Abstract
Increasing evidence shows that long non-coding RNAs (lncRNAs) are not “transcriptional noise” but function in a myriad of biological processes. As such, this rapidly growing class of RNAs is important in both development and disease. Vascular smooth muscle cells are integral cells of the blood vessel wall. They are responsible for relaxation and contraction of the blood vessel and respond to hemodynamic as well as environmental signals to regulate blood pressure. Pathophysiological changes to these cells such as hyperproliferation, hypertrophy, migration, and inflammation contribute to cardiovascular diseases (CVDs) such as restenosis, hypertension, and atherosclerosis. Understanding the molecular mechanisms involved in these pathophysiological changes to VSMCs is paramount to developing therapeutic treatments for various cardiovascular disorders. Recent studies have shown that lncRNAs are key players in the regulation of VSMC functions and phenotype and, perhaps also, in the development of VSMC-related diseases. This chapter describes our current understanding of the functions of lncRNAs in VSMCs. It highlights the emerging role of lncRNAs in VSMC proliferation and apoptosis, their role in contractile and migratory phenotype of VSMCs, and their potential role in VSMC disease states.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Thoracic Aortic Aneurysm
- Thoracic Aortic Aneurysm
- VSMC Proliferation
- Renal Proximal Tubular Epithelial Cell
- Human VSMCs
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Vascular smooth muscle cells (VSMCs) are integral components of the blood vessel wall. These highly specialized cells are responsible for contraction and relaxation of the vasculature in response to many signals and cues, including hemodynamic alterations, mechanical injury, growth factors, and ligand–receptor signaling (Owens 1995; Mack 2011; Lacolley et al. 2012). VSMCs are important for maintaining normal blood pressure, vessel integrity and function, and perturbations of their fully differentiated contractile states can contribute to the development and onset of vascular diseases. Specifically, inappropriate VSMC proliferation, cell growth, migration, and inflammatory signaling contribute to cardiovascular diseases (CVDs). For example, hyperproliferation and migration of VSMCs have been shown to lead to lesion formation in restenosis, atherosclerosis, and hypertension (Brasier et al. 2002).
Early work on VSMCs uncovered many protein signaling pathways, including classical G protein-mediated pathways and receptor tyrosine kinases, which regulate the response of VSMCs to environmental cues and growth factors. These include angiotensin II (Ang II) and platelet-derived growth factor (PDGF) (Mehta and Griendling 2007; Berk and Corson 1997). In recent years, it has become clear that non-protein mechanisms and post-transcriptional mechanisms, such as those mediated by small non-coding RNAs called microRNAs (miRNAs) (Maegdefessel et al. 2015) and long non-coding RNAs (lncRNAs), also function in normal and diseased VSMCs (Leung et al. 2013; Bell et al. 2014; Leung and Natarajan 2014). Non-coding RNAs have been at the forefront of research due to increasing evidence of their involvement in several cellular processes, their dysregulation in diseased states, and their potential to be novel therapeutic targets in the treatment of various diseases. In this chapter, we will briefly discuss the known roles of lncRNAs and then cover the recent literature that uncovers functions of lncRNA in VSMCs.
2 Non-coding RNAs
Since the discovery of miRNAs, it has become clear that non-coding RNAs can function in post-transcriptional regulation of gene expression (Arasu et al. 1991; Wightman et al. 1991; Lee et al. 1993; Bartel 2009). miRNAs are 20–25 nucleotide non-coding RNAs which regulate the stability and/or translation of specific target mRNAs based upon sequence specificity with the target 3′ UTR. These small RNAs have been shown to be important for normal development and act as fine-tuners of gene expression. Abnormal levels of miRNAs have been associated with numerous diseases including CVDs (Small and Olson 2011; Kataoka and Wang 2014). In VSMCs, miRNAs are important for many processes and phenotypes. For example, miR-143 and miR-145 have been shown to regulate normal VSMC differentiation and contractility (Cordes et al. 2009). The upregulation of miR-221 and miR-222, which target mRNAs of two key cyclin-dependent kinase inhibitors, p27Kip1 and p57Kip2, results in the migration and proliferation of VSMCs and reduces the expression of contractile genes (Liu et al. 2009).
