Abstract
Twin-arginine protein translocation systems (Tat) translocate fully folded and co-factor-containing proteins across biological membranes. In this review, we focus on the Tat pathway of Gram-positive bacteria. The minimal Tat pathway is composed of two components, namely a TatA and TatC pair, which are often complemented with additional TatA-like proteins. We provide overviews of our current understanding of Tat pathway composition and mechanistic aspects related to Tat-dependent cargo protein translocation. This includes Tat pathway flexibility, requirements for the correct folding and incorporation of co-factors in cargo proteins and the functions of known cargo proteins. Tat pathways of several Gram-positive bacteria are discussed in detail, with emphasis on the Tat pathway of Bacillus subtilis. We discuss both shared and unique features of the different Gram-positive bacterial Tat pathways. Lastly, we highlight topics for future research on Tat, including the development of this protein transport pathway for the biotechnological secretion of high-value proteins and its potential applicability as an antimicrobial drug target in pathogens.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Twin-Arginine Protein Translocation Pathway
The movement of substances across biological membranes is essential for the growth, replication and survival of all living cells. In order to translocate substances through these phospholipid bilayers a variety of diverse import and export systems have evolved. One class of compounds that need to be translocated across membranes are proteins. However, transporting proteins over a membrane is not enough to guarantee their proper function, as proteins require correct folding and often co-factors for full functionality. Consequently, there is an intimate relationship between the protein translocation process and protein folding. Protein translocation pathways ensure that substrates are folded either post-translocationally or pre-translocationally. In the case of the Sec pathway for protein secretion, the translocated proteins are folded after translocation (Tjalsma et al. 2000). In contrast, twin-arginine translocation (Tat) pathways are known specifically for the ability to move pre-folded and co-factor-containing proteins across membranes. Translocation of large globular and tightly folded proteins across a membrane is no small feat as the energy needed and the size of the membrane passage required is far greater than that needed for translocating a loosely folded polypeptide chain by the Sec pathway. Exactly how the Tat pathway is able to transport these globular proteins without breaking the cellular barrier or destroying transmembrane ion gradients (e.g. the proton-motive force) is perplexing and of great fundamental scientific interest.
The Tat pathway is evolutionarily conserved in all the kingdoms of life. It is present in 77 % of bacteria, in archaeal species and in the membranes of thylakoids in plants and cyanobacteria (Chaddock et al. 1995; Hutcheon and Bolhuis 2003; Simone et al. 2013). Remnants of the pathway have even been observed in sponges (Pett and Lavrov 2013a). The focus of this chapter is the Gram-positive bacterial Tat pathway, where the folded proteins are moved from the cytoplasm into the membrane, cell wall or extracellular milieu. Tat systems have been most extensively studied in the Gram-negative bacterium Escherichia coli, but also in the Gram-positive Bacillus and Corynebacterium species, and in pea thylakoids [reviewed in (Palmer and Berks 2012; Goosens et al. 2014b; Patel et al. 2014)].
What has become evident from comparing the various Tat systems is that they are broadly conserved with a high degree of similarity between proteins and mechanisms. For this reason, this review builds on and refers to observations made in Tat systems from other species in addition to Gram-positive bacteria.
2 Tat-Dependent Cargo
The number of Tat-dependent cargo proteins ranges from over 100 in Streptomyces species, to only a few in B. subtilis and Staphylococcus aureus and none in, for example, Lactobacillus species (Schaerlaekens et al. 2001, 2004b; Widdick et al. 2006; Joshi et al. 2010; Yamada et al. 2007; Biswas et al. 2009; Goosens et al. 2013; Bolotin et al. 2001; Kleerebezem et al. 2003). These Tat-dependent cargo proteins include secreted proteins, lipoproteins, cell wall-associated proteins and proteins that form components of larger extracytoplasmic complexes on the membrane surface (Widdick et al. 2011; Keller et al. 2012; Monteferrante et al. 2012b; Goosens et al. 2013; Miethke et al. 2013).
Cargo may be destined for the Tat pathway for numerous reasons. Many Tat-dependent substrates are known to require complex co-factors for activity and these are incorporated into the protein in the cytoplasm prior to membrane translocation. Certain other proteins that bind divalent metal ions with affinity ranges lower down in the Irving Williams series, such as Mn, may use the Tat pathway to avoid competing ions with higher binding affinities, such as Zn (Tottey et al. 2008; Monteferrante et al. 2012b). Extremophiles and archaea may need to fold the proteins prior to secretion due to the harsh external milieus in which they live (Bolhuis 2002; Rose et al. 2002). Further, some Tat-destined proteins form multi-protein complexes that are translocated in a hitchhiker or piggyback manner (Rodrigue et al. 1999; Friedrich et al. 2000; Wu et al. 2000a).
Tat substrates have been implicated in a wide range of cellular functions and in the case of pathogenic bacteria they have been associated with virulence, antibiotic resistance and antibacterial compounds (McDonough et al. 2005; De Buck et al. 2008; Weatherspoon-Griffin et al. 2011). Notably, certain industrially relevant proteins are difficult to produce due to co-factor—or disulphide-bond requirements and in some organisms, such as E. coli and Corynebacterium glutamicum, the Tat pathway has been successfully used for export of these types of proteins, including the alkaline phosphatase PhoA, carbohydrate oxidase, antibody fragments and human tissue plasminogen activator (DeLisa et al. 2003; Kim et al. 2005; Bruser 2007; Ribnicky et al. 2007; Panahandeh et al. 2008; Maurer et al. 2009; Matos et al. 2013; Scheele et al. 2013). Also, the Tat system of Gram-positive bacteria has been used to secrete small enzymes (De Keersmaeker et al. 2006; Kikuchi et al. 2006, 2008; Scheele et al. 2013). However, biotechnological applications have not yet taken full advantage of the potential of Gram-positive bacterial Tat systems. Although the ability to secrete complex cargo directly into the fermentation broth is enticing, production has been hampered by low yields possibly due to yet unidentified quality control requirements.
In addition to a folded state, Tat cargo proteins also have a unique ‘twin-arginine’ signal peptide signature. In principle, the N-terminal signal peptides of Tat substrates have a similar tripartite structure as the N-terminal signal peptides of Sec substrates; they are made up of a polar N-region, a hydrophobic H-region in the middle and a polar C-region next to the signal peptidase cleavage site (Tjalsma et al. 2004). However, a distinguishing feature of Tat signal peptides is the presence of two-arginine residues in the N-terminal region that form part of the consensus motif SRRxFLK where x is a polar amino acid (Berks et al. 2000; Stanley et al. 2000; Tjalsma et al. 2004). Compared to Sec signal peptides, the Tat signal peptides also tend to be longer, their N-terminal region is more positively charged (Tjalsma et al. 2000), and their H-region is slightly less hydrophobic (Cristobal et al. 1999). While the twin-arginine residues are conserved, mutation studies have shown that changes in the motif result in a variation of phenotypes, ranging from completely blocked to slowed down translocation of the cargo (Chaddock et al. 1995; DeLisa et al. 2002; Mendel et al. 2008).
Several Tat prediction software programs are available, including TatFind and PRED-TAT (Rose et al. 2002; Bendtsen et al. 2005; Bagos et al. 2010). However, although the signal peptide region is important for translocation, amino acid sequence motifs and patterns are not always reliable predictors, especially since Tat cargo has also been associated with piggyback or hitchhiker mechanisms in organisms like E. coli. Here, proteins without a signal peptide of their own bind to the Tat substrate possessing the Tat signal peptide and are exported as a complex by the machinery (Rodrigue et al. 1999; Wu et al. 2000a). Further, Sec–Tat substrate overlap has been shown to occur and sequence ambiguity can lead to false-positive identifications (Tjalsma et al. 2000; Jongbloed et al. 2002; Kouwen et al. 2009; Keller et al. 2012; Goosens et al. 2013). Therefore, although bioinformatic tools are invaluable for lead finding, potential Tat substrates need to be confirmed experimentally.
3 Tat Components and Processes
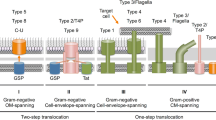
Genes for the Tat system are observed in 77 % of sequenced microbial genomes. The respective organisms typically contain a TatA and TatC pair (Fig. 1), which are often encoded by genes found within a single operon (Wu et al. 2000b; Yen et al. 2002; Simone et al. 2013). Such tatA-tatC operons are occasionally located in the vicinity of genes for Tat cargo proteins (Jongbloed et al. 2000, 2004; Biswas et al. 2009).
