Abstract
The development of CD4+ helper and CD8+ cytotoxic T-cells expressing the αβ form of the T-cell receptor (αβTCR) takes place in the thymus, a primary lymphoid organ containing distinct cortical and medullary microenvironments. While the cortex represents a site of early T-cell precursor development, and the positive selection of CD4+8+ thymocytes, the thymic medulla plays a key role in tolerance induction, ensuring that thymic emigrants are purged of autoreactive αβTCR specificities. In recent years, advances have been made in understanding the development and function of thymic medullary epithelial cells, most notably the subset defined by expression of the Autoimmune Regulator (Aire) gene. Here, we summarize current knowledge of the developmental mechanisms regulating thymus medulla development, and examine the role of the thymus medulla in recessive (negative selection) and dominant (T-regulatory cell) tolerance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Single Positive
- Lymphoid Tissue Inducer
- Thymic Medulla
- Thymic Microenvironment
- Lymphoid Tissue Inducer Cell
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
T-cells bearing the alpha–beta T-cell receptor complex (αβTCR) represent a critical cellular component of immune responses aimed at targeting a wide range of pathogens including bacteria and viruses. The development of αβT-cells occurs within the thymus, a process that is initiated following the entry of blood-borne lymphoid progenitors originating from the fetal liver or bone marrow (Anderson et al. 2007; Takahama 2006). Intrathymic T-cell development is a complex process, and involves a series of steps including T-cell commitment, proliferation, differentiation, selection, and migration. To accommodate this developmental program, the thymus consists of distinct T-cellular microenvironments in which thymocytes at particular developmental stages are housed. For example, immature T-cell precursors defined by their lack of expression of CD4 and CD8 are enriched in the subcapsular region, while their CD4+8+ progeny, representing the bulk of thymocytes, reside within the thymic cortex. In contrast, the thymus medulla provides a microenvironment for the most mature single positive (SP) CD4+ and CD8+ cells expressing high levels of the αβTCR. Importantly, these major regions of the thymus are further defined by the phenotypically and functionally distinct stromal cells that are contained within them, including cortical thymic epithelium (cTEC) and medullary thymic epithelium (mTEC) (Alves et al. 2009).
Current models of thymic function are based upon the idea that an ordered process of T-cell development occurs as a result of the sequential migration of developing thymocytes through these distinct stromal microenvironments, ensuring that they receive important signals and cell–cell interactions in an appropriate order and context (Petrie and Zuniga-Pflucker 2007). The primary aim of this review is to discuss the role of the thymus medulla in αβT-cell development. In particular, we will summarize the current knowledge of the cellular and molecular interactions that lead to thymic medulla formation, focusing on the processes involving maturation of mTEC. In addition, we will examine how thymic medullary environments contribute to both deletional and dominant self-tolerance mechanisms that operate upon the newly positively selected αβTCR repertoire.
2 Cellular Features of the Thymic Medulla
2.1 Medullary Hemopoietic Non-T Lineage Cells
While the major hemopoietic compartment of the thymic medulla consists of CD4+ and CD8+ αβTCRhi thymocytes generated as a result of positive selection in the thymic cortex, it also contains a variety of hemopoietic accessory cells that are linked to its function. Notably, thymic dendritic cells (tDC) are enriched in medullary areas and at the surrounding cortico-medullary junction. Given the role of tDC in purging the positively selected repertoire of potentially autoreactive specificities, such positioning is likely to be of importance in the screening of newly selected cells as they migrate from the cortex into the medulla. Interestingly, tDC are heterogeneous, suggestive of differing roles in thymocyte differentiation. Thus, in the adult thymus, three phenotypically distinct tDC subsets have been identified, namely plasmacytoid DC (pDC), and two subsets of conventional DC (cDC) that can be defined by CD8αlowCD11b+SIRPα+ and CD8αhighCD11b−SIRPα− phenotypes. Interestingly, these distinct tDC subsets have distinct developmental origins—while SIRPα− tDC are generated intrathymically from immature progenitors, both SIRPα+ tDC and pDC are recruited to the thymus from the periphery. Despite the known heterogeneity of tDC in the thymus, relatively little is known about their anatomical location and positioning, and the long-held view is that their location is limited to the medulla and surrounding cortico-medullary junction. Interestingly however, a study recently showed that despite the presence of abundant medullary-resident CD11c+ tDC, SIRPα+ tDC were notably absent from the medulla, and instead could be detected within thymic cortical regions, often in association with small vessels (Baba et al. 2009). Indeed, two-photon microscopy of explanted thymic tissue demonstrated the formation of interactions between thymocytes and tDC within the thymic cortex, again at regions containing capillaries (Ladi et al. 2008). Collectively, such observations argue against the notion that tDC are restricted to medullary regions and instead suggest that distinct tDC subsets can be specifically positioned within particular regions of the thymus, including the cortex. Moreover, multiple chemokine receptors have been highlighted in relation to tDC recruitment and positioning, including CCR2 (Baba et al. 2009), CCR7 (Ladi et al. 2008), CCR9 (Hadeiba et al. 2012), and XCR1 (Lei et al. 2011), suggesting that chemokine production from distinct intrathymic microenvironments is important in the context of tDC location and function.
While tDC are important mediators of intrathymic negative selection of autoreactive thymocytes, other hemopoietic accessory cells are directly linked to the development of thymic medullary microenvironments. In particular, Lymphoid Tissue inducer (LTi) cells are present within thymic medullary regions, and through their provision of TNFSF ligands such as RANKL, have been shown to stimulate the maturation of RANK+ mTEC progenitors (Rossi et al. 2007). Perhaps importantly, LTi cells, first reported as essential mediators of lymph node (LN) organogenesis in the embryonic period (Cupedo et al. 2002), are found in both the fetal and adult thymus in close association with mTEC. Moreover, analysis of LTi-deficient RORγ−/− mice at embryonic stages prior to the emergence of positively selected αβTCRhi thymocytes has provided direct evidence that LTi cells are key to the generation of the first cohorts of Aire+ mTEC (White et al. 2008), the development of which represents a critical step in the establishment of T-cell tolerance in the neonatal period (Guerau-de-Arellano et al. 2009). Unlike their well-documented role in fetal thymus, ascribing a specific role to LTi in the adult thymus has been difficult, particularly since mTEC abnormalities in RORγ-deficient mice could also be explained by defective αβT-cell development. However, a recent study (Dudakov et al. 2012) showed a link between LTi and regeneration of the adult thymus following experimental ablation. Thus, irradiation-induced thymic atrophy resulted in the enhanced production of IL-22 by RORγt+CCR6+NKp46− LTi cells, with IL-22 then operating directly on thymic epithelial compartments to promote their expansion. Given that the mTEC lineage can be separated into distinct developmental stages (Dooley et al. 2008; Gabler et al. 2007; Nishikawa et al. 2010; Rossi et al. 2007), while stages in the cTEC lineage have also been described (Nowell et al. 2011; Ripen et al. 2011; Shakib et al. 2009), it will be interesting to determine whether IL-22 exerts its effect on immature or mature TEC populations, or both. Finally, although thymic LTi have been shown to have shared a common RORγt+CD4+IL7Rα+RANKL+ phenotype with LTi in peripheral lymphoid tissues (Anderson et al. 2007), it is currently unclear whether thymus and LN harbor tissue-specific LTi subsets, or whether LTi populations are capable of trafficking between these tissues.
2.2 Medullary Thymic Epithelial Cells
Immunohistological analysis of thymic microenvironments is a widely used approach with which to dissect the cellular complexity of cortical and medullary areas, enabling the phenotypic definition of stromal compartments in both areas, most notably thymic epithelial cells (TEC). Tissue sections of adult thymus often show individual medullary regions embedded within a cortical matrix, although it is important to note that the thymus medulla as a whole represents a complex structure with seemingly separate medullary areas actually joined by interconnecting branches (Anderson et al. 2000). While individual medullary areas have been shown to occur as a result of the expansion and differentiation of single mTEC progenitors (Rodewald et al. 2001), it is not clear how the complex three-dimensional organization of the thymic medulla is controlled, although thymic vasculature has been proposed to play a role (Anderson et al. 2000).