miRNAs can also function as part of the response to VSMC growth and inflammatory cues such as Ang II signaling. Ang II is a small polypeptide hormone that regulates many processes in the vessel wall including vasoconstriction, inflammation, fibrosis, and cellular states (Mehta and Griendling 2007). Ang II signaling mediated by its type 1 and type 2 receptors results in the activation of signaling cascades which rapidly result in gene expression changes and ultimately physiological, as well as pathophysiological responses in VSMCs, including proliferation, fibrosis, and inflammation (Berk and Corson 1997). Recently, non-coding RNAs have been shown to mediate this Ang II response in VSMCs. Specifically, Ang II signaling induces upregulation of miR-132 and miR-212, which target PTEN (phosphatase and tensin homolog) mRNA (Jin et al. 2012). This repression of PTEN in VSMCs furthermore causes induction of pro-inflammatory monocyte chemoattractant protein-1 (MCP-1). In addition, increase in miR-132 enhances activation of CREB (cyclic AMP-responsive element binding protein) through increased phosphorylation (Jin et al. 2012).
Dysregulation of certain miRNAs in VSMCs can also contribute to increased inflammation related to the development and progression of diabetic vascular complications. Under diabetic conditions, miR-125b upregulates the pro-inflammatory response of VSMCs by targeting and downregulating key repressive histone methyltransferases (Villeneuve et al. 2010). These data also highlight crosstalk between non-coding RNAs and epigenetic mechanisms in chromatin. Similarly, miR-200 family members upregulated in VSMCs of diabetic mice also enhance the expression of inflammatory genes by targeting the E-box repressor Zeb1 to relieve repression (Reddy et al. 2012). Clearly, non-protein-coding RNAs are emerging as important regulators in VSMC functions, and the dysregulation of miRNAs can contribute to VSMC dysfunction leading to development of disease. Since miRNAs are usually highly conserved, are well preserved in biological fluids and formalin-fixed sections, and can be targeted by various antisense strategies, they are increasingly exploited as novel biomarkers or therapeutic targets for various diseases, including CVDs and diabetic vascular complications (Kato and Natarajan 2014; Kataoka and Wang 2014).
2.1 Long Non-coding RNAs
After the discovery of miRNAs, additional high-throughput sequencing efforts characterized thousands of more non-protein-coding RNAs that are longer than 200 nucleotides and are subsequently classified as lncRNAs. Some members of this class of RNAs are similar to protein-coding RNAs as they are processed by RNA polymerase II, 5′ capped, and can be also 3′ polyadenylated, but lack distinct open reading frames (Cabili et al. 2011; Guttman et al. 2010; Khalil et al. 2009). LncRNAs are generally expressed at much lower levels than protein-coding RNAs (Khalil et al. 2009). In contrast to miRNAs that have a distinct role in targeting mRNAs, members of this class of RNA have many diverse molecular and biological functions (Fig. 1) (Wapinski and Chang 2011; Moran et al. 2012) and are important for many if not all aspects of cell biology. The earliest known function of this class of RNAs is in the regulation of transcription of local genes. Xist, transcribed on the X chromosome in mouse and humans, functions to regulate the expression of genes on the X chromosomes in the process of X inactivation (Brown et al 1991; Penny et al. 1996). Xist RNAs, which are highly expressed from one of the two X chromosome copies, coat the local inactivated X and interact with Polycomb Repressive Complex 2 (PRC2) silencing complex to silence local gene transcription (Froberg et al. 2013; Zhao et al. 2008). Since the characterization of Xist, additional lncRNAs have been identified using high-throughput sequencing technologies and were found to be important for the regulation of gene transcription.
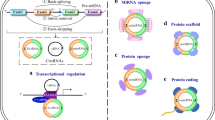
Molecular roles of lncRNAs. LncRNAs have myriad of molecular functions. Known roles include the following: a host transcripts for miRNAs, b molecular scaffolds for protein complexes known as ribonucleoproteins and chromatin remodeling complexes, c regulators of mRNA stability, d competitors of mRNAs targeted by miRNAs, and e cis regulators of gene expression
The first two lncRNAs found to regulate gene transcription include p15AS and p21 antisense (Morris et al. 2008; Yu et al. 2008). The former was shown to regulate the transcription of the overlapping p15 and the latter to regulate the transcription of p21, both by suppressing promoter activity. Other lncRNAs have also been found to interact with chromatin modifying complexes. One of these is HOTAIR transcript which interacts with both the PRC2 complex and the lysine-specific 1A/REST corepressor/RE1-silencing transcription factor (LSD1/REST/CoREST) (Tsai et al. 2010). Additional lncRNAs, such as linc-p21, have also been found to act as scaffolds for other types of proteins including hnRNPs (Huarte et al. 2010). Some lncRNAs were found to regulate local gene regulation through cis-acting function. In particular, enhancer-like RNAs were classified as lncRNAs which affect local transcription of nearby genes (Orom et al. 2010). One such RNA, ncRNA-a7, regulates a neighboring gene, Snai1, which is an important gene in cellular migration and the development of cancer (Orom et al. 2010). Further investigations have also described a set of lncRNAs called enhancer RNAs (eRNAs) which affect many biological processes including macrophage biology, p53-targeted gene expression, and estrogen receptor alpha-targeted gene expression (Melo et al. 2013; Li et al. 2013; Lam et al. 2013). These lncRNAs can interact with a variety of regulators involved in the control of local transcription including chromatin-modifying complexes and transcriptional activators (Fig. 1). One key molecular function of these lncRNAs is interacting and recruiting key protein complexes to local DNA.
