Abstract
The skin is the outermost organ of the body and is exposed to many kinds of external pathogens. To manage this, the skin contains multiple types of immune cells. To achieve sufficient induction of cutaneous adaptive immune responses, the antigen presentation/recognition in the skin is an essential process. Recent studies have expanded our knowledge of how T cells survey their cognate antigens in the skin. In addition, the formation of a lymphoid cluster, named inducible skin-associated lymphoid tissue (iSALT), has been reported during skin inflammation. Although iSALT may not be classified as a typical tertiary lymphoid organ, it provides specific antigen presentation sites in the skin. In this article, we provide an overview of the antigen presentation mechanism in the skin, with a focus on the development of iSALT and its function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The skin is the outermost organ of the body and is exposed to many kinds of external insults. To maintain the homeostasis of such an irritable tissue, the skin is equipped with two types of barrier systems: a physical barrier and an immunological barrier (Egawa and Kabashima 2016).
The physiological barrier of the skin is solid compared with that in mucous membranes, such as in tracheal and gastrointestinal tracts, because the skin needs to block the evaporation of internal water. It consists of the stratum corneum (a cornified layer of dead keratinocytes) and the tight junctions in the stratum granulosum, which are covered with the sebum secreted from sebaceous glands (Fig. 1). These barriers are robust and block molecules with a molecular weight greater than 1000; therefore, no pathogenic microorganism can invade the body through the intact physical skin barrier. In reality, however, there are many open seams in the physical barrier; the skin easily sustains small traumas and the skin appendages, such as hair follicles and sweat pores, lack the stratum corneum and can harbor microorganisms, known as the skin microbiome (Kabashima et al. 2019) (Fig. 1; left). The pathogenic microorganisms/molecules often invade the body through such “security holes” in the skin. To manage these invaders, the immune system keeps the skin under constant surveillance.
The immunological barrier of the skin consists of multiple types of immune cells (Fig. 1; right). Skin-resident innate immune cells, such as epidermal Langerhans cells (LCs), dermal dendritic cells (DCs), and macrophages, monitor the invasion of foreign antigens. Once they recognize the antigens, they produce inflammatory cytokines to cause inflammation. Upon inflammation, many immune cells, such as neutrophils, monocytes, and T cells, are recruited to the skin. Neutrophils and monocytes provide a quick but unspecific immune response, whereas T cells provide a delayed but antigen-specific immune reaction. For the latter reaction, antigen presentation to T cells in the skin is an essential process. To facilitate this, lymphoid structures can be formed in the skin in some pathogenic conditions (discussed later).
In this article, we will discuss the antigen presentation mechanism in the skin and focus on the function of a specific immunological unit, called inducible skin-associated lymphoid tissue (iSALT), which plays an essential role in the induction of cutaneous adaptive immunity (Natsuaki et al. 2014). We also review the formation of lymphoid clusters in the human skin in several pathogenic conditions.
2 The Concept of SALT
T cells play a central role in adaptive immunity. They are generated and mature in the primary lymphoid organs such as bone marrow and thymus. They then circulate in the blood and travel to the secondary lymphoid organs (SLOs), such as lymph nodes (LNs) and spleen, to survey for antigens. Once they recognize the cognate antigen in SLOs, they are expanded and mature into effector T cells, and acquire the nature to migrate peripheral organs such as the skin and gut. In some submucosal areas, the tertiary lymphoid organs (TLOs) are organized as “sentinel” lymphoid tissues to be on the alert for invading pathogens (Brandtzaeg et al. 1999). In humans, for example, the oral and nasal pharynx areas are monitored by the tonsils and adenoids, and lymphoid follicles are present in the normal bronchi. Single lymphoid follicles are also distributed throughout the intestine and, in the distal ileum, lymphoid follicles are grouped in large clusters termed Peyer’s patches. These tissues are known as mucosa-associated lymphoid tissue (MALT) and serve as antigen presentation sites in peripheral organs. As for skin, however, no lymphoid structures were reported until recently.
Around 1980, cutaneous immunologists elucidated some key findings; (1) LCs are bone marrow-derived and capable of antigen presentation, (2) a fraction of T cells display high skin tropism, and (3) epidermal cells markedly affect T cell maturation by producing multiple cytokines and chemokines (Stingl et al. 1978; Rubenfeld et al. 1981). Based on these findings, researchers proposed that lymphoid tissues analogous to MALT in submucosal areas may exist within the skin. They offered the term SALT (skin-associated lymphoid tissue) for these putative skin-associated tissues (Streilein 1978, 1983, 1985; Egawa and Kabashima 2011). As lymphoid structure was not found in the skin at that time, SALT was a conceptual tissue; however, this hypothesis proposes that the skin is not merely a physical barrier but also an essential component of the immune system, and that the antigen presentation in the skin is an important step in elicitation of acquired skin immune responses.
