Abstract
Despite its impact on global health, there is no vaccine available for the prevention of respiratory syncytial virus (RSV) infection. Failure to develop a licensed vaccine is not due to lack of effort, as numerous vaccine candidates have been characterized in preclinical and clinical studies spanning five decades. The vaccine candidates thus far explored can be generally divided into four categories: (1) whole inactivated virus, (2) replication competent, attenuated virus including recombinant viruses, (3) gene-based vectors, and (4) subunit and particulate forms of RSV antigens. The first clinically tested RSV vaccine candidate was a formalin-inactivated purified virus preparation administered to infants and children in the late 1960s. Due to the disastrous outcome of these trials and results of animal models investigating the mechanisms involved, there have been no further studies with inactivated RSV vaccines. Rather, efforts have focused on development of other approaches. In this chapter, we review the history and status of purified proteins, peptides, virus-like particles, virosomes, and nanoparticles and discuss their future potential.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Respiratory Syncytial Virus
- Newcastle Disease Virus
- Vaccine Candidate
- Respiratory Syncytial Virus Infection
- Human Papilloma Virus Vaccine
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Target Populations for RSV Vaccines
Human RSV infection affects different populations with variable severity, and each can be considered as a potential vaccine target group. RSV is the single most important cause of acute viral lower respiratory tract disease in infants and young children worldwide (Karron 2008), with an estimated 33.8 million RSV cases and 199,000 deaths worldwide in 2005 alone (Nair et al. 2010). The incidence and severity of RSV is greatest in the first 6 months of life, with hospitalization rates of 17/1000 infants in the USA. However, children 2–5 years of age, another target group, comprise a substantial proportion of RSV illness burden, and likely provide the reservoir for infection of newborns. The epidemiology and impact of RSV in the elderly, adults with underlying cardiopulmonary disease and hematologic stem cell and solid organ transplant recipients have also been described (Walsh et al. 1999). Estimates of RSV attributable deaths in adults in the USA range from 10,000 to 17,000 annually, with hospitalizations ranging from 14,000 to 177,000 (Falsey et al. 2005; Falsey and Walsh 2000; Han et al. 1999; Thompson et al. 2003; Zhou et al. 2012). Another vaccine target population is pregnant women because of the potential protective role of maternal antibodies for very young infants. Because each of these populations has notable differences in the properties and robustness of their immune responses and immunological history, it will be likely necessary to tailor RSV vaccines to each specific population.
2 Considerations for Types of RSV Vaccine Candidates
Live-attenuated virus vaccines are often considered, in general, the most effective due to their stimulation of broad innate and adaptive T and B cell immunity. However, they may cause disease in immunocompromised persons, an expanding population due to increased use of immune modulators for inflammatory diseases and cancer, the HIV pandemic, and organ transplantation. Recombinant live viruses, currently being developed for RSV (reviewed in the chapter by Karron 2008, this volume), likely similarly pose dangers to immunocompromised populations. Live virus vaccines in infants also raise concerns, particularly the ones that require intranasal inoculation such as those based on other respiratory viruses (reviewed in (Graham)). In addition, perhaps due to the immunological immaturity of young infants, some live-attenuated RSV vaccines may be incompletely attenuated in this population. Thus, infectious virus vaccine candidates, discussed in the chapter by Karron 2008 in this volume, may not always be appropriate for these populations.
In contrast, inactivated or protein subunit vaccines usually do not pose an immediate safety risk and are often the vaccine of choice for infants and immunocompromised populations. However, using classical methods for inactivated vaccine preparation, a formalin-inactivated preparation of purified virus (FI-RSV) not only failed to protect infants from infection, but also unexpectedly resulted in enhanced respiratory disease (ERD) upon subsequent infection with RSV (reviewed in Collins and Crowe 2007; Openshaw and Tregoning 2005). Eighty percent of infected infants vaccinated, when less than 6 months of age, were hospitalized and two died. In contrast, older RSV-experienced infants receiving this vaccine did not manifest ERD. The mechanisms responsible for this unusual response to a classical approach to vaccine preparation are not completely understood even after decades of research using animal models (see chapters by M.S. Boukhvalova and J.C.G. Blanco, P.J. Openshaw, R.J. Pickles and by G. Taylor, this volume). However, this experience has significantly affected the development of all inactivated or subunit RSV vaccine candidates and has heightened concerns about their safety, especially in sero-negative infants.
3 Challenges for Subunit and Particulate RSV Vaccine Development
Three interrelated problems have significantly and uniquely impeded RSV vaccine development, each of which must be overcome. First and foremost is safety, as discussed above. The ERD observed after FI-RSV vaccination was associated with poor neutralizing responses in infants, as well as in experimental animals, perhaps related, in part, to elimination of protective epitopes by formalin treatment (reviewed in Collins and Crowe 2007; Collins and Graham 2007). In addition, it has been reported that, in mice, FI-RSV immunization as well as immunization with purified F protein or UV-inactivated RSV, resulted in unbalanced Th2-biased cytokine responses and low affinity, poorly neutralizing antibodies (Delgado et al. 2009). Upon RSV challenge, immunized animals had weak CD8 + T cell responses, pronounced lung eosinophilia, and significant lung inflammation (reviewed in Collins and Crowe 2007; Collins and Graham 2007). It was proposed that inactivated viruses or purified proteins fail to adequately stimulate innate immune responses necessary for effective adaptive responses including affinity maturation necessary for effective neutralizing antibodies. This failure, for reasons that are still unclear, results in the immunopathology observed upon subsequent live virus infection (Delgado et al. 2009). In any event, the absence of the types of immune responses associated with enhanced disease is now considered a benchmark for development of a successful RSV vaccine.
A second critical issue is predicting and determining efficacy. Some vaccine candidates have proved to be highly protective in animal models, but failed to have equivalent protection in humans (reviewed in Power 2008). In infants, this problem may be related to immunological immaturity or the inhibitory effect of maternal antibodies. In other populations, the problem may be related to preexisting antibody or non-responsiveness of aging or a compromised immune system. It is also likely that there are undefined differences in stimulation of anti-RSV responses and in the character of these responses in animal model systems and in humans. The lack of an animal model that is directly translatable to human protection, coupled with the lack of clearly defined immune correlates of protection in different human populations, makes efficacy in human populations difficult to predict for new vaccine candidates.