Tat components and interactions of Tat complexes with their cargo. Tat protein translocases are essentially composed of two types of subunits, namely TatA-like proteins and TatC, which have distinct membrane topologies (upper panel). TatA-like proteins (indicated in orange and red) have an N out–C in topology and consist of two helical domains, one of which spans the membrane while the other one (amphipathic) is exposed to the cytoplasm. TatC is an integral membrane protein with six transmembrane domains and an N in–C in topology. The translocation process is believed to involve two major Tat complexes, namely a docking complex (middle panel) and a translocation complex (lower panel). The docking complex is composed of TatC and a TatA-like protein (red). In some organisms, such as E. coli, the latter TatA-like protein has a specialized docking function in which case it is referred to as TatB. Docking of cargo proteins (green) involves interaction of the twin-arginine signal peptide (purple) with TatC. Once the cargo protein has docked, a large number of TatA-like proteins (orange) are recruited to the translocation site, thereby forming the translocation complex
3.1 TatA, TatA-like and TatC Proteins
TatA and TatA-like proteins are small membrane proteins with an N-out C-in topology. They have a small N-terminal extracytoplasmic domain, a single transmembrane helix and an amphipathic helix that lies on the membrane surface or is partially embedded in the membrane on the cytoplasmic side (Fig. 1) (Lange et al. 2007; Hu et al. 2010; Walther et al. 2010). In numerous species, tatA genes have undergone multiple duplication events forming the tatA-like genes, and these are found in the main tat operon or elsewhere in the genome (Wu et al. 2000b; Yen et al. 2002). Some TatA-like proteins can simply be duplicate TatA proteins, such as TatE or TatA2 in Corynebacterium species, Streptomyces coelicolor, Salmonella enterica and E. coli (Sargent et al. 1999; Ikeda and Nakagawa 2003; Kalinowski et al. 2003; Nishio et al. 2003; Baglieri et al. 2012). In B. subtilis and C. glutamicum, the expression of the duplicate proteins TatAc and TatE, respectively, has been shown to assist the activity of the primary TatA protein (Kikuchi et al. 2006; Goosens et al. 2015). However, in some instances, duplicate TatA-like proteins (often referred to as TatB) have further undergone sequence divergence and functional specialization. This seems to have occurred independently numerous times. TatA-like proteins with divergent functions, such as TatB have been studied in E. coli, Streptomyces species and in the thylakoids of plant chloroplasts (where they are referred to as Hcf106). In these organisms, both TatA and TatB are needed for full translocation activity (Sargent et al. 1999; Mori and Cline 2001; Schaerlaekens et al. 2001; De Keersmaeker et al. 2005a). Intriguingly, certain TatA proteins (e.g. the TatA proteins from B. subtilis) are able to functionally replace both E. coli TatA and TatB in the TatA-B-C system (Barnett et al. 2008). This is even more remarkable since TatA shares only 20 % sequence similarity with TatB although the E. coli TatA and TatB are structurally the same (Hicks et al. 2003; Lange et al. 2007; Hu et al. 2010; Walther et al. 2010). Nevertheless, minor changes to E. coli TatA allow it to complement for TatB (Blaudeck et al. 2005; Barrett et al. 2007). Therefore, no clear definition exists that allows one to properly differentiate TatA from TatB, and in many sequence annotations where a second TatA-like protein has been defined as TatB, this annotation may be erroneous. Importantly, over 50 % of the Tat-encoding genomes sequenced to date specify only a TatA-TatC component (Simone et al. 2013). Accordingly, the concept that a core Tat system composed of a core TatA-TatC pair with various assistant TatA-like proteins is gaining more support. This view is backed by studies in B. subtilis, where a third TatA protein (TatAc) was shown to assist TatAy in protein translocation, and in Helicobacter pylori, Campylobacter jejuni, and C. glutamicum where a second TatA-like protein was shown to be essential only under some conditions of Tat-dependent protein translocation but not all (Kikuchi et al. 2006; Benoit and Maier 2014; Liu et al. 2014; Goosens et al. 2015; Oertel et al. 2015). Thus, in many organisms, it is not necessary to have a TatB as is found in E. coli and thylakoids, which have TatA, TatB and TatC subunits, each with their own function.
Unlike TatA, TatC proteins are large integral membrane proteins with six membrane-spanning domains (Fig. 1). TatC is central to the Tat pathway as is evident by the multiple protein interactions in which this protein is involved. The TatC transmembrane regions interact with other TatC proteins (Buchanan et al. 2002; Punginelli et al. 2007), but also with the cargo protein (Behrendt et al. 2004; Frobel et al. 2012). Both the cargo protein and TatA(-like) proteins (e.g. E. coli TatB) have been shown to interact with the membrane-embedded region of TatC and its conserved cytoplasmic loop (Buchanan et al. 2002; McDevitt et al. 2006; Schreiber et al. 2006; Holzapfel et al. 2007; Strauch and Georgiou 2007; Frobel et al. 2011; Zoufaly et al. 2012; Ma and Cline 2013; Blümmel et al. 2015). Although the extracytoplasmic loops do not share high sequence similarity, random mutagenesis studies showed that the conserved secondary structure is vital (Strauch and Georgiou 2007; Kneuper et al. 2012). Further, the C-terminal tail of TatC has been shown to be essential for successful Tat-dependent protein translocation in B. subtilis (Eijlander et al. 2009b).
3.2 Translocation Process
The mechanism of translocating cargo proteins via the Tat pathway has not been concretely defined. However, what is currently agreed upon is that there are at least two Tat complexes and at least three steps to the process (Cline and Mori 2001). The first step, the formation of a docking complex, is initiated when cargo proteins interact with a TatC and TatA(-like) complex at the membrane (Fig. 1) (Bolhuis et al. 2001; Whitaker et al. 2012). In E. coli and thylakoids, this is where the (TatA-like) TatB proteins perform their specialized functions. The signal peptide of the cargo protein directly interacts with TatC in the docking complex and is inserted into the membrane after proofreading (Cline and Mori 2001; Alami et al. 2003; Papish et al. 2003; Robinson et al. 2011; Frobel et al. 2012). In the second step, the translocation complex is formed. This occurs once the cargo protein has ‘docked’, and a large number of TatA proteins are recruited to the translocation site in a manner that is dependent on the proton-motive force, thereby forming the translocation complex (Fig. 1) (Mori and Cline 2002; Alami et al. 2003; Dabney-Smith et al. 2006). The final step is the translocation itself, but how this occurs is not clear. A recent study by Blümmel et al. (2015) indicates that TatBC oligomers can assemble into closed intramembrane substrate-binding cavities, where TatB monomers would form dome-like structures that are surrounded by TatC monomers. These TatBC complexes would bind the N-termini of TatA protomers facilitating contacts with TatB and membrane-inserted cargo proteins.
There are two popular translocation models, which are both speculative: the pore and the membrane-weakening models [reviewed in (Berks 2015 ; Patel et al. 2014)]. Both models are supported by data and, depending on their interpretation, some results are used to reinforce either. The pore model was conceived based upon single particle electron microscopy studies that showed TatA and TatA-like proteins self-assemble to form cup-like structures or pores with varying diameters (Gohlke et al. 2005; Oates et al. 2005; Beck et al. 2013). Consistent with this model, the E. coli TatA complexes have been shown to form ladders of multiple sizes in native gels (Gohlke et al. 2005; Oates et al. 2005; Beck et al. 2013) giving rise to the hypothesis that a pore made up of TatA proteins adapts its diameter to the globular cargo by varying the amount of TatA components (Fig. 1). The theory goes that TatA-cargo protein interactions within the cup-like TatA structure allow for translocation by the folding-in (trap-door mechanism) or twisting (iris mechanism) of the amphipathic helix of TatA up into the membrane (Berks et al. 2000; Gouffi et al. 2004; Gohlke et al. 2005; Walther et al. 2013). In contrast, the membrane-weakening model predicts that the TatA complexes observed by electron microscopy do not form a pore, but that the aggregates of TatA proteins form destabilised membrane regions that permit cargo passage (Bruser and Sanders 2003). Data that support this include the length of the TatA transmembrane region, which is too short to span the lipid bilayer (Rodriguez et al. 2013). Also, the B. subtilis TatAd complexes observed by single particle electron microscopy were structurally too small to represent pores that can accommodate a substrate (Beck et al. 2013). Other evidence not consistent with the pore model is that the large size variation and laddering effect seen in E. coli TatA complexes have not been convincingly observed for other TatA and TatA-like proteins (Baglieri et al. 2012; Monteferrante et al. 2012a; Walther et al. 2013). Moreover, NMR studies suggest that, because the TatA amphipathic helix is not flexible (Walther et al. 2010), the movement of the amphipathic helix into the membrane would have to be sudden and, most likely, disruptive. Another piece of evidence that seems to support the membrane-weakening model is the involvement of the phage shock protein PspA in Tat-dependent protein transport. For example, PspA has been implicated in the stabilization of the membrane under stress conditions (Darwin 2005; Vrancken et al. 2008) and in suppressing proton leakage (Kobayashi et al. 2007). PspA binds both E. coli TatA (Mehner et al. 2012) and phospholipids (Kobayashi et al. 2007) forming scaffold-like structures in the membrane (Standar et al. 2008). The possible involvement of PspA in Tat-dependent protein transport suggests that the translocation event induces stress. Importantly, expression of PspA has been shown to improve Tat-dependent protein secretion in both S. lividans and E. coli and, hence, its role in suppressing proton leakage that may occur in the Tat export process may be conserved in both Gram-positive and Gram-negative bacteria (DeLisa et al. 2004; Vrancken et al. 2007).
4 Cargo protein Processing and Quality Control
A defining feature of Tat is its inability to translocate incorrectly folded proteins. Although a small amount of flexibility has been described for small synthetic peptides (Hynds et al. 1998; Richter et al. 2007; Rocco et al. 2012), the system is known to have strict folding requirements regarding its native substrates. If a protein is not sufficiently folded or does not have its co-factors inserted, the translocation is prevented and the protein is degraded (Jack et al. 2004; Kolkman et al. 2008; Matos et al. 2008). Protein folding, co-factor insertion and quality control prior to Tat complex interactions are therefore considered important for cargo translocation. The quality control step has been shown to occur at the docking complex (Buchanan et al. 2008; Panahandeh et al. 2008; Frobel et al. 2012; Rocco et al. 2012). Further evidence of quality control prior to docking complex formation has been clearly described in E. coli, where a number of substrate-specific chaperones have been identified (Oresnik et al. 2001; Jack et al. 2004). However, homologous chaperones have not been characterized in other organisms, and it remains unclear which factors may be involved in pre-translocational protein folding and quality control prior to docking-complex interactions in Gram-positive bacteria. Nonetheless, in B. subtilis the Tat-dependent QcrA protein was shown to undergo quality control at two subcellular locations, in the cytoplasm and membrane, and on two different pre-QcrA intermediates. While neither of the pre-QcrA proteins were translocated, pre-QcrA quality control occurred both at the membrane, where the Tat-docking complex is shown to perform proofreading functions, and in the cytoplasm via an as-yet-unknown mechanism (Goosens et al. 2014a). Quantitative proteomic studies have implicated the membrane-targeting chaperone DnaJ and co-factor assembly protein SufS with the Tat pathway in B. subtilis (Albrecht et al. 2011; Goosens et al. 2013; Castanie-Cornet et al. 2014). However, functional studies are still required to confirm these links. What has clearly been shown is a direct interaction between the B. subtilis TatAd protein and the soluble chemoreceptor HemAT, and between TatAd and the putative pentose transporter CsbC within the membrane. Not only do HemAT and CsbC individually interact with TatAd, but they are also essential for the secretion of the TatAd-specific cargo protein PhoD (Monteferrante et al. 2013). Exactly what the roles of these proteins in the PhoD quality control and Tat-dependent export pathway are remained unclear.