3 Antigen Presentation Mechanism in the Skin
After the development of the concept of SALT, focus was placed on the antigen presentation mechanism in the skin. As the majority of skin-infiltrating T cells are memory-phenotype, and naïve T cells and B cells are almost absent in the skin (Clark et al. 2006), the antigen presentation process in the skin should be substantially different from that in the LNs and MALTs. Recent studies with transgenic animals in combination with intravital imaging techniques have extensively expanded our knowledge of the biology of cutaneous antigen-presenting cells (APCs) and skin-resident/homing T cells. In this section, we will overview how cells in the skin induce inflammation against pathogen invasion and how T-cell-mediated adaptive immunity is initiated in the skin.
3.1 Keratinocytes as an Initiator of Skin Inflammation
When keratinocytes are injured or recognize pathogens mainly through pattern recognition receptors, such as Toll-like receptors, they produce several kinds of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1α/β, IL-33, and thymic stromal lymphopoietin (TSLP) (Ansel et al. 1990; Leyva-Castillo et al. 2013; Meephansan et al. 2013). These cytokines induce the expression of E- and P-selectin and intercellular adhesion molecule 1 (ICAM-1) on vascular endothelial cells that promote the extravasation of blood-circulating lymphocytes (Brinkman et al. 2013). Phagocytes, such as neutrophils and monocytes, appear in the skin as the primary treatment, and a small number of T cells arrive slightly later to scan for their cognate antigens. Proinflammatory cytokines produced by keratinocytes also activate skin-resident immune cells, including LCs and DCs. For example, TSLP from keratinocytes promotes the migration of LCs to the skin-draining LNs and enhances the induction of Th2-type immune responses (Nakajima et al. 2012). These functions of keratinocytes as an initiator of skin inflammation suggest that keratinocytes are not only a producer of the physical barrier, but also an inducer of the immunological barrier.
3.2 Cutaneous Antigen-Presenting Cells
The antigens that breach the physical barrier of the skin are then captured by the second-line immunological barrier, cutaneous DCs. DCs are a diverse family of cells that play an essential role in linking the innate and adaptive immune systems (Dress et al. 2018). In general, four cell types are classified into the DC family in the skin: epidermal LCs, conventional DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs. cDCs can be further subdivided into the cDC1 and cDC2 subsets on the basis of subset-specific gene expression profiles, their dependence on different transcription factors, and unique subset functions (Schlitzer et al. 2015; Guilliams et al. 2016).
Epidermal LCs were previously thought to play a major role in antigen presentation not only in the LNs but also in the skin (Toews et al. 1980; Grabbe and Schwarz 1998). However, novel depletion systems of LCs (and dermal DCs) have challenged this classic LC paradigm (Bennett et al. 2005; Kissenpfennig et al. 2005). When LCs were selectively depleted, the contact hypersensitivity (CHS) response, a classical mouse model for type IV hypersensitivity, was not (Kissenpfennig et al. 2005; Bursch et al. 2007; Wang et al. 2008) or only marginally attenuated (Bennett et al. 2005). On the other hand, the depletion of LCs caused impaired CD8+ T cell activation in the skin in a graft-versus-host disease model (Bennett et al. 2011). These reports suggest that the role of LCs as cutaneous APCs differs depending on the pathogenic conditions.
The antigen-presenting role of cDCs is also controversial. Several studies found that the depletion of both LCs and cDC1 markedly reduced the CHS response (Bursch et al. 2007; Wang et al. 2008), suggesting that cDC1, but not LCs, plays an important role during CHS. However, another study reported that Batf3-deficient mice, which lack cDC1 in the skin, exhibited a normal CHS phenotype (Edelson et al. 2010), suggesting compensation of its function by other DCs. We recently demonstrated that when all subsets of cutaneous DCs were depleted, the elicitation of CHS was abrogated, whereas selective depletion of LCs or cDC1 did not attenuate the elicitation of CHS (Natsuaki et al. 2014). These results suggest the dominant role of cDC2 in T cell activation in the skin, at least during CHS.
In addition to DCs, several other skin-resident cells have been proposed to function in antigen presentation in the skin. Mast cells and basophils participate in antigen presentation and promote Th2-type inflammatory responses by acquiring MHC class II molecules from DC, termed “trogocytosis” (Dudeck et al. 2017; Miyake et al. 2017). In addition, the deletion of MHC class I molecule on radio-resistant cells impaired the activation of CD8+ T cells in CHS (Ono et al. 2018), suggesting the possible involvement of vascular endothelial cells (Kish et al. 2011) and keratinocytes (Gaspari and Katz 1988; Kim et al. 2009) as APCs in the skin, although in vivo evidence is lacking. As co-stimulatory molecules are not required for the activation of effector T cells (Krummel et al. 1999), non-professional APCs in the skin may carry out the APC function in a context-dependent manner.