A third but related problem is an understanding of requirements for long-lived and memory responses to RSV. One of the hallmarks of RSV is the observation that humans experience repeated infection caused by the same virus serogroup multiple times over several years or even within the same season (reviewed in Hall 2001; Power 2008). The reasons for the failure of RSV infection to protect against subsequent infection are not clear but the inadequate immune response to RSV natural infection illustrates a major problem to be overcome in RSV vaccine development. As noted by Pulendran and Ahmed (2011), an optimally effective RSV vaccine must stimulate immune responses that are more protective and more durable than those resulting from natural infection (Pulendran and Ahmed Pulendran and Ahmed 2011). Indeed, many RSV vaccine candidates have failed to stimulate long-term protective responses (discussed in Hall 2001; Power 2008), illustrating the lack of knowledge of immune mechanisms required to generate long-term, protective anti-RSV immune responses in humans.
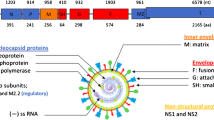
Another issue that also should be addressed in any vaccine candidate is the existence of two major serotypes of RSV, designated group A and B, each of which has 5–6 genotypes (reviewed in Collins and Crowe 2007; Collins and Graham 2007; and chapters by C.B. Hall et al. and by E.H. Choi et al. this volume). The G proteins of different RSV strains demonstrate significant antigenic diversity while the F proteins are virtually identical. Thus, inclusion of F protein in any vaccine candidate is usually considered necessary in order to cover both serotypes.
4 Subunit and Particulate Vaccine Candidates Overview
The primary goal of immunization with vaccines is the stimulation of protective antibody responses. Indeed, the only currently effective prophylaxis for RSV disease is a humanized monoclonal antibody specific for RSV F protein (Cardenas et al. 2005). While too expensive for use in general populations, this reagent clearly demonstrates that serum antibodies specific to the RSV F protein, while not preventing infection, can reduce severity and underscores the importance of humoral immune responses to this virus and, in particular, the importance of antibodies to the F protein. The protective role of G protein antibodies is less clear although recent studies have suggested that antibodies specific to the conserved central domain of the G protein are protective in animal models and prevent ERD stimulated by FI-RSV in those systems (Simard et al. 1995; Zhang et al. 2010).
Protein subunit and particulate vaccine candidates for RSV fall into three categories, intact purified F protein or both F and G proteins, peptide fragments of G protein, and particles of various types containing F and/or G proteins (summarized in Table 1). All candidates have been characterized in preclinical studies in model systems, usually in mice and/or in cotton rats, and a few in primate models. Many of the earlier candidates have also been tested in phase I or II human clinical trials although for various reasons have failed to move forward to large-scale phase III efficacy trials. These candidates will be described with an emphasis on the reasons for their failure to progress and their potential for future development (Fig. 1).
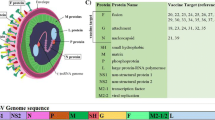
Particulate RSV vaccine candidates figure illustrates four types of particulate RSV vaccine candidates described in detail. VLP, virus-like particles. Red, RSV F protein sequences; blue, RSV G protein sequences; green, NDV sequences; purple, RSV M sequences; gray, influenza M1 protein; light blue, RSV N protein; black line, E. coli RNA
5 Subunit Vaccine Candidates
5.1 Purified Protein Vaccine Candidates
Following the failed FI-RSV trials, the next vaccine candidates to be developed were purified F protein (PFP) isolated from RSV infected VERO cells. Three versions, PFP-1, PFP-2, and PFP-3 (Table 1), were developed sequentially, each with increasing purity. PFP-1 was isolated by affinity chromatography using a monoclonal antibody and contained up to 10 % contaminating G protein. PFP-2 and 3 were purified by ion exchange chromatography and were 98–99 % pure. The F proteins were combined with either aluminum hydroxide (PFP-1, PFP-2) or aluminum phosphate (PFP-3) as adjuvant. Characterization of the conformation of the F protein in these preparations is not reported, thus it is unknown if the antigen is composed of pre-fusion or post fusion mature forms or unfolded, immature forms or a mixture of these forms. It is unknown if the protein was aggregated into rosettes as often the case in purified preparations of paramyxovirus F proteins (Calder et al. 2000).
Limited preclinical studies in animal models are described for these early candidates. Some reports suggest that they may prime for ERD upon subsequent RSV challenge (Delgado et al. 2009; Murphy et al. 1990) although this conclusion has been challenged (Hildreth et al. 1993). There have been, however, a number of clinical trials of these vaccine candidates in various human populations, primarily seropositive children and older adults. For example, placebo-controlled trials of PFP-1 in seropositive children (and two inadvertently included sero-negative children) (Belshe et al. 1993; Paradiso et al. 1994; Piedra et al. 1995; Tristram et al. 1993) and of PFP-2 in RSV experienced children with bronchopulmonary dysplasia (Groothuis et al. 1998) showed generally fourfold or greater increases in serum neutralizing antibody titers with few adverse reactions. Notably, PFP-1, but not PFP-2 or 3, also induced significant anti-G responses. Although there was a trend toward a reduction of RSV infection in vaccine recipients during the subsequent winter, the small study size precludes firm conclusions (Falsey and Walsh 1996). However, a meta-analysis of six double-blind randomized control trials with PFP was reported in 2002 (Simoes et al. 2001). The authors concluded that the vaccinees had a statistically significant reduction in the incidence of all RSV infections, but not in more serious lower respiratory tract infection. Despite these findings, the authors of this meta-analysis questioned the validity of this conclusion due to possible bias in reporting of negative studies. PFP-2 was also evaluated in pregnant women with the goal of enhancing transmission of RSV-specific IgG to their newborns (Munoz et al. 2003). Immunized mothers had only modest increases in anti-RSV serum titers, as did their newborns. Again, subject numbers were too small to assess efficacy. A much larger phase II clinical trial of PFP-3 in older RSV seropositive children with cystic fibrosis showed a robust increase in serum antibody titers and neutralizing antibody titers but no significant reduction in the incidence of RSV infection (Piedra et al. 2003). Finally, PFP-2 was shown to be immunogenic and safe in two phase I trials in healthy and frail elderly adults (Falsey and Walsh 1996, 1997). Importantly, none of the above studies found evidence of ERD although no formal studies in sero-negative children have been reported. Nevertheless, the lingering specter of the FI-RSV experience and the inconsistent results from animal models of ERD with these vaccine candidates have inhibited their study in newborn infants. The lack of an indication of efficacy of these vaccines in other human populations has also discouraged their further development.