It has also been demonstrated that lncRNAs affect gene expression via several post-transcriptional regulatory mechanisms (Fig. 1). They can function as competing RNAs which can deplete miRNAs from their target RNAs. For example, in muscle differentiation linc-MD1, RNA competes with two miRNAs, miR-135 and miR-133, which target MEF2C and MAML1 mRNAs, respectively. With the expression of linc-MD1, miR-135 and miR-133 are titrated from MEF2C and MAML1 mRNAs and prevented from inducing mRNA degradation (Cesana et al. 2011). Aberrant expression of linc-MD1 has been found in patients with Duchenne muscular dystrophy, highlighting the importance of lncRNAs in muscular disorders. In addition to modulating levels of miRNAs, lncRNAs can also serve as host genes of miRNAs (Fig. 1). It is estimated that 10 % of lncRNAs host miRNAs (Consortium et al. 2007; Kapranov et al. 2007). Transcription of lncRNAs can thus directly alter the level of miRNAs. There is also evidence that lncRNAs can directly interact with mRNAs to affect their stabilization. For example, TINCR (Terminal differentiation-induced ncRNA) binds to target RNAs through a 25-nucleotide motif sequence and regulates the stability of its targets. Lack of this interaction results in abnormal epidermal differentiation (Kretz et al. 2013). In recent years, lncRNAs have been increasingly implicated in various disease states and hence evaluated as potential therapeutic targets (Kataoka and Wang 2014; Kato and Natarajan 2014).
3 LncRNAs in Vascular Smooth Muscle Cells
The study of lncRNAs in VSMCs has been relatively underexplored compared to other tissues types. Since VSMC growth and differentiation is critical for normal and pathophysiological states of the vessel wall, a study of lncRNAs could shed new insights into their roles in VSMC biology and functions. Here, we describe recent studies which have just begun to uncover the role of lncRNAs in these very important cell types and their potential role in human disease (Fig. 2).
VSMC processes regulated by LncRNAs. LncRNAs reported to date (in red) that function in VSMC proliferation, apoptosis, contraction, and migration. Lnc-Ang362, regulated by Ang II, is the host gene for miR-221 and miR-222, which regulate VSMC proliferation. BRG1 regulates HIF-AS1 inducing apoptosis and reducing cell proliferation. p53 and lincRNA-p21 regulate each other to promote or reduce cell proliferation and apoptosis. SENCR transcripts promote VSMC contraction and reduce VSMC migration. HAS2-AS1 regulates HAS2 transcription to promote extracellular matrix remodeling in VSMCs
3.1 Rats
One of the first studies to investigate lncRNAs in VSMCs described lncRNA mediation of Ang II signaling in rat VSMCs (Leung et al. 2013). In this study, we performed genome-wide chromatin profiling of two post-translational histone modifications associated with active transcription, histone H3 lysine 4 trimethylation (H3K4me3), and histone H3 lysine 36 trimethylation (H3K4me36), along with transcriptome profiling. These parallel experiments allowed us to comprehensively characterize lncRNAs that are expressed in rat VSMCs as well as those that are differentially expressed under Ang II treatment. In total, 466 lncRNAs were found to be expressed in control and/or Ang II-treated VSMCs, and of those, 29 lncRNAs were significantly regulated by Ang II. We further investigated the role of one novel lncRNA, Lnc-Ang362, which is located in proximity to miR-221 and miR-222. These two proximal miRNAs are co-expressed and have been shown to be involved in the response of VSMC to Ang II. Two key features led us to hypothesize that this lncRNA and two miRNAs are co-regulated as follows: (1) Lnc-Ang362 is upregulated in response to Ang II which is concordant with the expression of the two miRNAs and (2) the chromatin profile for this locus indicated one RNA polymerase II initiation site for the lncRNA and the miRNAs (i.e., one H3K4me3-enriched locus at the 5′ end of Lnc-Ang362 locus and continuous H3K36me3 enrichment across the locus including the miR-221 and miR-222 loci). To investigate the potential of these RNAs to be co-regulated, short interfering RNAs (siRNAs) were employed to reduce the levels of Lnc-Ang362. In response to the siRNA-mediated reduction of Lnc-Ang362, the two miRNAs were downregulated. Lnc-Ang362 was therefore classified as the host transcript for the two miRNAs, and these investigations uncovered a novel mechanism by which Ang II regulates the expression of these two miRNA. The siRNA-mediated reduction of the Lnc-Ang362 was also able to reduce VSMC proliferation which is likely to occur through the downstream action of the two miRNAs. As noted earlier, these miRNAs have been shown to be involved in VSMC proliferation and the development of neointimal lesions (Liu et al. 2009).