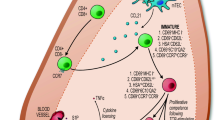
The cTEC and mTEC compartments are identified by both shared and lineage restricted molecules (Fig. 1). Many of the reagents that are used to define TEC immunohistologically, in addition to the pan-epithelial marker EpCAM1 (Nelson et al. 1996), recognize cytokeratin family members, structural proteins that reflect the differing morphology of cTEC and mTEC compartments (Farr and Braddy 1989). Thus, unlike their cTEC counterparts, mTEC are often defined by expression of cytokeratin-5 and cytokeratin-14, and lack of expression of cytokeratin-8/18 (Klug et al. 1998, 2002). In addition, antibodies that recognize unknown molecules expressed by mTEC include ERTR5 (Van Vliet et al. 1984) and MTS10 (Godfrey et al. 1990), while the fucose binding lectins Tetragonolobus Purpureas Agglutinin (TPA), and Ulex Europeus Agglutinin (UEA) also demonstrate selective reactivity with the thymic medulla in tissue sections (Farr and Anderson 1985). However, it is not entirely clear from this type of tissue section analysis whether such reagents reflect ‘pan-mTEC’ markers that react with the whole mTEC compartment, or whether distinct mTEC subsets exist. Perhaps importantly, immunohistochemical analysis of the mTEC compartment can be further complemented by flow cytometric analysis of enzymatically disaggregated thymus preparations. Although analysis of TEC compartments following enzymatic digestion can be limited by the sensitivity of cell surface molecules (Izon et al. 1994; Seach et al. 2012), a panel of markers has emerged that is now widely used in association with enzymatic digestion. Thus, total TEC are frequently defined as CD45−EpCAM1+, which can be further subdivided on the basis of cell surface expression of Ly51, enabling the discrimination of Ly51+ cTEC and Ly51− mTEC compartments. Within the mTEC lineage, an additional panel of molecules including CD40, CD80, MHC class II, and Aire reveal distinct subsets including CD80−MHCIIlow and CD80+MHCIIhi cells, often referred to as mTEClow and mTEChi (Derbinski et al. 2001; Gray et al. 2006; Hubert et al. 2008; Rossi et al. 2007). Until recently, the relevance of phenotypically distinct mTEC subsets was not clear. However, many studies now show that mTEC represent a dynamic thymic compartment, that can be defined by precursor-product relationships with a turnover time of 2–3 weeks for the mature mTEC population (Gabler et al. 2007). Functional analysis of the developmental relationships of distinct mTEC subsets will be discussed in Sect. 3.
Shared and lineage restricted markers of cortical and medullary thymic epithelial cells. Panels of markers used in both immunohistochemical and flow cytometric analysis are frequently used to study cTEC and mTEC lineages. While some molecules are common to both, others enable the discrimination of these discrete lineages. However, it is important to note that it is currently unclear how expression of these markers relates to distinct immature progenitors and mature stages within TEC lineages
2.3 Non-epithelial Mesenchymal Stroma
The thymus is an epithelial–mesenchymal tissue, and during early stages of thymus organogenesis, the inner epithelial rudiment is surrounded by a layer of mesenchyme derived from the neural crest (Manley and Blackburn 2003; Rodewald 2008). Within the adult thymus, several cell fate-mapping studies have now shown that much of the mesenchyme present is of neural crest origin, where it is associated with epithelium and the endothelium of the thymus vasculature (Foster et al. 2008; Muller et al. 2008; Yamazaki et al. 2005). During thymus development, mesenchymal cells that form the thymic capsule penetrate the epithelial core, separating it into lobules via trabeculae. In addition to its mesenchymal components, vascularization of the developing thymus occurs after anlage formation, culminating in a complex network of both blood and lymphatic vessels (Odaka et al. 2006) that are composed of perivascular cells and endothelium. Thus, a panel of markers including smooth muscle actin, ERTR7, and desmin has been used to define histological organization of non-epithelial medulla stroma (Odaka 2009), while flow cytometric analysis using the markers podoplanin, Ly51, and PDGFRα reveals complex heterogeneity in mesenchymal subsets (Jenkinson et al. 2007; Muller et al. 2005). While further analysis of the functional importance of these distinct compartments is required, it is interesting to note that thymic mesenchyme can act as both positive and negative regulators of TEC expansion, through their control of the Retinoic Acid and Fibroblast Growth Factor pathways (Jenkinson et al. 2003; Sitnik et al. 2012).
The corticomedullary junction (CMJ) represents an important area with respect to vasculature, with both the entry of lymphoid progenitors and the exit of mature thymocytes taking place at this site (Porritt et al. 2003). Indeed, the perivascular spaces of blood vessels at the CMJ contain c-Kit+ T-cell precursors and CD4+ and CD8+ thymocytes (Mori et al. 2007), with neural crest derived pericytes controlling the emigration of the latter via their production of sphingosine-1-phosphate (S1P), a ligand for sphingosine-1-phosphate receptor-1 (S1PR1) expressed by mature thymocytes (Zachariah and Cyster 2010). Additionally, a non-epithelial conduit system has been identified in human thymus, which represents a network of inter-connecting tubules containing multiple basement membrane components including laminin-5, collagen type IV and perlecan (Drumea-Mirancea et al. 2006). Interestingly, such a network is reminiscent of the conduit system present within the T-zone of the lymph node and spleen, further highlighting the similarities between the thymic medulla and compartments within secondary lymphoid tissues (Derbinski and Kyewski 2005). While the functional importance of the thymic medullary conduit system remains unclear, its diameter appears too small to enable transport of cells (Drumea-Mirancea et al. 2006), leaving open the possibility that by acting as a transport network for small molecules such as antigen and chemokines, it plays a role in medullary thymocyte migration and tolerance induction.
3 Development of Thymic Medullary Epithelium
3.1 Defining mTEC Progenitors
Although the mTEC compartment has been shown to share a common bipotent progenitor with the cTEC (Bleul et al. 2006; Rossi et al. 2006), relatively little is known about the mechanisms controlling the emergence of cells that are committed to the mTEC lineage from this progenitor pool. Recently however the possible role of FoxN1, a transcription factor representing a master regulator of TEC differentiation (Blackburn et al. 1996; Nehls et al. 1994, 1996), has been evaluated through analysis of TEC development in FoxN1-deficient nude mice and a panel of mice expressing FoxN1 at varying levels (Nowell et al. 2011). Interestingly, these findings suggested that the mTEC lineage might emerge from the bipotent TEC progenitor stage via a mechanism occurring independently of FoxN1. Given that bipotent TEC progenitors persist within the FoxN1-deficient thymus rudiment at least until the postnatal stages (Bleul et al. 2006), these findings suggest that FoxN1 may be selectively required downstream of the emergence of mTEC progenitors, perhaps through controlling their survival as well as differentiation.
The first data demonstrating the existence of mTEC committed progenitors involved functional clonal analyses in the absence of a defined phenotype (Rodewald et al. 2001). Subsequent attempts to define and then directly isolate mTEC committed progenitors have often relied upon use of markers typically associated with the mature mTEC lineage in the context of the developing embryonic thymus, so the accurate phenotype of these cells, and the separation of immature and mature mTEC remains obscure. For example, claudin-3 and claudin-4, tight junction components expressed by mTEC in the adult thymus, have been shown to identify TEC within the early thymus anlage that are also reactive with the mTEC markers MTS10 and UEA1 (Hamazaki et al. 2007). Perhaps most importantly, purified Claudin3/4hi embryonic TEC were shown to give rise to mature Aire+ mTEC in precursor-product experiments involving reaggregate thymus organ cultures (RTOC), providing the first phenotypic definition of mTEC progenitors (Hamazaki et al. 2007). In other studies, analysis of the mTEC compartment using CD80 and MHCII expression showed that during embryonic thymus development, CD80−MHCIIlow ‘mTEClow’ cells appear prior to the emergence of CD80+MHCIIhi ‘mTEChi’ cells, suggesting a possible precursor-product relationship between these populations (Gabler et al. 2007; Rossi et al. 2007). Importantly, direct analysis of mTEC development using RTOC experiments demonstrated that mTEClow were able to give rise to their more mature mTEChi counterparts, including the subset expressing Aire (Gabler et al. 2007; Rossi et al. 2007). Further, mTEClow and mTEChi subsets are also present in the adult thymus (Gray et al. 2006), with BrdU labeling experiments providing evidence of the continued generation of mTEChi from mTEClo cells in the postnatal thymus, with a turnover time of 2–3 weeks for mTEChi cells (Gabler et al. 2007). Collectively, these studies were important as they highlighted distinct developmental stages within mTEC, and provided direct indications that the epithelial component of the thymus medulla represents a dynamic cellular microenvironment undergoing constant renewal. Importantly however, it is perhaps important to note that precursor-product analysis of mTEC has frequently focused on events that culminate in generation of the Aire+ subset. Thus, it remains possible that other mature mTEC subsets exist that are not linked to the same Aire-expressing pathway, and which could be generated via a separate mTEC progenitor pool. Whether such a subset resides within the mTEClow population requires a more detailed phenotypic and functional analysis of these poorly defined cells.
3.2 Cellular and Molecular Regulation of the mTEC Compartment
A normal program of T-cell development and selection depends upon sequential interactions between thymocytes and stromal cells in the cortex and then the medulla. Importantly, studies in the late 1980s provided the first indications that growth and formation of the thymic medulla was, in turn, influenced by developing thymocytes. For example, analysis of thymic microenvironments following disruption of thymic hemopoietic compartments by either irradiation (Adkins et al. 1988) or treatment with the immunosuppressant cyclosporin A (Kanariou et al. 1989) was shown to have a dramatic, and reversible, impact on mTEC. Critically, subsequent experiments showed that the transplantation of WT hemopoietic progenitors into SCID mice corrected their severely disorganized thymic epithelial microenvironments (Shores et al. 1991), providing the first evidence that signals from hemopoietic cells directly influenced thymic epithelial cell development. Other studies showed that peripheral T-cells (Surh et al. 1992) and SP thymocytes could also regulate the mTEC compartment, a process requiring αβTCR expression (Palmer et al. 1993; Shores et al. 1994). Such observations were collectively described as a ‘thymus crosstalk’ process, (van Ewijk et al. 1994), during which interaction with, and signals from, developing thymocytes are required for the formation of thymic epithelial microenvironments.