Subsequently, candidates containing both F and G proteins as well as M protein have been characterized in two different studies (Falsey et al. 2008; Langley et al. 2009) of elderly populations. This protein mix was co-purified from RSV grown in VERO cells. It has since been reported by others that RSV G protein made in VERO cells lacks the carboxyl terminal sequences and is, thus, a truncated version of the authentic G protein (Kwilas et al. 2009). The effect of this deletion on immune responses is unknown. Furthermore, the conformation of the F protein was uncharacterized. This vaccine formulation was reported by Murphy and colleagues not to induce ERD upon RSV challenge in cotton rats (Murphy et al. 1989), in contrast to their results with PFP. In phase I and II clinical trials, the protein mix was formulated with and without alum, with both studies reporting better F, G and neutralizing antibody responses without alum. One possible interpretation of this result is that alum alters the conformation of the proteins, thus interfering with stimulation of protective neutralizing antibodies, a consideration in evaluating all RSV vaccine candidates with alum. While these studies were specifically designed to test safety and immunogenicity and were not powered to test efficacy, there was little indication that the vaccine protected from subsequent RSV infection. However, there was the suggestion that risk of RSV infection was lowered in those with the highest serum antibody. At this time, there is no indication that the F, G, M protein mixture has been further developed as a vaccine in either adults or infants.
5.2 Peptide Vaccine Candidates
Other subunit vaccine candidates have been formulations that contain various peptide sequences primarily from the G protein. BBG2Na is a peptide with sequences from amino acid 130-230 of the G protein fused to the albumin-binding domain of the Streptococcus G protein, which served as carrier. The protein was made in E. coli and formulated in alum. In mice, this antigen resulted in antibody responses, and immunization of cotton rats with this antigen protected against lower respiratory tract infection but not upper respiratory tract infection. Studies of responses in healthy, seropositive humans (18–45 years) to different doses after intramuscular inoculation were also reported by Power et al. (2001). At the highest dose, the vaccine candidate showed no evidence of adverse reactions and stimulated >4 fold increases in serum antibody titers and >2 fold increases in neutralization titers. Thus the vaccine appeared to be safe and immunogenic in RSV sero-positive adults. As reported by Murata (2009), trials in the elderly also stimulated antibody responses but their rapid declined diminished interest in further development of this candidate for elderly populations. Furthermore, subsequent studies in rhesus macaques showed evidence of Th2 skewed responses as determined by IL13 levels and eosinophils in lungs of challenged animals (de Waal et al. 2004) and protective efficacy was also unclear. In addition, clinical phase III trials of BBG2Na were halted due to adverse events in a small number of individuals (cited in Nguyen et al. 2012). More recently, it was reported that these adverse reactions were due to the Streptococcus component (BB component), and work has explored the use of diphtheria toxin fragments as a carrier (Nguyen et al. 2012). These results have stimulated renewed interest in sequences from this region (amino acids 130–230) of the G protein as a vaccine or a component of a vaccine candidate.
It has been shown that passive transfer of anti-G protein antibodies directed to the central cysteine rich region of the ectodomain sequence (amino acids 174–187) is protective in mice (for example Miao et al. 2009; Trudel et al. 1991). The G protein ectodomain includes a motif analogous to a motif responsible for the binding of the CX3C chemokine fractalkine to its receptor (Tripp et al. 2001). Fractalkine is involved in migration of immune cells, mainly CD8+ and NK T cells, to sites of inflammation. It has been suggested that the soluble form of G protein acts as a fractalkine antagonist and is involved in immune evasion by the virus, ultimately resulting in disease (Tripp 2004). Tripp, Anderson, and colleagues have shown that antibodies specific to this CX3C sequence motif block the binding of G protein to the CX3C receptor, and that immunization with a peptide encoding the CX3C motif protected mice from RSV challenge and decreased pulmonary inflammation (Zhang et al. 2010). These studies have recently been extended showing some cross reaction of antibodies raised to this region to RSV-B G protein in mice (Choi et al. 2012) indicating that inclusion of this region of the G protein in vaccine candidates may increase protective responses to both RSV A and B.
Other studies in mice and cotton rats utilized an antigen made in insect cells that was a fusion of most of the ectodomain sequences from both the F and G proteins, designated F-G. Other details of the structure of this fusion protein are not available. Studies in cotton rats of the F-G antigen showed low levels of neutralizing antibodies, only partial protection from challenge, and possible evidence of ERD (Connors et al. 1992). A modified version of F-G linked to cholera toxin subunit B was also studied, and when given intranasally or via intramuscular injection, induced serum antibody responses, but only intranasal inoculation resulted in protection (Oien et al. 1993; Oien et al. 1994). No human clinical trials have been reported.
6 Particulate Vaccine Candidates
6.1 Virus-Like Particles (VLPs) as Vaccines for RSV
6.1.1 General Properties of VLPs
VLPs are particles with sizes similar to authentic virus and contain repeating protein arrays that mimic those of infectious viruses (Jennings and Bachmann 2008) and account, in part, for the potent immunogenicity of viruses (Jennings and Bachmann 2008). VLPs are released from cells expressing viral structural proteins. The viral surface glycoproteins are folded and inserted into the VLP membranes typical of infectious virus, thus antigenicity of VLPs is likely very similar to live virus. No inactivation is required, thus important epitopes are retained and new ones are not likely generated. Since VLPs are assembled without a genome, VLPs cannot replicate and spread from cell to cell typical of an infectious virus. Nor is there is any chance of reversion to virulence.
Immune responses to VLPs are usually quite robust. Not only do they stimulate neutralizing antibody, but, because of their particulate nature, VLPs are taken up and processed for presentation by both MHC class II and class I, by cross presentation pathways, resulting in broad range of T cell responses (reviewed in Jennings and Bachmann 2008) at least in murine systems. There is evidence that VLPs are also potent stimulators of innate responses since no adjuvant is required for robust immune responses in most systems. Indeed, VLPs are described as “self adjuvanting” (Grgacic and Anderson 2006; Ludwig and Wagner 2007). All these responses usually translate into vaccine candidates that provide good protection of experimental animals from challenge by live, virulent viruses (reviewed in Jennings and Bachmann 2008; Kang et al. 2009). The memory responses to VLPs are less well characterized.