These data highlight the importance of key lncRNAs in VSMC biology and the influence of lncRNAs in the response of VSMCs to environmental cues. Furthermore, Lnc-Ang362 is just one of the many lncRNAs that are regulated by Ang II in rat VSMCs (Leung et al. 2013) which indicates that several other unidentified lncRNAs may also be important for Ang II biology, other related growth factor actions, and ultimately the regulation of VSMC functions pertinent to CVD. Unlike miRNAs, lncRNAs display lesser conservation across species. Hence, the lncRNAs expressed in rat VSMCs must be further examined for similar expression profiles and actions in human VSMCs to determine relevance to human CVD.
3.2 Humans
Since the first study with rat VSMCs, a few studies have been performed to investigate the role of lncRNAs in human VSMCs and their potential influence on VSMC differentiation as well as the development of human vascular disease. Initial studies focused on roles of previously identified lncRNAs, including H19 (Han et al. 1996) and ANRIL (Congrains et al. 2012), on human VSMC function and atherosclerosis development. Recently, it was discovered that expression of the non-coding natural antisense transcript for hyaluronan (HA) synthase 2 (HAS2-AS1) in human atherectomy specimens correlates directly with lesion severity (Vigetti et al. 2014). HAS2-AS1 partially overlaps with HAS2 exon 1 and promoter regions, was initially identified in several tumor cell lines (Chao and Spicer 2005), and has previously been shown to stabilize HAS2 mRNA in renal proximal tubular epithelial cells (Michael et al. 2011). HAS2 is responsible for HA synthesis, and previous studies have implicated HA vascular deposition with extracellular matrix remodeling, vessel wall thickening, and neointima formation (Riessen et al. 1996; Chai et al. 2005). Because HAS2 is upregulated by O-GlcNAcylation (Vigetti et al. 2012), Vigetti et al. sought to identify a possible role for HAS2-AS1 in CVD. Interestingly, they found that HAS1-AS1 enrichment was required for HAS2 upregulation in human aortic smooth muscle cells upon O-GlcNAcylation, but not through mRNA stability as previously identified in other cell types. Rather, induction of O-GlcNAcylation caused NFκB-dependent accumulation of HAS-AS1 transcripts, which induced chromatin opening at the promoter of HAS2 allowing increased HAS2 transcription. This novel mechanism of HAS2-AS1 function in VSMCs highlights the varying physiological roles lncRNAs can have are dependent on tissues in which they are expressed.
Only within the past year have researchers forayed into the subject of human VSMC-selective lncRNAs. Bell and colleagues investigated novel lncRNAs with potential functions in human VSMCs (Bell et al. 2014). Using RNA sequencing, they first identified lncRNAs that were enriched in human coronary artery smooth muscle cells (HCASMCs). One of these lncRNAs, a multi-exonic lncRNA named SENCR, resides within the first intron of FL1 in an antisense orientation. There are two distinct isoforms of SENCR, with SENCR_V1 exhibiting much broader expression than SENCR_V2. Further analyses using high-resolution RNA FISH revealed that the transcript is cytoplasmic and depicts low levels of expression in human umbilical vein endothelial cells (HUVECs). To investigate the potential function of SENCR, Bell and colleagues knocked down the transcript using siRNAs. This knockdown of SENCR did not influence the expression of FL1, indicating that the lncRNA, unlike Lnc-Ang362, does not act in cis. To identify the function of SENCR in an unbiased manner, the investigators performed RNA sequencing of HCASMCs after SENCR knockdown. They discovered that with the reduction in SENCR transcripts, many contractile genes, including those associated with regulation of MYOCD, an important transcriptional regulator of VSMC contractile gene expression, were downregulated and cell migration genes were upregulated. Phenotypically, HCASMCs with reduced expression of SENCR displayed increase in cell migration in scratch wound and Boyden chamber assays. Therefore, these studies demonstrated that SENCR is likely to be involved in maintaining a normal, non-motile contractile phenotype in SMCs, thereby uncovering a novel lncRNA-mediated mechanism in regulating VSMC contractility.