Although the studies above provided information on the cellular source of the molecules that promote mTEC development and medullary growth, the nature of the signals provided by developing thymocytes and/or additional hemopoietic cells was, until recently poorly understood. However, several studies had noted that mice harboring mutations in several genes critical in the NF-κB signaling, including TRAF6 (Akiyama et al. 2005), NIK (Kajiura et al. 2004), and RelB (Burkly et al. 1995; Heino et al. 2000; Weih et al. 1995; Zuklys et al. 2000) displayed mTEC abnormalities. Such phenotypes often included reduced/absent Aire expression and a failure to establish T-cell tolerance, suggesting that cell surface receptors expressed by mTEC that utilize the NF-kB signaling cascade could be critical molecular components of thymus medulla crosstalk. Interestingly, the development of secondary lymphoid tissues is known to involve multiple members of the Tumor Necrosis Factor Receptor SuperFamily (TNFRSF) (Weih and Caamano 2003), whose ligands are expressed by lymphoid cells, raising the possibility that a similar axis might also be involved in formation of medullary thymic microenvironments (Anderson et al. 2007; Derbinski and Kyewski 2005). Indeed, many studies have now shown the expression of various TNFRSF members by mTEC, some of which have been shown to play a direct role during mTEC development. Of these, Lymphotoxinβ Receptor (LTβR, TNFRSF3), CD40 (TNFRSF5), and RANK (TNFRSF11a) remain the best studied. For example, studies on LTβR−/− mice have demonstrated a reduction in mTEC numbers and medullary disorganization, which is associated with abnormalities in thymocyte emigration and autoimmunity. Importantly, although initial studies (Chin et al. 2003) suggested that the LT-LTβR axis was linked to the generation of Aire-expressing mTEC, other studies showed this not to be the case (Martins et al. 2008; Venanzi et al. 2007). Rather, LTβR appears to be involved in mechanisms controlling the expression of Aire-independent Tissue Restricted Antigens (TRAs), as well as the chemokines CCL19 and CCL21 (Chin et al. 2006; Seach et al. 2008; Zhu et al. 2007). Importantly however, as well as being expressed by mTEC, LTβR is also detectable within cTEC and MTS15+ fibroblasts (Hikosaka et al. 2008; Seach et al. 2008). So, it remains unclear which features of LTβR deficiency are a direct result of absence of LTβR expression by mTEC, or whether abnormalities can occur indirectly as a result of absence of expression in other thymic stromal compartments. In relation to the involvement of LTβR in thymocyte-TEC crosstalk, several studies now show that LTα and LTβ are expressed by mature SP thymocytes as compared to their CD4+8+ precursors (Boehm et al. 2003; White et al. 2008), indicating a crosstalk process involving positively selected thymocytes. Interestingly however, the absence of LIGHT, an additional LTβR ligand does not appear to play a role in mTEC development. Moreover, mTEC abnormalities in LTβR−/− mice are more severe as compared to LTβ−/−LIGHT−/− double deficient mice (Boehm et al. 2003), perhaps suggesting additional unknown ligands for LTβR that are expressed by thymocytes and which operate during mTEC development.
Both CD40 and RANK have been shown to play key roles in the generation of the Aire+ subset of mTEC. While CD40 is expressed by both cTEC and mTEC, RANK expression is higher in the latter (Hikosaka et al. 2008; Rossi et al. 2007; Shakib et al. 2009). Moreover, absence of RANK expression leads to a dramatic reduction in the frequency of Aire+ mTEC, in both the fetal and adult thymus, with combined RANK/CD40 deficiency in the adult reducing this mTEC compartment further (Akiyama et al. 2008; Rossi et al. 2007). RANK deficiency and RANK/CD40 double deficiency also results in the onset of T-cell mediated autoimmunity, highlighting the importance of these TNFRSF molecules during intrathymic tolerance induction (Akiyama et al. 2008; Rossi et al. 2007). Several studies have investigated the cellular sources of RANKL and CD40L in relation to thymocyte crosstalk and thymic medulla formation, in both the fetal and adult thymus (Anderson and Takahama 2012). In the fetal thymus, we showed that RANKL expression maps to subsets of cells belonging to the innate immune system, including RORγt+ Lymphoid Tissue Inducer (LTI) cells (Rossi et al. 2007), and invariant Vγ5+TCR dendritic epidermal T-cell progenitors (Roberts et al. 2012). Interestingly, the involvement of the innate immune system during thymus medulla formation is active at developmental stages prior to positive selection of the αβTCR repertoire (White et al. 2008), meaning that the crosstalk processes in the fetal and adult thymus medulla are distinct. Given the importance of Aire expression during neonatal tolerance (Guerau-de-Arellano et al. 2009), these findings suggest a scenario in which the innate immune system helps in the control of tolerance induction of the nascent TCR repertoire by ensuring efficient generation of mTEC compartments in the embryo.
In the adult thymus, as with LTβR ligands, positively selected thymocytes in particular CD4+8− cells, act as sources of RANKL and CD40L (Hikosaka et al. 2008; Irla et al. 2008). Relevant to this, we have recently shown (Desanti et al. 2012) that RANKL and CD40L expression map to different subsets and developmental stages within the intrathymic CD4+8− compartment. Thus, recently selected CD69+CD4+8− cells are enriched for RANKL+ cells, while CD40L expression is linked to more mature CD69−CD4+8− thymocytes. Moreover, FoxP3+ Regulatory T-cells present in the thymus express RANKL but not CD40L, demonstrating that thymocyte crosstalk in the development of the mTEC compartment involves distinct interactions with multiple CD4+8− subsets.
While the above studies highlight cellular and molecular control involving the generation of Aire+ mTEC, and although the CD80+ mTEC subset which contains Aire expressing cells (Gray et al. 2006), have a turnover time of 2–3 weeks (Gabler et al. 2007), less is known about events occurring during late stage mTEC development. Indeed, uncertainty exists regarding possible stages of mTEC development post-Aire expression, and the role of Aire itself during mTEC development. Several models have been put forward to explain mTEC developmental programmes in relation to the timing of Aire expression. For example, based on cell fate mapping studies utilizing Aire-Cre transgenic mice, Aire+CD80+ mTEC were shown to progress to an Aire−CD80low stage (Nishikawa et al. 2010), suggestive of mTEC maturation post-Aire expression, and arguing against the idea that Aire directly promotes mTEC apoptosis (Gray et al. 2007). Interestingly, other studies described a small subset of Aire− mTEC that expressed involucrin, a marker of both keratinocyte terminal differentiation and Hassalls Corpuscles, concentric whorls of cells thought to represent end-stage medullary epithelium (Yano et al. 2008). Given that the frequency of involucrin+ mTEC are reduced in the Aire−/− thymus (Yano et al. 2008), such observations support the ‘Terminal Differentiation’ model of mTEC development, in which Aire plays a role during end stage mTEC development (Matsumoto 2011). Indeed, involucrin+ mTEC were reduced in the absence of LTβR signaling (White et al. 2010), suggesting further crosstalk mechanisms operating during post-Aire expression in mTEC. However, it is also important to note that other studies analyzing the disruption of mTEC development in Aire−/− mice have suggested that Aire is required during earlier stages of the mTEC developmental program (Dooley et al. 2008). In this ‘Developmental Model’, Aire may be involved in regulating the developmental choice of mTEC progenitors, a process that then impacts upon TRA expression within the thymic medulla (Gillard and Farr 2005). For example, Aire controls mTEC expression of a panel of transcription factors, including Oct4 and Nanog, typically associated with progenitor cells (Gillard et al. 2007). Importantly, while these models provide distinct views on the timing of Aire expression in the mTEC lineage, they collectively highlight the importance of Aire during normal thymus medulla development. While further studies are required, recent analyses suggest that Aire expression by mTEC is limited to a single window of 1–2 days (Wang et al. 2012), which may help to provide a clearer picture of the timing and role of Aire expression in relation to early and late mTEC developmental stages.
4 Functions of the Thymic Medulla
4.1 Medullary Thymic Epithelium and Central Tolerance Induction
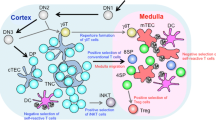
TCR gene recombination occurs in a seemingly random manner leading to the generation of a highly diverse T-cell repertoire. While this provides a clear benefit in terms of the capacity of T-cells to recognize and respond to diverse pathogenic challenge, a potential negative aspect of this mode of TCR determination lies in the generation of T-cells bearing receptors capable of both recognizing and becoming activated by self-antigen. T-cell activation against antigens expressed by tissues of the host leads to the highly undesirable outcome of T-cell orchestrated autoimmune disease. In order to combat the potentially destructive generation and subsequent escape of autoreactive T-cells into the periphery, thymic medullary microenvironments provide several layers of tolerance induction, including that of deletional tolerance, acting to purge autoreactive T-cell clones from the naïve repertoire via negative selection (Fig. 2).