Not all virus systems yield VLPs at levels sufficient for their use as immunogens. Indeed, VLP release from cells expressing the structural proteins of RSV has been reported to be very inefficient (McGinnes et al. 2010; Teng and Collins 1998). To overcome this problem, two different approaches, summarized below, have been reported both of which take advantage of the very efficient release of VLPs formed with core proteins of other viruses.
6.1.2 Newcastle Disease Virus-Based VLPs
One type of RSV VLP vaccine candidate is based on Newcastle disease virus (NDV) VLPs. Expression of the structural proteins of NDV, another paramyxovirus, results in extremely efficient release of VLPs and these VLPs (ND VLPs) (Pantua et al. 2006) stimulated, without adjuvant, robust, neutralizing anti-NDV antibodies and CD8+ and CD4+ T cell immune responses in mice that were comparable to responses to virus (McGinnes et al. 2010). These VLPs have been used as a platform to construct particles containing ectodomains of the RSV glycoprotein ectodomains (McGinnes et al. 2011; Murawski et al. 2010). Efficient incorporation of the RSV glycoproteins into ND VLPs was achieved by constructing chimera protein genes composed of the sequences encoding the ectodomains of G or F proteins fused to the sequences encoding the transmembrane (TM) and cytoplasmic (CT) domains of the NDV HN or F glycoprotein, respectively. Upon expression in cells of the chimera protein genes along with the NDV M (membrane or matrix) protein and NP (nucleocapsid protein) genes, the NDV domains in the chimera proteins specifically interact with the NDV NP and M protein resulting in very efficient incorporation of the chimera protein into VLPs. Using this approach, VLPs containing the RSV G protein ectodomain or VLPs containing ectodomains of both the RSV G and F proteins have been generated (McGinnes et al. 2011; Murawski et al. 2010).
The VLPs containing the RSV G (Murawski et al. 2010) or both the G and F proteins (McGinnes et al. 2011) demonstrated striking effectiveness as a vaccine for RSV in mice. A single intramuscular immunization of BALB/c mice with either VLP stimulated, without adjuvant, antibody levels that were comparable to or higher than responses to infectious RSV delivered by intranasal inoculation to mimic natural infection (McGinnes et al. 2011). The ratios of IgG subtypes during infection or immunization have been used as one indicator of Th1 or Th2 biased immune responses (for example Delgado et al. 2009). VLP immunization resulted in anti-G protein and anti-F protein IgG2a/IgG1 ratios that indicated a predominant Th1 immune response. The neutralizing antibody titers after immunization with VLPs containing only the G protein were relatively weak (Murawski et al. 2010). However, after a single immunization with VLPs containing both glycoproteins, the neutralizing antibody titers were robust (McGinnes et al. 2011). Immunization with either VLP completely protected mice from virus replication upon live virus challenge. Neither VLP stimulated ERD after RSV challenge as determined by inflammation around blood vessels, airways, and in interstitial spaces (McGinnes et al. 2011; Murawski et al. 2010).
Assessment of long-term immune responses to VLPs containing both G and F protein in mice have also shown that these particles can stimulate durable serum neutralizing antibody levels and long-lived memory responses, properties important for an effective RSV vaccine (Schmidt et al. 2012). BALB/c mice immunized with a single dose of VLPs, without adjuvant, generated stable neutralizing antibody titers that lasted for 14 months whereas those of RSV immunized animals declined significantly by 3 months. This finding was reinforced by detection of significant levels of long-lived, bone marrow associated anti-F protein antibody secreting cells in VLP immunized mice while none were detected in mice immunized with an RSV infection. In addition, VLPs stimulated memory responses while RSV infection did not, as determined by adoptive transfer of splenic B cells from VLP immunized mice into immunodeficient Rag-/- mice. After transfer, the recipient mice had significant levels of anti-F and anti-G protein serum IgG antibody responses that were protective upon RSV challenge. In contrast, transfer of splenic B cells from RSV immunized mice produced no detectable serum antibody in the recipients nor could these mice inhibit RSV replication upon virus challenge. Thus these VLPs promise to be more effective than natural infection, a requirement for a successful vaccine.
6.1.3 Influenza-Based RSV VLPs
Expression of baculovirus encoded influenza M1 (matrix) protein in SF9 insect cells results in efficient release of VLPs (Latham and Galarza 2001). Taking advantage of this observation, Quan et al. (2011) produced influenza M1 based VLPs containing an RSV glycoprotein by co-expressing influenza M1 and either the RSV G or F proteins. The RSV glycoproteins were presumably passively incorporated into M1 containing particles released from the insect cells. In mice, these particles stimulated anti-F or anti-G antibody responses, both of which were neutralizing. Antibodies, particularly after both a prime and boost immunization were predominantly IgG2a suggesting Th1 biased immune responses. Furthermore, immunization with either of these particles provided protection from RSV challenge. Surprisingly, immunization with G protein containing VLPs showed higher neutralization titers and marginally better protection from challenge than the F protein containing VLPs. Whether this difference between the two VLPs is related to antigen dose is unclear since the amounts of F or G proteins in these particles were not reported. The safety of these VLPs, as determined by analysis of markers of ERD upon RSV challenge of immunized animals was not assessed. In addition, the authors have not reported construction of VLPs containing both RSV glycoproteins. Clinical trials of these VLPs as RSV vaccine candidates have not been reported.
6.2 Virosomes
Another type of particulate RSV vaccine candidate recently described is a virosome (Kamphuis et al. 2012; Stegmann et al. 2010). Virosomes are defined as phospholipid vesicles containing viral glycoproteins. The recently described RSV virosomes contain both RSV F and G proteins and were formed by reconstituting solublized virus envelopes with various combinations of phosphatidyl choline, phosphatidyl serine, cholesterol, and sphingomyelin. The particles formed were relatively homogeneous with a diameter from 70–130 nm. These particles have also been reconstituted to include the adjuvants MPL, a TLR-4 agonist, or P3CSK4, a TLR-2 agonist. The particles stimulated robust anti-RSV antibody responses and neutralizing antibody responses. Without inclusion of either adjuvant, the particles stimulated predominantly a Th2 response as determined by IgG2a/IgG1 ratios, and by IFNγ and IL5 levels. However, inclusion of either adjuvant shifted the response to a more balanced one, but MPL was more effective in promoting this shift.