Whereas lncRNAs can mediate normal functions including contractile gene expression in VSMCs, it is also becoming clear that aberrant levels of lncRNAs are associated with aberrant cell growth and disease progression in human cells. Below are recent examples of VSMC-expressed lncRNAs related to human vascular disease.
Apoptosis of VSMCs in the aortic media can lead to thoracic aortic aneurysms (TAA). Wang and colleagues therefore examined the expression of Brahma-related gene 1 (BRG1), a component of the SWI/SNF chromatin remodeling complex and a mediator of apoptosis in VSMC, in aortic specimens from TAA patients and found that BRG1 was expressed at significantly higher levels in TAA specimens compared to control (Wang et al. 2014). Further, overexpression of BRG1 in cultured VSMCs caused an increased rate of apoptosis, higher levels of apoptosis-promoting gene caspase 3, downregulation of anti-apoptotic gene Bcl2, and concomitant decrease in VSMC proliferation. Since BRG1 controls gene expression by altering chromatin remodeling and structure, the researchers investigated the potential that lncRNAs were serving as regulators of chromatin remodeling through BRG1. 95 apoptosis-related lncRNAs were screened for expression changes upon modulation of BRG1 levels. LncRNA HIF1-AS1 expression was modulated by changes in BRG1 levels. Knockdown of HIF1A-AS1 in VSMCs caused lower caspase 3 levels and increased Bcl2 expression, as well as increased cell proliferation rate. These data suggest that HIF1-AS1 may play a role in the pathology of TAA and VSMC dysfunction.
In addition to aneurysms, lncRNAs expressed in VSMCs can function in the development of atherosclerosis. In investigating the role of p53, Wu and colleagues characterized the potential role of a lncRNA named lincRNA-p21, a member of the p53 pathway which is known to interact with p53 repressive complex hnRNP-K to cause reduction of many p53 targets (Huarte et al. 2010; Wu et al. 2014). The authors initially examined atherosclerotic plaques from ApoE-/- mice fed a high fat diet and found reduced levels of lincRNA-p21 transcripts when compared to wild-type mice. Further, inhibition of lincRNA-p21 transcript expression increased cell proliferation, improved viability, and decreased apoptosis in both RAW264.7 mouse macrophage cell line and human VSMCs (HVSMC). Global gene expression analysis after lincRNA-p21 transcript knockdown revealed downregulation of many p53 targets, indicating a role for this lncRNA in regulating p53 activity. Indeed, RNA immunopreciptation experiments show a direct interaction between lincRNA-p21 and the p53 antagonist MDM2. Furthermore, p53-specific ChIP-seq revealed lincRNA-p21 negatively affects the recruitment of p53 to its target promoters and enhancers. Interestingly, given that lincRNA-p21 is a transcriptional target of p53 itself, these data suggest a negative feedback loop in the lincRNA-p21/p53 axis. In vivo experiments using the murine carotid artery injury model showed that injection of lincRNA-p21 siRNA-expressing vector can cause a dramatic increase in the severity of neointima formation, including intima-media thickness, increased Ki67+ prevalence, and decreased apoptosis. Consistent with in vitro studies, p300/p53 interaction was reduced, while MDM2/p53 interaction increased, causing repression of p53 target genes. The expression of lincRNA-p21 was found to be reduced by 50 % in human coronary artery tissues collected from patients suffering from coronary artery disease and atherosclerosis demonstrating relevance to human disease (Wu et al. 2014).
4 Conclusions
LncRNAs have been in the forefront of molecular biology in recent years due to their numerous biological functions and potential as novel therapeutic targets. As such, we have begun to understand their molecular functions and how these processes affect the development of disease. VSMCs are important cells that mediate normal vascular processes as well as the development of vascular diseases such as restenosis, hypertension, and atherosclerosis. As highlighted in this review, lncRNAs are novel mediators of normal VSMC processes such as contraction and migration, as well as in VSMC dysfunction in response to pathophysiological stimuli such as Ang II. We are also beginning to learn that abnormal lncRNA expression can be associated with human vascular disease, implying that lncRNAs are perhaps mediating the development and onset of CVDs. There is a flurry of recent reports demonstrating the involvement of several lncRNAs in cardiac hypertrophy, heart failure, and heart functions (Klattenhoff et al. 2013; Grote et al. 2013; Ishii et al. 2006; Han et al. 2014) which are not discussed in this review.