Medullary thymic microenvironments regulate CD4 thymocyte maturation and selection via multiple mechanisms. Newly selected CD4+8− T-cells interact with both Aire+ mTEC and thymic dendritic cells during medullary maturation. Conventional CD4 thymocytes undergo a series of maturational steps (SP1-4), where semi-mature autoreactive T-cell clones are deleted at an immature stage (SP1-3) in part via Aire-dependent tissue restricted antigens generated and presented either directly by mTEC or indirectly via antigen transfer and presentation by thymic DC. Generation of SP4 CD4 thymocytes relies upon Aire+ mTEC and subsequent emigration occurs 4 days post-selection in an S1P-dependent manner via blood vessels at the CMJ BV. Medullary APC interaction additionally drives pT-Reg induction in a TCR- and CD80/86-dependent manner leading to the generation of Foxp3+ T-Reg that exit thymus 5 days post-selection
As described previously, the key cellular mediators of intrathymic central tolerance induction primarily constitute medullary thymic epithelium and thymic dendritic cells acting in concert. The clear requirement for medullary thymic epithelium in the induction of central tolerance via negative selection of autoreactive thymocytes is evidenced from several mutant mouse models demonstrating defective mTEC development and associated onset of autoimmune disease (Akiyama et al. 2005; Burkly et al. 1995; Nitta et al. 2011; Rossi et al. 2007). Following positive selection, maturing thymocytes demonstrate directed migration into medullary microenvironments where they spend a calculated 4–5 days scanning both mTEC and dendritic cells (McCaughtry et al. 2007). Interestingly, self-antigen presented to developing thymocytes includes multiple sources, comprising both peripheral self-antigen transported into the thymus by peripheral DC subsets and self-Ag generated from intrathymic microenvironments (Baba et al. 2009; Hadeiba et al. 2012; Klein et al. 2011).
Pivotal to the efficient role of mTEC in screening developing T-cells for autoreactive specificities is the precise array of self-antigens expressed by mTEC against which T-cells are tested for high affinity recognition and subsequent deletion. A key paradox in the intrathymic screening of TCR specificities is how T-cells, while anatomically restricted to thymic microenvironments during development, are exposed to the breadth of self-antigens normally associated with particular peripheral tissues. A series of refined experiments have gradually begun to unravel this contradiction of anatomical compartmentalization of T-cells and peripheral self-antigens. Primarily, it was discovered that mTEC possessed a highly unusual characteristic of being able to express a diverse array of antigens normally associated with defined peripheral tissues (Derbinski et al. 2001). This remarkable ability of mTEC to mimic the antigen profile of an array of different tissue types led to the search for specific molecular mechanisms regulating this unusual functional capacity. Significantly, several lines of evidence led to the discovery of the role of the transcriptional regulator Aire (Auto-Immune REgulator) in the control of mTEC peripheral tissue antigen expression. Notably, human patients demonstrating a mutation in Aire exhibit autoimmune disease, termed autoimmune polyglandular syndrome type-1 (APS-1) or autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) (Mathis and Benoist 2009). Generation of mutant mouse strains lacking fully functional Aire protein demonstrated a broadly similar spectrum of autoimmune disease manifestation, providing a useful murine model to study the role of Aire in the appearance of associated autoimmune disease (Anderson et al. 2002; Ramsey et al. 2002). Investigation of the cellular expression pattern of Aire showed it was primarily restricted to thymic tissue, and moreover intrathymic expression was limited to a sub-population of mTEC (Heino et al. 2000; Nagamine et al. 1997). Importantly, lack of Aire expression by mTEC in murine knockout models directly resulted in reduced expression of specific peripheral tissue antigens and resulted in the generation of targeted autoimmune disease (Anderson et al. 2002; Ramsey et al. 2002). Direct evidence that mTEC Aire-mediated expression of ectopic peripheral tissue antigens played a central role in thymic deletional tolerance was subsequently demonstrated whereby deletion of potentially autoreactive transgenic T-cell clones capable of recognizing pancreatic-associated self-antigen with a high degree of affinity was driven in an Aire-dependent manner (Liston et al. 2003). The exquisite sensitivity of mTEC-mediated deletion of autoreactive T-cells was later demonstrated via elegant experiments demonstrating that the reduced expression of a single restricted antigen specifically associated with ocular tissue by mTEC could manifest in highly targeted auto-immunity targeted toward the eye (DeVoss et al. 2006).
It would therefore appear clear that mTEC-mediated expression of antigens associated with peripheral tissues plays a pivotal role in the enforcement of central tolerance through the deletion of autoreactive T-cell clones. However, while Aire would appear to control the expression of a large array of peripheral tissue antigens, it is important to note that not all ectopic peripheral tissue antigen expression within mTEC is Aire-dependent (Anderson et al. 2002; Derbinski et al. 2005). Initial experiments have indicated that at a least a portion of Aire-independent antigens are influenced by signaling through the lymphotoxin pathway (Seach et al. 2008), it still remains to be determined precisely how the complete array of intrathymic TRA are regulated within the mTEC compartment.
A key question in the efficiency of thymic negative selection is posed by how large cohorts of developing T-cells are successfully screened by a relatively minor fraction of mTEC. Compounding this high ratio of thymocyte to mTEC distribution is the high selective distribution of any single given peripheral tissue antigen. In this regard, it has previously been estimated that any individual TRA is expressed by less than 5 % of total Aire-positive mTEC, which themselves comprise a minor fraction of total mTEC (Derbinski et al. 2001, 2008). In order to effectively delete autoreactive T-cell clones, several coordinated mechanisms appear to operate in order to ensure an imposition of central tolerance upon thymocytes. While mTEC are essential cellular production units for TRA a sharing of labor exists between mTEC and tDC in the presentation of self-antigen. Transfer of mTEC-derived antigen would appear to occur in a directional manner from mTEC to tDC interestingly including both intracellular and cell surface expressed antigen (Koble and Kyewski 2009). However, as yet the precise mechanism of how mTEC-derived self-antigen is transferred to DC for presentation to T-cells remains currently unknown. The absolute necessity of tDC in the contribution to negative selection is suggested both by conditional deletion of CD11c-positive cells via targeted diphtheria toxin susceptibility leading to fatal autoimmunity and absence of DC-MHC expression leading to inefficient transgenic T-cell deletion against an mTEC-associated neo-antigen (Gallegos and Bevan 2004; Ohnmacht et al. 2009). The contribution of DC to negative selection likely facilitates the spreading of particular self-antigens within thymic microenvironments, such antigen spread may particularly be of note in regard to recent studies indicating a rather anatomically restricted range of intramedullary T-cell migration (Le Borgne et al. 2009).
While transfer of antigen from mTEC to DC likely plays an important role in the spreading of peripheral tissue antigen and presentation to developing T-cells, direct presentation of antigen by mTEC additionally seems to shape the TCR repertoire. Recent experiments have demonstrated that negative selection of transgenic CD4 T-cells was impaired when MHC class II expression was selectively reduced on mTEC (Hinterberger et al. 2010). Such findings clearly imply a direct role of mTEC in autonomous presentation of antigen to T-cells, in addition to acting as a peripheral tissue antigen reservoir for co-operative transfer to tDC. Outstanding questions in relation to medullary enforcement of negative selection remain, particularly regarding the specific routes of peptide generation and loading of endogenous self-antigens into MHC II pathways in medullary thymic epithelial cells. In addition, it is interesting to note that the major fraction of thymic DC are comprised of the CD205-expressing subset, being peripherally-associated with a capacity to present exogenous antigen into both MHC class-II, and -I via cross-presentation pathways and being capable of tolerance induction (Bonifaz et al. 2002, 2004). Whether such a proportional makeup of tDC reflects a functional association with transfer of antigen from mTEC and presentation to both CD4 and CD8 T-cells remains unclear. Further, as compared to peripherally equivalent DC, thymic resident DC demonstrate an enhanced capacity for antigen cross-presentation and T-cell cross-priming in the absence of DC activating factors (Dresch et al. 2011), suggesting that thymic microenvironments may uniquely influence the efficiency of antigen presentation via as yet undefined cellular and molecular mechanisms.
4.2 Foxp3 Regulatory T-Cell Development
While it would appear clear that deletional tolerance mediated by thymic medullary cellular microenvironments is essential for the removal of newly generated autoreactive T-cell clones as described above, the effectiveness of such tolerizing mechanisms does not appear to be one hundred percent efficient. The leakiness in the efficiency of T-cell negative selection can be clearly revealed by murine models lacking T-regulatory cell (T-Reg) populations. In the absence of Foxp3-dependent T-Reg, autoreactive T-cell clones normally present in the peripheral T-cell pool become apparent, with their unopposed activation rapidly leading to the generation of catastrophic and fatal systemic autoimmunity (Kim et al. 2007). Early experimentation demonstrating fatal autoimmunity in neonatal mice, having undergone early stage thymectomy, among other data, presented initial evidence that thymic microenvironments were essential for the generation of suppressive CD4 T-cells (Josefowicz et al. 2012). Subsequently, the requirement for thymic microenvironments in development of T-Reg was found to strictly depend upon the selection of T-Reg by intrathymic self-antigen expression (Itoh et al. 1999; Jordan et al. 2001). The notion that TCR specificity may influence Foxp3+ T-Reg generation combines several pieces of evidence, including the finding that TCR usage of conventional versus regulatory T-cells indicated partially differential specificity with a low degree of overlay, at least in the context of an experimentally limited TCR repertoire (Hsieh et al. 2004; Pacholczyk et al. 2006). The question subsequently arising from the proposition that T-Reg are developmentally selected by TCR specificity for cognate self-antigen is how potential self-reactivity leads to a T-Reg fate versus the induction of apoptosis via negative selection. The primary notion in this regard involves a role for the strength of TCR self-reactivity, such that the selection of T-Reg occurs at an intermediate level between the low degrees of self-peptide:self-MHC required for positive selection and the high level of self-reactivity driving negative selection (Liston and Rudensky 2007). Directly in support of this theory, experiments utilizing microRNA-mediated MHC class II suppression in mTEC, resulting in a quantitative reduction in antigen presentation, resulted in the enhanced induction of T-Reg and a corresponding decline in negative selection (Hinterberger et al. 2010), implying that avidity plays a determining role in thymic T-Reg development. Interestingly, evidence from Nurr77-GFP mice, where levels of GFP expression correlate with intensity of TCR signal strength, indicate that thymic T-Reg would appear to experience a higher level of stimulation through their TCR than Foxp3-negative conventional T-cells (Moran et al. 2011).