3.3 T Cell Recruitment to the Skin
To mediate their effector functions, most lymphocytes, except for B cells, need to home into the affected peripheral tissues before their immunomodulatory functions can be initiated. Upon inflammation, effector T cells are recruited to the skin with limited antigen dependency (Honda et al. 2014). T cells migrate into the inflamed tissues by scanning vascular endothelial cells displaying molecular signatures required for precise spatial homing. After activation and differentiation within the secondary lymphoid organs, the effector T cells downregulate lymph node homing molecules like CD62L and CCR7 and upregulate molecules specific for their homing into peripheral tissues (Masopust and Schenkel 2013).
Recent studies suggest that effector T cells express homing molecules specific for organs from where the antigenic insult originated. T cells primed in the skin-draining lymph nodes upregulate E- and P-selectin ligands (Tietz et al. 1998; Hirata et al. 2002), CCR4 and/or CCR10, which are required for skin homing (Soler et al. 2003; Masopust and Schenkel 2013). Analogous to vitamin A-mediated upregulation of gut homing molecules (Mora et al. 2008), DCs can process vitamin D present in the skin to its active metabolite 1,25-dihydroxyvitamin D3, which facilitates the induction of CCR10, and concomitant downregulation of α4β7 and CCR9 (Sigmundsdottir et al. 2007; Masopust and Schenkel 2013). Expression of CCL17 by skin venules and CCL27 by epidermal keratinocytes helps in homing CCR4+ and CCR10+ T cells to the skin, respectively (Reiss et al. 2001; Homey et al. 2002; Soler et al. 2003). These studies provide mechanistic insight into how the site of priming can imprint homing molecules on effector T cells.
3.4 Antigen Survey by T Cells in the Skin
After homing into the inflamed tissue, T cells have to search for rare antigen-bearing target cells before effector functions can be initiated. Intravital imaging studies have revealed that effector T cells entering the interstitium of the skin exhibit a polarized cell shape, characterized by the formation of a leading edge and a uropod, and migrate at high velocities (Matheu et al. 2008; Egawa et al. 2011; Honda et al. 2014). During interstitial migration, T cells constantly integrate molecular cues provided by surrounding cells. Unlike neutrophils and DCs, T cells are less dependent on chemoattractant gradients. During interstitial migration, they demonstrate a “stop and go” behavior reminiscent of naïve T cells in the LNs (Miller et al. 2002; Kawakami et al. 2005; Munoz et al. 2014; Weninger et al. 2014). This behavior may provide an effective strategy for screening large regions of the tissue.
Once they encounter their cognate antigens, effector T cells initiate stable contact with APCs and are activated to produce inflammatory cytokines. Upon activation, effector CD4+ T cells can produce large-scale cytokine gradients. For example, in the Leishmania major infection model, effector CD4+ T cells interact with infected APCs, resulting in migratory arrest and T-cell-mediated production of interferon-γ (IFN-γ), leading to the generation of IFN-γ gradients up to 80 μm away from the T cell-APC interaction site (Muller et al. 2012). On the other hand, cytotoxic effector CD8+ T cells induce target cell apoptosis by different means, including the release of cytokines and cytotoxic mediators such as perforin and granzymes. Although the migratory behavior of effector CD8+ T cell populations within inflamed tissues is best described as random migration, several studies have observed T cell navigation along the extracellular matrix and other anatomical structures. Intravital imaging of subcutaneous tumors has revealed that CD8+ T cells migrate along dermal collagen fibers and blood vessels (Mrass et al. 2006; Boissonnas et al. 2007).
Of note, after infiltration into the skin, a part of the T cells returns back to the draining LNs via afferent lymphatics. Classic lymph recirculation studies in sheep have demonstrated that effector/memory CD4+ T cells comprise a major portion of lymphocytes in afferent lymph (Mackay et al. 1988, 1990). T cell egress from the skin is dependent on CCR7 (Bromley et al. 2005; Debes et al. 2005), similarly to other leukocytes such as DCs or neutrophils (Randolph 2001; Ng et al. 2011). Endothelial cells of the afferent lymph vessels constitutively express the CCR7 ligand CCL21 (Debes et al. 2005). Moreover, a recent study employing photo-convertible Kaede transgenic mice, which serves an in vivo cell-tracking system, reported that the cell migration from peripheral tissues to the draining LNs was markedly increased after inflammation (Tomura et al. 2010). Importantly, approximately half of the CD4+ T cells that return back from the inflamed site are regulatory T cells (Tregs). These observations suggest that Tregs preferentially infiltrate or are induced in peripheral tissue during the resolution phase of inflammation.