It has been suggested that the Tat-associated quality control is linked to a pool of cytoplasmic TatA (Pop et al. 2003; De Keersmaeker et al. 2005a, b; Schreiber et al. 2006; Westermann et al. 2006; De Keersmaeker et al. 2007; Frielingsdorf et al. 2008). In this model, the cytoplasmic TatA of B. subtilis, Streptomyces lividans or thylakoids interacts with cargo prior to translocation and guides it to the docking complex in the membrane. Also, overexpressed TatA molecules have been observed to form distinct tubes in the cytoplasm (Berthelmann et al. 2008). However, since the cytoplasmic TatA-cargo protein interaction has only been observed under induced circumstances and the presence of TatA in the cytoplasm has not been shown consistently under all experimental methodologies, future studies will need to verify the possible quality control function of cytoplasmic TatA (Wexler et al. 2000; Barnett et al. 2008; Leake et al. 2008; Barnett et al. 2009; Ridder et al. 2009).
Other steps in the quality control of Tat-dependent cargo proteins occur during or shortly after membrane translocation. In particular, these include the removal of the twin-arginine signal peptide by signal peptidases, which has been widely observed (Jongbloed et al. 2004; Luke et al. 2009; Widdick et al. 2011; Goosens et al. 2013). In addition to signal peptidases, extracytoplasmic proteases have also been shown to effect the Tat-dependent cargo protein EfeB in B. subtilis. EfeB directly interacts with and requires the cell wall-bound protease WprA for processing (Monteferrante et al. 2013), but is degraded by extracellular proteases in the growth medium (Krishnappa et al. 2012). EfeB forms part of a membrane-bound complex with the EfeU and EfeO proteins, and the association with extracellular proteases is an indication of a possible assembly proofreading mechanism (Monteferrante et al. 2013).
5 Flexibility of the Tat System Between Different Organisms
Expression of B. subtilis Tat components in E. coli leads to Tat-dependent export and functionally replaces the Tat pathway in E. coli (Barnett et al. 2008, 2009; Monteferrante et al. 2012a; van der Ploeg et al. 2012). Single B. subtilis tatA genes are able to functionally replace both the E. coli TatA and TatB proteins (Barnett et al. 2008; Monteferrante et al. 2012a; Beck et al. 2013). However, when similar interspecies experiments were performed in a B. subtilis background, complementation was not that simple. Although the Tat systems from Bacillus cereus and Listeria monocytogenes were functional in B. subtilis, the Tat system from S. aureus was barely active in B. subtilis (Barnett et al. 2008, 2009; van der Ploeg et al. 2011a, 2012). These differences suggest that the Tat pathway alone is not enough to ensure complete translocation and possible chaperone or quality control mechanisms in E. coli and S. aureus do not match up with those in B. subtilis.
Interspecies variations have also been observed with regard to the export of cargo proteins. The addition of a Tat signal peptide to a cargo protein has allowed for heterologous Tat-dependent translocation in many cases, but this does not always equally prove successful in all genetic backgrounds and with all cargo (Thiemann et al. 2006; Meissner et al. 2007; Kikuchi et al. 2008; Widdick et al. 2008; Scheele et al. 2013). Environmental salt (i.e. NaCl) conditions also affected the Tat-dependent export of cargo that was heterologously expressed in B. subtilis suggesting external conditions and media may affect Tat-dependent secretion (van der Ploeg et al. 2011a, 2012). However, the environmental salt concentration did not significantly affect the amount of Tat-dependently translocated QcrA in B. subtilis (Goosens et al. 2015). The influence of salt on the translocation of other cargo is therefore not necessarily an intrinsic Tat effect.
6 Monoderm Gram-Positive Bacterial Tat Systems
Bacterial phyla are broadly defined by the physical properties of the outer layer of their cell structure. In most cases, bacteria are classified by the outcome of so-called Gram staining. The Gram staining procedure was developed in the late nineteenth century and works by interaction of the stain with the peptidoglycan of the cell wall. The stain is either retained by the peptidoglycan, giving cells a purple colour, or washed out. Accordingly, this gave rise to the common nomenclature of Gram-positive bacteria where the stain is retained, or Gram-negative bacteria where the stain is not retained. The Gram-positive bacteria have a single plasma membrane surrounded by a thick outer cell wall composed of peptidoglycan (i.e. a monoderm cell envelope). In contrast, Gram-negative bacteria have a double membrane with a peptidoglycan layer in between (i.e. a diderm cell envelope). Although the Gram-staining-based nomenclature is generally a good indicator of the physical properties of the outer layer of cells, it can be ambiguous. Some bacteria stain positive, but do in fact have a diderm cell envelope structure. Such bacteria include mycobacteria, corynebacteria, rhodococci and nocardiae. The Tat systems of these diderm Gram-positive bacteria will not be detailed here, as they have been reviewed previously (Goosens et al. 2014b) and not much new information has become available since this review was published.
6.1 Bacillus subtilis
Bacillus subtilis is the major Gram-positive model organism with an extensive array of genetic tools, including in-depth genomic, transcriptomic and proteomic insights (Kunst et al. 1997; Tjalsma et al. 2000; Eymann et al. 2004; Wolff et al. 2007; Buescher et al. 2012; Nicolas et al. 2012). A number of Bacillus species are biotechnologically relevant. B. subtilis, Bacillus licheniformis and Bacillus amyloliquefaciens, for example, have the ability to secrete large titres of proteins, qualify for the Qualified Presumption of Safety (QPS) status of the European Food Safety Authority, and many of their products have a Generally Recognized As Safe (GRAS) status from the US Food and Drug Administration. Furthermore, B. subtilis becomes naturally competent, thereby allowing for easy genetic modification (Tjalsma et al. 2000; van Dijl et al. 2002; Graumann 2011).
The B. subtilis Tat system is one of the most extensively studied Tat systems within the field, because it has unique characteristics in particular relating to gene duplication. As indicated above, duplication of TatA and TatA-like proteins is a common feature in most Tat systems. However, the duplication of TatC proteins is rare except in Bacillus species, where multiple isoforms of TatC have been observed (Jongbloed et al. 2000; Yen et al. 2002; Simone et al. 2013).
The core progenitor operon in B. subtilis is tatAy-tatCy (Simone et al. 2013). This operon has been duplicated and, consequently, there is a second separate tat operon named tatAd-tatCd. Thus, B. subtilis Tat is specified by two separate operons, which are expressed at different times and, under normal conditions, do not share substrate specificity (Jongbloed et al. 2004; Eijlander et al. 2009a; Nicolas et al. 2012; Goosens et al. 2013). The predominant Tat pathway is TatAy-TatCy and, although an early bioinformatics analysis has predicted up to 69 potential Tat-dependent substrates, only three substrates have been confirmed to be strictly TatAy-TatCy-dependent, namely EfeB, QcrA and YkuE (Tjalsma et al. 2000; Jongbloed et al. 2002, 2004; Monteferrante et al. 2012b; Goosens et al. 2013). The genes for the second B. subtilis Tat pathway, TatAd-TatCd, are found next to the gene for its only known substrate, the phosphate acquisition protein PhoD. Accordingly, the tatAd-tatCd operon is only expressed under low-phosphate conditions (Eder et al. 1996; Jongbloed et al. 2000, 2004; Nicolas et al. 2012).
Apart from duplicating the whole tatAy-tatCy operon, the Tat system of B. subtilis has a further tatA duplication, namely tatAc. This third tatA gene is located elsewhere on the chromosome, and it is expressed constitutively under numerous conditions (Nicolas et al. 2012). Although it was investigated in several studies, a physiological role for TatAc has, until recently, remained enigmatic (Tjalsma et al. 2000; Jongbloed et al. 2002, 2004; Eijlander et al. 2009a; Nicolas et al. 2012). TatAc is unable to form an active translocon when paired with TatC proteins in B. subtilis (Eijlander et al. 2009a; Goosens et al. 2015). However, when expressed in E. coli, TatAc formed functional translocases with either E. coli TatBC, B. subtilis TatCd or B. subtilis TatCy (Monteferrante et al. 2012a; Beck et al. 2013). This difference illustrates the interpathway flexibility of E. coli and further suggests potentially different quality control or chaperone activities in the Tat pathways of B. subtilis and E. coli. Yeast two-hybrid (Y2H) protein–protein interaction studies have shown that not only does TatAc interact with itself and the B. subtilis TatA proteins, but it also directly interacts with HemAT (Monteferrante et al. 2013). HemAT was in turn shown to be essential for the Tat-dependent secretion of PhoD, which therefore suggested a functional role for TatAc in B. subtilis (Monteferrante et al. 2013). A functional role for TatAc as a Tat-assistance protein was confirmed when it was shown to permit the translocation of EfeB in cells with an impaired TatAy function, despite the fact that TatAc was unable to replace TatAy (Goosens et al. 2015). It thus seems that TatAc, the third TatA-like protein of B. subtilis, reflects an intermediate evolutionary step in TatA-TatB specialization. In this scenario, the presently available data suggest that the defective TatAy protein has a role that is comparable to that of E. coli TatB, while TatAc has a role similar to that of E. coli TatA. Altogether, it can be concluded that the core Tat translocon in B. subtilis is composed of a TatAy-TatCy pair and that the TatAc protein has a non-essential assistant role in translocation. For example, TatAc could allow for more efficient cargo-Tat protein–protein interactions, and it might improve the overall efficiency of the Tat pathway (Goosens et al. 2015).
All confirmed B. subtilis Tat-dependent cargo proteins are known to contain co-factors, thereby emphasizing their need for the Tat pathway (Schneider and Schmidt 2005; Monteferrante et al. 2012b; Miethke et al. 2013; Rodriguez et al. 2014). Further, QcrA contains a disulphide bond in addition to its iron-sulphur cluster (Iwata et al. 1996; Link et al. 1996; Schmidt and Shaw 2001; Hunsicker-Wang et al. 2003). Of note, QcrA has been observed to be a Tat-dependent substrate in a wide-range of organisms (Molik et al. 2001; Bachmann et al. 2006; De Buck et al. 2007; Goosens et al. 2013; Pett and Lavrov 2013b; Oertel et al. 2015). Both QcrA and EfeB form part of larger extracytoplasmic complexes, where the QcrA-B-C proteins form the cytochrome bc 1 complex, whereas the EfeU-O-B proteins form an iron uptake system. This organization of Tat-dependent substrate proteins into larger complexes further suggests that the Tat pathway assists in protein complex assembly (Yu et al. 1995; Schneider and Schmidt 2005; Miethke et al. 2013; Sousa et al. 2013). The extracellular protease WprA also directly affects EfeB and indirectly influences YkuE (Monteferrante et al. 2013). The action of WprA may thus be associated with this complex maturation.