Future investigations into the role of lncRNAs expressed in human diseased vascular tissue biopsies have the potential to illuminate new VSMC-specific, lncRNA-based biomarkers and mechanisms that could someday be translated into new treatment options for CVD. Furthermore, examination of single nucleotide polymorphisms (SNPs) within or near lncRNA genomic sites can potentially provide important genetic information to complement data emanating from genome-wide association studies of human CVDs. This is because such SNPs can alter the biological and epigenetic mechanisms of actions of these non-coding RNAs to influence the expression of disease-related genes.
The study of lncRNAs in VSMCs is still in its infancy, and many questions remain as to the degree by which these transcripts affect VSMC function and whether they can be effective therapeutic targets in the treatment of diseases associated with Ang II or growth factors, VSMC dysfunction, and CVDs. However, with the characterization of additional lncRNAs in VSMCs and those related to CVDs, at the very least, they may be used as biomarkers for clinical diagnosis and prognosis. Recently, there have been approaches to effectively modulate lncRNA levels by various chemical approaches in vitro that can be extrapolated to in vivo models (Kato and Natarajan 2014; Kataoka and Wang 2014). Some antisense oligonucleotide-based techniques have been able to modulate lncRNA levels in mouse models (Wheeler et al. 2012), pointing toward a potential therapeutic strategy or experimental technique for studying lncRNAs in vivo. Overall, lncRNA research, while still in the early stages, represents a fast moving and novel area of investigation, and future studies will further illuminate our understanding of VSMC biology that could in turn help exploit these intriguing molecules for therapeutic purposes.
Abbreviations
- VSMCs:
-
Vascular smooth muscle cells
- Ang II:
-
Angiotensin II
- lncRNA:
-
Long non-coding RNA
- miRNA:
-
microRNA
- CVDs:
-
Cardiovascular diseases
- HCASMCs:
-
Human coronary artery smooth muscle cells.
References
Arasu P, Wightman B, Ruvkun G (1991) Temporal regulation of lin-14 by the antagonistic action of two other heterochronic genes, lin-4 and lin-28. Genes Develop 5(10):1825–1833
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233. doi:10.1016/j.cell.2009.01.002
Bell RD, Long X, Lin M, Bergmann JH, Nanda V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D, Miano JM (2014) Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol 34(6):1249–1259. doi:10.1161/ATVBAHA.114.303240
Berk BC, Corson MA (1997) Angiotensin II signal transduction in vascular smooth muscle: role of tyrosine kinases. Circ Res 80(5):607–616
Brasier AR, Recinos A 3rd, Eledrisi MS (2002) Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22(8):1257–1266
Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF (1991) A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349:38–44
Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, Rinn JL (2011) Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Develop 25(18):1915–1927. doi:10.1101/gad.17446611
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I (2011) A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147(2):358–369. doi:10.1016/j.cell.2011.09.028
Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen LM, Wogensen L (2005) Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis (Circulation research). Circ Res 96(5):583–591. doi:10.1161/01.RES.0000158963.37132.8b
Chao H, Spicer AP (2005) Natural antisense mRNAs to hyaluronan synthase 2 inhibit hyaluronan biosynthesis and cell proliferation. J Biol Chem 280(30):27513–27522. doi:10.1074/jbc.M411544200
Congrains A, Kamide K, Katsuya T, Yasuda O, Oguro R, Yamamoto K, Ohishi M, Rakugi H (2012) CVD-associated non-coding RNA, ANRIL, modulates expression of atherogenic pathways in VSMC. Biochem Biophys Res Commun 419(4):612–616. doi:10.1016/j.bbrc.2012.02.050
Consortium EP, Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaoz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Loytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, Program NCS, Baylor College of Medicine Human Genome Sequencing C, Washington University Genome Sequencing C, Broad I, Children’s Hospital Oakland Research I, Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Shahab A, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Zhang X, Xu M, Haidar JN, Yu Y, Ruan Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrimsdottir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, de Jong PJ (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447(7146):799–816. doi:10.1038/nature05874
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460(7256):705–710. doi:10.1038/nature08195