The precise developmental timing of T-Reg generation has led to multiple lines of experimentation. While the induction of T-Reg was proposed to occur within the CD4+8+ fraction, being associated with cortical thymic localization and cortical cellular interactions including cTEC (Bensinger et al. 2001; Liston et al. 2008), subsequent studies have disputed the developmental significance of Foxp3+ T-Reg generated within cortically restricted CD4+8+ stages (Lee and Hsieh 2009), instead suggesting that T-Reg in the main are generated following the transition to a CD4 SP (SP) stage. The cellular interactions leading to Foxp3 Treg generation therefore likely follow CD4 SP transition into thymic medullary environments, as mediated by CCR7 guided migration (Ueno et al. 2004). While the defining hallmark of T-Reg can be considered to be Foxp3 expression, it has been previously discovered that Foxp3+CD25+ intrathymic T-Reg appear to be derived from a Foxp3−CD25+ sub-population encompassing T-Reg progenitors (pT-Reg), as demonstrated by precursor-product experiments analyzing development of pT-Reg in vivo (Lio and Hsieh 2008). Interestingly, Foxp3−CD25+ pT-Reg selected by TCR-directed interaction with self-antigen were subsequently found to develop independently of TCR stimulation following their initial specification (Lio and Hsieh 2008). While this subsequent developmental step is proposed to be TCR-independent, evidence points to a cytokine-dependence of T-Reg maturation beyond the initial Foxp3+CD25+ stage, including signaling through Il-2 and IL-15 (Burchill et al. 2007), however the precise involvement of TCR-independent signaling in directing Foxp3+ T-Reg maturation versus maintenance and survival remains unclear.
The differential ability of thymic medullary resident APC to dictate T-Reg induction has formed the basis for several experimental studies attempting to identify the key players in this important process. Primarily, both mTEC and thymic DC would appear to be able to efficiently induce T-Reg generation, as indicated by experimental systems providing selective absence and restriction of antigen expression to either population of APC (Aschenbrenner et al. 2007; Proietto et al. 2008; Spence and Green 2008). Thus, the capacity to efficiently select T-Reg does not reside within any single thymic APC population. While the ability of medullary-resident APC would seem to be promiscuous in the ability to select Foxp3 T-Reg, the antigen array responsible for selecting such cells remains unclear. The relatively small zonal territory of T-cells in medullary epithelium and their propensity to demonstrate increased dwelling time with medullary APC following recognition of cognate antigen, in a transgenic TCR model (Le Borgne et al. 2009), may fit with the interesting finding that the efficiency of intrathymic T-Reg development is highly dependent upon competition of specific T-cell clones for selecting antigen (Bautista et al. 2009). Such a balance of competition for selecting ligand in relation to induction of a T-Reg fate may play a pivotal role in determining the frequency of developing T-cells undergoing either negative selection, T-Reg fate induction, or progression as a conventional naïve T-cell. Further, the finding that the frequency of given TCR clones within the T-cell compartment is required to be below 1 % for efficient Treg generation, at least in the context of monoclonal transgenic T-cells, (Bautista et al. 2009), may suggest that a major fraction of thymic T-Reg are specifically selected by infrequently expressed antigen. Such scarce selecting antigen may potentially reflect peripheral tissue antigens expressed by mTEC, presenting the possibility that thymically derived T-Reg may display preferential specificity toward defined tissue-associated antigens rather than broadly expressed ubiquitous self-antigen. However, it should be noted that recent experiments studying the role of thymic niche availability in the regulation of T-Reg generation in a polyclonal T-cell compartment have come to the opposing conclusion that niche availability does not limit T-Reg generation (Romagnoli et al. 2012). It therefore remains unclear precisely how the proportion of T-cells entering the T-Reg pathway is intrathymically regulated.
In addition to antigen presentation, provision of co-stimulation via the CD28:CD80/86 axis plays a key role in T-Reg generation, with an absence of CD28-mediated co-stimulation leading to a highly depleted T-Reg population (Tai et al. 2005). As expected from promiscuous T-Reg induction influenced by self-antigen presentation, again expression of CD80/86 on either mTEC or hemopoietic cells, including tDC, is equally able to induce T-Reg development (Roman et al. 2010). The ability of medullary APC subsets to influence T-Reg induction may therefore depend upon their ability to present self-antigen in conjunction with defined co-stimulation, rather than perhaps being absolutely dependent upon the provision of unique cell-specific signals or self-antigen arrays limited for instance solely to mTEC. While both mTEC and tDC are able to induce T-Reg generation in a quantitative fashion, it remains unclear whether any qualitative differences exist between mTEC versus tDC specified T-Reg. In this regard, it could be speculated that the spectrum of T-Reg clones induced by mTEC interaction may differ from those generated via extrathymically derived CD8−Sirpa+ tDC which are able to transport peripheral antigen, including blood-borne antigens, into the thymus (Baba et al. 2009; Li et al. 2009). Finally, while thymic DC are globally capable of efficient T-Reg induction, in vitro studies have proposed that differences may exist in the efficiency of extrathymically CD8−Sirpa+ and intrathymically generated CD8+Sirpa− DC to induce T-Reg (Proietto et al. 2008). Of particular note, in an in vitro model, CD8−Sirpa+ DC were proposed to demonstrate a superior capacity to instruct T-Reg induction potentially by virtue of increased maturity phenotype, including MHC class II and CD80/86 expression levels, again suggesting that the ability of APC to induce signals through the TCR with a particular strength may link their ability to efficiently induce T-Reg. In addition, CD8−Sirpa+ DC are proposed to selectively produce the chemokines CCL17 and CCL22 compared to CD8+Sirpa− DC, potentially enhancing their ability to interact with newly selected CD4 SP thymocytes bearing the cognate chemokine receptor CCR4 (Proietto et al. 2008). The correct localization and ability of medullary resident APC to efficiently interact with thymocytes is further highlighted by findings in mice lacking expression of the Aire-dependent chemokine XCL1. XCL1-deficient mice demonstrate aberrant intrathymic DC positioning, albeit at normal total numbers, and display a corresponding reduction in T-Reg development with associated autoimmune disease (Lei et al. 2011). Together such findings highlight that the correct anatomical organization and positioning of medullary thymic cellular components likely plays a key role in influencing the efficiency of T-Reg development.
4.3 Post-Selection Thymocyte Differentiation
Upon entry of newly selected T-cells into medullary microenvironments, a period of medullary residency is essential to ensure sufficient screening of CD4+ and CD8+ thymocytes potentially preventing the escape of autoreactive clones into the periphery. It would therefore appear logical that specific mechanisms may operate in order to ensure that maturing SP thymocytes are retained within thymic medulla for a sustained period of time. Analysis of SP thymocyte populations, particularly CD4 thymocytes, has clearly demonstrated a distinct series of phenotypic subsets proposed to reflect differential maturational states. Following positive selection CD4 SP thymocytes were initially described to demonstrate a heterogeneous mix of phenotypes, being primarily split into an immature and mature subset based on CD24 (heat-stable antigen) and Qa-2 expression (Ramsdell et al. 1991; Vicari et al. 1994; Wilson et al. 1988). While such immature and mature SP thymocyte subsets appeared to display differential responsiveness to external stimuli, including susceptibility to negative selection being associated with immature-type SP stages (Kishimoto and Sprent 1997), evidence of progressive maturation has only recently been directly presented. Specifically, four clearly defined subsets of CD4 thymocytes, termed SP1-4, defined as CD69+Qa2−6C10− (SP1), CD69+Qa2−6C10+ (SP2), CD69−Qa2−6C10− (SP3), and CD69−Qa2+6C10− (SP4) were phenotypically identified in murine thymus. Direct in vivo injection of traceable SP1 CD4 thymocytes into adult murine thymus provided strong evidence for SP CD4 thymocyte maturation occurring in a regulated sequential fashion (Li et al. 2007).