Most of the observed phenotypes associated with Tat-deficiencies have been linked directly to the known substrates, i.e. PhoD is required under phosphate starvation and EfeB is required under conditions of iron deficiency and low salt (Jongbloed et al. 2000; van der Ploeg et al. 2011b). However, quantitative proteomic studies revealed that numerous proteins associated with motility and biofilm formation were decreased in tatAy-tatCy deficient strains, leading to the identification of an, as-yet, not-well-understood Tat-associated delayed biofilm formation phenotype (Goosens et al. 2013). Most studies investigating Tat have used Western blotting techniques to validate Tat-dependency of substrates. Although this remains the golden standard and a powerful tool, it does not give an indication of whether the protein is correctly folded and active. In the B. subtilis studies, the activity of cargo proteins was determined using the alkaline phosphatase activity of YkuE (Monteferrante et al. 2012b) and the ferric iron uptake to assess EfeB activity (Miethke et al. 2013; Goosens et al. 2015). EfeB is a hemoprotein that oxidizes ferrous iron to ferric iron for uptake via EfeU and EfeO. For this reason, EfeB stimulates growth under microaerobic conditions where ferrous iron is more abundant. In addition, EfeB was shown to have an important role in the protection against cell envelope stress through the elimination of reactive oxygen species that are generated in the presence of ferrous iron (Miethke et al. 2013).
6.2 Streptomyces
Streptomyces species are found naturally in the soil where they often form mycelia. These bacteria have become workhorses for industry as they can be used for the high-level production of various antibiotics and secreted proteins (Anne et al. 2012). The Tat system is a major contributor to overall protein secretion in these species with numbers of potential substrates ranging between 100 and 189 (Widdick et al. 2006; Joshi et al. 2010; Palmer and Berks 2012). The Tat system in Streptomyces species is composed of at least three Tat components, where the genes for a minimal TatA-TatC system are found clustered and the gene for an extra TatA-like protein (depending on the species, these are called TatB or TatA2) is located elsewhere on the chromosome (Schaerlaekens et al. 2001; Palmer and Berks 2012). Crosstalk between the Sec and Tat pathways has been observed in the sense that Sec-dependent secretion was enhanced by a mutated Tat system or reduced by an overexpressed Tat system (Schaerlaekens et al. 2004a; De Keersmaeker et al. 2006).
In S. lividans, optimal secretion of the Tat-dependent substrates xylanase C and tyrosinase occurred when all three Tat components were present. Secretion did still occur when a single TatA or TatB protein was paired with TatC, albeit at lowered efficiency. It was therefore concluded that the TatA-like proteins in Streptomyces were unable to fully functionally replace each other and that each must have a specialized function (De Keersmaeker et al. 2005a). A very interesting observation made in S. lividans was that both TatA and TatB proteins were detected in the cytoplasm under native conditions (De Keersmaeker et al. 2005b), and when expression was induced these TatA-like proteins apparently interacted with Tat-dependent cytoplasmic pre-proteins (De Keersmaeker et al. 2005a, 2007).
6.3 Staphylococcus
Not all Staphylococcus species have a Tat pathway. However, this pathway has been identified in Staphylococcus haemolyticus, Staphylococcus carnosus, Staphylococcus lugdunensis and S. aureus (Biswas et al. 2009). The staphylococcal Tat pathway is composed of a single TatA–TatC pair. This Tat pathway has been investigated for biotechnological applications in S. carnosus and, although shown to secrete heterologous proteins, it was considered inadequate for the required applications (Thiemann et al. 2006; Meissner et al. 2007). To date, only one native Tat-dependent staphylococcal substrate, FepB, has been confirmed, and inactivation of the Tat system did not show any global changes in protein secretion profiles (Yamada et al. 2007; Biswas et al. 2009). FepB is an iron-dependent peroxidase encoded by the fepABC operon, and the corresponding complex is very similar to the iron-scavenging EfeUOB complexes in E. coli, B. subtilis and L. monocytogenes (Biswas et al. 2009; Miethke et al. 2013; Turlin et al. 2013). Interestingly, in a mouse kidney abscess model, the bacterial load of tat or fepB mutant strains was shown to be decreased, thereby pointing at a physiologically relevant role of Tat-dependent export of FepB in staphylococcal disease (Biswas et al. 2009).
6.4 Listeria monocytogenes
Listeria monocytogenes is a saprophytic bacterium that, once it has entered the food chain, becomes a dangerous food-borne pathogen. The Listeria Tat system is composed of a TatA–TatC pair (Desvaux and Hebraud 2006; Machado et al. 2013). In strains where tat genes were deleted, no significant changes in cell viability or virulence have been described (Machado et al. 2013; Halbedel et al. 2014). Bioinformatic analysis predicted two potential Tat-dependent substrates, namely FepB (Lmo0367) and a FabF-like protein (Lmo2201) (Desvaux and Hebraud 2006). However, when these proteins were tagged and expressed neither was Tat-dependent (Halbedel et al. 2014). Nonetheless, the FepB signal peptide was shown to confer Tat-dependent secretion in the S. lividans agarase reporter assay, and both a tatC and fepB mutant strain displayed decreased overall ferric reductase activity (Widdick et al. 2008; Tiwari et al. 2015). Hence, there is evidence of a FepB-Tat association, but none of the currently available data confirms a direct Tat-dependency. The fepB gene is co-transcribed in an iron-induced fepCAB operon, which is also Fur-regulated (Ledala et al. 2010; Tiwari et al. 2015). Although the tat operon is transcribed in the early exponential phase in rich medium, it is Fur-regulated and highly induced under iron starvation conditions (Ledala et al. 2010; Machado et al. 2013; Tiwari et al. 2015). Therefore, it is conceivable that, in order to detect the possible Tat-dependency of FepB, environmental conditions with low iron availability may be required, or as in B. subtilis, other environmental conditions such as low salt (van der Ploeg et al. 2011b; Goosens et al. 2015). In fact, the Listeria fepCAB operon is highly reminiscent of the Tat-associated fepABC and efeUOB operons in S. aureus and B. subtilis, respectively. Accordingly, there appears to be a conservation of the Tat requirement in these iron-scavenging complexes.
6.5 Streptococcus
The majority of Streptococcus species studied have no identifiable Tat components. However, genes encoding a TatA and TatC protein have been identified in Streptococcus sanguinis and Streptococcus thermophilus. Intriguingly, in both S. sanguinis and S. thermophilus these tat genes are localized in close genomic proximity to three genes that resemble the efeUOB, fepCAB and fepABC operons of B. subtilis, Listeria and Staphylococcus, respectively. Further, in both Streptococcus species, the EfeB-like iron-dependent peroxidase contains a twin-arginine motif in the signal peptide. In the facultative anaerobe S. thermophilus, EfeB was shown to be translocated by the Tat system, and mutation of efeB or tatC resulted in decreased growth under aerobic conditions, suggesting that the respective proteins have a role in protecting the cell against oxidative stress (Xu et al. 2007; Zhang et al. 2015).
7 Conclusion
The investigations on the Tat pathways of Gram-positive bacteria, as described in this review, suggest a general association between the Tat pathway and iron-scavenging complexes, phosphate acquisition and respiratory complexes. Intriguingly, phylogenetic analyses of TatC showed that 89 % of species that have TatC are either facultative aerobes, or facultative or obligate anaerobes, while only 11 % are obligate aerobes (Simone et al. 2013). Thus, the majority of organisms with a Tat system find themselves in anaerobic environments. Of note, anaerobic bacteria and anaerobic growth are, over all, relatively poorly characterized. It thus seems likely that the full potential of the Tat pathway and the spectrum of biological functions that it fulfils are currently substantially underappreciated. With this in mind, an important challenge for future Tat-related research could be the exploration of this pathway in the microbiota of the human gut. Here bacteria, many of which are Firmicutes, need to thrive and survive in a challenging anaerobic environment that continuously changes depending on the ingestion of different nutrients by the host, continuous flow-through and influx of oxygen from the gut epithelium (Khan et al. 2012). Indeed, sequence analyses have shown that various dominant gut microbes do contain tat genes, and it would be interesting to explore their functions and find out whether they are conditionally essential.
The Tat system is known to be essential in only a few bacteria (Palmer and Berks 2012), including Mycobacterium tuberculosis where Tat has been shown to be important for drug resistance and virulence (Raynaud et al. 2002; McDonough et al. 2005). Yet, gene essentiality is often condition-dependent and this also applies to some tat genes as exemplified in B. subtilis, where the absence of the tatAy-tatCy operon leads to severe growth impairment in salt- or iron-depleted environments. This conditional essentiality implies that Tat is a potentially druggable target in notoriously drug-resistant pathogens, such as M. tuberculosis.
Today, there are various areas in the Tat field that merit further research, some of which have been touched upon in the present review. For example, this applies to the condition-dependent regulation of tat gene expression, including possible roles of antisense RNAs and small non-coding regulatory RNAs. In this respect, it is worth mentioning recent studies, showing that the non-coding RNA Mcr7 of M. tuberculosis modulates TatC expression, thereby serving as an intriguing new Tat secretion control mechanism (Solans et al. 2014). Other major knowledge gaps concerning the Tat pathways of Gram-positive bacteria relate to the chaperones that guide cargo folding and cofactor insertion, quality control of cargo prior to membrane translocation, the actual mechanism of Tat-mediated translocation of cargo across the membrane, and post-translocational cargo processing and quality control. Research into these areas will be important, not only to enrich our fundamental understanding of protein translocation mechanisms, but also to open up the enigmatic Tat pathway for the biotechnological secretion of high-value proteins, and to explore the potential of Tat as an antimicrobial drug target.