Froberg JE, Yang L, Lee JT (2013) Guided by RNAs: X-inactivation as a model for lncRNA function. J Mol Biol 425(19):3698–3706. doi:10.1016/j.jmb.2013.06.031
Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A, Macura K, Blass G, Kellis M, Werber M, Herrmann BG (2013) The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Develop Cell 24(2):206–214. doi:10.1016/j.devcel.2012.12.012
Guttman M, Garber M, Levin JZ, Donaghey J, Robinson J, Adiconis X, Fan L, Koziol MJ, Gnirke A, Nusbaum C, Rinn JL, Lander ES, Regev A (2010) Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol 28(5):503–510. doi:10.1038/nbt.1633
Han DK, Khaing ZZ, Pollock RA, Haudenschild CC, Liau G (1996) H19, a marker of developmental transition, is reexpressed in human atherosclerotic plaques and is regulated by the insulin family of growth factors in cultured rabbit smooth muscle cells. J Clin Invest 97(5):1276–1285. doi:10.1172/jci118543
Han P, Li W, Lin CH, Yang J, Shang C, Nurnberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien HC, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HS, Quertermous T, Chang CP (2014) A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514(7520):102–106. doi:10.1038/nature13596
Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL (2010) A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142(3):409–419. doi:10.1016/j.cell.2010.06.040
Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, Saito S, Nakamura Y, Tanaka T (2006) Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 51(12):1087–1099. doi:10.1007/s10038-006-0070-9
Jin W, Reddy MA, Chen Z, Putta S, Lanting L, Kato M, Park JT, Chandra M, Wang C, Tangirala RK, Natarajan R (2012) Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells. J Biol Chem 287(19):15672–15683. doi:10.1074/jbc.M111.322669
Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR (2007) RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 316(5830):1484–1488. doi:10.1126/science.1138341
Kataoka M, Wang DZ (2014) Non-coding RNAs including miRNAs and lncRNAs in cardiovascular biology and disease. Cells 3(3):883–898. doi:10.3390/cells3030883
Kato M, Natarajan R (2014) Diabetic nephropathy–emerging epigenetic mechanisms. Nat Rev Nephrol 10(9):517–530. doi:10.1038/nrneph.2014.116
Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, Rinn JL (2009) Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Nat Acad Sci USA 106(28):11667–11672. doi:10.1073/pnas.0904715106
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA (2013) Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152(3):570–583. doi:10.1016/j.cell.2013.01.003
Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, Lee CS, Flockhart RJ, Groff AF, Chow J, Johnston D, Kim GE, Spitale RC, Flynn RA, Zheng GX, Aiyer S, Raj A, Rinn JL, Chang HY, Khavari PA (2013) Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 493(7431):231–235. doi:10.1038/nature11661
Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB (2012) The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 95(2):194–204. doi:10.1093/cvr/cvs135
Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, Lee CY, Watt A, Grossman TR, Rosenfeld MG, Evans RM, Glass CK (2013) Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 498(7455):511–515. doi:10.1038/nature12209
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75(5):843–854
Leung A, Natarajan R (2014) Noncoding RNAs in vascular disease. Curr Opin Cardiol 29(3):199–206. doi:10.1097/HCO.0000000000000054
Leung A, Trac C, Jin W, Lanting L, Akbany A, Saetrom P, Schones DE, Natarajan R (2013) Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res 113(3):266–278. doi:10.1161/CIRCRESAHA.112.300849
Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, Oh S, Kim HS, Glass CK, Rosenfeld MG (2013) Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498(7455):516–520. doi:10.1038/nature12210
Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C (2009) A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 104(4):476–487. doi:10.1161/CIRCRESAHA.108.185363
Mack CP (2011) Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 31(7):1495–1505. doi:10.1161/ATVBAHA.110.221135
Maegdefessel L, Rayner KJ, Leeper NJ (2015) MicroRNA regulation of vascular smooth muscle function and phenotype: early career committee contribution. Arterioscler Thromb Vasc Biol 35(1):2–6. doi:10.1161/ATVBAHA.114.304877
Mehta PK, Griendling KK (2007) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Amer J Physiol Cell Physiol 292(1):C82–C97. doi:10.1152/ajpcell.00287.2006
Melo CA, Drost J, Wijchers PJ, van de Werken H, de Wit E, Oude Vrielink JA, Elkon R, Melo SA, Leveille N, Kalluri R, de Laat W, Agami R (2013) eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol Cell 49(3):524–535. doi:10.1016/j.molcel.2012.11.021