Initial estimates of SP thymocyte dwell time within medullary microenvironments proposed a timespan in the region of 14 days (Egerton et al. 1990), although contrasting studies suggested that newly generated naïve thymocytes were found to emigrate following just 2 days post intrathymic BrdU labeling (Tough and Sprent 1994). A key question that related to this potential discrepancy in proposed length of medullary residency was how exit from thymic microenvironments was regulated. Two potential mechanisms proposed opposing models of either a random exit of SP thymocytes at multiple stages of maturation (lucky-dip) versus a linear, hierarchical mode whereby thymic exit was restricted to the most mature cells (Scollay and Godfrey 1995). Subsequent experiments utilizing a novel RAG2-GFP reporter mice, whereby GFP expression levels correlated with thymocyte maturation, directly demonstrated that SP thymocytes spent a relatively brief period of 4 days within thymic microenvironments prior to their export (McCaughtry et al. 2007). In addition, it was further substantiated that thymic egress was found to be limited to the most mature SP thymocytes (Li et al. 2007; McCaughtry et al. 2007). Such a relatively short time of SP thymocyte medullary habitation presumably equates to a highly efficient process of autoreactive T-cell screening against correspondingly rare cognate self-antigen, including peripheral tissue antigens. In addition, the window for negative selection would appear to be even shorter than the 4-day intramedullary window, assuming that susceptibility to negative selection is enhanced within immature SP CD4 thymocytes defined by CD24hi (Kishimoto and Sprent 1997), again further narrowing the time frame in which negative selection is effective (Weinreich and Hogquist 2008). In direct relation to the efficiency of thymocyte negative selection, titration experiments using reaggregate thymic organ culture techniques demonstrated that thymic DC are still able to mediate efficient negative selection even at 1 % of total cell numbers, emphasizing the efficiency of DC as potent mediators of negative selection (Anderson et al. 1998), potentially demonstrating the highly efficient nature whereby autoreactive T-cell clones can be screened within thymic medullary microenvironments. However, it is also possible that the relatively tight temporal availability of negative selection susceptibility may correspond with the potential escape of autoreactive T-cell clones into the peripheral repertoire as may occur in Treg-deficient mice (Kim et al. 2007). The extent to which autoreactive T-cells are able to escape negative selection in the adult steady-state thymus warrants further investigation, further, whether extended medullary dwell time of developing thymocytes at a negative selection susceptible stage could influence the efficiency of negative selection poses an additional point of interest. Of note, a recent study has demonstrated an increased intrathymic dwell time for newly generated T-Reg compared to conventional T-cells (Romagnoli et al. 2012). The mechanisms responsible for this discrepancy between these two related T-cell sub-lineages remain unknown, as does the functional significance, if any, of this phenomenon.
If maturation and exit of SP thymocytes is dependent upon a linear, hierarchical model, it follows that specific mechanisms must tightly regulate the selective ability of the most mature thymocytes to be released into the periphery. Indeed, the ability of thymocytes to exit thymus into the periphery was clearly shown to be highly dependent upon the action of the transcription factors Foxo1 and KLF2 (Bai et al. 2007; Carlson et al. 2006; Kerdiles et al. 2009) at least in part, regulating the expression of the cell surface receptor S1PR1 (Allende et al. 2004; Matloubian et al. 2004). Notably, expression of S1PR1 directs chemoattraction toward a gradient of S1P predominantly present in blood but also potentially produced by vascular endothelium and modulated by perivascular cells in the thymus leading to the observed exit of mature thymocytes at blood vessels located at the cortico-medullary junction (Pham et al. 2010; Zachariah and Cyster 2010). In direct correlation with the progressive maturation of SP1 > SP4 thymocytes, gradual increased expression of the previously mentioned factors, including S1PR1, has been recently reported (Teng et al. 2011). Of interest, while it seems apparent that the most mature SP thymocytes selectively exit the thymus, recent thymus emigrants still appear to require further maturation events in the periphery, as demonstrated by progressive Qa-2 upregulation and a notable decrease in proliferative response as compared to more mature naïve T-cells (Boursalian et al. 2004). Whether the developmental prompts facilitating such final maturation post-thymic exits are unique to peripheral environments or are shared with those of the thymic medulla is yet to be fully determined.
Assuming that ordered SP thymocyte maturation correspondingly determines the regulated exit of mature cells as described previously, it is of particular interest to determine whether this linear development occurs via thymocyte-autonomous process or is influenced by external medullary microenvironmental factors. Intrathymic injection of SP1 thymocytes into adult thymus has been observed to follow a maturation time of 2–3 days (Li et al. 2007), however it was also reported that a subset of mature SP4 thymocytes were found to reside within the host thymus for a period up to 7 days (Li et al. 2007), suggesting that while SP maturation correlates with a functional capacity to emigrate, additional influences extrinsic to thymocytes may impose upon the timing of thymocyte exit.
In relation to the question surrounding control of SP maturation, interesting in vitro data provided evidence that isolated immature SP1 thymocytes were capable of progression through maturation stages SP1 > SP2 > SP3 in the absence of additional cellular support, including mTEC, however IL-7 was found to be critical for the survival of such isolated cells in vitro (Li et al. 2007). However, investigation of this potential regulatory axis revealed that absence of functional IL-7 signaling in vivo did not lead to an impairment in the maturation of post-selection SP thymocytes (Weinreich et al. 2011), suggesting that while IL-7 is sufficient to facilitate SP survival it would not appear to be essential in order to drive differentiation and maturation. Further analysis of the thymic microenvironment-dependent developmental requirements for SP3 > SP4 transition identified a stage-specific requirement for medullary microenvironments as RelB-deficient mice (Burkly et al. 1995), that display a drastic impairment in mTEC generation, were found to lack SP3 > SP4 development (Li et al. 2007). In addition, analysis of Aire-deficient mice again revealed defective generation of the final stage of SP4 maturation. However, it remains unclear precisely how Aire+ mTEC influence SP4 thymocyte maturation.
Aire expression, in addition to regulating ectopic peripheral antigen expression, also appears to control additional aspects of mTEC biology, including chemokine expression such as CCR7 and CCR4 ligands (Laan et al. 2009) potentially influencing the capacity of developing SP thymocytes to localize and interact with mTEC. While Aire regulates chemokines associated with the attraction of mature thymocytes, it also controls the expression of DC attractants including XCL1, which play an essential role in regulating the efficiency of DC-mediated thymocyte selection and interaction (Lei et al. 2011). Further, while RelB-deficient mice display mTEC-intrinsic defects, DC also demonstrate altered representation within thymic microenvironments both due to mTEC-deficiencies and due to a DC-intrinsic influence of RelB (Burkly et al. 1995; Wu et al. 1998). Further experiments investigating the complex cellular interplay required for regulated SP thymocyte maturation leading to efficient deletional tolerance, Treg induction, and appropriate thymocyte maturation leading to tightly regulated thymic export will provide valuable insights into our current understanding of how medullary thymic microenvironments function.
5 Conclusions
The thymic medulla represents a key site in intrathymic αβT-cell development, by controlling the fate of thymocytes that have undergone positive selection in the thymic cortex. The induction of T-cell tolerance in the medulla is controlled by multiple mechanisms: central tolerance results in the elimination of autoreactive αβTCR specificities, while the development of Foxp3+ Regulatory T-cells ensures that dominant tolerance can occur within peripheral body tissues. An increasing body of evidence supports the idea that these mechanisms of T-cell tolerance require both medullary epithelial cells, including the Aire+ subset, and thymic dendritic cells that act in concert to shape the developing T-cell receptor repertoire. While some aspects of thymus medulla function are beginning to be defined, further studies are required to investigate several key aspects that remain poorly understood, including the identity and requirements of mTEC progenitors, the role of Aire in thymus medulla organization and mTEC development, and the importance of mTEC in post-selection maturation of conventional αβT-cells. Perhaps most importantly, how the medulla controls the balance between negative selection and T-Reg production that ultimately results in a self-tolerant state is poorly understood. Gaining a better understanding of these features of thymic medulla function should help in identifying the cellular and molecular basis of T-cell mediated autoimmunity, and could inform future therapeutic strategies aimed at its treatment. A recent study from our laboratory has shown that medullary thymic epithelial cells are essential for the development of Foxp3+ T-Reg but are not required for continued development of conventional CD4+ thymocytes (Cowan et al 2013)
References
Adkins B, Gandour D, Strober S, Weissman I (1988) Total lymphoid irradiation leads to transient depletion of the mouse thymic medulla and persistent abnormalities among medullary stromal cells. J Immunol 140:3373–3379
Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J (2005) Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science 308:248–251
Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J (2008) The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29:423–437
Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279:52487–52492
Alves NL, Huntington ND, Rodewald HR, Di Santo JP (2009) Thymic epithelial cells: the multi-tasking framework of the T cell “cradle”. Trends Immunol 30:468–474
Anderson G, Takahama Y (2012) Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol 33:256–263
Anderson G, Partington KM, Jenkinson EJ (1998) Differential effects of peptide diversity and stromal cell type in positive and negative selection in the thymus. J Immunol 161:6599–6603