3.5 Antigen Recognition by Resident Memory T Cells in the Skin
Although T cells have a dynamic migratory nature, a number of studies suggested that some of the tissue-infiltrating effector T cells never return to the circulation (Shin and Iwasaki 2013; Mueller et al. 2014). This non-circulating memory T cell subset is called tissue-resident memory T cells (TRM). TRM have been identified in several non-lymphoid tissues, such as the skin, gut, lung, brain, and female reproductive tract (Gebhardt et al. 2009; Masopust et al. 2010; Wakim et al. 2010), although the longevity of TRM greatly differs among tissues. For example, skin TRM in mice persist for over a year (Mackay et al. 2012), whereas lung TRM are maintained for a few months (Wu et al. 2014).
Of note, the distribution and migration of CD4+ TRM and CD8+ TRM differ significantly in the skin. Previous studies with herpes simplex virus (HSV) skin infection elucidated that after pathogen clearance, antigen-specific CD4+ TRM redistribute within the dermis, whereas CD8+ TRM localize to the epidermis (Gebhardt et al. 2011; Mackay et al. 2012) (Fig. 2). CD4+ TRM have amoeboid morphology and actively migrate throughout the dermis, whereas CD8+ TRM exhibit a dendritic morphology and are almost sessile. However, during vaccinia virus skin infections, CD8+ TRM were found in the dermis as well as the epidermis, and these CD8+ TRM were found not only in the infected site, but also in the non-infected regions within the tissue (Jiang et al. 2012).
A schematic representation of the skin depicting the presence of different leukocyte subsets before and after Herpes simplex virus (HSV) infection. In naïve skin, epidermis is populated with sessile Langerhans cells and dendritic epithelial T cells (DETCs), whereas the dermis is populated with dermal DCs, CD4+ T cells, dermal γδ T cells, and a few CD8+ T cells. After the resolution of HSV infection, a subset of antigen-specific CD8+ TRM cells resides in the epidermis, whereas antigen-specific CD4+ TRM clusters reside in the dermis
There is evidence suggesting that rapid control of infection at peripheral sites requires the presence of TRM. Upon viral re-infection, TRM respond to antigen and produce proinflammatory cytokines, such as IFN-γ, within hours, whereas circulating memory T cells re-enter the infected site within 2 days but do not produce IFN-γ until 5 days after the challenge (Iijima and Iwasaki 2014). TRM-derived cytokines activate local innate immunity by driving antiviral/antibacterial genes, DC maturation, NK cells activation, and VCAM-1 expression on the blood endothelium (Ariotti et al. 2014; Schenkel et al. 2014). Therefore, TRM function as antigen-specific sensors and provide robust site-specific immunity. The presence of TRM in epidermis should be important in maintaining immunity against HSV and human immunodeficiency virus (HIV), as both of these viruses commonly begin as local infections in the genital tract in a limited population of infected cells. In such situations, TRM-mediated rapid initiation of the antiviral state at the site of entry is essential for preventing re-infections.
4 The Concept of iSALT
In contrast to mucosal boundary tissues, such as gut and bronchi, in which TLOs can be observed even in the non-pathogenic condition, no lymphocytic cluster is observed in normal skin. This is probably due to the anatomical difference between the skin and mucosal boundary tissues; external antigens hardly reach the skin through the thick physical barrier, the stratum corneum. Therefore, it has been generally considered that skin-infiltrating T cells randomly migrate in the skin to scan for cognate antigens, and that individual T cell-APC interaction is important for T cell activation in the skin.
Recently, however, the formation of a leukocyte-clustering structure was found during skin inflammation (Natsuaki et al. 2014). Unlike MALT, these leukocyte clusters are not found in the steady state and are inducible during the development of acquired immune response. Thus, this cluster was named inducible skin-associated lymphoid tissue (iSALT), similar to inducible bronchus-associated lymphoid tissue (iBALT) in the lung (Moyron-Quiroz et al. 2004). In this section, we will overview the mechanism of iSALT formation, especially during the CHS response.
4.1 iSALT Formation in CHS
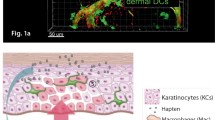
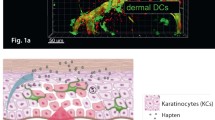
The CHS response is induced by the epicutaneous application of a hapten, a small molecule that can bind to self-proteins in the skin and acquire antigenicity. Intravital imaging studies have demonstrated that upon hapten application to the skin, T cells and dermal DCs form a lymphocytic cluster, termed iSALT, within 6 h (Natsuaki et al. 2014) (Fig. 3). iSALT disappears within days if there are no antigen-specific effector T cells in the body (the sensitization phase). In contrast, in the presence of antigen-specific effector T cells (the elicitation phase), iSALT persists for weeks. In the presence of antigens, iSALT was able to induce CD8+ T cell proliferation and reactivation.
iSALT is formed not only in response to hapten application, but also with other stimuli such as tape-stripping and bacterial infections. Furthermore, it was demonstrated that IL-1α produced by keratinocytes is essential for their induction (Natsuaki et al. 2014), suggesting that iSALT formation itself is an innate response.