Abbreviations
- Sec pathway:
-
General Secretory pathway
- Tat:
-
Twin-arginine translocation
- NMR:
-
Nuclear magnetic resonance
- Y2H:
-
Yeast two-hybrid
References
Alami M, Luke I, Deitermann S, Eisner G, Koch HG, Brunner J, Muller M (2003) Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol Cell 12(4):937–946
Albrecht AG, Peuckert F, Landmann H, Miethke M, Seubert A, Marahiel MA (2011) Mechanistic characterization of sulfur transfer from cysteine desulfurase SufS to the iron-sulfur scaffold SufU in Bacillus subtilis. FEBS Lett 585(3):465–470
Anne J, Maldonado B, Van Impe J, Van Mellaert L, Bernaerts K (2012) Recombinant protein production and streptomycetes. J Biotechnol 158(4):159–167
Bachmann J, Bauer B, Zwicker K, Ludwig B, Anderka O (2006) The Rieske protein from Paracoccus denitrificans is inserted into the cytoplasmic membrane by the twin-arginine translocase. FEBS J 273(21):4817–4830
Baglieri J, Beck D, Vasisht N, Smith CJ, Robinson C (2012) Structure of TatA paralog, TatE, suggests a structurally homogeneous form of Tat protein translocase that transports folded proteins of differing diameter. J Biol Chem 287(10):7335–7344
Bagos PG, Nikolaou EP, Liakopoulos TD, Tsirigos KD (2010) Combined prediction of Tat and Sec signal peptides with hidden Markov models. Bioinformatics 26(22):2811–2817
Barnett JP, Eijlander RT, Kuipers OP, Robinson C (2008) A minimal Tat system from a gram-positive organism: a bifunctional TatA subunit participates in discrete TatAC and TatA complexes. J Biol Chem 283(5):2534–2542
Barnett JP, van der Ploeg R, Eijlander RT, Nenninger A, Mendel S, Rozeboom R, Kuipers OP, van Dijl JM, Robinson C (2009) The twin-arginine translocation (Tat) systems from Bacillus subtilis display a conserved mode of complex organization and similar substrate recognition requirements. FEBS J 276(1):232–243
Barrett CM, Freudl R, Robinson C (2007) Twin arginine translocation (Tat)-dependent export in the apparent absence of TatABC or TatA complexes using modified Escherichia coli TatA subunits that substitute for TatB. J Biol Chem 282(50):36206–36213
Beck D, Vasisht N, Baglieri J, Monteferrante CG, van Dijl JM, Robinson C, Smith CJ (2013) Ultrastructural characterisation of Bacillus subtilis TatA complexes suggests they are too small to form homooligomeric translocation pores. Biochim Biophys Acta 1833:1811–1819
Behrendt J, Standar K, Lindenstrauss U, Bruser T (2004) Topological studies on the twin-arginine translocase component TatC. FEMS Microbiol Lett 234(2):303–308
Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S (2005) Prediction of twin-arginine signal peptides. BMC Bioinformatics 6:167
Benoit SL, Maier RJ (2014) Twin-arginine translocation system in Helicobacter pylori: TatC, but not TatB, is essential for viability. mBio 5(1):e01016–01013
Berks BC (2015) The twin-arginine protein translocation pathway. Annu Rev Biochem 84:843–864
Berks BC, Sargent F, Palmer T (2000) The Tat protein export pathway. Mol Microbiol 35(2):260–274
Berthelmann F, Mehner D, Richter S, Lindenstrauss U, Lunsdorf H, Hause G, Bruser T (2008) Recombinant expression of tatABC and tatAC results in the formation of interacting cytoplasmic TatA tubes in Escherichia coli. J Biol Chem 283(37):25281–25289
Biswas L, Biswas R, Nerz C, Ohlsen K, Schlag M, Schafer T, Lamkemeyer T, Ziebandt AK, Hantke K, Rosenstein R, Gotz F (2009) Role of the twin-arginine translocation pathway in Staphylococcus. J Bacteriol 191(19):5921–5929
Blaudeck N, Kreutzenbeck P, Muller M, Sprenger GA, Freudl R (2005) Isolation and characterization of bifunctional Escherichia coli TatA mutant proteins that allow efficient tat-dependent protein translocation in the absence of TatB. J Biol Chem 280(5):3426–3432
Blümmel AS, Haag LA, Eimer E, Müller M, Fröbel J (2015) Initial assembly steps of a translocase for folded proteins. Nat Commun 6:7234
Bolhuis A (2002) Protein transport in the halophilic archaeon Halobacterium sp. NRC-1: a major role for the twin-arginine translocation pathway? Microbiology 148(Pt 11):3335–3346
Bolhuis A, Mathers JE, Thomas JD, Barrett CM, Robinson C (2001) TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli. J Biol Chem 276(23):20213–20219
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Sorokin A (2001) The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res 11(5):731–753
Bruser T (2007) The twin-arginine translocation system and its capability for protein secretion in biotechnological protein production. Appl Microbiol Biotechnol 76(1):35–45
Bruser T, Sanders C (2003) An alternative model of the twin arginine translocation system. Microbiol Res 158(1):7–17
Buchanan G, de Leeuw E, Stanley NR, Wexler M, Berks BC, Sargent F, Palmer T (2002) Functional complexity of the twin-arginine translocase TatC component revealed by site-directed mutagenesis. Mol Microbiol 43(6):1457–1470
Buchanan G, Maillard J, Nabuurs SB, Richardson DJ, Palmer T, Sargent F (2008) Features of a twin-arginine signal peptide required for recognition by a Tat proofreading chaperone. FEBS Lett 582(29):3979–3984
Buescher JM, Liebermeister W, Jules M, Uhr M, Muntel J, Botella E, Hessling B, Kleijn RJ, Le Chat L, Lecointe F, Mader U, Nicolas P, Piersma S, Rugheimer F, Becher D, Bessieres P, Bidnenko E, Denham EL, Dervyn E, Devine KM, Doherty G, Drulhe S, Felicori L, Fogg MJ, Goelzer A, Hansen A, Harwood CR, Hecker M, Hubner S, Hultschig C, Jarmer H, Klipp E, Leduc A, Lewis P, Molina F, Noirot P, Peres S, Pigeonneau N, Pohl S, Rasmussen S, Rinn B, Schaffer M, Schnidder J, Schwikowski B, Van Dijl JM, Veiga P, Walsh S, Wilkinson AJ, Stelling J, Aymerich S, Sauer U (2012) Global network reorganization during dynamic adaptations of Bacillus subtilis metabolism. Science 335(6072):1099–1103
Castanie-Cornet MP, Bruel N, Genevaux P (2014) Chaperone networking facilitates protein targeting to the bacterial cytoplasmic membrane. Biochim Biophys Acta 1843(8):1442–1456
Chaddock AM, Mant A, Karnauchov I, Brink S, Herrmann RG, Klosgen RB, Robinson C (1995) A new type of signal peptide: central role of a twin-arginine motif in transfer signals for the delta pH-dependent thylakoidal protein translocase. EMBO J 14(12):2715–2722
Cline K, Mori H (2001) Thylakoid DeltapH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J Cell Biol 154(4):719–729
Cristobal S, de Gier JW, Nielsen H, von Heijne G (1999) Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J 18(11):2982–2990
Dabney-Smith C, Mori H, Cline K (2006) Oligomers of Tha4 organize at the thylakoid Tat translocase during protein transport. J Biol Chem 281(9):5476–5483
Darwin AJ (2005) The phage-shock-protein response. Mol Microbiol 57(3):621–628
De Buck E, Vranckx L, Meyen E, Maes L, Vandersmissen L, Anne J, Lammertyn E (2007) The twin-arginine translocation pathway is necessary for correct membrane insertion of the Rieske Fe/S protein in Legionella pneumophila. FEBS Lett 581(2):259–264
De Buck E, Lammertyn E, Anne J (2008) The importance of the twin-arginine translocation pathway for bacterial virulence. Trends Microbiol 16(9):442–453
De Keersmaeker S, Van Mellaert L, Lammertyn E, Vrancken K, Anne J, Geukens N (2005a) Functional analysis of TatA and TatB in Streptomyces lividans. Biochem Biophys Res Commun 335(3):973–982
De Keersmaeker S, Van Mellaert L, Schaerlaekens K, Van Dessel W, Vrancken K, Lammertyn E, Anne J, Geukens N (2005b) Structural organization of the twin-arginine translocation system in Streptomyces lividans. FEBS Lett 579(3):797–802
De Keersmaeker S, Vrancken K, Van Mellaert L, Lammertyn E, Anne J, Geukens N (2006) Evaluation of TatABC overproduction on Tat- and Sec-dependent protein secretion in Streptomyces lividans. Arch Microbiol 186(6):507–512
De Keersmaeker S, Vrancken K, Van Mellaert L, Anne J, Geukens N (2007) The Tat pathway in Streptomyces lividans: interaction of Tat subunits and their role in translocation. Microbiology 153(Pt 4):1087–1094
DeLisa MP, Samuelson P, Palmer T, Georgiou G (2002) Genetic analysis of the twin arginine translocator secretion pathway in bacteria. J Biol Chem 277(33):29825–29831
DeLisa MP, Tullman D, Georgiou G (2003) Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc Natl Acad Sci USA 100(10):6115–6120
DeLisa MP, Lee P, Palmer T, Georgiou G (2004) Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway. J Bacteriol 186(2):366–373
Desvaux M, Hebraud M (2006) The protein secretion systems in Listeria: inside out bacterial virulence. FEMS Microbiol Rev 30(5):774–805
Eder S, Shi L, Jensen K, Yamane K, Hulett FM (1996) A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology 142:2041–2047
Eijlander RT, Jongbloed JD, Kuipers OP (2009a) Relaxed specificity of the Bacillus subtilis TatAdCd translocase in Tat-dependent protein secretion. J Bacteriol 191(1):196–202
Eijlander RT, Kolbusz MA, Berendsen EM, Kuipers OP (2009b) Effects of altered TatC proteins on protein secretion efficiency via the twin-arginine translocation pathway of Bacillus subtilis. Microbiology 155(Pt 6):1776–1785
Eymann C, Dreisbach A, Albrecht D, Bernhardt J, Becher D, Gentner S, Le Tam T, Buttner K, Buurman G, Scharf C, Venz S, Volker U, Hecker M (2004) A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics 4(10):2849–2876
