogenesis of influenza. J Antimicrob Chemother 44 Suppl B:3–9
Zeltins A (2013) Construction and characterization of virus-like particles: a review. Mol Biotechnol 53(1):92–107
Zhang X, Wei M, Pan H, Lin Z, Wang K, Weng Z, Zhu Y, Xin L, Zhang J, Li S, Xia N et al (2014) Robust manufacturing and comprehensive characterization of recombinant hepatitis e virus-like particles in hecolin(®). Vaccine 32(32):4039–4050
Zhang S, Akmar LZ, Bailey F, Rath BA, Alchikh M, Schweiger B, Lucero MG, Nillos LT, Kyaw MH, Kieffer A, Tong S et al (2020) Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis 222(Supplement_7):S680–S687
Funding
This work was supported by grants from the Ministry of Health and Welfare, Republic of Korea (HV20C0085, HV20C0142), and the National Research Foundation of Korea (NRF) (2018R1A6A1A03025124).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chu, KB., Quan, FS. (2021). Virus-Like Particle Vaccines Against Respiratory Viruses and Protozoan Parasites. In: Gill, H.S., Compans, R.W. (eds) Nanoparticles for Rational Vaccine Design. Current Topics in Microbiology and Immunology, vol 433. Springer, Cham. https://doi.org/10.1007/82_2021_232
Download citation
DOI: https://doi.org/10.1007/82_2021_232
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85066-1
Online ISBN: 978-3-030-85067-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)