4.2 iSALT Formation Around Post-capillary Venules
Dermal blood vessels can be divided into four different parts with distinct functions: arteries, capillaries, post-capillary venules (PCVs), and venules. Among them, PCVs have a unique property that is particularly important during inflammation. Adjacent blood endothelial cells are sealed with tight junctions and adherence junctions, and limit the passage of plasma proteins larger than 70 kDa (Egawa et al. 2013). Importantly, this vascular permeability is variable only at PCVs. Upon inflammation, hyper-permeability is induced on PCVs, leading to extravasation of albumin (70 kDa) and immunoglobulins (150 kDa) into the dermal interstitium, suggesting that PCVs are specific portal sites for humoral immunity into the skin under inflammatory conditions (Fig. 4).
Intravital studies also demonstrated that PCVs are specific portal sites for cellular immunity. In PCVs, blood endothelial cells are surrounded by pericytes and macrophages, and mast cells are located nearby. Previous studies using a mouse Staphylococcus aureus infection model revealed that these cellular units around PCVs are important for neutrophil extravasation in the dermis (Abtin et al. 2014). As for T cells, intravital imaging studies and immunohistochemical analyses revealed that most iSALT was formed around PCVs (Honda and Kabashima 2016; Kogame et al. 2017) (Fig. 5) and in the absence of iSALT formation, T cell infiltration to the skin is impaired. These findings suggest that cellular units around PCVs play an import role in lymphocyte recruitment into the skim. In particular, recent studies revealed indispensable roles of perivascular macrophages (PVMs) in this process.
A schema of iSALT formation during CHS. Keratinocytes contact with hapten induces the release of IL-1 in the skin, which activates perivascular macrophages that subsequently attract dermal DCs via CXCR2 to form clusters. In the absence of antigen-specific memory T cells, DC clustering is a transient event, and hapten-carrying DCs migrate to the skin-draining LNs. In the presence of antigen-specific effector T cells, T cells are activated and skin inflammation is promptly induced
4.3 Indispensable Role of Perivascular Macrophages in iSALT Formation
An intravital imaging study using DPE-GFP mice revealed that approximately 40% of venules are surrounded by PVMs (Abtin et al. 2014). Upon S. aureus infection, PVMs highly express the neutrophil-attracting chemokines Cxcl1 and Cxcl2, and neutrophils extravasate in the vicinity of PVMs. PVMs are depleted when they are exposed to S. aureus-producing exotoxin, a-hemolysin, and under this condition, neutrophil recruitment to the skin is significantly suppressed (Abtin et al. 2014).
PCVs also play an essential role during iSALT formation and T cell activation in the skin (Natsuaki et al. 2014). Cell-type specific depletion studies found that mast cells, T cells, B cells, and basophils were dispensable for iSALT formation by hapten application, but when macrophages were depleted, iSALT formation was abrogated. Further analysis revealed that IL-1α produced by keratinocytes upon external insults stimulates M2-like macrophages around PCVs, which then produce Cxcl2 and recruit dermal DCs to the cluster to form iSALT. Leukotriene B4, a lipid mediator, also mediates the cluster formation by promoting DC migration (Sawada et al. 2015). Subsequently, effector T cells accumulated in the cluster are presented antigens by dermal DCs and initiate proliferation and activation. In the cluster, both dermal DC subsets (Kashem et al. 2017), i.e., CD103+ cDC1 and CD11b+ cDC2, are detected (Okada et al. 2016). It is currently unclear, however, which dermal DC subsets mediate antigen presentation in the cluster (Ono et al. 2018). Each DC subset in the cluster may function in a compensatory manner like DCs in the sensitization phase (Honda et al. 2010). The blockade of CXCL2 and IL-1 receptor signaling impairs iSALT formation and effector T cell activation, suggesting that iSALT is an essential structure for antigen presentation in the skin.
5 iSALT Formation in Humans
It remains unclear whether iSALT formation and functions in T cell activation in human skin are analogous to those in mice. In many inflammatory skin diseases, including eczema, psoriasis, and drug eruptions, perivascular leukocyte infiltrations are frequently observed by histological examination. In allergic contact dermatitis in humans, T cell-DC clusters are found in the dermis and are accompanied by vesicle formation in the epidermis, a marker of eczema, suggesting T cell activation and subsequent cytokine production occur above the cluster (Natsuaki et al. 2014).