Friedrich CG, Quentmeier A, Bardischewsky F, Rother D, Kraft R, Kostka S, Prinz H (2000) Novel genes coding for lithotrophic sulfur oxidation of Paracoccus pantotrophus GB17. J Bacteriol 182(17):4677–4687
Frielingsdorf S, Jakob M, Klosgen RB (2008) A stromal pool of TatA promotes Tat-dependent protein transport across the thylakoid membrane. J Biol Chem 283(49):33838–33845
Frobel J, Rose P, Muller M (2011) Early contacts between substrate proteins and TatA translocase component in twin-arginine translocation. J Biol Chem 286(51):43679–43689
Frobel J, Rose P, Lausberg F, Blummel AS, Freudl R, Muller M (2012) Transmembrane insertion of twin-arginine signal peptides is driven by TatC and regulated by TatB. Nat Commun 3:1311
Gohlke U, Pullan L, McDevitt CA, Porcelli I, de Leeuw E, Palmer T, Saibil HR, Berks BC (2005) The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc Natl Acad Sci USA 102(30):10482–10486
Goosens VJ, Otto A, Glasner C, Monteferrante CC, van der Ploeg R, Hecker M, Becher D, van Dijl JM (2013) Novel twin-arginine translocation pathway-dependent phenotypes of Bacillus subtilis unveiled by quantitative proteomics. J Proteome Res 12(2):796–807
Goosens VJ, Monteferrante CG, van Dijl JM (2014a) Co-factor insertion and disulfide bond requirements for twin-arginine translocase-dependent export of the Bacillus subtilis Rieske protein QcrA. J Biol Chem 289(19):13124–13131
Goosens VJ, Monteferrante CG, van Dijl JM (2014b) The Tat system of Gram-positive bacteria. Biochim Biophys Acta 1843(8):1698–1706
Goosens VJ, De-San-Eustaquio-Campillo A, Carballido-Lopez R, van Dijl JM (2015) A Tat menage a trois—the role of Bacillus subtilis TatAc in twin-arginine protein translocation. Biochim Biophys Acta 1853(10 Pt A):2745–2753
Gouffi K, Gerard F, Santini CL, Wu LF (2004) Dual topology of the Escherichia coli TatA protein. J Biol Chem 279(12):11608–11615
Graumann P (2011) Bacillus: Cellular and Molecular Biology. Caister Academic Press, Wymondham, United Kingdom
Halbedel S, Reiss S, Hahn B, Albrecht D, Mannala GK, Chakraborty T, Hain T, Engelmann S, Flieger A (2014) A systematic proteomic analysis of Listeria monocytogenes house-keeping protein secretion systems. Mol Cell Proteomics 13(11):3063–3081
Hicks MG, de Leeuw E, Porcelli I, Buchanan G, Berks BC, Palmer T (2003) The Escherichia coli twin-arginine translocase: conserved residues of TatA and TatB family components involved in protein transport. FEBS Lett 539(1–3):61–67
Holzapfel E, Eisner G, Alami M, Barrett CM, Buchanan G, Luke I, Betton JM, Robinson C, Palmer T, Moser M, Muller M (2007) The entire N-terminal half of TatC is involved in twin-arginine precursor binding. Biochemistry 46(10):2892–2898
Hu Y, Zhao E, Li H, Xia B, Jin C (2010) Solution NMR structure of the TatA component of the twin-arginine protein transport system from gram-positive bacterium Bacillus subtilis. J J Am Chem Soc 132(45):15942–15944
Hunsicker-Wang LM, Heine A, Chen Y, Luna EP, Todaro T, Zhang YM, Williams PA, McRee DE, Hirst J, Stout CD, Fee JA (2003) High-resolution structure of the soluble, respiratory-type Rieske protein from Thermus thermophilus: analysis and comparison. Biochemistry 42(24):7303–7317
Hutcheon GW, Bolhuis A (2003) The archaeal twin-arginine translocation pathway. Biochem Soc Trans 31(Pt 3):686–689
Hynds PJ, Robinson D, Robinson C (1998) The sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J Biol Chem 273(52):34868–34874
Ikeda M, Nakagawa S (2003) The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62(2–3):99–109
Iwata S, Saynovits M, Link TA, Michel H (1996) Structure of a water soluble fragment of the ‘Rieske’ iron-sulfur protein of the bovine heart mitochondrial cytochrome bc1 complex determined by MAD phasing at 1.5 A resolution. Structure (London, England: 1993) 4(5):567–579
Jack RL, Buchanan G, Dubini A, Hatzixanthis K, Palmer T, Sargent F (2004) Coordinating assembly and export of complex bacterial proteins. EMBO J 23(20):3962–3972
Jongbloed JD, Martin U, Antelmann H, Hecker M, Tjalsma H, Venema G, Bron S, van Dijl JM, Muller J (2000) TatC is a specificity determinant for protein secretion via the twin-arginine translocation pathway. J Biol Chem 275(52):41350–41357
Jongbloed JD, Antelmann H, Hecker M, Nijland R, Bron S, Airaksinen U, Pries F, Quax WJ, van Dijl JM, Braun PG (2002) Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J Biol Chem 277(46):44068–44078
Jongbloed JD, Grieger U, Antelmann H, Hecker M, Nijland R, Bron S, van Dijl JM (2004) Two minimal Tat translocases in Bacillus. Mol Microbiol 54(5):1319–1325
Joshi MV, Mann SG, Antelmann H, Widdick DA, Fyans JK, Chandra G, Hutchings MI, Toth I, Hecker M, Loria R, Palmer T (2010) The twin arginine protein transport pathway exports multiple virulence proteins in the plant pathogen Streptomyces scabies. Mol Microbiol 77(1):252–271
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Kramer R, Linke B, McHardy AC, Meyer F, Mockel B, Pfefferle W, Puhler A, Rey DA, Ruckert C, Rupp O, Sahm H, Wendisch VF, Wiegrabe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 104(1–3):5–25
Keller R, de Keyzer J, Driessen AJ, Palmer T (2012) Co-operation between different targeting pathways during integration of a membrane protein. J Cell Biol 199(2):303–315
Khan MT, Duncan SH, Stams AJ, van Dijl JM, Flint HJ, Harmsen HJ (2012) The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J 6(8):1578–1585
Kikuchi Y, Date M, Itaya H, Matsui K, Wu LF (2006) Functional analysis of the twin-arginine translocation pathway in Corynebacterium glutamicum ATCC 13869. Appl Environ Microbiol 72(11):7183–7192
Kikuchi Y, Itaya H, Date M, Matsui K, Wu LF (2008) Production of Chryseobacterium proteolyticum protein-glutaminase using the twin-arginine translocation pathway in Corynebacterium glutamicum. Appl Microbiol Biotechnol 78(1):67–74
Kim JY, Fogarty EA, Lu FJ, Zhu H, Wheelock GD, Henderson LA, DeLisa MP (2005) Twin-arginine translocation of active human tissue plasminogen activator in Escherichia coli. Appl Environ Microbiol 71(12):8451–8459
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RM, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A 100(4):1990–1995
Kneuper H, Maldonado B, Jager F, Krehenbrink M, Buchanan G, Keller R, Muller M, Berks BC, Palmer T (2012) Molecular dissection of TatC defines critical regions essential for protein transport and a TatB-TatC contact site. Mol Microbiol 85(5):945–961
Kobayashi R, Suzuki T, Yoshida M (2007) Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol Microbiol 66(1):100–109
Kolkman MA, van der Ploeg R, Bertels M, van Dijk M, van der Laan J, van Dijl JM, Ferrari E (2008) The twin-arginine signal peptide of Bacillus subtilis YwbN can direct either Tat- or Sec-dependent secretion of different cargo proteins: secretion of active subtilisin via the B. subtilis Tat pathway. Appl Environ Microbiol 74(24):7507–7513
Kouwen TR, van der Ploeg R, Antelmann H, Hecker M, Homuth G, Mader U, van Dijl JM (2009) Overflow of a hyper-produced secretory protein from the Bacillus Sec pathway into the Tat pathway for protein secretion as revealed by proteogenomics. Proteomics 9(4):1018–1032
Krishnappa L, Monteferrante CG, van Dijl JM (2012) Degradation of the Twin-Arginine Translocation Substrate YwbN by Extracytoplasmic Proteases of Bacillus subtilis. Appl Environ Microbiol 78(21):7801–7804
Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Codani JJ, Connerton IF, Danchin A (1997) The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390(6657):249–256
Lange C, Muller SD, Walther TH, Burck J, Ulrich AS (2007) Structure analysis of the protein translocating channel TatA in membranes using a multi-construct approach. Biochim Biophys Acta 1768(10):2627–2634
Leake MC, Greene NP, Godun RM, Granjon T, Buchanan G, Chen S, Berry RM, Palmer T, Berks BC (2008) Variable stoichiometry of the TatA component of the twin-arginine protein transport system observed by in vivo single-molecule imaging. Proc Natl Acad Sci USA 105(40):15376–15381
Ledala N, Sengupta M, Muthaiyan A, Wilkinson BJ, Jayaswal RK (2010) Transcriptomic response of Listeria monocytogenes to iron limitation and Fur mutation. Appl Environ Microbiol 76(2):406–416
Link TA, Saynovits M, Assmann C, Iwata S, Ohnishi T, von Jagow G (1996) Isolation, characterisation and crystallisation of a water-soluble fragment of the Rieske iron-sulfur protein of bovine heart mitochondrial bc1 complex. Eur J Biochem 237(1):71–75
Liu YW, Hitchcock A, Salmon RC, Kelly DJ (2014) It takes two to tango: two TatA paralogues and two redox enzyme-specific chaperones are involved in the localization of twin-arginine translocase substrates in Campylobacter jejuni. Microbiology 160(Pt 9):2053–2066
Luke I, Handford JI, Palmer T, Sargent F (2009) Proteolytic processing of Escherichia coli twin-arginine signal peptides by LepB. Arch Microbiol 191(12):919–925
Ma X, Cline K (2013) Mapping the signal peptide binding and oligomer contact sites of the core subunit of the pea twin arginine protein translocase. Plant Cell 25(3):999–1015
Machado H, Lourenco A, Carvalho F, Cabanes D, Kallipolitis BH, Brito L (2013) The Tat pathway is prevalent in Listeria monocytogenes lineage II and is not required for infection and spread in host cells. J Mol Microbiol Biotechnol 23(3):209–218