Particles with adjuvants included stimulated protective responses as determined by virus lung titers after challenge. Importantly, the adjuvanted particles showed no evidence of ERD upon virus challenge in either mice or cotton rats as assessed by cytokine secretion and lung histology. Thus these virosomes show promise as potential vaccine candidates. No clinical trials have been reported. In addition, the conformation of the F protein in the particles has not been characterized.
6.3 Nanoparticles
Another RSV vaccine candidate is described as a nanoparticle (Patent application #WO 2010/077717A1). This particle is composed entirely of a mutant version of the RSV F protein, which was synthesized in baculovirus-infected insect cells and purified by column chromatography. These particles are described as rosettes of F protein trimers that are 20–40 nm in diameter. The conformation of the F protein has not been reported. How this form of F protein differs from previously characterized purified protein vaccine candidates (PFP-1, 2, and 3) is unclear. Results of preclinical trials in either mice or cotton rats have not been reported, and, importantly, nor have safety studies in mice or cotton rats. However, phase 1 clinical trials of the material have recently been conducted (clinical trials.gov, NCT 01290419).
7 Considerations for Future Development of Subunit and Particulate RSV Vaccines
7.1 Overview
There are a number of lessons from past preclinical and, particularly, clinical trials of subunit vaccine candidates. In addition, results of recent studies of RSV protein structure and function and the immunology of RSV infections and vaccine candidates suggest important modifications to future RSV vaccine candidates, modifications that could significantly impact their safety and efficacy and, ultimately, their approval for use in human populations. Studies of potential improvements should minimally consider F protein conformation, G protein conformation, adjuvant incorporation, and supramolecular structures containing repeating arrays of antigens. Because murine or cotton rat immune responses are likely different than human responses, there must be careful assessment of the human immune response, perhaps informed by studies in humanized murine systems.
7.2 Role of Conformation of F Protein in Effective Antibody Stimulation
The paramyxovirus F protein is folded into a metastable conformation and upon fusion activation refolds into the post fusion conformation, which is structurally very different from the pre-fusion form (see chapter by McLellan et al. (2011) this volume; Yin et al. 2005; Yin et al. 2006). Given current models of paramyxovirus fusion, it is logical to assume that optimally neutralizing antibodies should bind to pre-fusion F protein in order to block virus entry, and that an effective vaccine candidate should contain the pre-fusion form of the protein. Recent structural studies of the RSV F protein demonstrate that at least two neutralizing monoclonal antibody binding sites, including the Palivizumab epitope, are accessible on the post fusion form of the protein, suggesting that post fusion forms should stimulate at least a subset of the potential protective neutralizing antibodies (McLellan et al. 2011; Swanson et al. 2011), at least in mice. However, it has also been reported that a significant proportion of the neutralizing antibodies in human immune sera do not bind to the post fusion form of the protein (Magro et al. 2012) raising the possibility that there are human neutralizing antibody binding sites on the pre-fusion form not present on the post fusion form. Thus, it remains to be determined which of the two forms of the F protein is the optimal antigen for inclusion in human vaccine candidates. Conformational intermediates between the pre and post fusion forms should also be considered. Presumably, the PFP vaccine candidates tested in the past were primarily in the post fusion form and stimulated only a subset of neutralizing antibodies, a possibility that could reduce their protective effect, as noted in clinical trials. Formulation of future vaccine candidates should focus on inclusion of the pre-fusion or a conformational intermediate form of F protein.
7.3 G Protein Conformation
Recent studies have indicated that antibodies to the central region of the G protein ectodomain have a significant role in protective responses to RSV (Zhang et al 2010). There is virtually no information about the conformation of the G protein or any conformational changes that may take place during virus entry. A more detailed understanding of the conformation of this protein and any changes that occur during virus attachment and entry could suggest more directed approaches for the inclusion of a form of the G protein that will stimulate effective antibodies to this central region. Perhaps future vaccine candidates should present only peptides from this region of the G protein rather than the intact protein.
7.4 Adjuvants
Stimulation of innate immunity is necessary for affinity maturation of antibodies and long-term T and B cell responses (reviewed in Bessa et al. 2010; Guay et al. 2007; Lanzavecchia and Sallusto 2007; Pasare and Medzhitov 2005). Adjuvants stimulate innate immunity and are often included in vaccines to enhance adaptive immune responses. Different adjuvants stimulate through different innate immune response pathways with different outcomes on adaptive immunity. The only licensed adjuvant that has been tested with RSV vaccine candidates is alum. Indeed, until just recently, alum is the only adjuvant licensed for use in vaccines in the US. While the pathways stimulated by alum are only recently described, precise mechanisms responsible for enhancement of antibody responses by alum are still not clearly defined (reviewed in Lambrecht et al. 2009). It is clear, however, that alum stimulates primarily Th2 responses (Lambrecht et al. 2009). Thus, alum is not the appropriate adjuvant for subunit RSV vaccine candidates, especially for use in young infants, since predominantly Th2 responses are associated with ERD. In addition, many subunit vaccine candidates discussed above are less likely to induce T cell responses, particularly Th1 responses, and optimal adjuvants will be those that increase stimulation of Th1 responses. Indeed, over the past decade, subunit or inactivated RSV vaccines combined with many different adjuvants, particularly TLR agonists, have shown significantly improved adaptive immune responses in animal models. For example, in a head-to-head comparison of alum and TLR agonists, it was recently reported that inclusion of TLR 4, 3, and 7 agonists with UV-inactivated RSV promoted antibody affinity maturation and protective responses without ERD while inclusion of alum did not (Delgado et al. 2009).
Recently one TLR4 agonist, MPL, has been approved for use in the HPV vaccine. However, a number of other TLR agonists are in phase I-III clinical trials (Steinhagen et al. 2011). Furthermore, recent reports clearly show that combinations of TLR agonists synergize to enhance adaptive immunity (Kasturi et al. 2011; Napolitani et al. 2005; Querec et al. 2006) to a noninfectious, particulate influenza vaccine candidate suggesting that inclusion of several different adjuvants in vaccine candidates may be optimal. Another adjuvant included in influenza vaccines used in Europe is MF59, an oil in water emulsion that improves immunogenicity. However, the mechanism of action of this adjuvant is not entirely understood (Lambrecht et al. 2009). Testing of these adjuvants and combinations of adjuvants with RSV vaccine candidates, including older ones such as the PFP, will likely significantly improve efficacy and yield formulations with acceptable properties in at least certain target populations. Inclusion of these new adjuvants with newer particulate RSV vaccine candidates will potentially further enhance the effectiveness of these candidates.