In psoriasis patients, the existence of high endothelial venules (HEV), a characteristic structure of TLOs, has been reported (Lowe et al. 1995), and clusters of DC-LAMP+ DCs and T cells have been detected in the dermis (Zaba et al. 2007) with abundant expression of CCL19, a ligand for CR7, and CCL20 (Mitsui et al. 2012; Kim et al. 2014). CCL20 is implicated in the formation and function of MALT via the chemoattraction of CCR6+ lymphocytes and DCs. This cluster observed in psoriatic lesions disappears after treatment with TNF-α inhibitors (Zaba et al. 2007), suggesting the fundamental role of TNF-α in the maintenance of the cluster.
iSALT-like structures were also found in the skin lesions of secondary syphilis infection (Kogame et al. 2017). Of note, these lymphatic clusters contain CXCL13+ cells. CXCL13 is a marker of follicular helper T cells and is an important chemokine responsible for the formation and maintenance of lymphatic clusters with B cells. Indeed, spotty infiltration of B cells is observed in the skin lesions of secondary syphilis infection. In melanoma patients, clusters with TLO features, such as the existence of HEV, T cells, B cells, and mature DCs, have been detected in the extratumoral area with tumor regression or favorable overall survival (Ladanyi et al. 2007; Martinet et al. 2012). Lymphoid follicles are also reported in the lesional skin of cutaneous lupus erythematosus (Arps and Patel 2013) and lymphoproliferative diseases such as Kimura’s disease (Kung et al. 1984). Although the functional significance of the leukocyte clusters/lymphoid follicles in the skin remains unclear, these structures may play important roles in the promotion or regulation of disease development.
6 Future Remarks
TLOs play important roles in host protection and the development of pathogenic conditions in non-lymphoid peripheral tissues (Dieu-Nosjean et al. 2014; Pitzalis et al. 2014; Colbeck et al. 2017). TLOs have not yet been clearly defined, but they should fulfill several characteristics: (1) the existence of distinct T and B cell compartments, (2) a follicular reticular cell (FRC) network, (3) peripheral node addressins (PNAd)+ HEVs, (4) lymphatic vasculature, and (5) evidence of class switching (Dieu-Nosjean et al. 2014). Most TLOs are not genetically programmed and do not develop postnatally. Lymphotoxin, lymphoid chemokines (CCL19, CCL21, CXCL13), TNF-α, and the receptor activator of nuclear factor kappa-B ligand (RANKL) play essential roles in the development of TLOs.
Based on these structural criteria, iSALT may not be classified as a typical TLO because of the absence of B cells, naïve T cells, HEV, and the FRC network. In addition, the involvement of lymphoid chemokines has not yet been fully clarified in iSALT. From a functional point of view, however, iSALT possesses the key features of a TLO in that it offers efficient sites for effector T cell activation; some researchers thus use the term TLO for iSALT, focusing on this point (Neyt et al. 2012; Colbeck et al. 2017).
Although both MALT and iSALT provide defined sites for antigen presentation within peripheral organs, there should be distinct functional differences between these tissues. MALT contains significant numbers of B cells and forms lymph follicles, whereas virtually all lymphocytes in the iSALT are T cells. MALT contains HEVs and serves as an entry point for naïve T cells, suggesting that it is equipped to provide a field for antigen presentation to naïve T cells and other SLOs. In contrast, HEVs are rare in the skin and most of the T cells recruited to the skin are considered to be antigen-experienced effector T cells. Thus, SALT may act as a peripheral lymphoid tissue to provide a function distinct from other secondary/tertiary lymphoid organs, including MALT.
Although studies on iSALT function have progressed, we lack a comprehensive understanding of the skin in health and diseases. It is unclear which immune cells and/or non-immune cells interact with each other at which immune response time point (e.g., the acute, chronic, and resolution stages) and where in the skin. Considering the fundamental differences between mouse and human skin is another important challenge. Evaluation of these points may lead to a breakthrough in the understanding of the immunological mechanisms of cutaneous immune responses and healthy skin homeostasis.