Matos CF, Robinson C, Di Cola A (2008) The Tat system proofreads FeS protein substrates and directly initiates the disposal of rejected molecules. EMBO J 27(15):2055–2063
Matos CF, Robinson C, Alanen HI, Prus P, Uchida Y, Ruddock LW, Freedman RB, Keshavarz-Moore E (2013) Efficient export of prefolded, disulfide-bonded recombinant proteins to the periplasm by the Tat pathway in Escherichia coli CyDisCo strains. Biotechnol Prog 30(2):281–290
Maurer C, Panahandeh S, Moser M, Muller M (2009) Impairment of twin-arginine-dependent export by seemingly small alterations of substrate conformation. FEBS Lett 583(17):2849–2853
McDevitt CA, Buchanan G, Sargent F, Palmer T, Berks BC (2006) Subunit composition and in vivo substrate-binding characteristics of Escherichia coli Tat protein complexes expressed at native levels. FEBS J 273(24):5656–5668
McDonough JA, Hacker KE, Flores AR, Pavelka MS Jr, Braunstein M (2005) The twin-arginine translocation pathway of Mycobacterium smegmatis is functional and required for the export of mycobacterial beta-lactamases. J Bacteriol 187(22):7667–7679
Mehner D, Osadnik H, Lunsdorf H, Bruser T (2012) The Tat system for membrane translocation of folded proteins recruits the membrane-stabilizing Psp machinery in Escherichia coli. J Biol Chem 287(33):27834–27842
Meissner D, Vollstedt A, van Dijl JM, Freudl R (2007) Comparative analysis of twin-arginine (Tat)-dependent protein secretion of a heterologous model protein (GFP) in three different Gram-positive bacteria. Appl Microbiol Biotechnol 76(3):633–642
Mendel S, McCarthy A, Barnett JP, Eijlander RT, Nenninger A, Kuipers OP, Robinson C (2008) The Escherichia coli TatABC system and a Bacillus subtilis TatAC-type system recognise three distinct targeting determinants in twin-arginine signal peptides. J Mol Biol 375(3):661–672
Miethke M, Monteferrante CG, Marahiel MA, van Dijl JM (2013) The Bacillus subtilis EfeUOB transporter is essential for high-affinity acquisition of ferrous and ferric iron. Biochim Biophys Acta 1833:2267–2278
Molik S, Karnauchov I, Weidlich C, Herrmann RG, Klosgen RB (2001) The Rieske Fe/S protein of the cytochrome b6/f complex in chloroplasts: missing link in the evolution of protein transport pathways in chloroplasts? J Biol Chem 276(46):42761–42766
Monteferrante CG, Baglieri J, Robinson C, van Dijl JM (2012a) TatAc, the third TatA subunit of Bacillus subtilis, can form active twin-arginine translocases with the TatCd and TatCy subunits. Appl Environ Microbiol 78(14):4999–5001
Monteferrante CG, Miethke M, van der Ploeg R, Glasner C, van Dijl JM (2012b) Specific targeting of the metallophosphoesterase YkuE to the bacillus cell wall requires the twin-arginine translocation system. J Biol Chem 287(35):29789–29800
Monteferrante CG, Mackichan C, Marchadier E, Prejean MV, Carballido-Lopez R, van Dijl JM (2013) Mapping the twin-arginine protein translocation network of Bacillus subtilis. Proteomics 13(5):800–811
Mori H, Cline K (2001) Post-translational protein translocation into thylakoids by the Sec and DeltapH-dependent pathways. Biochim Biophys Acta 1541(1–2):80–90
Mori H, Cline K (2002) A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid [Delta]pH/Tat translocase. J Cell Biol 157(2):205–210
Nicolas P, Mader U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Fogg MJ, Fromion V, Goelzer A, Hansen A, Hartig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Le Chat L, Lecointe F, Lewis P, Liebermeister W, March A, Mars RA, Nannapaneni P, Noone D, Pohl S, Rinn B, Rugheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stulke J, Wiegert T, Devine KM, Wilkinson AJ, van Dijl JM, Hecker M, Volker U, Bessieres P, Noirot P (2012) Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335(6072):1103–1106
Nishio Y, Nakamura Y, Kawarabayasi Y, Usuda Y, Kimura E, Sugimoto S, Matsui K, Yamagishi A, Kikuchi H, Ikeo K, Gojobori T (2003) Comparative complete genome sequence analysis of the amino acid replacements responsible for the thermostability of Corynebacterium efficiens. Genome Res 13(7):1572–1579
Oates J, Barrett CM, Barnett JP, Byrne KG, Bolhuis A, Robinson C (2005) The Escherichia coli twin-arginine translocation apparatus incorporates a distinct form of TatABC complex, spectrum of modular TatA complexes and minor TatAB complex. J Mol Biol 346(1):295–305
Oertel D, Schmitz S, Freudl R (2015) A TatABC-Type Tat translocase is required for unimpaired aerobic growth of Corynebacterium glutamicum ATCC13032. PLoS ONE 10(4):e0123413
Oresnik IJ, Ladner CL, Turner RJ (2001) Identification of a twin-arginine leader-binding protein. Mol Microbiol 40(2):323–331
Palmer T, Berks BC (2012) The twin-arginine translocation (Tat) protein export pathway. Nat Rev Microbiol 10(7):483–496
Panahandeh S, Maurer C, Moser M, DeLisa MP, Muller M (2008) Following the path of a twin-arginine precursor along the TatABC translocase of Escherichia coli. J Biol Chem 283(48):33267–33275
Papish AL, Ladner CL, Turner RJ (2003) The twin-arginine leader-binding protein, DmsD, interacts with the TatB and TatC subunits of the Escherichia coli twin-arginine translocase. J Biol Chem 278(35):32501–32506
Patel R, Smith SM, Robinson C (2014) Protein transport by the bacterial Tat pathway. Biochim Biophys Acta 1843(8):1620–1628
Pett W, Lavrov DV (2013a) The twin-arginine subunit C in Oscarella: origin, evolution, and potential functional significance. Integr Comp Biol 53(3):495–502
Pett W, Lavrov DV (2013b) The twin-arginine subunit C in oscarella: origin, evolution, and potential functional significance. Integr Comp Biol 53(3):495–502
Pop OI, Westermann M, Volkmer-Engert R, Schulz D, Lemke C, Schreiber S, Gerlach R, Wetzker R, Muller JP (2003) Sequence-specific binding of prePhoD to soluble TatAd indicates protein-mediated targeting of the Tat export in Bacillus subtilis. J Biol Chem 278(40):38428–38436
Punginelli C, Maldonado B, Grahl S, Jack R, Alami M, Schroder J, Berks BC, Palmer T (2007) Cysteine scanning mutagenesis and topological mapping of the Escherichia coli twin-arginine translocase TatC Component. J Bacteriol 189(15):5482–5494
Raynaud C, Guilhot C, Rauzier J, Bordat Y, Pelicic V, Manganelli R, Smith I, Gicquel B, Jackson M (2002) Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol Microbiol 45(1):203–217
Ribnicky B, Van Blarcom T, Georgiou G (2007) A scFv antibody mutant isolated in a genetic screen for improved export via the twin arginine transporter pathway exhibits faster folding. J Mol Biol 369(3):631–639
Richter S, Lindenstrauss U, Lucke C, Bayliss R, Bruser T (2007) Functional Tat transport of unstructured, small, hydrophilic proteins. J Biol Chem 282(46):33257–33264
Ridder AN, de Jong EJ, Jongbloed JD, Kuipers OP (2009) Subcellular localization of TatAd of Bacillus subtilis depends on the presence of TatCd or TatCy. J Bacteriol 191(13):4410–4418
Robinson C, Matos CF, Beck D, Ren C, Lawrence J, Vasisht N, Mendel S (2011) Transport and proofreading of proteins by the twin-arginine translocation (Tat) system in bacteria. Biochim Biophys Acta 1808(3):876–884
Rocco MA, Waraho-Zhmayev D, DeLisa MP (2012) Twin-arginine translocase mutations that suppress folding quality control and permit export of misfolded substrate proteins. Proc Natl Acad Sci USA 109(33):13392–13397
Rodrigue A, Chanal A, Beck K, Muller M, Wu LF (1999) Co-translocation of a periplasmic enzyme complex by a hitchhiker mechanism through the bacterial tat pathway. J Biol Chem 274(19):13223–13228
Rodriguez F, Rouse SL, Tait CE, Harmer J, De Riso A, Timmel CR, Sansom MS, Berks BC, Schnell JR (2013) Structural model for the protein-translocating element of the twin-arginine transport system. Proc Natl Acad Sci U S A 110(12):E1092–1101
Rodriguez F, Lillington J, Johnson S, Timmel CR, Lea SM, Berks BC (2014) Crystal structure of the Bacillus subtilis phosphodiesterase PhoD reveals an iron and calcium-containing active site. J Biol Chem 289(45):30889–30899
Rose RW, Bruser T, Kissinger JC, Pohlschroder M (2002) Adaptation of protein secretion to extremely high-salt conditions by extensive use of the twin-arginine translocation pathway. Mol Microbiol 45(4):943–950
Sargent F, Stanley NR, Berks BC, Palmer T (1999) Sec-independent protein translocation in Escherichia coli. A distinct and pivotal role for the TatB protein. J Biol Chem 274(51):36073–36082
Schaerlaekens K, Schierova M, Lammertyn E, Geukens N, Anne J, Van Mellaert L (2001) Twin-arginine translocation pathway in Streptomyces lividans. J Bacteriol 183(23):6727–6732
Schaerlaekens K, Lammertyn E, Geukens N, De Keersmaeker S, Anne J, Van Mellaert L (2004a) Comparison of the Sec and Tat secretion pathways for heterologous protein production by Streptomyces lividans. J Biotechnol 112(3):279–288
Schaerlaekens K, Van Mellaert L, Lammertyn E, Geukens N, Anne J (2004b) The importance of the Tat-dependent protein secretion pathway in Streptomyces as revealed by phenotypic changes in tat deletion mutants and genome analysis. Microbiology 150(Pt 1):21–31
Scheele S, Oertel D, Bongaerts J, Evers S, Hellmuth H, Maurer KH, Bott M, Freudl R (2013) Secretory production of an FAD cofactor-containing cytosolic enzyme (sorbitol-xylitol oxidase from Streptomyces coelicolor) using the twin-arginine translocation (Tat) pathway of Corynebacterium glutamicum. Microb Biotechnol 6(2):202–206