Anderson M, Anderson SK, Farr AG (2000) Thymic vasculature: organizer of the medullary epithelial compartment? Int Immunol 12:1105–1110
Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D (2002) Projection of an immunological self shadow within the thymus by the aire protein. Science 298:1395–1401
Anderson G, Lane PJ, Jenkinson EJ (2007) Generating intrathymic microenvironments to establish T-cell tolerance. Nat Rev Immunol 7:954–963
Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L (2007) Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8:351–358
Baba T, Nakamoto Y, Mukaida N (2009) Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol 183:3053–3063
Bai A, Hu H, Yeung M, Chen J (2007) Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol 178:7632–7639
Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS (2009) Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol 10:610–617
Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM (2001) Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med 194:427–438
Blackburn CC, Augustine CL, Li R, Harvey RP, Malin MA, Boyd RL, Miller JF, Morahan G (1996) The nu gene acts cell-autonomously and is required for differentiation of thymic epithelial progenitors. Proc Natl Acad Sci U S A 93:5742–5746
Bleul CC, Corbeaux T, Reuter A, Fisch P, Monting JS, Boehm T (2006) Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature 441:992–996
Boehm T, Scheu S, Pfeffer K, Bleul CC (2003) Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med 198:757–769
Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM (2002) Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 196:1627–1638
Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM (2004) In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 199:815–824
Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ (2004) Continued maturation of thymic emigrants in the periphery. Nat Immunol 5:418–425
Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA (2007) IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 178:280–290
Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D (1995) Expression of relB is required for the development of thymic medulla and dendritic cells. Nature 373:531–536
Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC (2006) Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442:299–302
Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX (2003) Lymphotoxin pathway directs thymic Aire expression. Nat Immunol 4:1121–1127
Chin RK, Zhu M, Christiansen PA, Liu W, Ware C, Peltonen L, Zhang X, Guo L, Han S, Zheng B, Fu YX (2006) Lymphotoxin pathway-directed, autoimmune regulator-independent central tolerance to arthritogenic collagen. J Immunol 177:290–297
Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G (2013) The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med 210:675–681
Cupedo T, Kraal G, Mebius RE (2002) The role of CD45+CD4+CD3− cells in lymphoid organ development. Immunol Rev 189:41–50
Derbinski J, Kyewski B (2005) Linking signalling pathways, thymic stroma integrity and autoimmunity. Trends Immunol 26:503–506
Derbinski J, Schulte A, Kyewski B, Klein L (2001) Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol 2:1032–1039
Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B (2005) Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med 202:33–45
Derbinski J, Pinto S, Rosch S, Hexel K, Kyewski B (2008) Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci U S A 105:657–662
Desanti GE, Cowan JE, Baik S, Parnell SM, White AJ, Penninger JM, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G (2012) Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J Immunol 189:5519–5526
DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS (2006) Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med 203:2727–2735
Dooley J, Erickson M, Farr AG (2008) Alterations of the medullary epithelial compartment in the Aire-deficient thymus: Implications for programs of thymic epithelial differentiation. J Immunol 181:5225–5232
Dresch C, Ackermann M, Vogt B, de Andrade Pereira B, Shortman K, Fraefel C (2011) Thymic but not splenic CD8(+) DCs can efficiently cross-prime T cells in the absence of licensing factors. Eur J Immunol 41:2544–2555
Drumea-Mirancea M, Wessels JT, Muller CA, Essl M, Eble JA, Tolosa E, Koch M, Reinhardt DP, Sixt M, Sorokin L, Stierhof YD, Schwarz H, Klein G (2006) Characterization of a conduit system containing laminin-5 in the human thymus: a potential transport system for small molecules. J Cell Sci 119:1396–1405
Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR (2012) Interleukin-22 drives endogenous thymic regeneration in mice. Science 336:91–95
Egerton M, Scollay R, Shortman K (1990) Kinetics of mature T-cell development in the thymus. Proc Natl Acad Sci U S A 87:2579–2582
Farr AG, Anderson SK (1985) Epithelial heterogeneity in the murine thymus: fucose-specific lectins bind medullary epithelial cells. J Immunol 134:2971–2977
Farr AG, Braddy SC (1989) Patterns of keratin expression in the murine thymus. Anat Rec 224:374–378
Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M (2008) Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol 180:3183–3189
Gabler J, Arnold J, Kyewski B (2007) Promiscuous gene expression and the developmental dynamics of medullary thymic epithelial cells. Eur J Immunol 37:3363–3372
Gallegos AM, Bevan MJ (2004) Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med 200:1039–1049
Gillard GO, Farr AG (2005) Contrasting models of promiscuous gene expression by thymic epithelium. J Exp Med 202:15–19
Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG (2007) Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J Immunol 178:3007–3015
Godfrey DI, Izon DJ, Tucek CL, Wilson TJ, Boyd RL (1990) The phenotypic heterogeneity of mouse thymic stromal cells. Immunology 70:66–74
Gray DH, Abramson J, Benoist C, Mathis D (2007) Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med 204:2521–2528
Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL (2006) Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108:3777–3785
Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D (2009) Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med 206:1245–1252
Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC (2012) Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity 36:438–450
Hamazaki Y, Fujita H, Kobayashi T, Choi Y, Scott HS, Matsumoto M, Minato N (2007) Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol 8:304–311
Heino M, Peterson P, Sillanpaa N, Guerin S, Wu L, Anderson G, Scott HS, Antonarakis SE, Kudoh J, Shimizu N, Jenkinson EJ, Naquet P, Krohn KJ (2000) RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur J Immunol 30:1884–1893
Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y (2008) The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29:438–450
Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L (2010) Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol 11:512–519
Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY (2004) Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 21:267–277
Hubert FX, Kinkel SA, Webster KE, Cannon P, Crewther PE, Proeitto AI, Wu L, Heath WR, Scott HS (2008) A specific anti-Aire antibody reveals aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol 180:3824–3832
Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W (2008) Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29:451–463
Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S (1999) Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 162:5317–5326
Izon DJ, Nieland JD, Godfrey DI, Boyd RL, Kruisbeek AM (1994) Flow cytometric analysis reveals unexpected shared antigens between histologically defined populations of thymic stromal cells. Int Immunol 6:31–39
Jenkinson WE, Jenkinson EJ, Anderson G (2003) Differential requirement for mesenchyme in the proliferation and maturation of thymic epithelial progenitors. J Exp Med 198:325–332
Jenkinson WE, Rossi SW, Parnell SM, Jenkinson EJ, Anderson G (2007) PDGFRalpha-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood 109:954–960
Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ (2001) Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2:301–306
Josefowicz SZ, Lu LF, Rudensky AY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30:531–564
Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, Takahama Y, Sakaguchi S, Mitani T, Matsumoto M (2004) NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol 172:2067–2075
Kanariou M, Huby R, Ladyman H, Colic M, Sivolapenko G, Lampert I, Ritter M (1989) Immunosuppression with cyclosporin A alters the thymic microenvironment. Clin Exp Immunol 78:263–270
Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM (2009) Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol 10:176–184
Kim JM, Rasmussen JP, Rudensky AY (2007) Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8:191–197
Kishimoto H, Sprent J (1997) Negative selection in the thymus includes semimature T cells. J Exp Med 185:263–271
Klein L, Hinterberger M, von Rohrscheidt J, Aichinger M (2011) Autonomous versus dendritic cell-dependent contributions of medullary thymic epithelial cells to central tolerance. Trends Immunol 32:188–193
Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER (1998) Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci U S A 95:11822–11827
Klug DB, Carter C, Gimenez-Conti IB, Richie ER (2002) Thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J Immunol 169:2842–2845
Koble C, Kyewski B (2009) The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med 206:1505–1513
Laan M, Kisand K, Kont V, Moll K, Tserel L, Scott HS, Peterson P (2009) Autoimmune regulator deficiency results in decreased expression of CCR4 and CCR7 ligands and in delayed migration of CD4+ thymocytes. J Immunol 183:7682–7691
Ladi E, Schwickert TA, Chtanova T, Chen Y, Herzmark P, Yin X, Aaron H, Chan SW, Lipp M, Roysam B, Robey EA (2008) Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. J Immunol 181:7014–7023
Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK, Robey EA (2009) The impact of negative selection on thymocyte migration in the medulla. Nat Immunol 10:823–830
Lee HM, Hsieh CS (2009) Rare development of Foxp3+ thymocytes in the CD4+CD8+ subset. J Immunol 183:2261–2266
Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt Rde W, Nitta T, Takahama Y (2011) Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med 208:383–394
Li J, Li Y, Yao JY, Jin R, Zhu MZ, Qian XP, Zhang J, Fu YX, Wu L, Zhang Y, Chen WF (2007) Developmental pathway of CD4+CD8− medullary thymocytes during mouse ontogeny and its defect in Aire−/− mice. Proc Natl Acad Sci U S A 104:18175–18180
Li J, Park J, Foss D, Goldschneider I (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med 206:607–622
Lio CW, Hsieh CS (2008) A two-step process for thymic regulatory T cell development. Immunity 28:100–111
Liston A, Rudensky AY (2007) Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol 19:176–185
Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC (2003) Aire regulates negative selection of organ-specific T cells. Nat Immunol 4:350–354
Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY (2008) Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A 105:11903–11908
Manley NR, Blackburn CC (2003) A developmental look at thymus organogenesis: where do the non-hematopoietic cells in the thymus come from? Curr Opin Immunol 15:225–232
Martins VC, Boehm T, Bleul CC (2008) Ltbetar signaling does not regulate Aire-dependent transcripts in medullary thymic epithelial cells. J Immunol 181:400–407
Mathis D, Benoist C (2009) Aire. Annu Rev Immunol 27:287–312
Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427:355–360
Matsumoto M (2011) Contrasting models for the roles of Aire in the differentiation program of epithelial cells in the thymic medulla. Eur J Immunol 41:12–17
McCaughtry TM, Wilken MS, Hogquist KA (2007) Thymic emigration revisited. J Exp Med 204:2513–2520
Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA (2011) T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 208:1279–1289
Mori K, Itoi M, Tsukamoto N, Kubo H, Amagai T (2007) The perivascular space as a path of hematopoietic progenitor cells and mature T cells between the blood circulation and the thymic parenchyma. Int Immunol 19:745–753
Muller SM, Terszowski G, Blum C, Haller C, Anquez V, Kuschert S, Carmeliet P, Augustin HG, Rodewald HR (2005) Gene targeting of VEGF-A in thymus epithelium disrupts thymus blood vessel architecture. Proc Natl Acad Sci U S A 102:10587–10592
Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR (2008) Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol 180:5344–5351
Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N (1997) Positional cloning of the APECED gene. Nat Genet 17:393–398
Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T (1994) New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 372:103–107
Nehls M, Kyewski B, Messerle M, Waldschutz R, Schuddekopf K, Smith AJ, Boehm T (1996) Two genetically separable steps in the differentiation of thymic epithelium. Science 272:886–889
Nelson AJ, Dunn RJ, Peach R, Aruffo A, Farr AG (1996) The murine homolog of human Ep-CAM, a homotypic adhesion molecule, is expressed by thymocytes and thymic epithelial cells. Eur J Immunol 26:401–408
Nishikawa Y, Hirota F, Yano M, Kitajima H, Miyazaki J, Kawamoto H, Mouri Y, Matsumoto M (2010) Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med 207:963–971
Nitta T, Ohigashi I, Nakagawa Y, Takahama Y (2011) Cytokine crosstalk for thymic medulla formation. Curr Opin Immunol 23:190–197
Nowell CS, Bredenkamp N, Tetelin S, Jin X, Tischner C, Vaidya H, Sheridan JM, Stenhouse FH, Heussen R, Smith AJ, Blackburn CC (2011) Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet 7:e1002348
Odaka C (2009) Localization of mesenchymal cells in adult mouse thymus: their abnormal distribution in mice with disorganization of thymic medullary epithelium. J Histochem Cytochem 57:373–382
Odaka C, Morisada T, Oike Y, Suda T (2006) Distribution of lymphatic vessels in mouse thymus: immunofluorescence analysis. Cell Tissue Res 325:13–22
Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D (2009) Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med 206:549–559
Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L (2006) Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity 25:249–259
Palmer DB, Viney JL, Ritter MA, Hayday AC, Owen MJ (1993) Expression of the alpha beta T-cell receptor is necessary for the generation of the thymic medulla. Dev Immunol 3:175–179
Petrie HT, Zuniga-Pflucker JC (2007) Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol 25:649–679
Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG (2010) Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 207:17–27
Porritt HE, Gordon K, Petrie HT (2003) Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med 198:957–962
Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L (2008) Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A 105:19869–19874
Ramsdell F, Jenkins M, Dinh Q, Fowlkes BJ (1991) The majority of CD4+8− thymocytes are functionally immature. J Immunol 147:1779–1785
Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L (2002) Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet 11:397–409
Ripen AM, Nitta T, Murata S, Tanaka K, Takahama Y (2011) Ontogeny of thymic cortical epithelial cells expressing the thymoproteasome subunit beta5t. Eur J Immunol 41:1278–1287
Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, Cunningham AF, Paolino M, Penninger JM, Simon AK, Nitta T, Ohigashi I, Takahama Y, Caamano JH, Hayday AC, Lane PJ, Jenkinson EJ, Anderson G (2012) Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity 36:427–437
Rodewald HR (2008) Thymus organogenesis. Annu Rev Immunol 26:355–388
Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C (2001) Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature 414:763–768
Romagnoli P, Dooley J, Enault G, Vicente R, Malissen B, Liston A, van Meerwijk JP (2012) The thymic niche does not limit development of the naturally diverse population of mouse regulatory T lymphocytes. J Immunol 189:3831–3837
Roman E, Shino H, Qin FX, Liu YJ (2010) Cutting edge: Hematopoietic-derived APCs select regulatory T cells in thymus. J Immunol 185:3819–3823
Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ (2006) Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature 441:988–991
Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, Lane PJ, Anderson G (2007) RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 204:1267–1272
Scollay R, Godfrey DI (1995) Thymic emigration: conveyor belts or lucky dips? Immunol Today 16:268–273, discussion 273–274
Seach N, Ueno T, Fletcher AL, Lowen T, Mattesich M, Engwerda CR, Scott HS, Ware CF, Chidgey AP, Gray DH, Boyd RL (2008) The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J Immunol 180:5384–5392
Seach N, Wong K, Hammett M, Boyd RL, Chidgey AP (2012) Purified enzymes improve isolation and characterization of the adult thymic epithelium. J Immunol Methods 385:23–34
Shakib S, Desanti GE, Jenkinson WE, Parnell SM, Jenkinson EJ, Anderson G (2009) Checkpoints in the development of thymic cortical epithelial cells. J Immunol 182:130–137
Shores EW, Van Ewijk W, Singer A (1991) Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. Eur J Immunol 21:1657–1661
Shores EW, Van Ewijk W, Singer A (1994) Maturation of medullary thymic epithelium requires thymocytes expressing fully assembled CD3-TCR complexes. Int Immunol 6:1393–1402
Sitnik KM, Kotarsky K, White AJ, Jenkinson WE, Anderson G, Agace WW (2012) Mesenchymal cells regulate retinoic acid receptor-dependent cortical thymic epithelial cell homeostasis. J Immunol 188:4801–4809
Spence PJ, Green EA (2008) Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A 105:973–978
Surh CD, Ernst B, Sprent J (1992) Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J Exp Med 176:611–616
Tai X, Cowan M, Feigenbaum L, Singer A (2005) CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 6:152–162
Takahama Y (2006) Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 6:127–135
Teng F, Zhou Y, Jin R, Chen Y, Pei X, Liu Y, Dong J, Wang W, Pang X, Qian X, Chen WF, Zhang Y, Ge Q (2011) The molecular signature underlying the thymic migration and maturation of TCRalphabeta+ CD4+ CD8 thymocytes. PLoS One 6:e25567
Tough DF, Sprent J (1994) Turnover of naive- and memory-phenotype T cells. J Exp Med 179:1127–1135
Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y (2004) CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med 200:493–505
van Ewijk W, Shores EW, Singer A (1994) Crosstalk in the mouse thymus. Immunol Today 15:214–217
Van Vliet E, Melis M, Van Ewijk W (1984) Monoclonal antibodies to stromal cell types of the mouse thymus. Eur J Immunol 14:524–529
Venanzi ES, Gray DH, Benoist C, Mathis D (2007) Lymphotoxin pathway and Aire influences on thymic medullary epithelial cells are unconnected. J Immunol 179:5693–5700
Vicari A, Abehsira-Amar O, Papiernik M, Boyd RL, Tucek CL (1994) MTS-32 monoclonal antibody defines CD4+8− thymocyte subsets that differ in their maturation level, lymphokine secretion, and selection patterns. J Immunol 152:2207–2213
Wang X, Laan M, Bichele R, Kisand K, Scott HS, Peterson P (2012) Post-Aire maturation of thymic medullary epithelial cells involves selective expression of keratinocyte-specific autoantigens. Front Immunol 3:19
Weih F, Caamano J (2003) Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev 195:91–105
Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R (1995) Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell 80:331–340
Weinreich MA, Hogquist KA (2008) Thymic emigration: when and how T cells leave home. J Immunol 181:2265–2270
Weinreich MA, Jameson SC, Hogquist KA (2011) Postselection thymocyte maturation and emigration are independent of IL-7 and ERK5. J Immunol 186:1343–1347
White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, Jenkinson EJ, Anderson G (2008) Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct-cellular input. Eur J Immunol 38:942–947
White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, Narendran P, Pfeffer K, Nitta T, Takahama Y, Caamano JH, Lane PJ, Jenkinson EJ, Anderson G (2010) Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol 185:4769–4776
Wilson A, Day LM, Scollay R, Shortman K (1988) Subpopulations of mature murine thymocytes: properties of CD4−CD8+ and CD4+CD8− thymocytes lacking the heat-stable antigen. Cell Immunol 117:312–326
Wu L, D’Amico A, Winkel KD, Suter M, Lo D, Shortman K (1998) RelB is essential for the development of myeloid-related CD8alpha− dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity 9:839–847
Yamazaki H, Sakata E, Yamane T, Yanagisawa A, Abe K, Yamamura KI, Hayashi SI, Kunisada T (2005) Presence and distribution of neural crest-derived cells in the murine developing thymus and their potential for differentiation. Int Immunol 17:549–558
Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, Ikawa T, Satoh R, Kawamoto H, Mouri Y, Matsumoto M (2008) Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med 205:2827–2838
Zachariah MA, Cyster JG (2010) Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 328:1129–1135
Zhu M, Chin RK, Tumanov AV, Liu X, Fu YX (2007) Lymphotoxin beta receptor is required for the migration and selection of autoreactive T cells in thymic medulla. J Immunol 179:8069–8075
Zuklys S, Balciunaite G, Agarwal A, Fasler-Kan E, Palmer E, Hollander GA (2000) Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED). J Immunol 165:1976–1983
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Anderson, G. et al. (2013). Mechanisms of Thymus Medulla Development and Function. In: Boehm, T., Takahama, Y. (eds) Thymic Development and Selection of T Lymphocytes. Current Topics in Microbiology and Immunology, vol 373. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2013_320
Download citation
DOI: https://doi.org/10.1007/82_2013_320
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40251-7
Online ISBN: 978-3-642-40252-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)