Abbreviations
- APCs:
-
Antigen-presenting cells
- cDCs:
-
Conventional dendritic cells
- CHS:
-
Contact hypersensitivity
- DCs:
-
Dendritic cells
- FRC:
-
Follicular reticular cell
- iSALT:
-
Inducible skin-associated lymphoid tissue
- LCs:
-
Langerhans cells
- LNs:
-
Lymph nodes
- MALT:
-
Mucosa-associated lymphoid tissue
- PCVs:
-
Post-capillary venules
- pDCs:
-
Plasmacytoid DCs
- PVMs:
-
Perivascular macrophages
- SALT:
-
Skin-associated lymphoid tissue
- SLO:
-
Secondary lymphoid organs
- TLO:
-
Tertiary lymphoid organ
- Tregs:
-
Regulatory T cells
- TRM:
-
Resident memory T cells
References
Abtin A et al (2014) Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol 15(1):45
Ansel J et al (1990) Cytokine modulation of keratinocyte cytokines. J Invest Dermatol 94(6):s101–s107
Ariotti S et al (2014) T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science 346(6205):101–105
Arps DP, Patel RM (2013) Lupus profundus (panniculitis): a potential mimic of subcutaneous panniculitis-like T-cell lymphoma. Arch Pathol Lab Med 137(9):1211–1215
Bennett CL et al (2011) Langerhans cells regulate cutaneous injury by licensing CD8 effector cells recruited to the skin. Blood 117(26):7063–7069
Bennett CL et al (2005) Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol 169(4):569–576
Boissonnas A et al (2007) In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med 204(2):345–356
Brandtzaeg P et al (1999) Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol Today 20(3):141–151
Brinkman CC et al (2013) Peripheral tissue homing receptor control of naive, effector, and memory CD8 T cell localization in lymphoid and non-lymphoid tissues. Front Immunol 4:241
Bromley SK et al (2005) Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol 6(9):895–901
Bursch LS et al (2007) Identification of a novel population of Langerin + dendritic cells. J Exp Med 204(13):3147–3156
Clark RA et al (2006) The vast majority of CLA + T cells are resident in normal skin. J Immunol 176(7):4431–4439
Colbeck EJ et al (2017) Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol 8:1830
Debes GF et al (2005) Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 6(9):889–894
Dieu-Nosjean MC et al (2014) Tertiary lymphoid structures in cancer and beyond. Trends Immunol 35(11):571–580
Dress RJ et al (2018) Homeostatic control of dendritic cell numbers and differentiation. Immunol Cell Biol
Dudeck J et al (2017) Mast cells acquire MHCII from dendritic cells during skin inflammation. J Exp Med 214(12):3791–3811
Edelson BT et al (2010) Peripheral CD103 + dendritic cells form a unified subset developmentally related to CD8alpha + conventional dendritic cells. J Exp Med 207(4):823–836
Egawa G et al (2011) In vivo imaging of T-cell motility in the elicitation phase of contact hypersensitivity using two-photon microscopy. J Invest Dermatol 131(4):977–979
Egawa G, Kabashima K (2011) Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J Invest Dermatol 131(11):2178–2185
Egawa G, Kabashima K (2016) Multifactorial skin barrier deficiency and atopic dermatitis: essential topics to prevent the atopic march. J Allergy Clin Immunol 138(2):350–358. e351
Egawa G et al (2013) Intravital analysis of vascular permeability in mice using two-photon microscopy. Sci Rep 3:1932
Gaspari AA, Katz SI (1988) Induction and functional characterization of class II MHC (Ia) antigens on murine keratinocytes. J Immunol 140(9):2956–2963
Gebhardt T et al (2009) Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10(5):524–530
Gebhardt T et al (2011) Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477(7363):216–219
Grabbe S, Schwarz T (1998) Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today 19(1):37–44
Guilliams M et al (2016) Unsupervised high-dimensional analysis aligns dendritic cells across tissues and species. Immunity 45(3):669–684
Hirata T et al (2002) P-, E-, and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J Immunol 169(8):4307–4313
Homey B et al (2002) CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med 8(2):157–165
Honda T et al (2010) Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol 125(5):1154–1156 e1152
Honda T et al (2014) Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 40(2):235–247
Honda T, Kabashima K (2016) Novel concept of iSALT (inducible skin-associated lymphoid tissue) in the elicitation of allergic contact dermatitis. Proc Jpn Acad Ser B Phys Biol Sci 92(1):20–28
Iijima N, Iwasaki A (2014) T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346(6205):93–98
Jiang X et al (2012) Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483(7388):227–231
Kabashima K et al (2019) The immunological anatomy of the skin. Nat Rev Immunol 19(1):19–30
Kashem SW et al (2017) Antigen-presenting cells in the skin. Annu Rev Immunol 35:469–499
Kawakami N et al (2005) Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med 201(11):1805–1814
Kim BS et al (2009) Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis. J Invest Dermatol 129(12):2805–2817
Kim TG et al (2014) Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J Invest Dermatol 134(5):1462–1465
Kish DD et al (2011) Hapten application to the skin induces an inflammatory program directing hapten-primed effector CD8 T cell interaction with hapten-presenting endothelial cells. J Immunol 186(4):2117–2126
Kissenpfennig A et al (2005) Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22(5):643–654
Kogame T et al (2017) Possible inducible skin-associated lymphoid tissue (iSALT)-like structures with CXCL13(+) fibroblast-like cells in secondary syphilis. Br J Dermatol 177(6):1737–1739
Krummel MF et al (1999) Differential coupling of second signals for cytotoxicity and proliferation in CD8+ T cell effectors: amplification of the lytic potential by B7. J Immunol 163(6):2999–3006