7.5 Supramolecular Structure of the Antigens
It is increasingly recognized that immunization with particulate antigens often results in more robust antibody responses, in more efficient maturation of high affinity antibodies, and in enhanced development of T cell responses compared to responses to purified proteins. Indeed, there are two particulate vaccines licensed for use, the vaccine for the human papilloma virus (HPV) and the vaccine for hepatitis B virus (HBV). The HPV vaccine is a virus-like particle (VLP) composed of the major capsid structural protein and is assembled into a structure very similar to the virus particle. The HBV vaccine is the envelope protein embedded in a lipid vesicle resembling, to some extend, the virus particle. Preclinical studies of particulate vaccine candidates for RSV, described above, show promise, some of which do not require addition of adjuvant.
Potentially other types of particles may also be developed for future candidates. Indeed, particles, described as microparticles, have recently been reported as a potential vaccine candidate for RSV. These 10-nm polyphosphazene microparticles were composed of poly[di(sodium carboxylatoethylphenoxyl)] phosphazene and contained the truncated secreted form of F protein as well as TLR agonists (Garlapati et al. 2012). Nanoparticles composed of the influenza HA protein as well as TLR agonists encapsulated in 300-nm particles composed of poly(D,L-lactic-co-glycolic acid) have been reported as potent influenza vaccine candidates (Kasturi et al. 2011). These could potentially be developed for RSV vaccine candidates.
Another type of nanoparticle reported contains the RSV N protein and a carboxyl terminal fragment of the P protein (Roux et al. 2008) and is an alternative approach to RSV vaccines. N protein sequence includes human specific RSV T cell epitopes. It has been reported that CD8 T cells are important for protection from vaccine induced eosinophilia (Hussell et al. 1997, 1998) thus the goal of these studies was to explore the potential role of N protein induced T cell responses in protective responses. The N protein, expressed in E. coli, formed 15 nM particles composed of a ring of 10–11 N proteins associated with 70 nucleotide fragments of E. coli RNA. In adult mice, intranasal immunization with this particle resulted in N protein specific antibody, CD4 and CD8 T cell responses, and protection from RSV replication upon challenge as determined by quantitative PCR of NP sequences in lungs (Roux et al. 2008). However, in neonatal mice (Remot et al. 2012), while there was some protection, there was evidence for ERD and a Th2 biased immune response upon RSV challenge. Inclusion of the adjuvant CpG only partially eliminated ERD. Thus, the efficacy and safety of this type of vaccine candidate must be further optimized and assessed in preclinical trials.
Thus, future endeavors that focus on defining the most appropriate form of particulate antigen for RSV vaccines are warranted. A consideration is that many of the early vaccine candidates, notably PFPs, were purified proteins mixed with alum. This combination does result in particulate multivalent structures (Tritto et al. 2009) yet these particles were not very effective vaccines in humans. It is likely that the protein antigens in alum are not organized into repeating arrays typical of a virus. Thus, the organization of the viral antigens in any particulate vaccine candidate may need to be carefully considered.
7.6 Human Immune Responses to Vaccine Candidates
A major difficulty in developing an effective RSV vaccine has been the failure to translate the positive results obtained in rodent models to humans (Power 2008). It is possible that RSV vaccine candidate stimulation of innate and B cell immunity in humans is different than in model animal systems (Lanzavecchia and Sallusto 2009). Definition of immune responses to vaccine candidates in humans will be a key to the development of an effective human vaccine. The recent development of humanized murine systems for vaccine testing (Schmidt et al. 2008; Shultz et al. 2007; Zhang et al. 2007) should facilitate these studies. Not only will formulations most favorable for human innate immunity and B cell stimulation be identified but also optimal routes, schedules, and doses of immunization can be tested.
8 Conclusions
Existing data suggest that subunit vaccines, despite primarily inducing antibody without T cell responses, are safe and relatively immunogenic in adults of all ages, although efficacy will need to be demonstrated and may require enhanced immunogenicity. Similarly, this approach should also be safe and effective in the RSV-experienced older child. However, subunit vaccines will need to reliably induce balanced Th1/Th2 responses as well as neutralizing antibodies and T cells if they are to be successfully developed for use in very young RSV-naïve infants, the primary group in need of a vaccine. VLPs may be particularly successful for this group as they can induce T cell immunity.
Given the history and current status of subunit and particulate vaccine candidates summarized here as well as considerations described above, one may predict that an optimal RSV vaccine candidate will include the pre-fusion form of the F protein and a conformation of G protein or G protein fragment that exposes the central conserved region of the protein. These proteins should be assembled in a virus-sized particle in an organized array typical of an infectious virus. In addition, a combination of adjuvants targeting different innate immunity pathways will likely increase the affinity maturation of antibodies as well as induction of long-lived antibody secreting bone marrow plasma cells and memory B cells. Testing of new candidates should include neonatal rodents, humanized murine systems, as well as different human target populations. Approval of any of subunit or particulate vaccine candidates for use in humans will depend upon optimization of efficacy and safety in these model systems. Approval for vaccines targeted to adults and sero-positive children will be far less problematic than those targeted for sero-negative infants.