Michael DR, Phillips AO, Krupa A, Martin J, Redman JE, Altaher A, Neville RD, Webber J, Kim MY, Bowen T (2011) The human hyaluronan synthase 2 (HAS2) gene and its natural antisense RNA exhibit coordinated expression in the renal proximal tubular epithelial cell. J Biol Chem 286(22):19523–19532. doi:10.1074/jbc.M111.233916
Moran VA, Perera RJ, Khalil AM (2012) Emerging functional and mechanistic paradigms of mammalian long non-coding RNAs. Nucl Acids Res 40(14):6391–6400. doi:10.1093/nar/gks296
Morris KV, Santoso S, Turner AM, Pastori C, Hawkins PG (2008) Bidirectional transcription directs both transcriptional gene activation and suppression in human cells. PLoS Genet 4(11):e1000258. doi:10.1371/journal.pgen.1000258
Orom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q, Guigo R, Shiekhattar R (2010) Long noncoding RNAs with enhancer-like function in human cells. Cell 143(1):46–58. doi:10.1016/j.cell.2010.09.001
Owens GK (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75(3):487–517
Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N (1996) Requirement for Xist in X chromosome inactivation. Nature 379(6561):131–137. doi:10.1038/379131a0
Reddy MA, Jin W, Villeneuve L, Wang M, Lanting L, Todorov I, Kato M, Natarajan R (2012) Pro-inflammatory role of microrna-200 in vascular smooth muscle cells from diabetic mice. Arterioscler Thromb Vasc Biol 32(3):721–729. doi:10.1161/ATVBAHA.111.241109
Riessen R, Wight TN, Pastore C, Henley C, Isner JM (1996) Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation 93(6):1141–1147
Small EM, Olson EN (2011) Pervasive roles of microRNAs in cardiovascular biology. Nature 469(7330):336–342. doi:10.1038/nature09783
Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329(5992):689–693. doi:10.1126/science.1192002
Vigetti D, Deleonibus S, Moretto P, Bowen T, Fischer JW, Grandoch M, Oberhuber A, Love DC, Hanover JA, Cinquetti R, Karousou E, Viola M, D’Angelo ML, Hascall VC, De Luca G, Passi A (2014) Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J Biol Chem 289(42):28816–28826. doi:10.1074/jbc.M114.597401
Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M, Bartolini B, Hascall VC, Tammi M, De Luca G, Passi A (2012) Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J Biol Chem 287(42):35544–35555. doi:10.1074/jbc.M112.402347
Villeneuve LM, Kato M, Reddy MA, Wang M, Lanting L, Natarajan R (2010) Enhanced levels of microRNA-125b in vascular smooth muscle cells of diabetic db/db mice lead to increased inflammatory gene expression by targeting the histone methyltransferase Suv39h1. Diabetes 59(11):2904–2915. doi:10.2337/db10-0208
Wang S, Zhang X, Yuan Y, Tan M, Zhang L, Xue X, Yan Y, Han L, Xu Z (2014) BRG1 expression is increased in thoracic aortic aneurysms and regulates proliferation and apoptosis of vascular smooth muscle cells through the long non-coding RNA HIF1A-AS1 in vitro. Eur J Cardiothorac Surg Off J Eur Assoc Cardiothorac Surg. doi:10.1093/ejcts/ezu215
Wapinski O, Chang HY (2011) Long noncoding RNAs and human disease. Trends Cell Biol 21(6):354–361. doi:10.1016/j.tcb.2011.04.001
Wightman B, Burglin TR, Gatto J, Arasu P, Ruvkun G (1991) Negative regulatory sequences in the lin-14 3′-untranslated region are necessary to generate a temporal switch during Caenorhabditis elegans development. Genes Develop 5(10):1813–1824
Wheeler TM, Leger AJ, Pandey SK, MacLeod AR, Nakamori M, Cheng SH, Wentworth BM, Bennett CF, Thornton CA (2012) Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature 488(7409):111–115. doi:10.1038/nature11362
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, Zeng C (2014) LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 130(17):1452–1465. doi:10.1161/CIRCULATIONAHA.114.011675
Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, Cui H (2008) Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature 451(7175):202–206. doi:10.1038/nature06468
Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322(5902):750–756. doi:10.1126/science.1163045
Acknowledgments
We gratefully acknowledge funding from the National Institutes of Health, NHLBI and NIDDK (R01 HL106089 (RN), R01 DK 065073 (RN), T32DK007571 (to AL), and K01 DK104993 (AL)). The authors thank Dustin Schones for critically reading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Leung, A., Stapleton, K., Natarajan, R. (2015). Functional Long Non-coding RNAs in Vascular Smooth Muscle Cells. In: Morris, K. (eds) Long Non-coding RNAs in Human Disease. Current Topics in Microbiology and Immunology, vol 394. Springer, Cham. https://doi.org/10.1007/82_2015_441
Download citation
DOI: https://doi.org/10.1007/82_2015_441
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23906-4
Online ISBN: 978-3-319-23907-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)