Kung IT et al (1984) Kimura’s disease: a clinico-pathological study of 21 cases and its distinction from angiolymphoid hyperplasia with eosinophilia. Pathology 16(1):39–44
Ladanyi A et al (2007) Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother 56(9):1459–1469
Leyva-Castillo JM et al (2013) TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol 133(1):154–163
Lowe PM et al (1995) The endothelium in psoriasis. Br J Dermatol 132(4):497–505
Mackay CR et al (1988) Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J Exp Med 167(6):1755–1765
Mackay CR et al (1990) Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med 171(3):801–817
Mackay LK et al (2012) Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA 109(18):7037–7042
Martinet L et al (2012) High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 1(6):829–839
Masopust D et al (2010) Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207(3):553–564
Masopust D, Schenkel JM (2013) The integration of T cell migration, differentiation and function. Nat Rev Immunol 13(5):309–320
Matheu MP et al (2008) Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity 29(4):602–614
Meephansan J et al (2013) Expression of IL-33 in the epidermis: the mechanism of induction by IL-17. J Dermatol Sci 71(2):107–114
Miller MJ et al (2002) Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296(5574):1869–1873
Mitsui H et al (2012) Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease-related genes in focal regions of psoriasis vulgaris skin lesions. J Invest Dermatol 132(6):1615–1626
Miyake K et al (2017) Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc Natl Acad Sci U S A 114(5):1111–1116
Mora JR et al (2008) Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8(9):685–698
Moyron-Quiroz JE et al (2004) Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10(9):927–934
Mrass P et al (2006) Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med 203(12):2749–2761
Mueller SN et al (2014) Tissue-resident T cells: dynamic players in skin immunity. Front Immunol 5:332
Muller AJ et al (2012) CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity 37(1):147–157
Munoz MA et al (2014) T cell migration in intact lymph nodes in vivo. Curr Opin Cell Biol 30:17–24
Nakajima S et al (2012) Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol 129(4):1048–1055. e1046
Natsuaki Y et al (2014) Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol 15(11):1064–1069
Neyt K et al (2012) Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol 33(6):297–305
Ng LG et al (2011) Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol 131(10):2058–2068
Okada T et al (2016) In vivo multiphoton imaging of immune cell dynamics. Pflugers Arch 468(11–12):1793–1801
Ono S et al (2018) Requirement of MHC class I on radioresistant cells for granzyme B expression from CD8(+) T cells in murine contact hypersensitivity. J Dermatol Sci
Pitzalis C et al (2014) Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 14(7):447–462
Randolph GJ (2001) Dendritic cell migration to lymph nodes: cytokines, chemokines, and lipid mediators. Seminars in immunology. Elsevier
Reiss Y et al (2001) CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med 194(10):1541–1547
Rubenfeld MR et al (1981) Induction of lymphocyte differentiation by epidermal cultures. J Invest Dermatol 77(2):221–224
Sawada YH, T, Hanakawa S, Nakamizo S, Murata T, Ueharaguchi-Tanada Y, Ono S, Amano W, Nakajima S, Egawa G, Tanizaki H, Otsuka A, Kitoh A, Dainichi T, Ogawa N, Kobayashi Y, Yokomizo T, Arita M, Nakamura M, Miyachi Y, Kabashima K (2015) Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J Exp Med (in press)
Schenkel JM et al (2014) T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346(6205):98–101
Schlitzer A et al (2015) Dendritic cells and monocyte-derived cells: two complementary and integrated functional systems. Seminars in Cell and Developmental Biology. Elsevier
Shin H, Iwasaki A (2013) Tissue-resident memory T cells. Immunol Rev 255(1):165–181
Sigmundsdottir H et al (2007) DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8(3):285–293
Soler D et al (2003) CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood 101(5):1677–1682
Stingl G et al (1978) Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol 121(5):2005–2013
Streilein JW (1978) Lymphocyte traffic, T-cell malignancies and the skin. J Invest Dermatol 71(3):167–171
Streilein JW (1983) Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol 80(Suppl):12s–16s
Streilein JW (1985) Circuits and signals of the skin-associated lymphoid tissues (SALT). J Invest Dermatol 85(1 Suppl):10s–13s
Tietz W et al (1998) CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol 161(2):963–970
Toews GB et al (1980) Langerhans cells: sentinels of skin associated lymphoid tissue. J Invest Dermatol 75(1):78–82
Tomura M et al (2010) Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J Clin Investig 120(3):883–893
Wakim LM et al (2010) Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA 107(42):17872–17879
Wang L et al (2008) Langerin expressing cells promote skin immune responses under defined conditions. J Immunol 180(7):4722–4727
Weninger W et al (2014) Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol 14(4):232–246
Wu T et al (2014) Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95(2):215–224
Zaba LC et al (2007) Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 204(13):3183–3194
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Egawa, G., Kabashima, K. (2020). Role of Lymphoid Structure in Skin Immunity. In: Kabashima, K., Egawa, G. (eds) Inducible Lymphoid Organs. Current Topics in Microbiology and Immunology, vol 426. Springer, Cham. https://doi.org/10.1007/82_2020_206
Download citation
DOI: https://doi.org/10.1007/82_2020_206
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51746-5
Online ISBN: 978-3-030-51747-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)