References
Belshe RB, Anderson EL, Walsh EE (1993) Immunogenicity of purified F glycoprotein of respiratory syncytial virus: clinical and immune responses to subsequent natural infection in children. J Infect Dis 168:1024–1029
Bessa J, Kopf M, Bachmann MF (2010) Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J Immunol 184:4615–4619
Calder LJ, Gonzales-Reyes L, Garcia-Barreno B, Wharton SA, Skehel JJ, Wiley DC, Melero JA (2000) Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology 271:122–131
Cardenas S, Auais A, Piedimonte G (2005) Palivizumab in the prophylaxis of respiratory syncytial virus infection. Expert Rev Anti Infect Ther 3:719–726
Choi Y, Mason CS, Jones LP, Crabtree J, Jorquera PA, Tripp RA (2012) Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C-CX3CR1 binding and cross-neutralize RSV A and B strains. Viral Immunol 25:193–203
Collins PL, Crowe JE (2007) Respiratory syncytial virus and metapneumovirus, 5th edn. LippincottWilliams and Wilkins, Philadelphia
Collins PL, Graham BS (2007) Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 82:2040–2055
Connors M, Collins PL, Firestone C-Y, Sotnikov AV, Waitze A, Davis AR, Hung PP, Chanock RM, Murphy BR (1992) Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia RSV recombinants or RSV. Vaccine 10:475–484
de Waal L, Power UF, Yuksel S, van Amerongen G, Nguyen TN, Niesters HGM, de Swart RL, Osterhaus ADME (2004) Evaluation of BBG2Na in infant macaques: specific immune responses after vaccination and RSV challenge. Vaccine 22:915–922
Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang H-Y, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP (2009) Lack of antibody affinity maturation due to poor toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 15:34–41
Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE (2005) Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759
Falsey AR, Walsh EE (1996) Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in ambulatory adults over age 60. Vaccine 14:1214–1218
Falsey AR, Walsh EE (1997) Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP-2) in the institutionalized elderly. Vaccine 15:1130–1132
Falsey AR, Walsh EE (2000) Respiratory syncytial virus infection in adults. Clin Microbiol Rev 13:371–384
Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, Gorse GJ, Edelman R, Hayden FG, McElhaney JE, Neuzil KM, Nichol KL, Simoes EAF, Wright PF, Sales VM-P (2008) Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (RSV) vaccines–nonadjuvanted vaccine or vaccine adjuvanted with alum–given concomitantly with influenza vaccine to high-risk elderly individuals. J Infect Dis 198:1317–1326
Garlapati S, Garg R, Brownlie R, Latimer L, Simko E, Hancock REW, Babiuk LA, Gerdts V, Potter A, van Drunen Littel-van den Hurk S (2012) Enhanced immune responses and protection by vaccination with respiratory syncytial virus fusion protein formulated with CpG oligodeoxynucleotide and innate defense regulator peptide in polyphosphazene microparticles. Vaccine 30:5206–5214
Graham BS (2012) Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev 239:149–166
Grgacic EVL, Anderson DA (2006) Virus-like particles: passport to immune recognition. Methods 40:60–65
Groothuis JR, King SJ, Hogerman DA, Paradiso PR, Simoes EAF (1998) Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis 177:467–469
Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E (2007) MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol 178:5124–5131
Hall CB (2001) Respiratory syncytial virus and parainfluenza virus. N Engl J Med 344:1917–1928
Han LL, Alexander JP, Anderson LJ (1999) Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis 179:25–30
Hildreth SW, Baggs RR, Brownstein DG, Castleman WL, Paradiso PR (1993) Lack of detectable enhanced pulmonary histopathology in cotton rats immunized with purified F glycoprotein of respiratory syncytial virus (RSV) when challenged at (3–6) months after immunization. Vaccine 11:615–618
Hussell T, Baldwin CJ, O’Garra A, Openshaw PJM (1997) CD8 + T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol 27:3341–3349
Hussell T, Georgiou A, Sparer TE, Matthews S, Pala P, Openshaw PJM (1998) Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol 161:6215–6222
Jennings GT, Bachmann MF (2008) The coming of age of virus-like particle vaccines. J Biol Chem 389:521–536
Kamphuis T, Meijerhof T, Stegmann T, Lederhofer J, Wilschut J, de Haan A (2012) Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid a in mice. PLoS ONE 7:e36812
Kang S-M, Song J-M, Quan F-S, Compans RW (2009) Influenza vaccines based on virus-like particles. Virus Res 143:140–146
Karron RA (2008) Respiratory syncytial virus and parainfluenza virus vaccines, 5th edn. Saunders-Elsevier, Philadelphia
Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B (2011) Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547
Kwilas S, Liesman RM, Zhang L, Walsh E, Pickles RJ, Peeples ME (2009) Respiratory syncytial virus grown in vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol 83:10710–10718
Lambrecht BN, Kool M, Willart MAM, Hammad H (2009) Mechanism of action of clinically approved adjuvants. Curr Opin Immunol 21:23–29
Langley JM, Sales V, McGeer A, Guasparini R, Predy G, Meekison W, Li M, Capellan J, Wang E (2009) A dose-ranging study of a subunit respiratory syncytial virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults greater than or equal to 65 years of age. Vaccine 27:5913–5919
Lanzavecchia A, Sallusto F (2007) Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol 19:268–274
Lanzavecchia A, Sallusto F (2009) Human B cell memory. Curr Opin Immunol 21:298–304
Latham T, Galarza JM (2001) Formation of wild type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J Virol 75:6154–6165
Ludwig C, Wagner R (2007) Virus-like particles–universal molecular toolboxes. Curr Opin Biotechnol 18:537–545
Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Terron MC, Melero JA, Palomo C (2012) Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci 109:3089–3094
McGinnes LW, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG (2011) Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol 85:366–377
McGinnes LW, Pantua H, Laliberte JP, Gravel KA, Jain S, Morrison TG (2010) Assembly and biological and immunological properties of Newcastle disease virus-like particles. J Virol 84:4513–4523
McLellan JS, Yang Y, Graham BS, Kwong PD (2011) Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol 85:7788–7796
Miao C, Radu GU, Caidi H, Tripp RA, Anderson LJ, Haynes LM (2009) Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab’)2 components mediated reduced pulmonary inflammation in mice. J Gen Virol 90:1119–1123
Munoz FM, Piedra PA, Glezen WP (2003) Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 21:3465–3467
Murata Y (2009) Respiratory syncytial virus vaccine development. Clin Lab Med 29:725–739
Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare M, Smith G, Heaton PM, Fraire A, Morrison TG (2010) Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice with no evidence of immunopathology. J Virol 84:1110–1123
Murphy BR, Sotnikov A, Paradiso PR, Hildreth SW, Jenson AB, Baggs RB, Lawrence L, Zubak JJ, Chanock RM, Beeler JA, Prince GA (1989) Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine 7:533–540
Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA (1990) Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine 8:497–502
Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EAF, Rudan I, Weber MW, Campbell H (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet 375:1545–1555
Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A (2005) Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol 6:769–776
Nguyen TN, Power UF, Robert A, J-Fo Haeuw, Helffer K, Perez A, Asin M-A, Corvaia N, Libon C (2012) The respiratory syncytial virus G protein conserved domain induces a persistent and protective antibody response in rodents. PLoS ONE 7:e34331
Oien NL, Brideau RJ, Thomsen DR, Homa FL, Wathen MW (1993) Vaccination with a heterologous respiratory syncytial virus chimeric FG glycoprotein demonstrates significant subgroup cross-reactivity. Vaccine 11:1040–1048
Oien NL, Brideau RJ, Walsh EE, Wathen MW (1994) Induction of local and systemic immunity against human respiratory syncytial virus using a chimeric FG glycoprotein and cholera toxin B subunit. Vaccine 12:731–735
Openshaw PJ, Tregoning JS (2005) Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev 18:541–555
Pantua HD, McGinnes LW, Peeples ME, Morrison TG (2006) Requirements for the assembly and release of Newcastle disease virus-like particles. J Virol 80:11062–11073
Paradiso PRP, Hildreth SWD, Hogerman DA, Speelman DJ, Lewin EBM, Oren JM, Smith DHM (1994) Safety and immunogenicity of a subunit respiratory syncytial virus vacine in children 24 to 48 months old. Pediatr Infect Dis J 13:792–798
Pasare C, Medzhitov R (2005) Control of B-cell responses by Toll-like receptors. Nature 438:364–368
Piedra PA, Cron SG, Jewell A, Hamblett N, McBride R, Palacio MA, Ginsberg R, Oermann CM, Hiatt PW (2003) Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine 21:2448–2460
Piedra PA, Glezen WP, Kasel JA, Welliver RC, Jewel AM, Rayford Y, Hogerman DA, Hildreth SW, Paradiso PR (1995) Safety and immunogenicity of the PFP vaccine against respiratory syncytial virus (RSV): the Western blot assay aids in distinguishing immune responses of the PFP vaccine from RSV infection. Vaccine 13:1095–1101
Power UF (2008) Respiratory syncytial virus (RSV) vaccines–two steps back for one leap forward. J Clin Virol 41:38–44
Power UF, Nguyen TN, Rietveld E, de Swart RL, Groen J, Osterhaus ADME, de Groot R, Corvaia N, Beck A, Bouveret-le-Cam N, Bonnefoy J-Y (2001) Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. J Infect Dis 184:1456–1460
Pulendran B, Ahmed R (2011) Immunological mechanisms of vaccination. Nat Immunol 12:509–517
Quan F-S, Kim Y, Lee S, Yi H, Kang S-M, Bozja J, Moore ML, Compans RW (2011) Virus-like particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis 204:987–995
Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B (2006) Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med 203:413–424
Remot A, Roux X, Dubuquoy C, Fix J, Bouet S, Moudjou M, Eleouet J-F, Riffault S, Petit-Camurdan A (2012) Nucleoprotein nanostructures combined with adjuvants adapted to the neonatal immune context: a candidate mucosal RSV vaccine. PLoS ONE 7:e37722
Roux X, Dubuquoy C, Durand G, Tran-Tolla T-L, Castagne N, Bernard J, Petit-Camurdan A, Eleouet J-F, Riffault S (2008) Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial virus. PLoS ONE 3:e1766
Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT (2008) Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS ONE 3:e3192
Schmidt MR, McGinnes LW, Kenward SA, Willems KN, Woodland RT, Morrison TG (2012) Long term and memory immune responses in mice against Newcastle disease virus-like particles containing respiratory syncytial virus glycoprotein ectodomains. J Virol 86:11654–11662
Shultz LD, Ishikawa F, Greiner DL (2007) Humanized mice in translational biomedical research. Nat Rev Immunol 7:118–130
Simard C, Nadon F, Seguin C, Trudel M (1995) Evidence that the amino acid region 124–203 of glycoprotein G from the respiratory synctial virus (RSV) constitutes a major part of the polypeptide domain that is involved in the protection against RSV infection. Antiviral Res 28:303–315
Simoes EAF, Tan DHS, Ohlsson A, Sales V, Wang EEL (2001) Respiratory syncytial virus vaccine: a systematic overview with emphasis on respiratory syncytial virus subunit vaccines. Vaccine 20:954–960
Stegmann T, Kamphuis T, Meijerhof T, Goud E, de Haan A, Wilschut J (2010) Lipopeptide-adjuvanted respiratory syncytial virus virosomes: a safe and immunogenic non-replicating vaccine formulation. Vaccine 28:5543–5550
Steinhagen F, Kinjo T, Bode C, Klinman DM (2011) TLR-based immune adjuvants. Vaccine 29:3341–3355
Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A (2011) Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci USA 108:9619–9624
Teng MN, Collins PL (1998) Identification of respiratory syncytial virus proteins required for formation and passage of helper dependent infectious particles. J Virol 72:5707–5716
Thompson W, Shay DK, Weintraub E et al (2003) Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186
Tripp RA (2004) Pathogenesis of respiratory syncytial virus infection. Viral Immunol 17:165–181
Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ (2001) CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2:732–738
Tristram DA, Welliver RC, Mohar CK, Hogerman DA, Hildreth SW, Paradiso P (1993) Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18–36 months old. J Infect Dis 167:191–195
Tritto E, Mosca F, De Gregorio E (2009) Mechanism of action of licensed vaccine adjuvants. Vaccine 27:3331–3334
Trudel M, Nadon F, Seguin C, Binz H (1991) Protection of BALB/c mice from respiratory syncytial virus infection by immunization with a synthetic peptide derived from the G glycoprotein. Virology 185:749–757
Walsh EE, Falsey AR, Hennessey PA (1999) Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care Med 160:791–795
Yin H-S, Paterson RG, Wen X, Lamb RA, Jardetzky TS (2005) Structure of the uncleaved ectodomain of the paramyxovirus (hPIV3) fusion protein. Proc Natl Acad Sci U S A 102:9288–9293
Yin H-S, Wen X, Paterson RG, Lamb RA, Jardetzky TS (2006) Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 439:38–44
Zhang L, Kovalev GI, Su L (2007) HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood 109:2978–2981
Zhang W, Choi Y, Haynes LM, Harcourt JL, Anderson LJ, Jones LP, Tripp RA (2010) Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus g protein reduces pulmonary inflammation and virus replication in mice. J Virol 84:1148–1157
Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P-Y, Steiner C, Abedi GR, Anderson LJ, Brammer L, Shay DK (2012) Hospitalizations Associated With Influenza and Respiratory Syncytial Virus in the United States, 1993–2008. Clin Infect Dis 54:1427–1436
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Morrison, T.G., Walsh, E.E. (2013). Subunit and Virus-Like Particle Vaccine Approaches for Respiratory Syncytial Virus. In: Anderson, L., Graham, B. (eds) Challenges and Opportunities for Respiratory Syncytial Virus Vaccines. Current Topics in Microbiology and Immunology, vol 372. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38919-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-38919-1_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38918-4
Online ISBN: 978-3-642-38919-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)