Schmidt CL, Shaw L (2001) A comprehensive phylogenetic analysis of Rieske and Rieske-type iron-sulfur proteins. J Bioenerg Biomembr 33(1):9–26
Schneider D, Schmidt CL (2005) Multiple Rieske proteins in prokaryotes: where and why? Biochim Biophys Acta 1710(1):1–12
Schreiber S, Stengel R, Westermann M, Volkmer-Engert R, Pop OI, Muller JP (2006) Affinity of TatCd for TatAd elucidates its receptor function in the Bacillus subtilis twin arginine translocation (Tat) translocase system. J Biol Chem 281(29):19977–19984
Simone D, Bay DC, Leach T, Turner RJ (2013) Diversity and evolution of bacterial twin arginine translocase protein, TatC, reveals a protein secretion system that is evolving to fit its environmental niche. PLoS ONE 8(11):e78742
Solans L, Gonzalo-Asensio J, Sala C, Benjak A, Uplekar S, Rougemont J, Guilhot C, Malaga W, Martin C, Cole ST (2014) The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog 10(5):e1004183
Sousa PM, Videira MA, Santos FA, Hood BL, Conrads TP, Melo AM (2013) The bc:caa3 supercomplexes from the Gram positive bacterium Bacillus subtilis respiratory chain: a megacomplex organization? Arch Biochem Biophys 537(1):153–160
Standar K, Mehner D, Osadnik H, Berthelmann F, Hause G, Lunsdorf H, Bruser T (2008) PspA can form large scaffolds in Escherichia coli. FEBS Lett 582(25–26):3585–3589
Stanley NR, Palmer T, Berks BC (2000) The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J Biol Chem 275(16):11591–11596
Strauch EM, Georgiou G (2007) Escherichia coli tatC mutations that suppress defective twin-arginine transporter signal peptides. J Mol Biol 374(2):283–291
Thiemann V, Saake B, Vollstedt A, Schafer T, Puls J, Bertoldo C, Freudl R, Antranikian G (2006) Heterologous expression and characterization of a novel branching enzyme from the thermoalkaliphilic anaerobic bacterium Anaerobranca gottschalkii. Appl Microbiol Biotechnol 72(1):60–71
Tiwari KB, Birlingmair J, Wilkinson BJ, Jayaswal RK (2015) Role of the twin-arginine translocase (tat) system in iron uptake in Listeria monocytogenes. Microbiology 161(Pt 2):264–271
Tjalsma H, Bolhuis A, Jongbloed JD, Bron S, van Dijl JM (2000) Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev 64(3):515–547
Tjalsma H, Antelmann H, Jongbloed JD, Braun PG, Darmon E, Dorenbos R, Dubois JY, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, van Dijl JM (2004) Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol Mol Biol Rev 68(2):207–233
Tottey S, Waldron KJ, Firbank SJ, Reale B, Bessant C, Sato K, Cheek TR, Gray J, Banfield MJ, Dennison C, Robinson NJ (2008) Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 455(7216):1138–1142
Turlin E, Debarbouille M, Augustyniak K, Gilles AM, Wandersman C (2013) Staphylococcus aureus FepA and FepB proteins drive heme iron utilization in Escherichia coli. PLoS ONE 8(2):e56529
van der Ploeg R, Barnett JP, Vasisht N, Goosens VJ, Pother DC, Robinson C, van Dijl JM (2011a) Salt sensitivity of minimal twin arginine translocases. J Biol Chem 286(51):43759–43770
van der Ploeg R, Mader U, Homuth G, Schaffer M, Denham EL, Monteferrante CG, Miethke M, Marahiel MA, Harwood CR, Winter T, Hecker M, Antelmann H, van Dijl JM (2011b) Environmental salinity determines the specificity and need for Tat-dependent secretion of the YwbN protein in Bacillus subtilis. PLoS ONE 6(3):e18140
van der Ploeg R, Monteferrante CG, Piersma S, Barnett JP, Kouwen TR, Robinson C, van Dijl JM (2012) High-salinity growth conditions promote Tat-independent secretion of tat substrates in Bacillus subtilis. Appl Environ Microbiol 78(21):7733–7744
van Dijl JM, Braun PG, Robinson C, Quax WJ, Antelmann H, Hecker M, Muller J, Tjalsma H, Bron S, Jongbloed JD (2002) Functional genomic analysis of the Bacillus subtilis Tat pathway for protein secretion. J Biotechnol 98(2–3):243–254
Vrancken K, De Keersmaeker S, Geukens N, Lammertyn E, Anne J, Van Mellaert L (2007) pspA overexpression in Streptomyces lividans improves both Sec- and Tat-dependent protein secretion. Appl Microbiol Biotechnol 73(5):1150–1157
Vrancken K, Van Mellaert L, Anne J (2008) Characterization of the Streptomyces lividans PspA response. J Bacteriol 190(10):3475–3481
Walther TH, Grage SL, Roth N, Ulrich AS (2010) Membrane alignment of the pore-forming component TatA(d) of the twin-arginine translocase from Bacillus subtilis resolved by solid-state NMR spectroscopy. J Am Chem Soc 132(45):15945–15956
Walther TH, Gottselig C, Grage SL, Wolf M, Vargiu AV, Klein MJ, Vollmer S, Prock S, Hartmann M, Afonin S, Stockwald E, Heinzmann H, Nolandt OV, Wenzel W, Ruggerone P, Ulrich AS (2013) Folding and self-assembly of the TatA translocation pore based on a charge zipper mechanism. Cell 152(1–2):316–326
Weatherspoon-Griffin N, Zhao G, Kong W, Kong Y, Morigen H, Andrews-Polymenis M McClelland, Shi Y (2011) The CpxR/CpxA two-component system up-regulates two Tat-dependent peptidoglycan amidases to confer bacterial resistance to antimicrobial peptide. J Biol Chem 286(7):5529–5539
Westermann M, Pop OI, Gerlach R, Appel TR, Schlormann W, Schreiber S, Muller JP (2006) The TatAd component of the Bacillus subtilis twin-arginine protein transport system forms homo-multimeric complexes in its cytosolic and membrane embedded localisation. Biochim Biophys Acta 1758(4):443–451
Wexler M, Sargent F, Jack RL, Stanley NR, Bogsch EG, Robinson C, Berks BC, Palmer T (2000) TatD is a cytoplasmic protein with DNase activity. No requirement for TatD family proteins in sec-independent protein export. J Biol Chem 275(22):16717–16722
Whitaker N, Bageshwar UK, Musser SM (2012) Kinetics of precursor interactions with the bacterial Tat translocase detected by real-time FRET. J Biol Chem 287(14):11252–11260
Widdick DA, Dilks K, Chandra G, Bottrill A, Naldrett M, Pohlschroder M, Palmer T (2006) The twin-arginine translocation pathway is a major route of protein export in Streptomyces coelicolor. Proc Natl Acad Sci USA 103(47):17927–17932
Widdick DA, Eijlander RT, van Dijl JM, Kuipers OP, Palmer T (2008) A facile reporter system for the experimental identification of twin-arginine translocation (Tat) signal peptides from all kingdoms of life. J Mol Biol 375(3):595–603
Widdick DA, Hicks MG, Thompson BJ, Tschumi A, Chandra G, Sutcliffe IC, Brulle JK, Sander P, Palmer T, Hutchings MI (2011) Dissecting the complete lipoprotein biogenesis pathway in Streptomyces scabies. Mol Microbiol 80(5):1395–1412
Wolff S, Antelmann H, Albrecht D, Becher D, Bernhardt J, Bron S, Buttner K, van Dijl JM, Eymann C, Otto A, Le Tam T, Hecker M (2007) Towards the entire proteome of the model bacterium Bacillus subtilis by gel-based and gel-free approaches. J Chromatogr B Analyt Technol Biomed Life Sci 849(1–2):129–140
Wu LF, Chanal A, Rodrigue A (2000a) Membrane targeting and translocation of bacterial hydrogenases. Arch Microbiol 173(5–6):319–324
Wu LF, Ize B, Chanal A, Quentin Y, Fichant G (2000b) Bacterial twin-arginine signal peptide-dependent protein translocation pathway: evolution and mechanism. J Mol Microbiol Biotechnol 2(2):179–189
Xu P, Alves JM, Kitten T, Brown A, Chen Z, Ozaki LS, Manque P, Ge X, Serrano MG, Puiu D, Hendricks S, Wang Y, Chaplin MD, Akan D, Paik S, Peterson DL, Macrina FL, Buck GA (2007) Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol 189(8):3166–3175
Yamada K, Sanzen I, Ohkura T, Okamoto A, Torii K, Hasegawa T, Ohta M (2007) Analysis of twin-arginine translocation pathway homologue in Staphylococcus aureus. Curr Microbiol 55(1):14–19
Yen MR, Tseng YH, Nguyen EH, Wu LF, Saier MH Jr (2002) Sequence and phylogenetic analyses of the twin-arginine targeting (Tat) protein export system. Arch Microbiol 177(6):441–450
Yu J, Hederstedt L, Piggot PJ (1995) The cytochrome bc complex (menaquinone:cytochrome c reductase) in Bacillus subtilis has a nontraditional subunit organization. J Bacteriol 177(23):6751–6760
Zhang C, Xin Y, Wang Y, Guo T, Lu S, Kong J (2015) Identification of a novel dye-decolorizing peroxidase, EfeBs, translocated by twin-arginine translocation system in Streptococcus thermophilus CGMCC 7.179. Appl Environ Microbiol 81(18):6108–6119
Zoufaly S, Frobel J, Rose P, Flecken T, Maurer C, Moser M, Muller M (2012) Mapping precursor-binding site on TatC subunit of twin arginine-specific protein translocase by site-specific photo cross-linking. J Biol Chem 287(16):13430–13441
Acknowledgements
VJG and JMvD were in parts supported by the CEU projects PITN-GA-2008-215524 (TranSys), LSHG-CT-2006-037469 and 244093, and the transnational SysMO initiative through projects BACELL SysMO1 and 2 with funding from the Research Council for Earth and Life Sciences of the Netherlands Organization for Scientific Research (NWO-ALW).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Goosens, V.J., van Dijl, J.M. (2016). Twin-Arginine Protein Translocation. In: Bagnoli, F., Rappuoli, R. (eds) Protein and Sugar Export and Assembly in Gram-positive Bacteria . Current Topics in Microbiology and Immunology, vol 404. Springer, Cham. https://doi.org/10.1007/82_2016_7
Download citation
DOI: https://doi.org/10.1007/82_2016_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56012-0
Online ISBN: 978-3-319-56014-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)